Removal Efficiency and Adsorption Kinetics of Methyl Orange from Wastewater by Commercial Activated Carbon
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
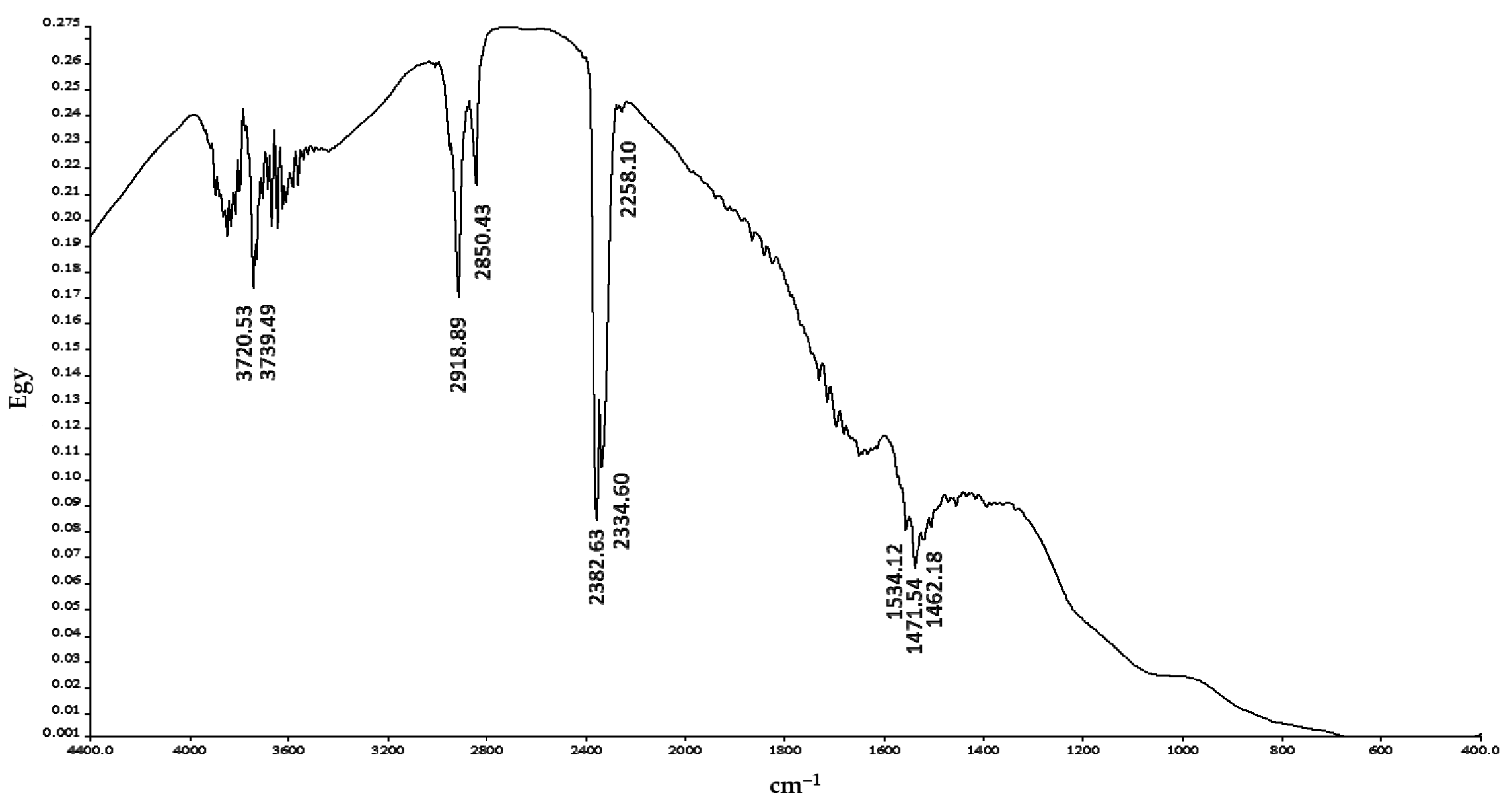
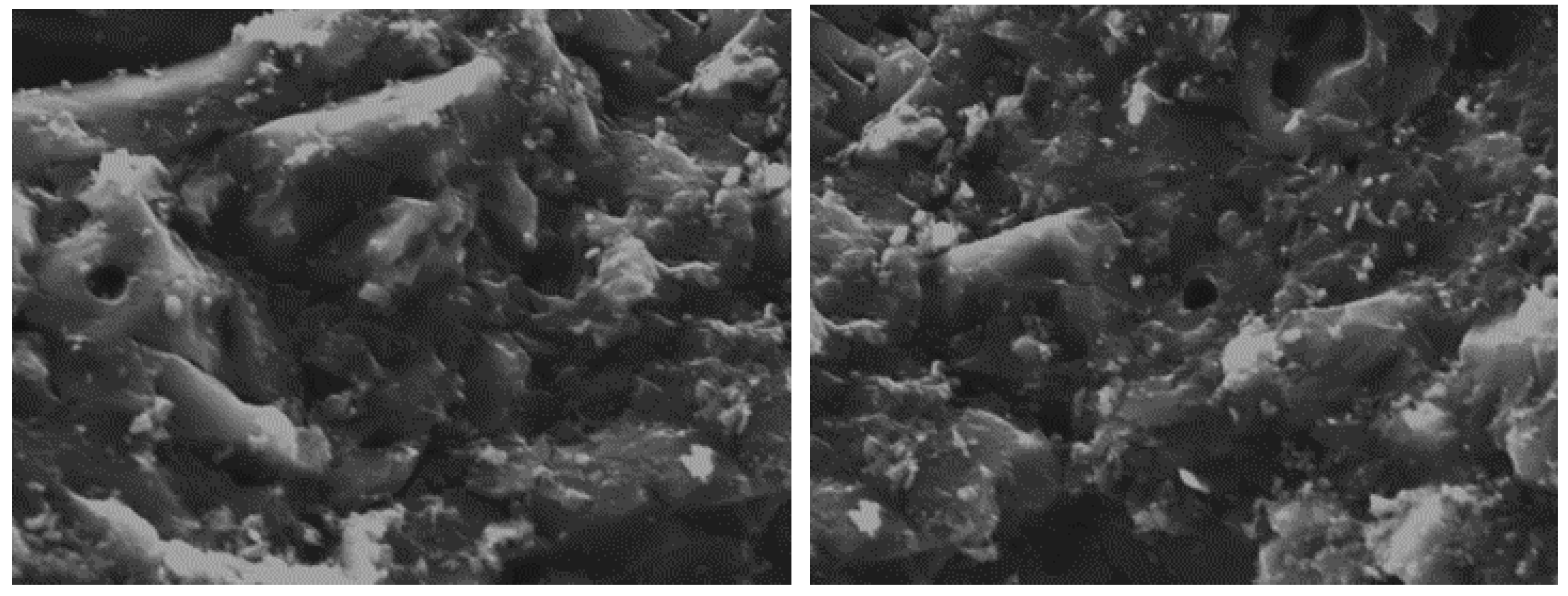
2.2. Characterization of Commercial AC
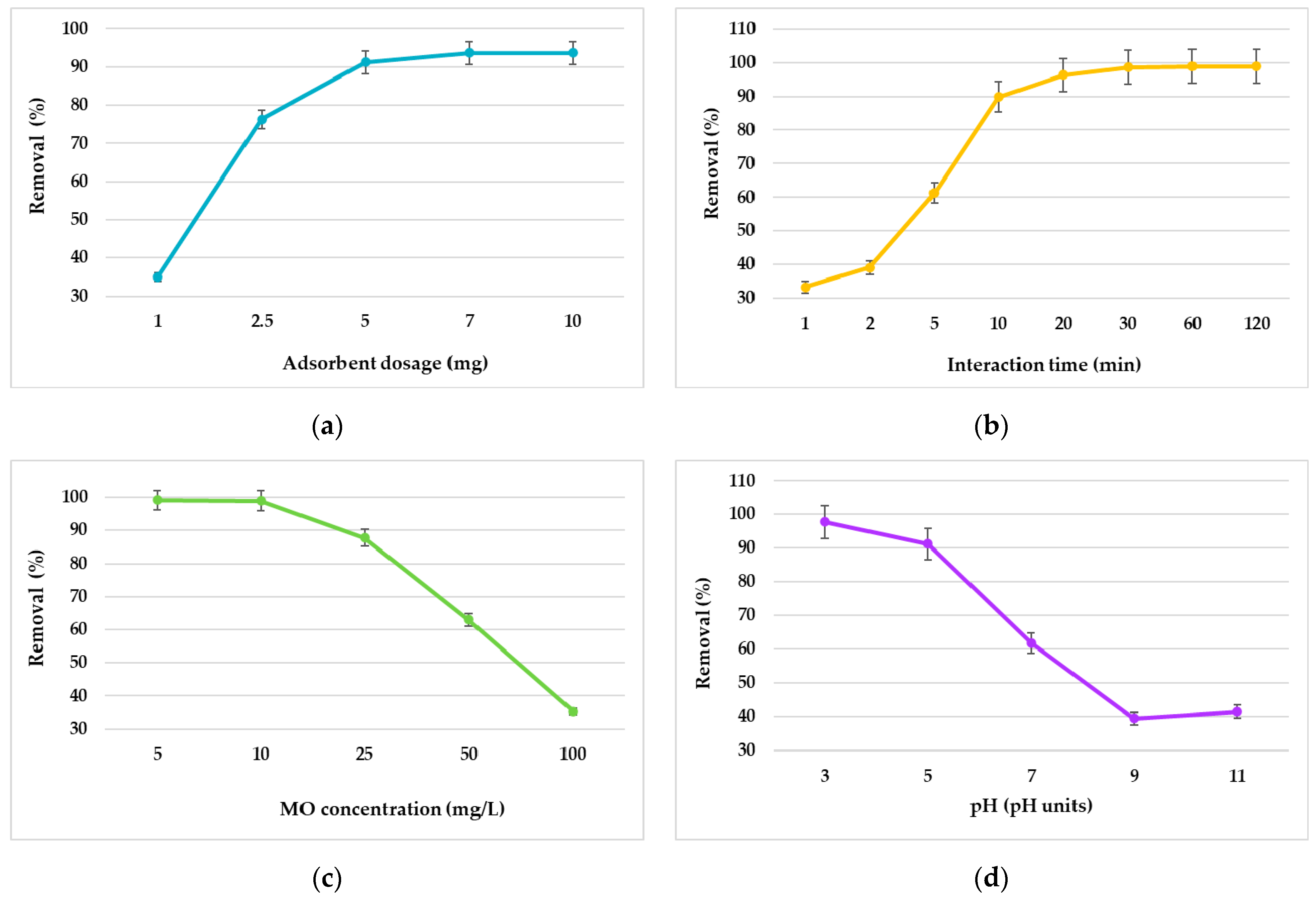
2.3. Adsorption Studies
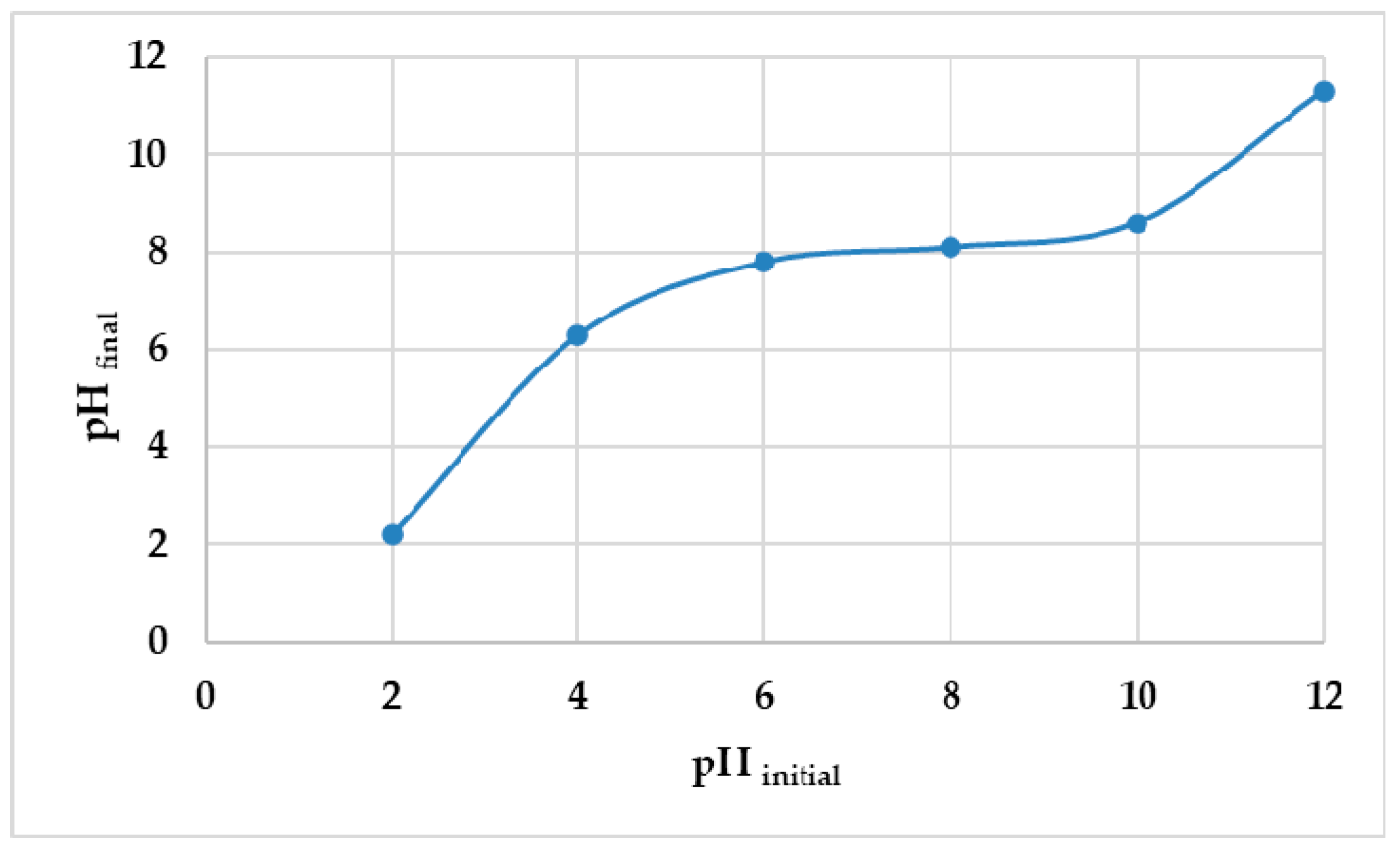
2.4. pHpzc
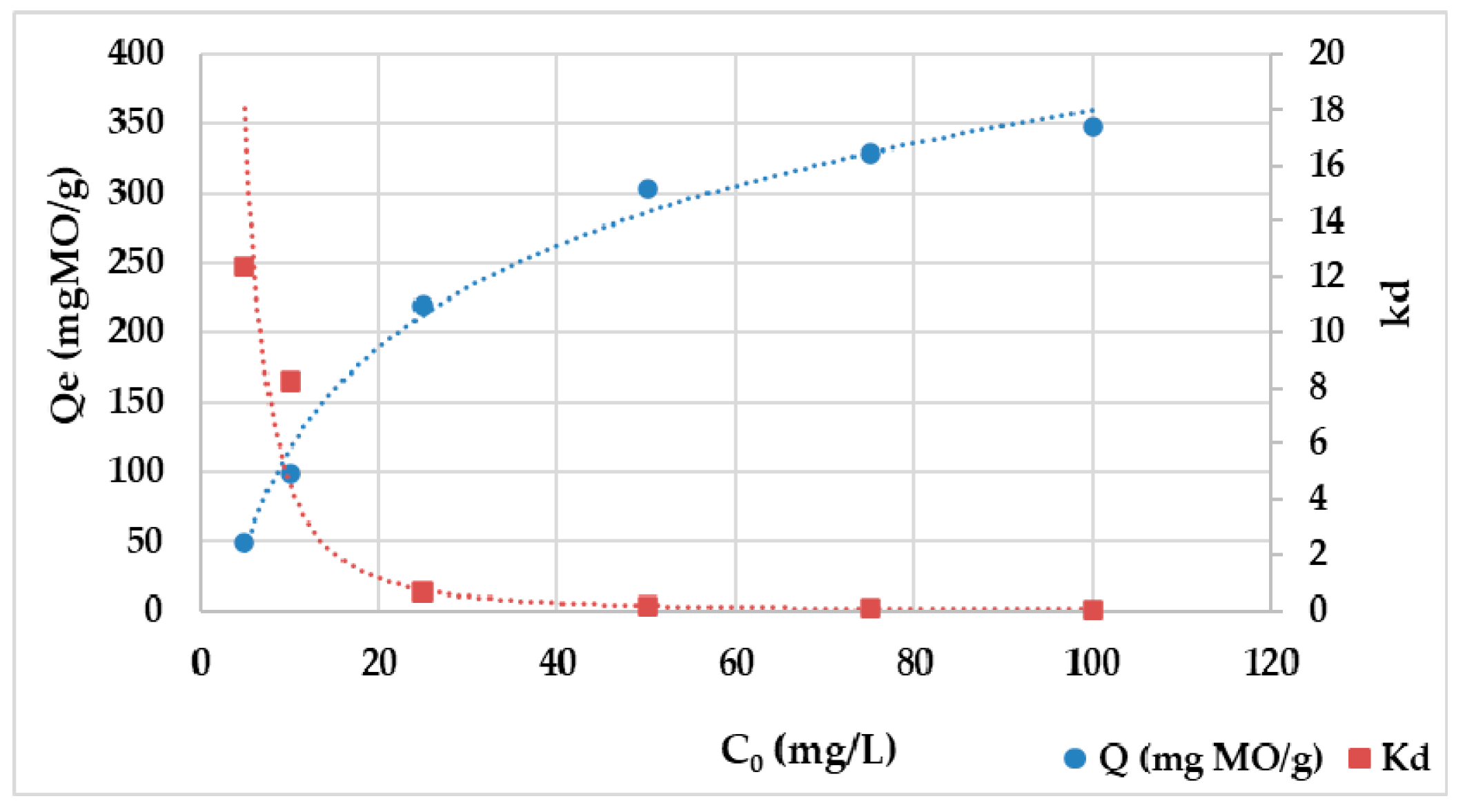
2.5. Adsorption Isotherms
2.6. Adsorption Kinetics
2.7. Thermodynamic Study
2.8. Instrumental Analysis
2.9. Quality Assurance and Quality Control
3. Results and Discussion
3.1. Chemical and Physical Characterization of the Adsorbent Material
3.2. Adsorption Study

3.3. Isothermal Models
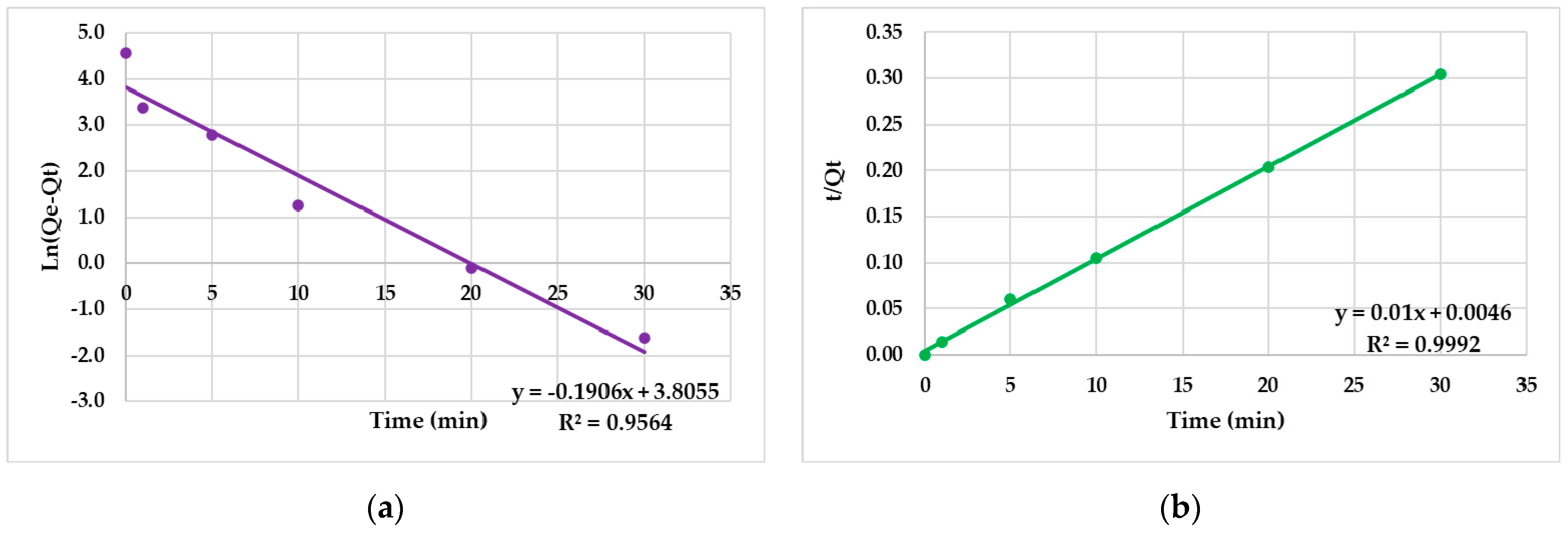
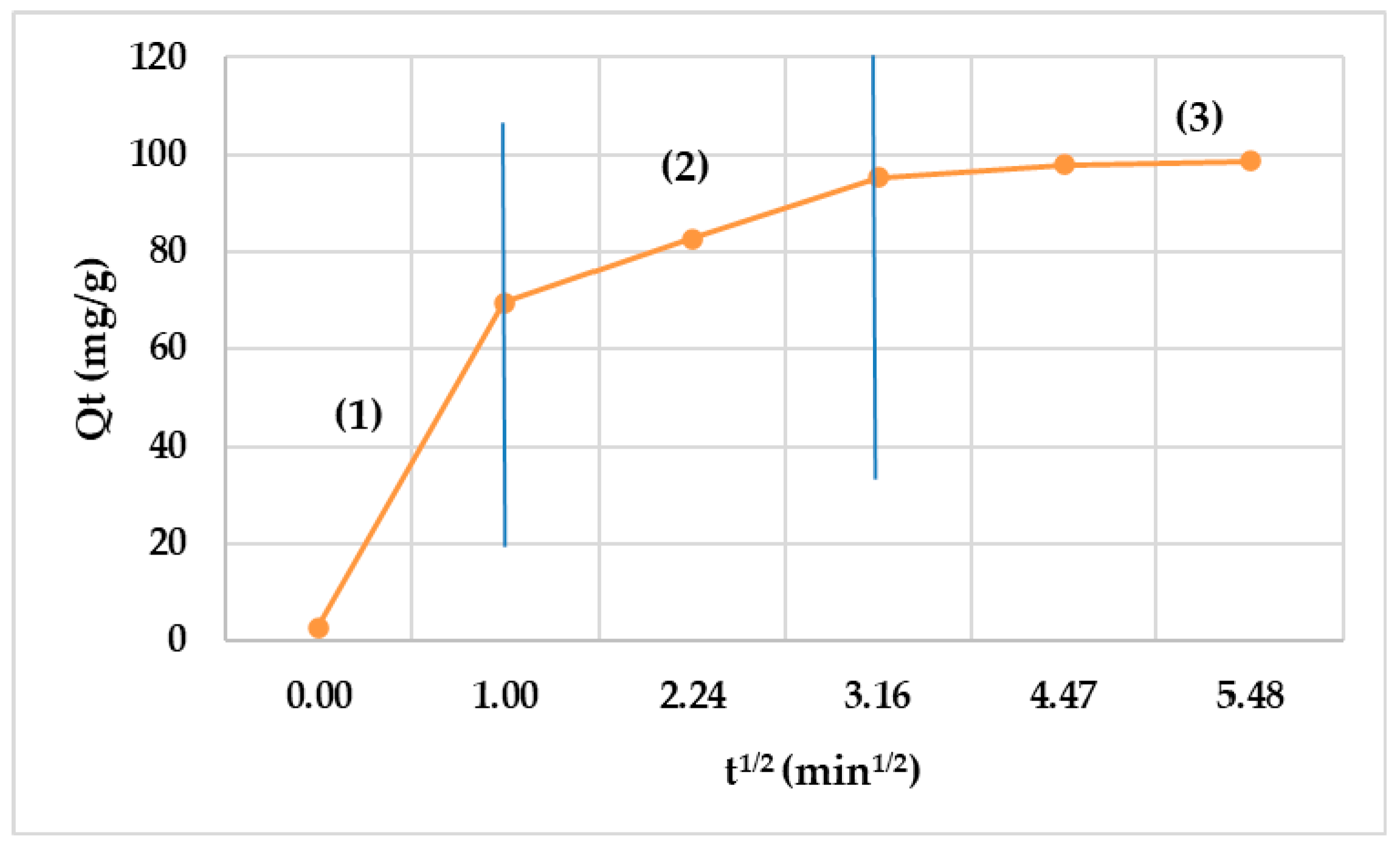
3.4. Kinetic Studies
3.5. Thermodynamic Study
3.6. Application for Real Wastewater Samples
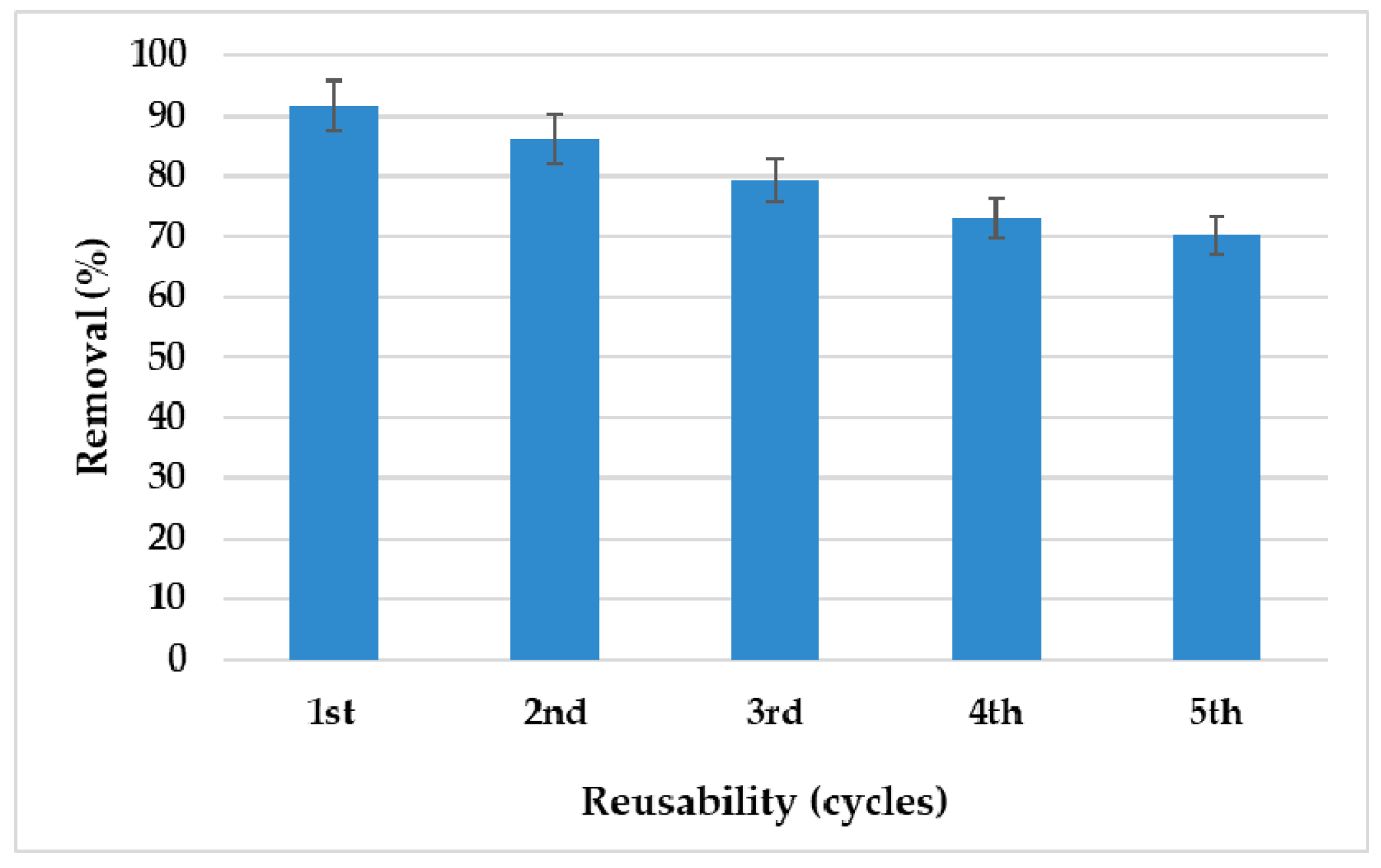
3.7. Desorption and Reusability
3.8. Adsorption Capabilities of the Commercial AC Material for Other Organic Compounds Removal
3.9. Comparison with Other Adsorbents
4. Conclusions and Future Work
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Al-Tohamy, R.; Ali, S.S.; Li, F.; Okasha, K.M.; Mahmoud, Y.A.-G.; Elsamahy, T.; Jiao, H.; Fu, Y.; Sun, J. A critical review on the treatment of dye-containing wastewater: Ecotoxicological and health concerns of textile dyes and possible remediation approaches for environmental safety. Ecotoxicol. Environ. Saf. 2022, 231, 1131. [Google Scholar] [CrossRef]
- Lellis, B.; Fávaro-Polonio, C.Z.; Pamphile, J.A.; Polonio, J.C. Effects of textile dyes on health and the environment and bioremediation potential of living organisms. Biotechnol. Res. Innov. 2019, 3, 275–290. [Google Scholar] [CrossRef]
- Tomar, T.; Kahandawala, N.; Kaur, J.; Thounaojam, L.; Choudhary, I.; Bera, S. Bioremediation of synthetic dyes from wastewater by using microbial nanocomposites: An emerging field for water pollution management. Biocatal. Agric. Biotechnol. 2023, 51, 102767. [Google Scholar] [CrossRef]
- Garcia, V.S.G.; de Freitas Tallarico, L.; Rosa, J.M.; Suzuki, C.F.; Roubicek, D.A.; Nakano, E.; Borrely, S.I. Multiple adverse effects of textile effluents and reactive Red 239 dye to aquatic organisms. Environ. Sci. Pollut. Res. 2021, 28, 63202–63214. [Google Scholar] [CrossRef]
- Tkaczyk, A.; Mitrowska, K.; Posyniak, A. Synthetic organic dyes as contaminants of the aquatic environment and their implications for ecosystems: A review. Sci. Total Environ. 2020, 717, 137222. [Google Scholar] [CrossRef]
- Mabuza, L.; Sonnenberg, N.; Marx-Pienaar, N. Natural versus synthetic dyes: Consumers’ understanding of apparel coloration and their willingness to adopt sustainable alternatives. Resour. Conserv. Recycl. Adv. 2023, 18, 200146. [Google Scholar] [CrossRef]
