Metal Accumulation and Tolerance of Energy Willow to Copper and Nickel under Simulated Drought Conditions
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Material and Experiment Set-Up
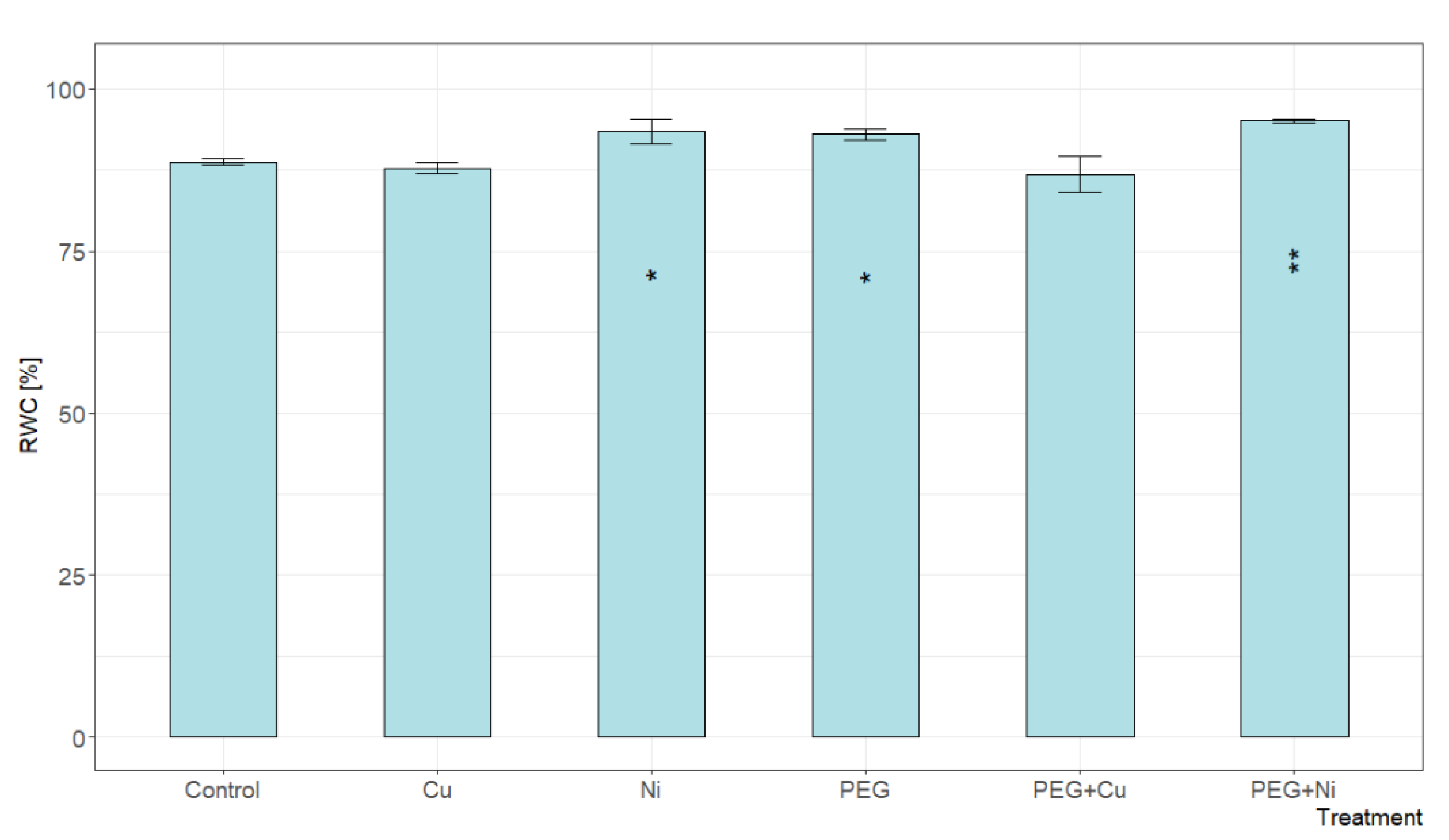
2.2. Relative Water Content of Leaves
2.3. Metal Content and Uptake Ratios
2.4. Elemental Analysis of Plant Material
2.5. Proline and Total Phenolic Content in Leaves
2.6. Statistical Analysis
3. Results
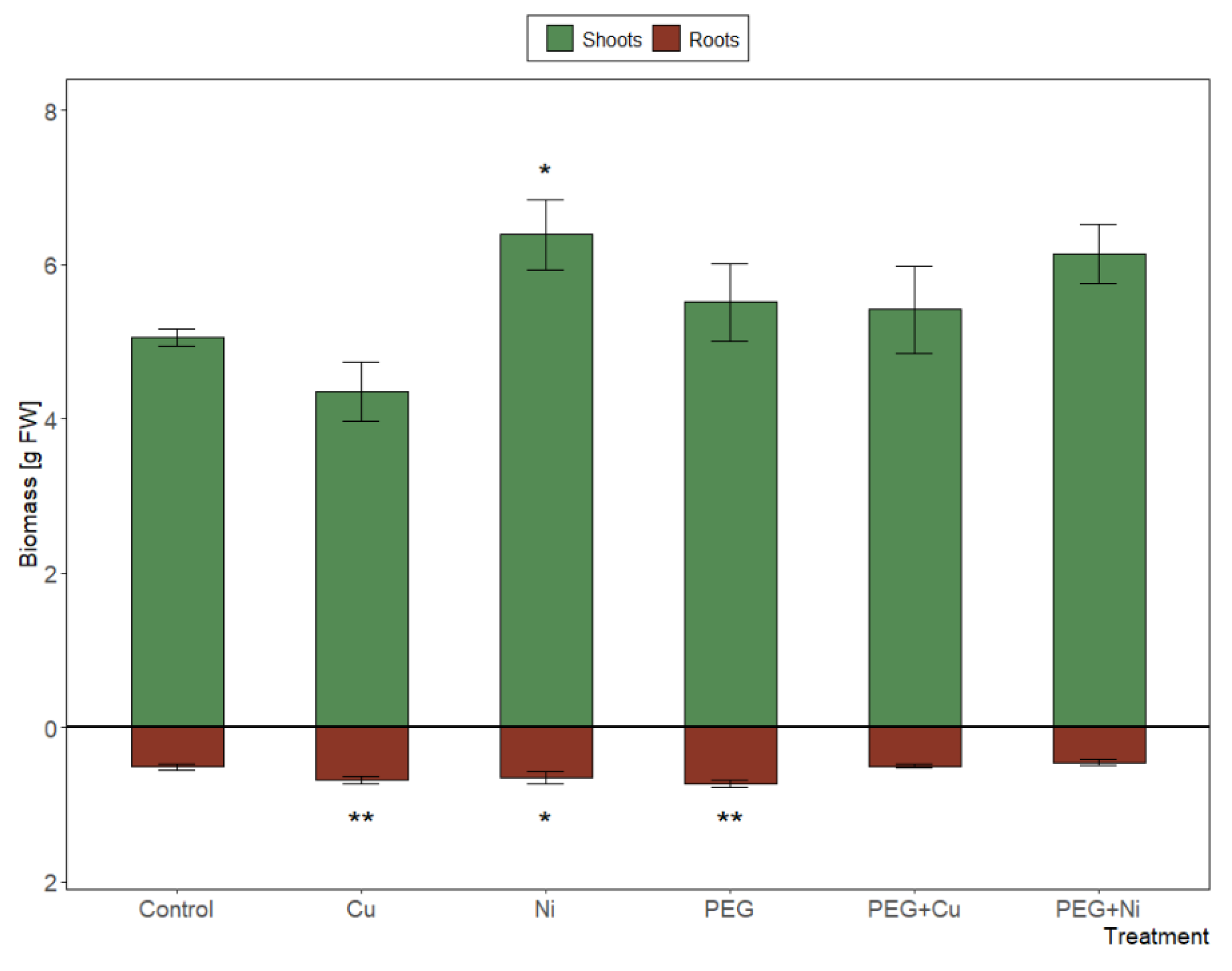
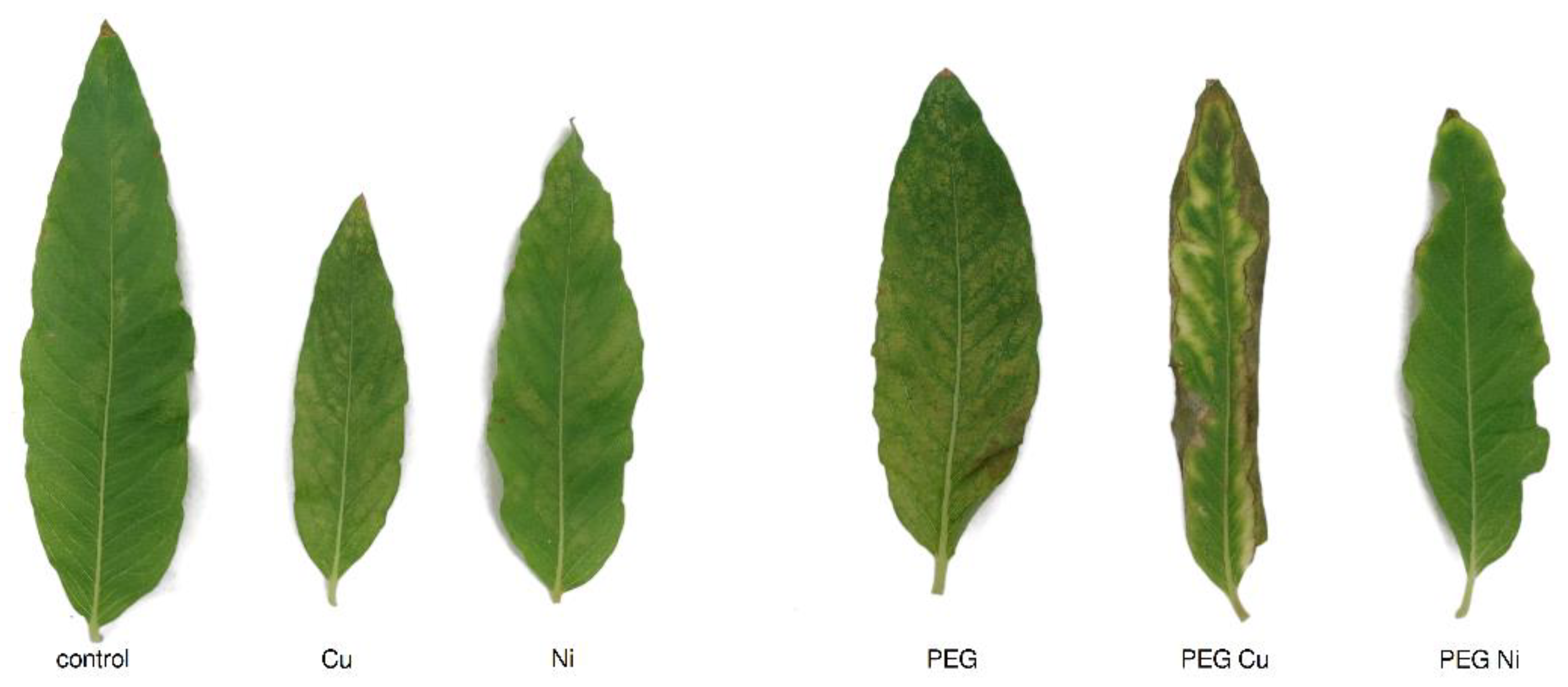
3.1. Plant Growth, Toxicity Symptoms and Relative Water Content
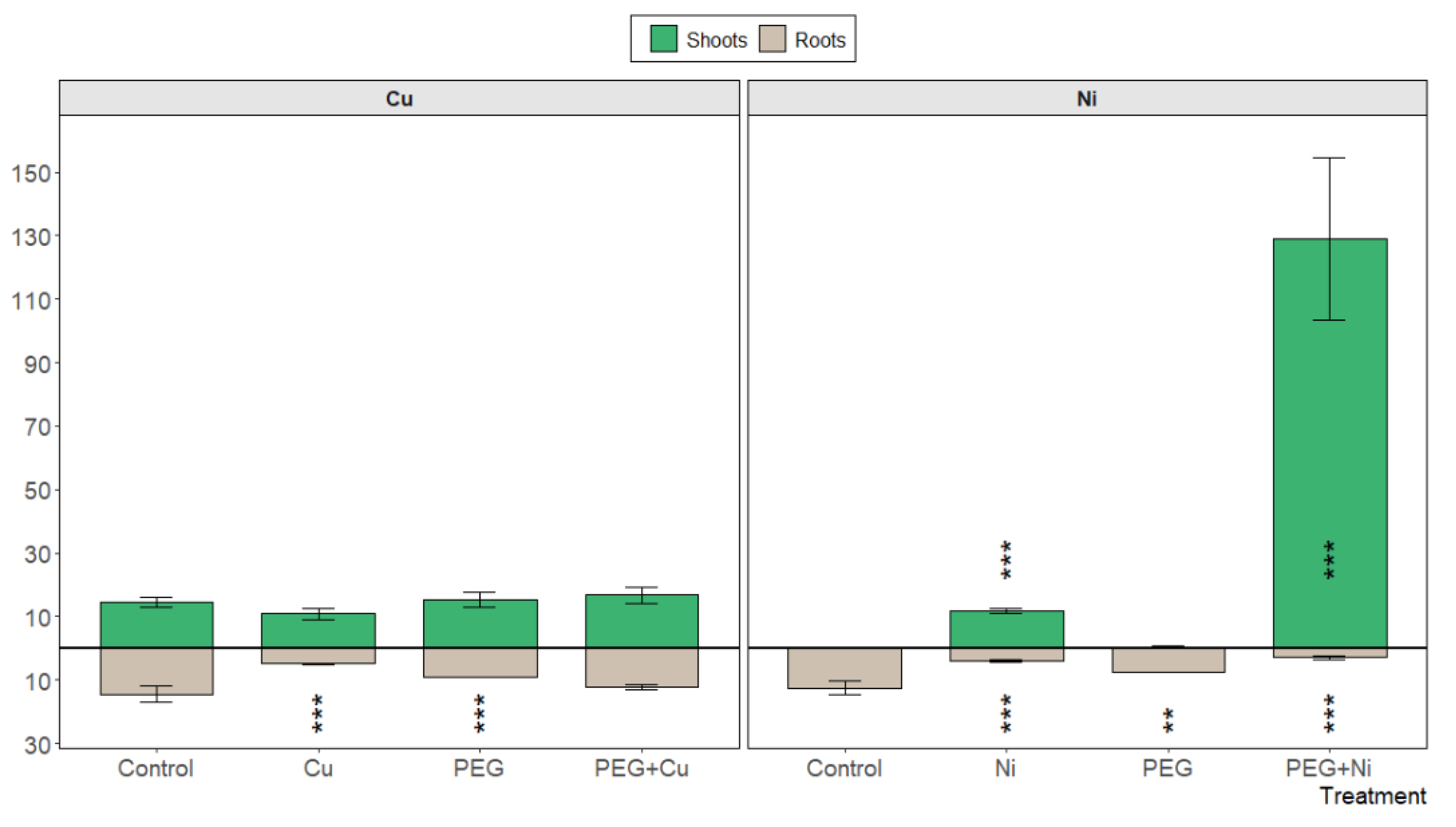
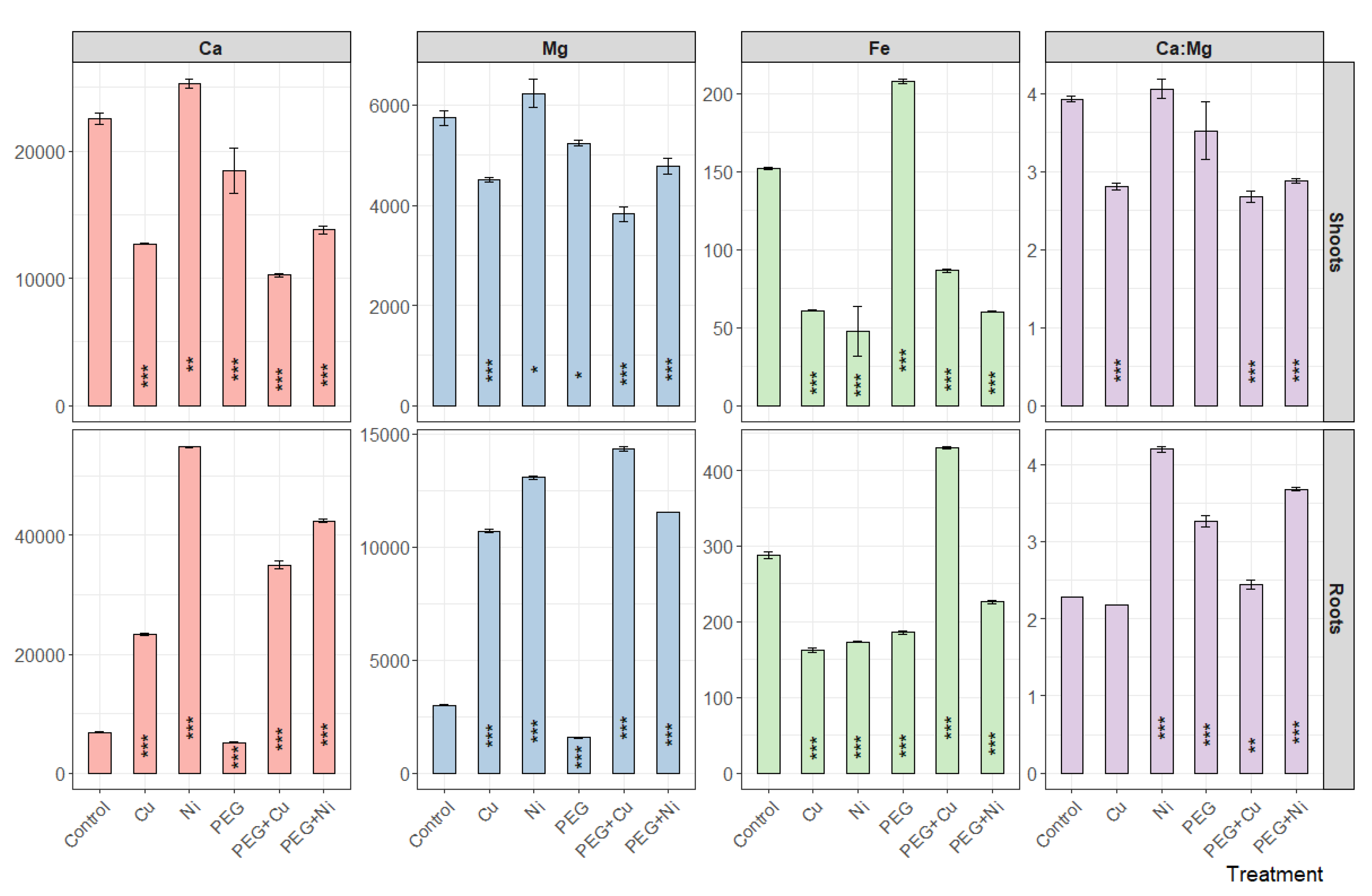
3.2. Metal Uptake and Mineral Nutrient Allocation
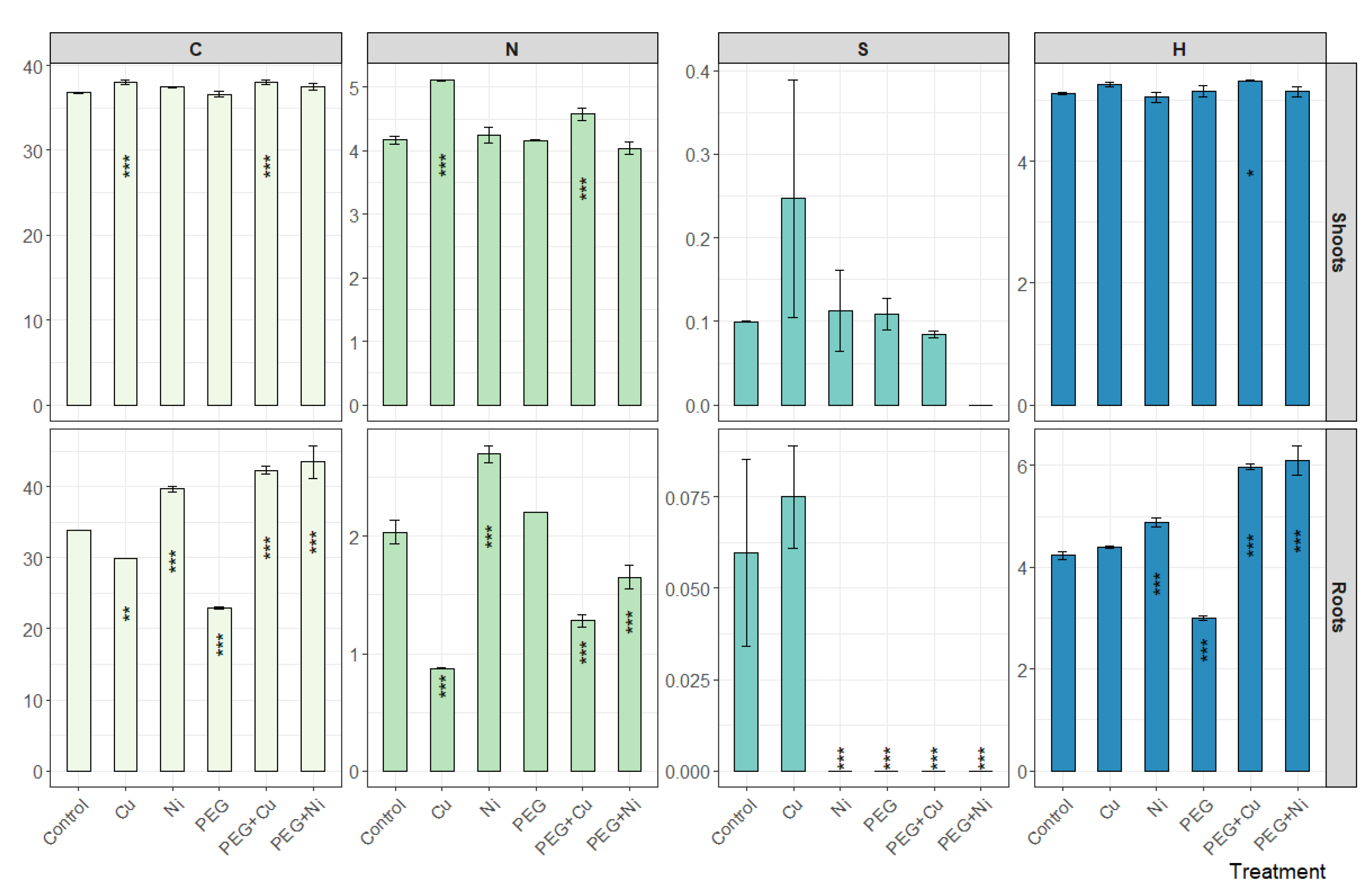
3.3. Elemental Composition of Roots and Shoots
3.4. Proline and Total Phenolic Contents in Leaves
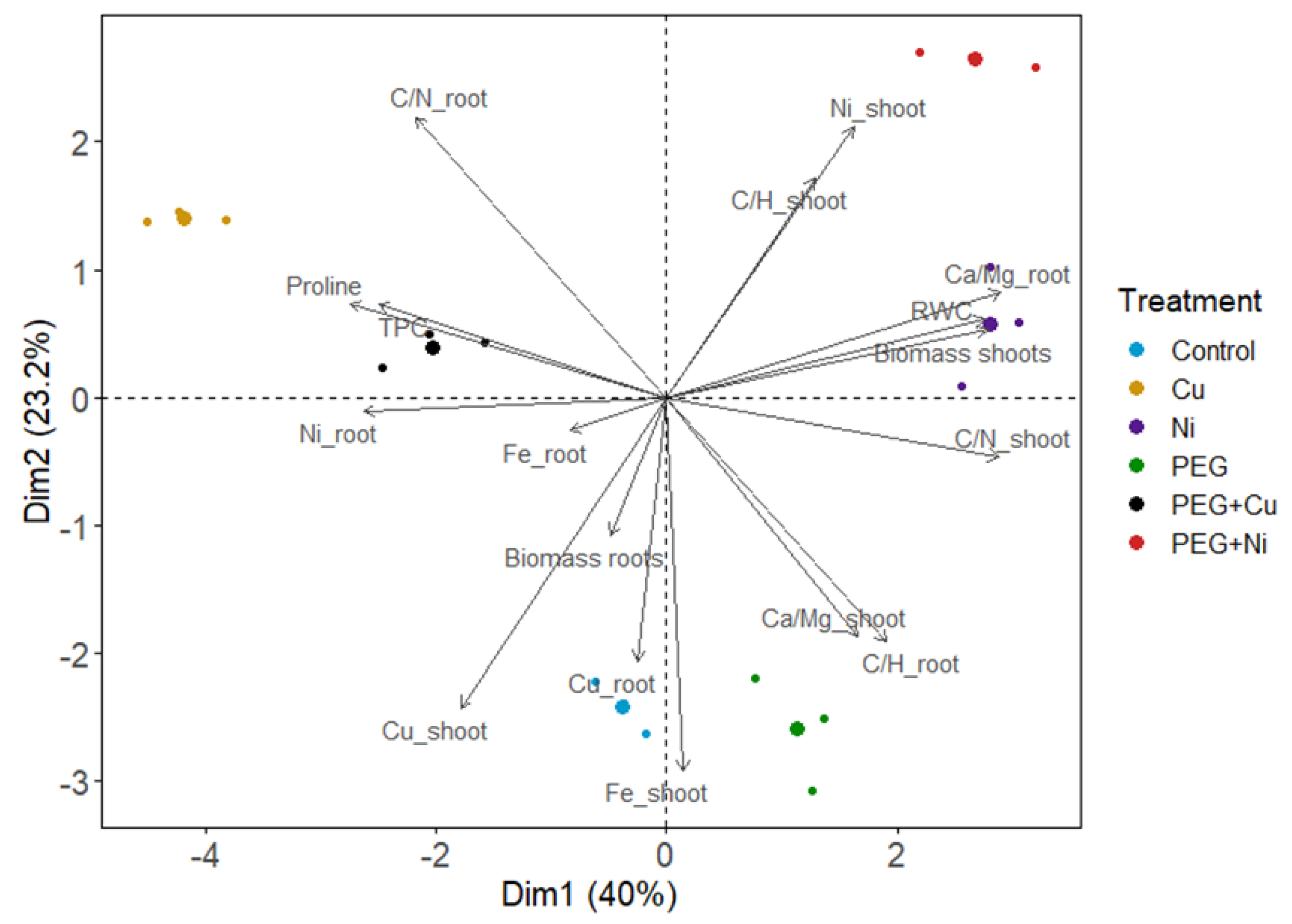
3.5. Principal Components Analysis (PCA)
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Rentier, G.; Lelieveldt, H.; Kramer, G.J. Varieties of coal-fired power phase-out across Europe. Energy Policy 2019, 132, 620–632. [Google Scholar] [CrossRef]
- Akerboom, S.; Botzen, W.; Buijze, A.; Michels, A.; van Rijswick, M. Meeting goals of sustainability policy: CO2 emission reduction, cost-effectiveness and societal acceptance. An analysis of the proposal to phase-out coal in the Netherlands. Energy Policy 2020, 138, 111210. [Google Scholar] [CrossRef]
- Stolarski, M.J.; Szczukowski, S.; Tworkowski, J.; Klasa, A. Yield, energy parameters and chemical composition of short-rotation willow biomass. Ind. Crops Prod. 2013, 46, 60–65. [Google Scholar] [CrossRef]
- Stolarski, M.J.; Szczukowski, S.; Tworkowski, J.; Krzyżaniak, M. Cost of heat energy generation from willow biomass. Renew. Energy 2013, 59, 100–104. [Google Scholar] [CrossRef]
