Less Is More: Seagrass Restoration Success Using Less Vegetation per Area
Abstract
:1. Introduction
2. Materials and Methods
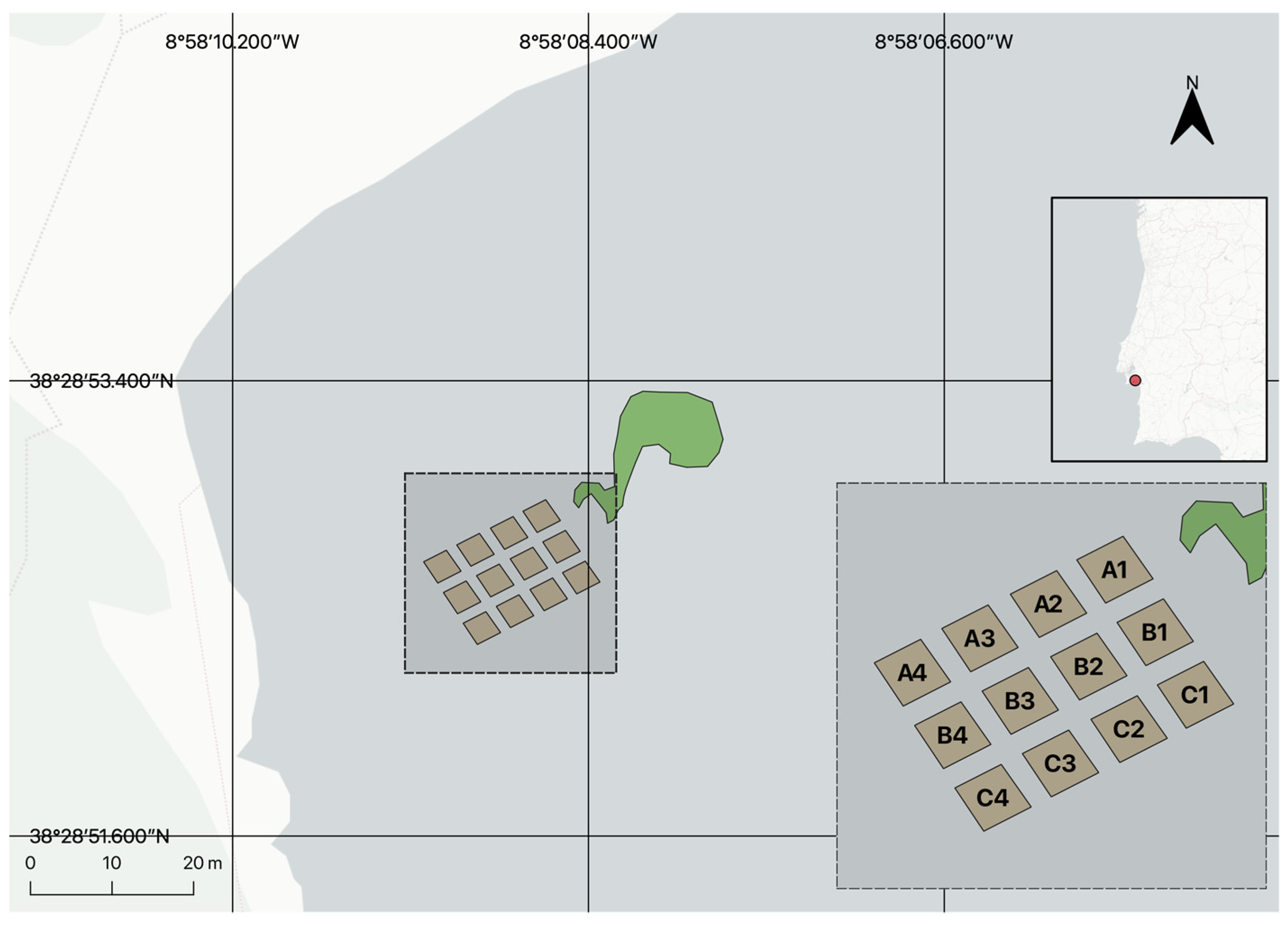
2.1. Site Description
2.2. Donor Population
2.3. Harvest Method and Transportation
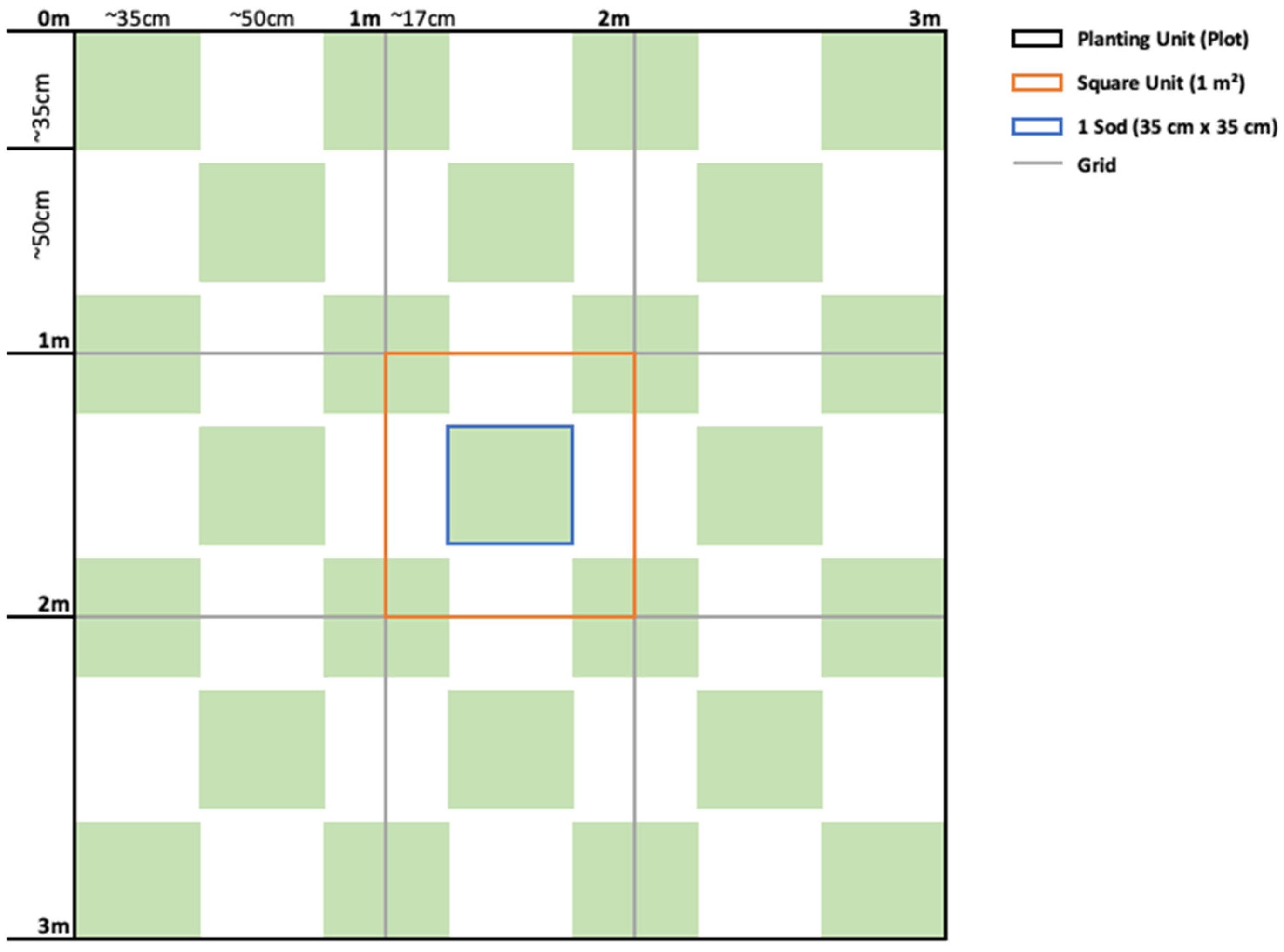
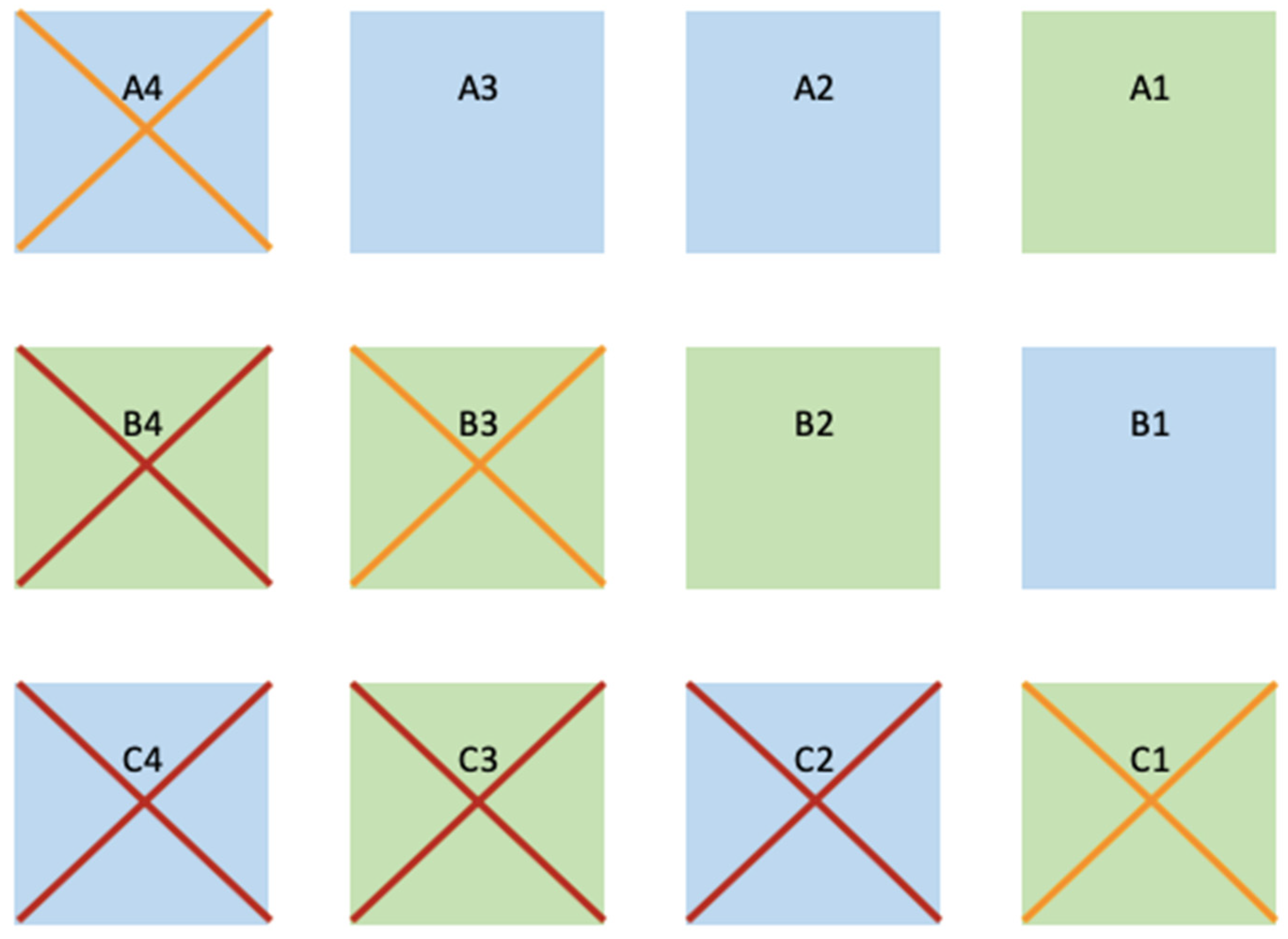
2.4. Transplant Methodology
2.5. Monitoring and Statistics
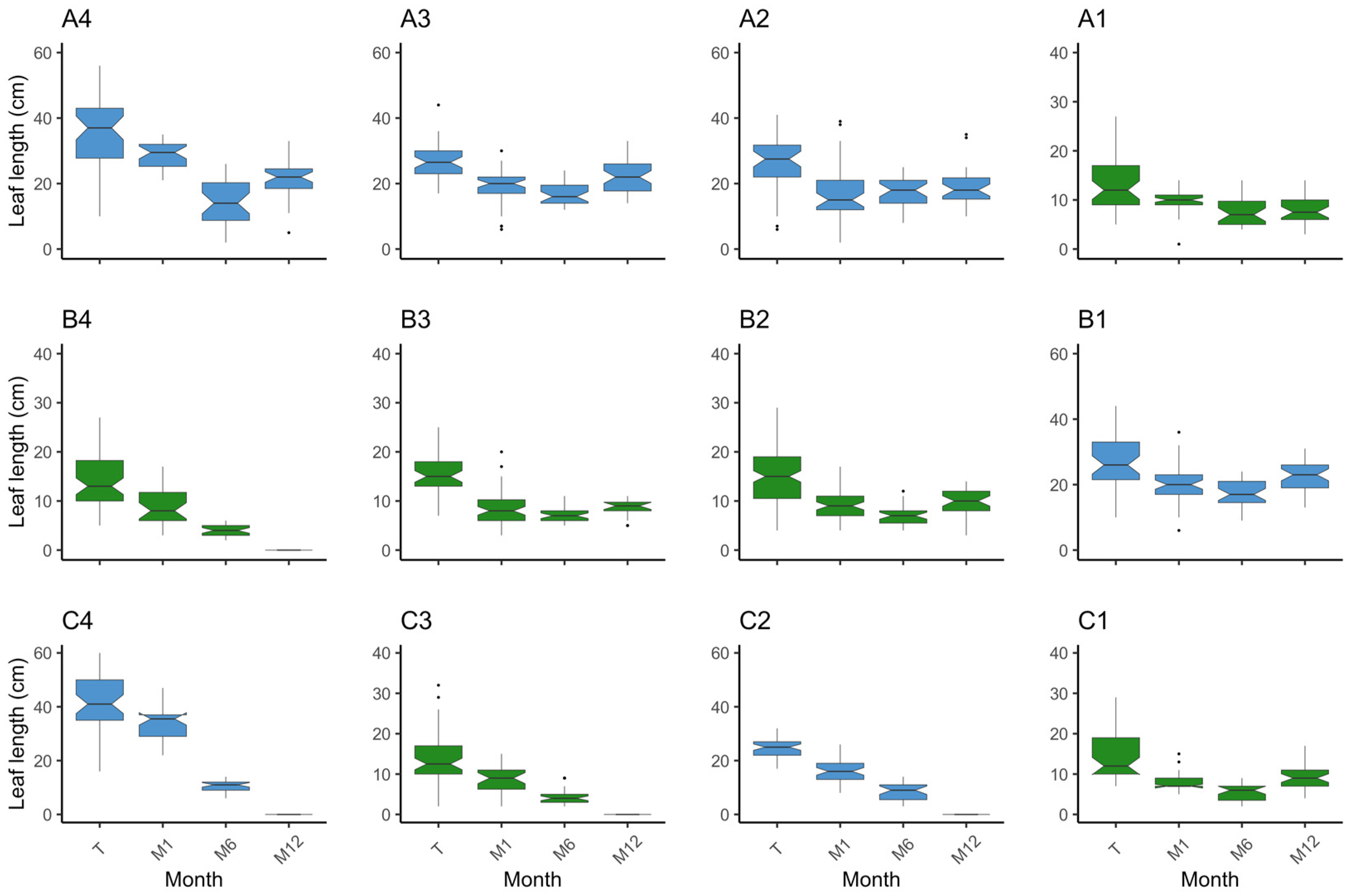
3. Results
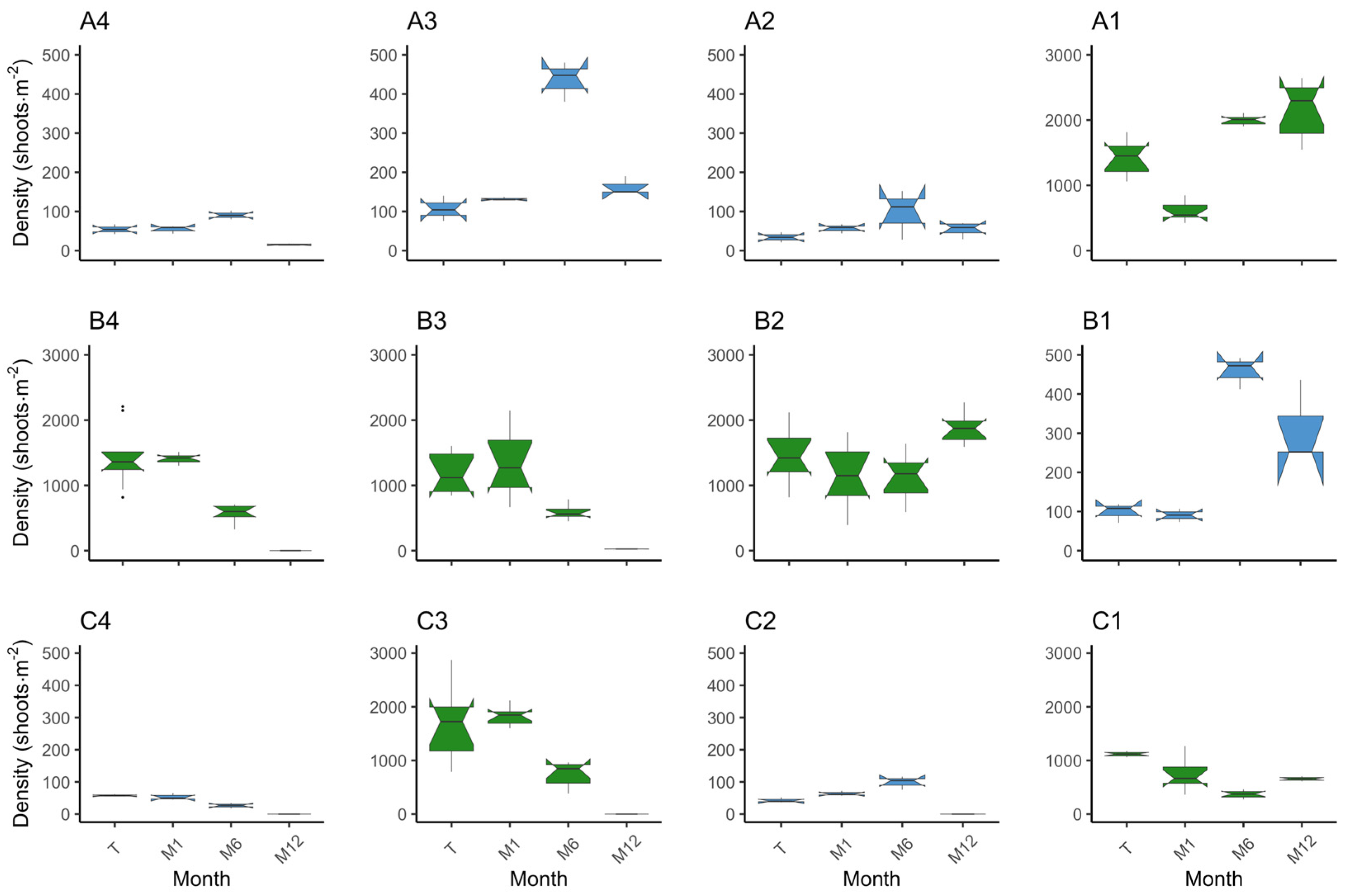
3.1. Plot Survival and Outcome
3.1.1. Z. marina
3.1.2. Z. noltei
3.2. Species Comparison on Methodology Success
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cullen-Unsworth, L.C.; Unsworth, R.K.F. Strategies to Enhance the Resilience of the World’s Seagrass Meadows. J. Appl. Ecol. 2016, 53, 967–972. [Google Scholar] [CrossRef]
- Green, E.P.; Short, F.T. World Atlas of Seagrasses, 1st ed.; University of California Press: Berkeley, CA, USA, 2003; p. 21. [Google Scholar]
- Cullen-Unsworth, L.C.; Nordlund, L.M.; Paddock, J.; Baker, S.; McKenzie, L.J.; Unsworth, R.K.F. Seagrass Meadows Globally as a Coupled Social-Ecological System: Implications for Human Wellbeing. Mar. Pollut. Bull. 2014, 83, 387–397. [Google Scholar] [CrossRef] [PubMed]