- Iwuozor, K.O.; Ighalo, J.O.; Emenike, E.C.; Ogunfowora, L.A.; Igwegbe, C.A. Adsorption of methyl orange: A review on adsorbent performance. Curr. Res. Green Sustain. Chem. 2021, 4, 100179. [Google Scholar] [CrossRef]
- Sugai, D.Y.; Benincá, C.; Zanoelo, E.F. Electrogenerated iron-based adsorbents: A case study of an azo dye removal viewed from a fundamental physico-chemical perspective. J. Chem. Eng. 2023, 454, 140129. [Google Scholar] [CrossRef]
- Chen, C.; Cao, Y.; Ali, A.; Toufouki, S.; Yao, S. How to apply terpenoid-based deep eutectic solvents for removal of antibiotics and dyes from water: Theoretical prediction, experimental validation and quantum chemical evaluation. Environ. Res. 2023, 231, 116180. [Google Scholar] [CrossRef]
- Gita, S.; Shukla, S.P.; Deshmukhe, G.; Singh, A.R.; Choudhury, T.G. Adsorption linked fungal degradation process for complete removal of Lanasyn olive dye using chemically valorized sugarcane bagasse. Waste Manag. Bull. 2023, 1, 29–38. [Google Scholar] [CrossRef]
- Chu, Y.; Zhu, S.; Wang, F.; Lei, W.; Xia, M.; Liao, C. Tyrosine-Immobilized Mont-morillonite: An Efficient Adsorbent for Removal of Pb2+ and Cu2+ from Aqueous Solution. J. Chem. Eng. Data 2019, 64, 3535–3546. [Google Scholar] [CrossRef]
- Zhu, S.; Chen, Y.; Khan, M.A.; Xu, H.; Wang, F.; Xia, M. Interfaces, In-Depth Study of Heavy Metal Removal by an Etidronic Acid-Functionalized Layered Double Hydroxide. Appl. Mater. 2022, 14, 7450–7463. [Google Scholar] [CrossRef]
- Amjad, S.; Shaukat, S.; Rahman, H.M.A.U.; Usman, M.; Farooqi, Z.H.; Nazar, M.F. Application of anionic-nonionic mixed micellar system for solubilization of methylene blue dye. J. Mol. Liq. 2023, 369, 120958. [Google Scholar] [CrossRef]
- Yusaf, A.; Usman, M.; Siddiq, M.; Bakhtiar, M.; Mansha, A.; Shaukat, S.; Rehman, H.F. Mixed micellar solubilization of Naphthol Green B followed by its removal from synthetic effluent by micellar-enhanced ultrafiltration under optimized conditions. Molecules 2022, 27, 6436. [Google Scholar] [CrossRef] [PubMed]
- Rehman, A.; Nisa, M.U.; Usman, M.; Ahmad, Z.; Bokhari, T.H.; Rahman, H.M.A.U.; Rasheed, A.; Kiran, L. Application of cationic-nonionic surfactant based nanostructured dye carriers: Mixed micellar solubilization. J. Mol. Liq. 2021, 326, 115345. [Google Scholar] [CrossRef]
- Debnath, S.; Das, R. Strong adsorption of CV dye by Ni ferrite nanoparticles for waste water purification: Fits well the pseudo second order kinetic and Freundlich isotherm model. Ceram. Int. 2023, 49, 16199–16215. [Google Scholar] [CrossRef]
- He, S.; Li, J.; Cao, X.; Xie, F.; Yang, H.; Wang, C.; Bittencourt, C.; Li, W. Regenerated cellulose/chitosan composite aerogel with highly efficient adsorption for anionic dyes. Int. J. Biol. Macromol. 2023, 244, 125067. [Google Scholar] [CrossRef]
- Chang, S.; Simeng, Y.; Henglong, T.; Shihao, F.; Zhu, L. Adsorption properties and interactions analysis of cyclodextrin-based polymer networks towards organic dyes. Carbohydr. Polym. Technol. Appl. 2023, 5, 100317. [Google Scholar] [CrossRef]