- Sahu, S.G.; Chakraborty, N.; Sarkar, P. Coal–biomass co-combustion: An overview. Renew. Sustain. Energy Rev. 2014, 39, 575–586. [Google Scholar] [CrossRef]
- Stolarski, M.J.; Krzyżaniak, M.; Łuczyński, M.; Załuski, D.; Szczukowski, S.; Tworkowski, J.; Gołaszewski, J. Lignocellulosic biomass from short rotation woody crops as a feedstock for second-generation bioethanol production. Ind. Crops Prod. 2015, 75, 66–75. [Google Scholar] [CrossRef]
- Su, T.; Zhao, D.; Khodadadi, M.; Len, C. Lignocellulosic biomass for bioethanol: Recent advances, technology trends, and barriers to industrial development. Curr. Opin. Green Sustain. Chem. 2020, 24, 56–60. [Google Scholar] [CrossRef]
- Urbaniak, M.; Wyrwicka, A.; Tołoczko, W.; Serwecińska, L.; Zieliński, M. The effect of sewage sludge application on soil properties and willow (Salix sp.) cultivation. Sci. Total Environ. 2017, 586, 66–75. [Google Scholar] [CrossRef]
- Schulz, U.; Brauner, O.; Gruß, H. Animal diversity on short-rotation coppices—A review. Landbauforsch. Volkenrode 2009, 59, 171–181. [Google Scholar]
- Volk, T.A.; Verwijst, T.; Tharakan, P.J.; Abrahamson, L.P.; White, E.H. Growing fuel: A sustainability assessment of willow biomass crops. Front. Ecol. Environ. 2004, 2, 411–418. [Google Scholar] [CrossRef]
- Marmiroli, M.; Pietrini, F.; Maestri, E.; Zacchini, M.; Marmiroli, N.; Massacci, A. Growth, physiological and molecular traits in Salicaceae trees investigated for phytoremediation of heavy metals and organics. Tree Physiol. 2011, 31, 1319–1334. [Google Scholar] [CrossRef]
- Prabha, J.; Kumar, M.; Tripathi, R. Opportunities and challenges of utilizing energy crops in phytoremediation of environmental pollutants: A review. Bioremediat. Environ. Sustain. 2021, 383–396. [Google Scholar] [CrossRef]
- Dimitriou, I.; Aronsson, P. Wastewater and sewage sludge application to willows and poplars grown in lysimeters—Plant response and treatment efficiency. Biomass Bioenergy 2011, 35, 161–170. [Google Scholar] [CrossRef]