- Nordlund, L.M.; Koch, E.W.; Barbier, E.B.; Creed, J.C. Seagrass Ecosystem Services and Their Variability across Genera and Geographical Regions. PLoS ONE 2016, 11, e0163091. [Google Scholar] [CrossRef]
- Duarte, C.M.; Losada, I.J.; Hendriks, I.E.; Mazarrasa, I.; Marbà, N. The Role of Coastal Plant Communities for Climate Change Mitigation and Adaptation. Nat. Clim. Chang. 2013, 3, 961–968. [Google Scholar] [CrossRef]
- Fourqurean, J.W.; Duarte, C.M.; Kennedy, H.; Marbà, N.; Holmer, M.; Mateo, M.A.; Apostolaki, E.T.; Kendrick, G.A.; Krause-Jensen, D.; McGlathery, K.J.; et al. Seagrass Ecosystems as a Globally Significant Carbon Stock. Nat. Geosci. 2012, 5, 505–509. [Google Scholar] [CrossRef]
- Short, F.T.; Polidoro, B.; Livingstone, S.R.; Carpenter, K.E.; Bandeira, S.; Bujang, J.S.; Calumpong, H.P.; Carruthers, T.J.B.; Coles, R.G.; Dennison, W.C.; et al. Extinction Risk Assessment of the World’s Seagrass Species. Biol. Conserv. 2011, 144, 1961–1971. [Google Scholar] [CrossRef]