- Elabboudi, M.; Bensalah, J.; Amri, A.E.; Azzouzi, N.E.; Srhir, B.; Lebkiri, A.; Zarrouk, A.; Rifi, E.H. Adsorption performance and mechanism of anionic MO dye by the adsorbent polymeric Amberlite®IRA-410 resin from environment wastewater: Equilibrium kinetic and thermodynamic studies. J. Mol. Struct. 2023, 1277, 134789. [Google Scholar] [CrossRef]
- Thamer, B.M.; Shaker, A.A.; Hameed, M.M.A.; Al-Enizi, A.M. Highly selective and reusable nanoadsorbent based on expansive clay-incorporated polymeric nanofibers for cationic dye adsorption in single and binary systems. J. Water Process. Eng. 2023, 54, 103918. [Google Scholar] [CrossRef]
- El-taweel, R.M.; Mohamed, N.; Alrefaey, K.A.; Husien, S.; Abdel-Aziz, A.B.; Salim, A.I.; Mostafa, N.G.; Said, L.A.; Fahim, I.S.; Radwan, A.G. A review of coagulation explaining its definition, mechanism, coagulant types, and optimization models; RSM, and ANN. Curr. Opin. Green Sustain. Chem. 2023, 6, 100358. [Google Scholar] [CrossRef]
- Ma, H.; Zhou, W.; Xu, X.; Zhu, X.; Wang, L.; Shi, X.; Jiang, M.; Li, C.; Li, T. Characterizing the removal of Pb2+ and Zn2+ from an acidic smelting wastewater using electrocatalytic internal Fe0/C micro-electrolysis. Sep. Purif. Technol. 2023, 317, 123874. [Google Scholar] [CrossRef]
- Pan, Z.F.; An, L. Removal of Heavy Metal from Wastewater Using Ion Exchange Membranes. In Applications of Ion Exchange Materials in the Environment; Ahamed, M.I., Asiri, A., Eds.; Springer: Cham, Switzerland, 2019. [Google Scholar] [CrossRef]
- Selvasembian, R.; Gwenzi, W.; Chaukura, N.; Mthembu, S. Recent advances in the polyurethane-based adsorbents for the decontamination of hazardous wastewater pollutants. J. Hazard. Mat. 2021, 417, 125960. [Google Scholar] [CrossRef]
- Adithya, G.T.; Rangabhashiyam, S.; Sivasankari, C. Lanthanum-iron binary oxide nanoparticles: As cost-effective fluoride adsorbent and oxygen gas sensor. Microchem. J. 2019, 148, 364–373. [Google Scholar] [CrossRef]
- Imessaoudene, A.; Cheikh, S.; Bollinger, J.-C.; Belkhiri, L.; Tiri, A.; Bouzaza, A.; El Jery, A.; Assadi, A.; Amrane, A.; Mouni, L. Zeolite Waste Characterization and Use as Low-Cost, Ecofriendly, and Sustainable Material for Malachite Green and Methylene Blue Dyes Removal: Box–Behnken Design, Kinetics, and Thermodynamics. Appl. Sci. 2022, 12, 7587. [Google Scholar] [CrossRef]
- Naseem, K.; Farooqi, Z.H.; Begum, R.; Rehman, M.Z.U.; Shahbaz, A.; Farooq, U.; Ali, M.; Rahman, H.M.A.U.; Irfan, A.; Al-Sehemi, A.G. Removal of Cadmium (II) from Aqueous Medium Using Vigna radiata Leave Biomass: Equilibrium Isotherms, Kinetics and Thermodynamics. Z. Fur Phys. Chem. 2019, 233, 669–690. [Google Scholar] [CrossRef]
- Boudechiche, N.; Fares, M.; Ouyahia, S.; Yazid, H.; Trari, M.; Sadaoui, Z. Comparative study on removal of two basic dyes in aqueous medium by adsorption using activated carbon from Ziziphus lotus stones. Microchem. J. 2019, 146, 1010–1018. [Google Scholar] [CrossRef]