- Scriba, C.; Lunguleasa, A.; Spirchez, C.; Ciobanu, V. Influence of INGER and TORDIS energetic willow clones planted on contaminated soil on the survival rates, yields and calorific value. Forests 2021, 12, 826. [Google Scholar] [CrossRef]
- Tőzsér, D.; Harangi, S.; Baranyai, E.; Lakatos, G.; Fülöp, Z.; Tóthmérész, B.; Simon, E. Phytoextraction with Salix viminalis in a moderately to strongly contaminated area. Environ. Sci. Pollut. Res. 2018, 25, 3275–3290. [Google Scholar] [CrossRef]
- Kuzovkina, Y.A.; Knee, M.; Quigley, M.F. Cadmium and Copper Uptake and Translocation in Five Willow (Salix L.) Species. Int. J. Phytoremediation 2004, 6, 269–287. [Google Scholar] [CrossRef]
- Drzewiecka, K.; Mleczek, M.; Gąsecka, M.; Magdziak, Z.; Budka, A.; Chadzinikolau, T.; Kaczmarek, Z.; Goliński, P. Copper and nickel co-treatment alters metal uptake and stress parameters of Salix purpurea × viminalis. J. Plant Physiol. 2017, 216, 125–134. [Google Scholar] [CrossRef]
- Haverkort, A.J.; Verhagen, A. Climate change and its repercussions for the potato supply chain. Potato Res. 2008, 51, 223–237. [Google Scholar] [CrossRef]
- Kapoor, D.; Bhardwaj, S.; Landi, M.; Sharma, A.; Ramakrishnan, M.; Sharma, A. The Impact of Drought in Plant Metabolism: How to Exploit Tolerance Mechanisms to Increase Crop Production. Appl. Sci. 2020, 10, 5692. [Google Scholar] [CrossRef]
- Naliwajski, M.; Skłodowska, M. The relationship between the antioxidant system and proline metabolism in the leaves of cucumber plants acclimated to salt stress. Cells 2021, 10, 609. [Google Scholar] [CrossRef]
- Osakabe, Y.; Osakabe, K.; Shinozaki, K.; Tran, L.S.P. Response of plants to water stress. Front. Plant Sci. 2014, 5, 86. [Google Scholar] [CrossRef] [PubMed]