- Waycott, M.; Duarte, C.M.; Carruthers, T.J.B.; Orth, R.J.; Dennison, W.C.; Olyarnik, S.; Calladine, A.; Fourqurean, J.W.; Heck, K.L.; Hughes, A.R.; et al. Accelerating Loss of Seagrasses across the Globe Threatens Coastal Ecosystems. Proc. Natl. Acad. Sci. USA 2009, 106, 12377–12381. [Google Scholar] [CrossRef]
- Curiel, D.; Pavelić, S.K.; Kovačev, A.; Miotti, C.; Rismondo, A. Marine Seagrasses Transplantation in Confined and Coastal Adriatic Environments: Methods and Results. Water 2021, 13, 2289. [Google Scholar] [CrossRef]
- Tan, Y.M.; Dalby, O.; Kendrick, G.A.; Statton, J.; Sinclair, E.A.; Fraser, M.W.; Macreadie, P.I.; Gillies, C.L.; Coleman, R.A.; Waycott, M.; et al. Seagrass Restoration Is Possible: Insights and Lessons from Australia and New Zealand. Front. Mar. Sci. 2020, 7, 617. [Google Scholar] [CrossRef]
- Calumpong, H.P.; Fonseca, M.S. Seagrass transplantation and other seagrass restoration methods. In Global Seagrass Research Methods, 1st ed.; Short, F.T., Coles, R.G., Eds.; Elsevier Science B.V.: Amsterdam, The Netherlands, 2001; Volume 1, pp. 425–443. [Google Scholar]