- Giannakoudakis, D.A.; Kyzas, G.Z.; Avranas, A.; Lazaridis, N.K. Multi-parametric adsorption effects of the reactive dye removal with commercial activated carbons. J. Mol. Liq. 2016, 213, 381–389. [Google Scholar] [CrossRef]
- Husien, S.; El-taweel, R.M.; Salim, A.I.; Fahim, I.S.; Said, L.A.; Radwan, A.G. Review of activated carbon adsorbent material for textile dyes removal: Preparation, and modelling. Cur. Res. Green Sustain. Chem. 2022, 5, 100325. [Google Scholar] [CrossRef]
- Azari, A.; Nabizadeh, R.; Nasseri, S.; Mahvi, A.H.; Mesdaghinia, A.R. Comprehensive systematic review and meta-analysis of dyes adsorption by carbon-based adsorbent materials: Classification and analysis of last decade studies. Chemosphere 2020, 250, 126238. [Google Scholar] [CrossRef] [PubMed]
- Mammar, A.C.; Mouni, L.; Bollinger, J.-C.; Belkhiri, L.; Bouzaza, A.; Assadi, A.A.; Belkacemi, H. Modeling and optimization of process parameters in elucidating the adsorption mechanism of Gallic acid on activated carbon prepared from date stones. Sep. Sci. Technol. 2019, 55, 3113–3125. [Google Scholar] [CrossRef]
- Hung, N.V.; Nguyet, B.T.M.; Nghi, N.H.; Thanh, N.M.; Quyen, N.D.V.; Nguyen, V.T.; Nhiem, D.N.; Khieu, D.Q. Highly effective adsorption of organic dyes from aqueous solutions on longan seed-derived activated carbon. Environ. Eng. Res. 2023, 28, 220116. [Google Scholar] [CrossRef]
- Kumar, M.; Tamilarasan, R. Modeling of experimental data for the adsorption of methyl orange from aqueous solution using a low cost activated carbon prepared from Prosopis julifl ora. Pol. J. Chem. Technol. 2013, 15, 29–39. [Google Scholar] [CrossRef]
- Ramutshatsha-Makhwedzha, D.; Mavhungu, A.; Moropeng, M.L.; Mbaya, R. Activated carbon derived from waste orange and lemon peels for the adsorption of methyl orange and methylene blue dyes from wastewater. Heliyon 2022, 8, e09930. [Google Scholar] [CrossRef] [PubMed]
- Shah, S.S.; Sharma, T.; Dar, B.A.; Bamezai, R.K. Adsorptive removal of methyl orange dye from aqueous solution using populous leaves: Insights from kinetics, thermodynamics and computational studies. Environ. Chem. Ecotoxicol. 2021, 3, 172–181. [Google Scholar] [CrossRef]
- Pirvu, F.; Covaliu-Mierla, C.I.; Paun, I.; Paraschiv, G.; Iancu, V. Treatment of Wastewater Containing Nonsteroidal Anti-Inflammatory Drugs Using Activated Carbon Material. Materials 2022, 15, 559. [Google Scholar] [CrossRef]
- Zhang, P.K.; Wang, L. Extended Langmuir equation for correlating multilayer adsorption equilibrium data. Sep. Purif. Technol. 2010, 70, 367–371. [Google Scholar] [CrossRef]
- Jeppu, G.P.; Clement, T.P. A modified Langmuir-Freundlich isotherm model for simulating pH-dependent adsorption effects. J. Contam. Hydrol. 2012, 129–130, 46–53. [Google Scholar] [CrossRef] [PubMed]