- Ngole, V.M.; Ekosse, G.I.E. Copper, nickel and zinc contamination in soils within the precincts of mining and landfilling environments. Int. J. Environ. Sci. Technol. 2012, 9, 485–494. [Google Scholar] [CrossRef]
- Simončič, A.; Sušin, J.; Šinkovec, M.; Leskovšek, R.; Čuš, F.; Žnidaršič Pongrac, V.; Baša Česnik, H. Twelve-year investigation of copper soil concentrations shows that vineyards are at risk. Acta Agric. Scand. Sect. B—Soil Plant Sci. 2017, 67, 381–394. [Google Scholar] [CrossRef]
- Tóth, G.; Hermann, T.; da Silva, M.R.; Montanarella, L. Heavy metals in agricultural soils of the European Union with implications for food safety. Environ. Int. 2016, 88, 299–309. [Google Scholar] [CrossRef] [PubMed]
- Helios, W. Effect of white clover (Trifolium repens L.) undersowing cultivation and nitrogen fertilization on weed infestation, biomass yield and its component, content and uptake of macroelements of willow (Salix viminalis L.). Agronomy 2021, 11, 786. [Google Scholar] [CrossRef]
- Kim, H.G.; Song, H.J.; Jeong, M.J.; Seo, Y.L.; Yang, J.K.; Yoo, S.B.; Choi, M.S. Bioethanol production by enzymatic saccharification of Salix viminalis var. gigantea biomass. Forest Sci. Technol. 2014, 10, 67–72. [Google Scholar] [CrossRef]
- Kuś, J.; Matyka, M. Productivity of selected crops planted for energy purposes depending on soil quality. Fragm. Agron. 2009, 26, 103–110. [Google Scholar]
- Osmolovskaya, N.; Shumilina, J.; Kim, A.; Didio, A.; Grishina, T.; Bilova, T.; Keltsieva, O.A.; Zhukov, V.; Tikhonovich, I.; Tarakhovskaya, E.; et al. Methodology of drought stress research: Experimental setup and physiological characterization. Int. J. Mol. Sci. 2018, 19, 4089. [Google Scholar] [CrossRef]
- Peršić, V.; Ament, A.; Antunović Dunić, J.; Drezner, G.; Cesar, V. PEG-induced physiological drought for screening winter wheat genotypes sensitivity–integrated biochemical and chlorophyll a fluorescence analysis. Front. Plant Sci. 2022, 13, 987702. [Google Scholar] [CrossRef]