- Paling, E.I.; Fonseca, M.; van Katwijk, M.M.; van Keulen, M. Seagrass Restoration. In Coastal Wetlands: An Integrated Ecosystem Approach, 1st ed.; Perillo, G., Wolanski, E., Cahoon, D., Brinson, M., Eds.; Elsevier Science: Amsterdam, The Netherlands, 2009; pp. 687–713. ISBN 978-008-093-213-2. [Google Scholar]
- Paling, E.I.; van Keulen, M.; Wheeler, K.D.; Phillips, J.; Dyhrberg, R. Influence of Spacing on Mechanically Transplanted Seagrass Survival in a High Wave Energy Regime. Restor. Ecol. 2003, 11, 56–61. [Google Scholar] [CrossRef]
- Paulo, D.; Cunha, A.H.; Boavida, J.; Serrão, E.A.; Gonçalves, E.J.; Fonseca, M. Open Coast Seagrass Restoration. Can We Do It? Large Scale Seagrass Transplants. Front. Mar. Sci. 2019, 6, 52. [Google Scholar] [CrossRef]
- Wegoro, J.; Pamba, S.; George, R.; Shaghude, Y.; Hollander, J.; Lugendo, B. Seagrass Restoration in a High-Energy Environment in the Western Indian Ocean. Estuar. Coast. Shelf Sci. 2022, 278, 108119. [Google Scholar] [CrossRef]
- Curiel, D.; Scarton, F.; Rismondo, A.; Marzocchi, M. Pilot transplanting project of Cymodocea nodosa and Zostera marina in the lagoon of Venice: Results and perspectives. Boll. Mus. Civ. St. Nat. Venezia 2005, 56, 25–40. [Google Scholar]