- Lagergren, B.K.S. Svenska Zur Theorie Der Sogenannten Adsorption Geloster Stofe, Kungliga Svenska Vetenskapsakademiens. Handlingar 1989, 24, 1–39. [Google Scholar]
- Olusegun, S.J.; Mohallem, N.D. Comparative adsorption mechanism of doxycycline and congo red using synthesized kaolinite supported CoFe2O4 nanoparticles. Environ. Pollut. 2020, 260, 114019. [Google Scholar] [CrossRef]
- Esvandi, Z.; Foroutan, R.; Peighambardoust, S.J.; Akbari, A.; Ramavandi, B. Uptake of anionic and cationic dyes from water using natural clay and clay/starch/MnFe2O4 magnetic nanocomposite. Surf. Interfaces 2020, 21, 100754. [Google Scholar] [CrossRef]
- Mahmoudi, E.; Azizkhani, S.; Mohammad, A.W.; Ng, L.Y.; Benamor, A.; Ang, W.L.; Ba-Abbad, M. Simultaneous removal of congo red and cadmium (II) from aqueous solutions using graphene oxide–silica composite as a multifunctional adsorbent. J. Environ. Sci. 2020, 98, 151–160. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, W.; Kang, X.; Zhang, C. Rapid and efficient recovery of silver with nanoscale zerovalent iron supported on high performance activated carbon derived from straw biomass. Environ. Pollut. 2019, 255, 113043. [Google Scholar] [CrossRef]
- Hu, Y.; Guo, T.; Ye, X.; Li, Q.; Guo, M.; Liu, H.; Wu, Z. Dye adsorption by resins: Effect of ionic strength on hydrophobic and electrostatic interactions. Chem. Eng. J. 2013, 228, 392–397. [Google Scholar] [CrossRef]
- Li, Y.; Du, Q.; Liu, T.; Sun, J.; Wang, Y.; Wu, S.; Wang, Z.; Xia, Y.; Xia, L. Methylene blue adsorption on graphene oxide/calcium alginate composites. Carbohydr. Polym. 2013, 95, 501–507. [Google Scholar] [CrossRef]
- Kanani-jazi, M.H.; Akbari, S. Journal of Environmental Chemical Engineering Amino-dendritic and carboxyl functionalized halloysite nanotubes for highly efficient removal of cationic and anionic dyes: Kinetic, isotherm, and thermodynamic studies. J. Environ. Chem. Eng. 2021, 9, 105214. [Google Scholar] [CrossRef]
- Tay, W.Y.; Ng, L.Y.; Ng, C.Y.; Sim, L.C. Removal of Methyl Red using Adsorbent Produced from Empty Fruit Bunches by Taguchi Approach. IOP Conf. Ser. Earth Environ. Sci. 2021, 945, 012014. [Google Scholar] [CrossRef]
- Dai, M. The Effect of Zeta Potential of Activate Carbon on the Adsorption of Dyes from Aqueous Solution. J. Colloid Interface Sci. 1994, 164, 223–228. [Google Scholar] [CrossRef]
- Aftab, R.A.; Zaidi, S.; Khan, A.A.P.; Usman, M.A.; Khan, A.Y.; Chani, M.T.S.; Asiri, A.M. Removal of congo red from water by adsorption onto activated carbon derived from waste black cardamom peels and machine learning modeling. Alex. Eng. J. 2023, 71, 355–369. [Google Scholar] [CrossRef]
- Bhomick, C.; Supong, A.; Baruah, M.; Pongener, C.; Sinha, D. Pine Cone biomass as an efficient precursor for the synthesis of activated biocarbon for adsorption of anionic dye from aqueous solution: Isotherm, kinetic, thermodynamic and regeneration studies. Sustain. Chem. Pharm. 2018, 10, 41–49. [Google Scholar] [CrossRef]