- Baker, A.J.M.; McGrath, S.P.; Sidoli, C.M.D.; Reeves, R.D. The possibility of in situ heavy metal decontamination of polluted soils using crops of metal-accumulating plants. Resour. Conserv. Recycl. 1994, 11, 41–49. [Google Scholar] [CrossRef]
- Weatherley, P.E.; Slatyer, R.O. Relationship between relative turgidity and diffusion pressure deficit in leaves. Nature 1957, 179, 1085–1086. [Google Scholar] [CrossRef]
- Baker, A.J. Accumulators and excluders-strategies in the response of plants to heavy metals. J. Plant Nutr. 1981, 3, 643–654. [Google Scholar] [CrossRef]
- Ma, L.Q.; Komar, K.M.; Tu, C.; Zhang, W.; Cai, Y.; Kennelley, E.D. A fern that hyperaccumulates arsenic. Nature 2001, 409, 579. [Google Scholar] [CrossRef] [PubMed]
- Woodrow, P.; Ciarmiello, L.F.; Annunziata, M.G.; Pacifico, S.; Iannuzzi, F.; Mirto, A.; D’Amelia, L.; Dell’Aversana, E.; Piccolella, S.; Fuggi, A.; et al. Durum wheat seedling responses to simultaneous high light and salinity involve a fine reconfiguration of amino acids and carbohydrate metabolism. Physiol. Plant. 2017, 159, 290–312. [Google Scholar] [CrossRef] [PubMed]
- Dewanto, V.; Wu, X.; Adom, K.K.; Liu, R.H. Thermal processing enhances the nutritional value of tomatoes by increasing total antioxidant activity. J. Agric. Food Chem. 2002, 50, 3010–3014. [Google Scholar] [CrossRef] [PubMed]
- Wickham, H. Elegant graphics for data analysis (ggplot2). In Applied Spatial Data Analysis R; Springer: New York, NY, USA, 2009; pp. 784–785. [Google Scholar]
- Frejowski, A.; Bondaruk, J.; Duda, A. Challenges and opportunities for end-of-life coal mine sites: Black-to-Green energy approach. Energies 2021, 14, 1385. [Google Scholar] [CrossRef]
- Perea-Moreno, M.A.; Samerón-Manzano, E.; Perea-Moreno, A.J. Biomass as renewable energy: Worldwide research trends. Sustainability 2019, 11, 863. [Google Scholar] [CrossRef]