- Turner, S.J.; Hewitt, J.E.; Wilkinson, M.R.; Morrisey, D.J.; Thrush, S.F.; Cummings, V.J.; Funnell, G. Seagrass Patches and Landscapes: The Influence of Wind-Wave Dynamics and Hierarchical Arrangements of Spatial Structure on Macrofaunal Seagrass Communities. Estuaries 1999, 22, 1016–1032. [Google Scholar] [CrossRef]
- Fonseca, M.S.; Bell, S.S. Influence of Physical Setting on Seagrass Landscapes. Mar. Ecol. Prog. Ser. 1998, 171, 109–121. [Google Scholar] [CrossRef]
- Katwijk, M.M.; Thorhaug, A.; Marbà, N.; Orth, R.J.; Duarte, C.M.; Kendrick, G.A.; Althuizen, I.H.J.; Balestri, E.; Bernard, G.; Cambridge, M.L.; et al. Global Analysis of Seagrass Restoration: The Importance of Large-scale Planting. J. Appl. Ecol. 2016, 53, 567–578. [Google Scholar] [CrossRef]
- Campbell, M.L. Getting the Foundation Right: A Scientifically Based Management Framework to Aid in the Planning and Implementation of Seagrass Transplant Efforts. Bull. Mar. Sci. 2002, 71, 1405–1414. [Google Scholar]
- Holling, C.S. Resilience and Stability of Ecological Systems. Annu. Rev. Ecol. Syst. 1973, 4, 1–23. [Google Scholar] [CrossRef]