- Langmuir, I. The adsorption of gases on plane surfaces of glass, mica and platinum. J. Am. Chem. Soc. 1918, 40, 1361–1403. [Google Scholar] [CrossRef]
- Freundlich, H.M.F. Over the adsorption in solution. J. Phys. Chem. 1906, 57, 385–471. [Google Scholar] [CrossRef]
- Ahmad, M.A.; Puad, N.A.A.; Bello, O.S. Kinetic, equilibrium and thermodynamic studies of synthetic dye removal using pomegranate peel activated carbon prepared by microwave-induced KOH activation. Water Res. Ind. 2014, 6, 18–35. [Google Scholar] [CrossRef]
- Mahamad, M.N.; Zaini, M.A.A.; Zakaria, Z.A. Preparation and characterization of activated carbon from pineapple waste biomass for dye removal. Int. Biodeterior. Biodegrad. 2015, 102, 274–280. [Google Scholar] [CrossRef]
- Nizam, N.U.M.; Hanafiah, M.M.; Mahmoudi, E.; Halim, A.A.; Mohammad, A.W. The removal of anionic and cationic dyes from an aqueous solution using biomass-based activated carbon. Sci. Rep. 2021, 11, 8623. [Google Scholar] [CrossRef]
- Foo, K.Y.; Hameed, B.H. Microwave-assisted preparation and adsorption performance of activated carbon from biodiesel industry solid reside: Influence of operational parameters. Bioresour. Technol. 2012, 103, 398–404. [Google Scholar] [CrossRef] [PubMed]
- Bouaziz, F.; Koubaa, M.; Kallel, F.; Chaari, F.; Driss, D.; Ghorbel, R.E.; Chaabouni, S.E. Efficiency of almond gum as a low cost adsorbent for methylene blue dye removal from aqueous solutions. Ind. Crop Prod. 2015, 74, 903–911. [Google Scholar] [CrossRef]
- Qiu, H.; Lv, L.; Pan, B.; Zhang, Q.; Zhang, W.; Zhang, Q. Critical review in adsorption kinetic models. J. Zhejiang Univ.—Sci. A 2009, 10, 716–724. [Google Scholar] [CrossRef]
- Foroutan, R.; Peighambardoust, S.J.; Peighambardoust, S.H.; Pateiro, M.; Lorenzo, J.M. Adsorption of Crystal Violet Dye Using Activated Carbon of LemonWood and Activated Carbon/Fe3O4 Magnetic Nanocomposite from Aqueous Solutions: A Kinetic, Equilibrium and Thermodynamic Study. Molecules 2021, 26, 2241. [Google Scholar] [CrossRef]
- Humpola, P.D.; Odetti, H.S.; Fertitta, A.E.; Vicente, J.L. Thermodynamic analysis of adsorption models of phenol in liquid phase on different activated carbons. J. Chil. Chem. Soc. 2013, 58, 1541–1544. [Google Scholar] [CrossRef]
- Mahmoudi, K.; Hosni, K.; Hamdi, N.; Srasra, E. Kinetics and equilibrium studies on removal of methylene blue and methyl orange by adsorption onto activated carbon prepared from date pits-A comparative study. Korean J. Chem. Eng. 2015, 32, 274–283. [Google Scholar] [CrossRef]
- Khattabi, E.H.E.L.; Rachdi, Y.; Bassam, R.; Mourid, E.H.; Naimi, Y.; Alouani, M.E.L.; Belaaouad, S. Enhanced elimination of methyl orange and recycling of an eco-friendly adsorbent activated carbon from aqueous solution. Russ. J. Phys. Chem. B 2021, 15 (Suppl. S2), S149–S159. [Google Scholar] [CrossRef]