- Stolarski, M.J.; Niksa, D.; Krzyżaniak, M.; Tworkowski, J.; Szczukowski, S. Willow productivity from small-and large-scale experimental plantations in Poland from 2000 to 2017. Renew. Sustain. Energy Rev. 2019, 101, 461–475. [Google Scholar] [CrossRef]
- Su, R.; Xie, T.; Yao, H.; Chen, Y.; Wang, H.; Dai, X.; Wang, Y.; Shi, L.; Luo, Y. Lead Responses and Tolerance Mechanisms of Koelreuteria paniculata: A Newly Potential Plant for Sustainable Phytoremediation of Pb-Contaminated Soil. Int. J. Environ. Res. Public Health 2022, 19, 14968. [Google Scholar] [CrossRef]
- Pouresmaieli, M.; Ataei, M.; Forouzandeh, P.; Azizollahi, P.; Mahmoudifard, M. Recent progress on sustainable phytoremediation of heavy metals from soil. J. Environ. Chem. Eng. 2022, 10, 108482. [Google Scholar] [CrossRef]
- Su, R.; Ou, Q.; Wang, H.; Dai, X.; Chen, Y.; Luo, Y.; Yao, H.; Ouyang, D.; Li, Z.; Wang, Z. Organic-inorganic composite modifiers enhance restoration potential of Nerium oleander L. to lead-zinc tailing: Application of phytoremediation. Environ. Sci. Pollut. Res. Int. 2023, 30, 56569–56579. [Google Scholar] [CrossRef] [PubMed]
- Fagnano, M.; Visconti, D.; Fiorentino, N. Agronomic approaches for characterization, remediation, and monitoring of contaminated sites. Agronomy 2020, 10, 1335. [Google Scholar] [CrossRef]
- Łapiński, D.; Wiater, J.; Szatyłowicz, E. The content of heavy metals in waste as an indicator determining the possibilities of their agricultural use. J. Ecol. Eng. 2019, 20, 225–230. [Google Scholar] [CrossRef] [PubMed]
- Kumar, V.; Pandita, S.; Sidhu, G.P.S.; Sharma, A.; Khanna, K.; Kaur, P.; Bali, A.S.; Setia, R. Copper bioavailability, uptake, toxicity and tolerance in plants: A comprehensive review. Chemosphere 2021, 262, 127810. [Google Scholar] [CrossRef]