- Govers, L.L.; Heusinkveld, J.H.T.; Gräfnings, M.L.E.; Smeele, Q.; van der Heide, T. Adaptive Intertidal Seed-Based Seagrass Restoration in the Dutch Wadden Sea. PLoS ONE 2022, 17, e0262845. [Google Scholar] [CrossRef]
- Marion, S.R.; Orth, R.J. Factors Influencing Seedling Establishment Rates in Zostera Marina and Their Implications for Seagrass Restoration. Restor. Ecol. 2010, 18, 549–559. [Google Scholar] [CrossRef]
- Orth, R.; McGlathery, K.; Cole, L. Eelgrass Recovery Induces State Changes in a Coastal Bay System. Mar. Ecol. Prog. Ser. 2012, 448, 173–176. [Google Scholar] [CrossRef]
- Sinclair, E.A.; Sherman, C.D.H.; Statton, J.; Copeland, C.; Matthews, A.; Waycott, M.; van Dijk, K.J.; Vergés, A.; Kajlich, L.; McLeod, I.M.; et al. Advances in Approaches to Seagrass Restoration in Australia. Ecol. Manag. Restor. 2021, 22, 10–21. [Google Scholar] [CrossRef]
- Matheson, F.E.; Reed, J.; Dos Santos, V.M.; Mackay, G.; Cummings, V.J. Seagrass Rehabilitation: Successful Transplants and Evaluation of Methods at Different Spatial Scales. N. Z. J. Mar. Freshwater. Res. 2017, 51, 96–109. [Google Scholar] [CrossRef]