- Yu, Y.; Qiao, N.; Wang, D.; Zhu, Q.; Fu, F.; Cao, R.; Wang, R.; Liu, W.; Xu, B. Fluffy honeycomb-like activated carbon from popcorn with high surface area and well-developed porosity for ultra-high efficiency adsorption of organic dyes. Bioresour. Technol. 2019, 285, 121340. [Google Scholar] [CrossRef]
- Islam, M.T.; Saenz-Arana, R.; Hernandez, C.; Guinto, T.; Ahsan, M.A.; Bragg, D.T.; Wang, H.; Alvarado-Tenorio, B.; Noveron, J.C. Conversion of waste tire rubber into a high-capacity adsorbent for the removal of methylene blue, methyl orange, and tetracycline from water. J. Environ. Chem. Eng. 2018, 6, 3070–3082. [Google Scholar] [CrossRef]
- Islam, M.S.; Ang, B.C.; Gharehkhani, S.; Afif, A.B.M. Adsorption capability of activated carbon synthesized from coconut shell. Carbon Lett. 2016, 20, 1–9. [Google Scholar] [CrossRef]
- Ahlawat, W.; Kataria, N.; Dilbaghi, N.; Hassan, A.A.; Kumar, S.; Kim, K.H. Carbonaceous nanomaterials as effective and efficient platforms for removal of dyes from aqueous systems. Environ. Res. 2020, 181, 108904. [Google Scholar] [CrossRef]
- Mohammadi, N.; Khani, H.; Gupta, V.K.; Amereh, E.; Agarwal, S. Adsorption process of methyl orange dye onto mesoporous carbon material-kinetic and thermodynamic studies. J. Colloid Interface Sci. 2011, 362, 457–562. [Google Scholar] [CrossRef]
- Rattanapan, S.; Srikram, J.; Kongsune, P. Adsorption of Methyl Orange on Coffee grounds Activated Carbon. Energy Procedia 2017, 138, 949–954. [Google Scholar] [CrossRef]
- Alardhi, S.M.; Albayati, T.M.; Alrubaye, J.M. Adsorption of the methyl green dye pollutant from aqueous solution using mesoporous materials MCM-41 in a fixed-bed column. Heliyon 2020, 6, e03253. [Google Scholar] [CrossRef]
- Ali, M.A.; Mubarak, M.F.; Keshawy, M.; Zayed, M.A.; Ataalla, M. Adsorption of Tartrazine anionic dye by novel fixed bed Core-Shell-polystyrene Divinylbenzene/Magnetite nanocomposite. Alex. Eng. J. 2022, 61, 1335–1352. [Google Scholar] [CrossRef]
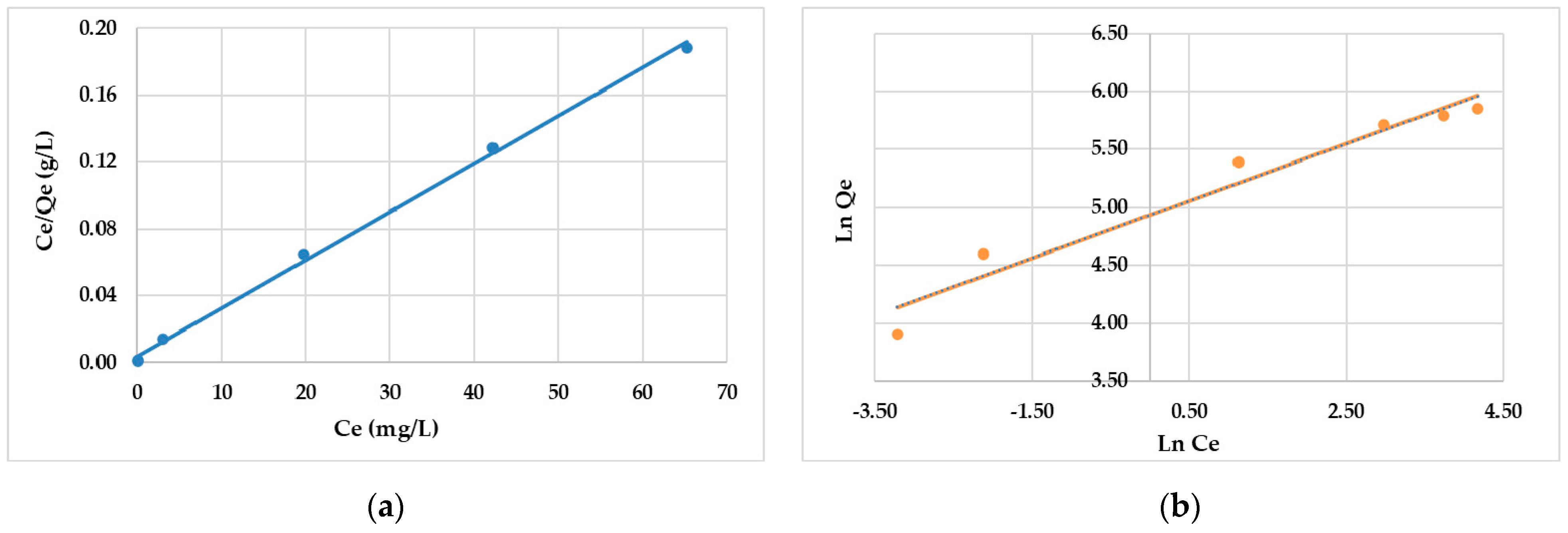
| Adsorbent | Langmuir | Freundlich | ||||||
|---|---|---|---|---|---|---|---|---|
| Qmax (mg/g) | KL (L/mg) | R2 | SD | KF (mg/g) | n | R2 | SD | |
| AC | 129.3 ± 4.13 | 0.0833 ± 0.003 | 0.9981 | 0.322 | 140 ± 3.22 | 4.05 | 0.9554 | 0.262 |
| Pseudo-first-order kinetics | k1 (min−1) | −0.006 ± 0.03 |
| R2 | 0.9564 | |
| Qe (exp) (mg/g) | 44.95 ± 1.61 | |
| SD | 0.353 | |
| Pseudo-second-order kinetics | k2 (g/mg × min) | 46 ± 1.62 |
| R2 | 0.9992 | |
| Qe (exp) (mg/g) | 100 ± 2.83 | |
| SD | 0.294 | |
| Intra-particle diffusion | Kid (g/mg × min) | 14.73 ± 0.58 |
| Cid | 34.3 ± 1.26 | |
| R2 | 0.6855 | |
| SD | 0.378 |
| T (K) | SD | KL (L/mg) | KL° × 105 (Dimensionless) | Ln KL° | ΔG° (KJ/mol) | ΔH° (KJ/mol) | ΔS° (J/mol) |
|---|---|---|---|---|---|---|---|
| 298 | 0.197 | 0.08 ± 0.003 | 0.27 ± 0.01 | 10.21 ± 0.43 | −32.87 ± 1.37 | −14.94 ± 2.45 | 60.16 ± 1.55 |
| 303 | 0.225 | 0.13 ± 0.005 | 0.41 ± 0.02 | 10.62 ± 0.45 | −33.17 ± 1.40 | ||