- Rai, S.; Singh, P.K.; Mankotia, S.; Swain, J.; Satbhai, S.B. Iron homeostasis in plants and its crosstalk with copper, zinc, and manganese. Plant Stress 2021, 1, 100008. [Google Scholar] [CrossRef]
- Martins, L.L.; Mourato, M.P. Effect of Excess Copper on Tomato Plants: Growth Parameters, Enzyme Activities, Chlorophyll, and Mineral Content. J. Plant Nutr. 2006, 29, 2179–2198. [Google Scholar] [CrossRef]
- Rehman, M.; Liu, L.; Wang, Q.; Saleem, M.H.; Bashir, S.; Ullah, S.; Peng, D. Copper environmental toxicology, recent advances, and future outlook: A review. Environ. Sci. Pollut. Res. 2019, 26, 18003–18016. [Google Scholar] [CrossRef]
- Krohling, C.A.; Eutrópio, F.J.; Bertolazi, A.A.; Dobbss, L.B.; Campostrini, E.; Dias, T.; Ramos, A.C. Ecophysiology of iron homeostasis in plants. Soil Sci. Plant Nutr. 2016, 62, 39–47. [Google Scholar] [CrossRef]
- Rout, G.R.; Sahoo, S. Role of iron in plant growth and metabolism. Rev. Agric. Sci. 2015, 3, 1–24. [Google Scholar] [CrossRef]
- Acosta-Gamboa, L.M.; Liu, S.; Langley, E.; Campbell, Z.; Castro-Guerrero, N.; Mendoza-Cozatl, D.; Lorence, A. Moderate to severe water limitation differentially affects the phenome and ionome of Arabidopsis. Funct. Plant Biol. 2017, 44, 94–106. [Google Scholar] [CrossRef]
- Sánchez-Rodríguez, E.; del Mar Rubio-Wilhelmi, M.; Cervilla, L.M.; Blasco, B.; Rios, J.J.; Leyva, R.; Romero, L.; Ruiz, J.M. Study of the ionome and uptake fluxes in cherry tomato plants under moderate water stress conditions. Plant Soil 2010, 335, 339–347. [Google Scholar] [CrossRef]
- D’Oria, A.; Courbet, G.; Billiot, B.; Jing, L.; Pluchon, S.; Arkoun, M.; Maillard, A.; Roux, C.P.; Trouverie, J.; Etienne, P.; et al. Drought specifically downregulates mineral nutrition: Plant ionomic content and associated gene expression. Plant Direct 2022, 6, e402. [Google Scholar] [CrossRef] [PubMed]