- Mokumo, M.F.; Adams, J.B.; von der Heyden, S. Investigating Transplantation as a Mechanism for Seagrass Restoration in South Africa. Restor. Ecol. 2023. [Google Scholar] [CrossRef]
- Ranwell, D.S.; Wyer, D.W.; Boorman, L.A.; Pizzey, J.M.; Waters, R.J. Zostera Transplants in Norfolk and Suffolk, Great Britain. Aquaculture 1974, 4, 185–198. [Google Scholar] [CrossRef]
- Fonseca, M.S. A Guide to Planting Sea Grasses in the Gulf of Mexico, 1st ed.; Sea Grant College Program, Texas A&M University: College Station, TX, USA, 1993; pp. 1–25. [Google Scholar]
- Cunha, A.H.; Assis, J.F.; Serrão, E.A. Seagrasses in Portugal: A Most Endangered Marine Habitat. Aquat. Bot. 2013, 104, 193–203. [Google Scholar] [CrossRef]
- Cunha, A.H.; Assis, J.; Serrão, E.A. Estimation of Available Seagrass Meadow Area in Portugal for Transplanting Purposes. J. Coast. Res. 2009, 56, 1100–1104. [Google Scholar]
- R Core Team. 2023. R: A Language and Environment for Statistical Computing (2023.3.0.386). MacOS. Austria: R Foundation for Statistical Computing. Available online: https://www.R-project.org/ (accessed on 28 June 2023).