| 308 | 0.266 | 0.34 ± 0.02 | 1.13 ± 0.05 | 11.63 ± 0.51 | −33.47 ± 1.46 | ||
| 313 | 0.301 | 0.72 ± 0.03 | 2.35 ± 0.09 | 12.37 ± 0.51 | −33.77 ± 1.40 | ||
| 318 | 0.358 | 1.62 ± 0.06 | 5.31 ± 0.21 | 13.18 ± 0.52 | −34.07 ± 1.35 | ||
| 323 | 0.432 | 3.55 ± 0.13 | 11.61 ± 0.44 | 13.97 ± 0.53 | −34.38 ± 1.30 |
| Wastewater Samples | Final Conc (mg/L) | Removal (%) |
|---|---|---|
| S1 | 1.22 ± 0.066 | 93.9 |
| S2 | 0.87 ± 0.054 | 95.7 |
| S3 | 1.47 ± 0.061 | 92.7 |
| S4 | 2.03 ± 0.095 | 89.9 |
| S5 | 0.66 ± 0.040 | 96.7 |
| Pharmaceutical Compounds | Adsorbent Dosage (g) | Conc (mg/L) | Solution pH (pH Units) | Removal Efficiencies (%) | Qmax (mg/g) |
|---|---|---|---|---|---|
| Ibuprofen | 1 | 1 | 6 | 96 | 0.70 |
| Acetaminophen | 98 | 0.64 | |||
| Diclofenac | 92 | 0.85 | |||
| Ketoprofen | 88 | 0.66 |
| Source of Activated Carbon | Adsorption Capacity (mg/g) | Removal Efficiency (%Re) | References |
|---|---|---|---|
| Dates pits | 434 | - | [62] |
| Commercial activated carbon | 113.6 | 90 | [63] |
| Prosopis juliflora bark | 10.29 | >90 | [34] |
| orange/citrus peels-activated carbon | 33 | 98.0 | [35] |
| Populous leaves charcoal | 90.4 | - | [36] |
| Longan seed- activated carbon | 464.6 | 95.8 | [33] |
| Popcorn—activated carbon | 969 | 48.5 | [64] |
| waste tire rubber—activated carbon | 588 | 80.0 | [65] |
| Coconut shell—activated carbon | 3000 | 100 | [66] |
| Activated carbon | 78.7 | 67 | [67] |
| SBA-15 | 294.1 | - | [68] |
| Coffee grounds | 558 | - | [69] |
| Commercial activated carbon | 129.3 | 97.8 | This work |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Serban, G.V.; Iancu, V.I.; Dinu, C.; Tenea, A.; Vasilache, N.; Cristea, I.; Niculescu, M.; Ionescu, I.; Chiriac, F.L. Removal Efficiency and Adsorption Kinetics of Methyl Orange from Wastewater by Commercial Activated Carbon. Sustainability 2023, 15, 12939. https://doi.org/10.3390/su151712939
Serban GV, Iancu VI, Dinu C, Tenea A, Vasilache N, Cristea I, Niculescu M, Ionescu I, Chiriac FL. Removal Efficiency and Adsorption Kinetics of Methyl Orange from Wastewater by Commercial Activated Carbon. Sustainability. 2023; 15(17):12939. https://doi.org/10.3390/su151712939
Chicago/Turabian StyleSerban, Gabriel Valentin, Vasile Ion Iancu, Cristina Dinu, Anda Tenea, Nicoleta Vasilache, Ionut Cristea, Marcela Niculescu, Ioana Ionescu, and Florentina Laura Chiriac. 2023. "Removal Efficiency and Adsorption Kinetics of Methyl Orange from Wastewater by Commercial Activated Carbon" Sustainability 15, no. 17: 12939. https://doi.org/10.3390/su151712939
APA StyleSerban, G. V., Iancu, V. I., Dinu, C., Tenea, A., Vasilache, N., Cristea, I., Niculescu, M., Ionescu, I., & Chiriac, F. L. (2023). Removal Efficiency and Adsorption Kinetics of Methyl Orange from Wastewater by Commercial Activated Carbon. Sustainability, 15(17), 12939. https://doi.org/10.3390/su151712939