- Pinheiro, J.C.; Bates, D.M.; R Core Team. Nlme: Linear and Nonlinear Mixed Effects Models (3.1-162); MacOS: New York, NY, USA, 2003. [Google Scholar]
- Pinheiro, J.C.; Bates, D.M. Mixed-Effects Models in S and S-PLUS, 1st ed.; Statistics and Computing; Springer-Verlag: New York, NY, USA, 2000; pp. 1–113. [Google Scholar]
- QGIS Department Team. QGIS Geographic Information System (3.10). 2020. Available online: https://qgis.org/en/site/ (accessed on 20 May 2023).
- Paulo, D.; Diekmann, O.; Ramos, A.A.; Alberto, F.; Serrão, E.A. Sexual Reproduction vs. Clonal Propagation in the Recovery of a Seagrass Meadow after an Extreme Weather Event. Sci. Mar. 2019, 83, 357–363. [Google Scholar] [CrossRef]
- Zimmerman, R.C.; Reguzzoni, J.L.; Alberte, R.S. Eelgrass (Zostera marina L.) Transplants in San Francisco Bay: Role of Light Availability on Metabolism, Growth and Survival. Aquat. Bot. 1995, 51, 67–86. [Google Scholar] [CrossRef]
- Borum, J. European Seagrasses: An Introduction to Monitoring and Management; Monitoring and Managing of European Seagrasses [EU Project]; The M&MS Project: Copenhagen, Denmark, 2004; ISBN 8789143213. [Google Scholar]
- Van Der Heide, T.; Van Nes, E.H.; Geerling, G.W.; Smolders, A.J.P.; Bouma, T.J.; Van Katwijk, M.M. Positive Feedbacks in Seagrass Ecosystems: Implications for Success in Conservatiooutln and Restoration. Ecosystems 2007, 10, 1311–1322. [Google Scholar] [CrossRef]
- Cunha, A.H.; Santos, R.P.; Gaspar, A.P.; Bairros, M.F. Seagrass Landscape-Scale Changes in Response to Disturbance Created by the Dynamics of Barrier-Islands: A Case Study from Ria Formosa (Southern Portugal). Estuar. Coast. Shelf. Sci. 2005, 64, 636–644. [Google Scholar] [CrossRef]
- Widdows, J.; Pope, N.D.; Brinsley, M.D.; Asmus, H.; Asmus, R.M. Effects of Seagrass Beds (Zostera noltii and Z. marina) on near-Bed Hydrodynamics and Sediment Resuspension. Mar. Ecol. Prog. Ser. 2008, 358, 125–136. [Google Scholar] [CrossRef]
- Bayraktarov, E.; Saunders, M.I.; Abdullah, S.; Mills, M.; Beher, J.; Possingham, H.P.; Mumby, P.J.; Lovelock, C.E. The Cost and Feasibility of Marine Coastal Restoration. Ecol. Appl. 2015, 26, 1055–1074. [Google Scholar] [CrossRef] [PubMed]
- Danovaro, R.; Aronson, J.; Cimino, R.; Gambi, C.; Snelgrove, P.V.R.; Van Dover, C. Marine Ecosystem Restoration in a Changing Ocean. Restor Ecol 2021, 29, e13432. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mourato, C.V.; Padrão, N.; Serrão, E.A.; Paulo, D. Less Is More: Seagrass Restoration Success Using Less Vegetation per Area. Sustainability 2023, 15, 12937. https://doi.org/10.3390/su151712937
Mourato CV, Padrão N, Serrão EA, Paulo D. Less Is More: Seagrass Restoration Success Using Less Vegetation per Area. Sustainability. 2023; 15(17):12937. https://doi.org/10.3390/su151712937
Chicago/Turabian StyleMourato, Carolina V., Nuno Padrão, Ester A. Serrão, and Diogo Paulo. 2023. "Less Is More: Seagrass Restoration Success Using Less Vegetation per Area" Sustainability 15, no. 17: 12937. https://doi.org/10.3390/su151712937
APA StyleMourato, C. V., Padrão, N., Serrão, E. A., & Paulo, D. (2023). Less Is More: Seagrass Restoration Success Using Less Vegetation per Area. Sustainability, 15(17), 12937. https://doi.org/10.3390/su151712937