- Schat, H. The role of phytochelatins in constitutive and adaptive heavy metal tolerances in hyperaccumulator and non-hyperaccumulator metallophytes. J. Exp. Bot. 2002, 53, 2381–2392. [Google Scholar] [CrossRef] [PubMed]
- Chandregowda, M.H.; Tjoelker, M.G.; Pendall, E.; Zhang, H.; Churchill, A.C.; Power, S.A. Belowground carbon allocation, root trait plasticity, and productivity during drought and warming in a pasture grass. J. Exp. Bot. 2023, 74, 2127–2145. [Google Scholar] [CrossRef]
- Canarini, A.; Kaiser, C.; Merchant, A.; Richter, A.; Wanek, W. Root Exudation of Primary Metabolites: Mechanisms and Their Roles in Plant Responses to Environmental Stimuli. Front. Plant Sci. 2019, 10, 157. [Google Scholar] [CrossRef]
- Szabados, L.; Savouré, A. Proline: A multifunctional amino acid. Trends Plant Sci. 2010, 15, 89–97. [Google Scholar] [CrossRef]
- Bündig, C.; Vu, T.H.; Meise, P.; Seddig, S.; Schum, A.; Winkelmann, T. Variability in osmotic stress tolerance of starch potato genotypes (Solanum tuberosum L.) as revealed by an in vitro screening: Role of proline, osmotic adjustment and drought response in pot trials. J. Agron. Crop Sci. 2017, 203, 206–218. [Google Scholar] [CrossRef]
- Lazcano-Ferrat, I.; Lovatt, C.J. Relationship between relative water content, nitrogen pools, and growth of Phaseolus vulgaris L. and P. acutifolius A. Gray during water deficit. Crop Sci. 1999, 39, 467–475. [Google Scholar] [CrossRef]
- Zouari, M.; Ahmed, C.B.; Elloumi, N.; Bellassoued, K.; Delmail, D.; Labrousse, P.; Ben Abdallah, F.; Rouina, B.B. Impact of proline application on cadmium accumulation, mineral nutrition and enzymatic antioxidant defense system of Olea europaea L. cv. Chemlali exposed to cadmium stress. Ecotoxicol. Environ. Saf. 2016, 128, 195–205. [Google Scholar] [CrossRef]
- Drzewiecka, K.; Mleczek, M.; Gąsecka, M.; Magdziak, Z.; Goliński, P.; Chadzinikolau, T. Copper phytoextraction with Salix purpurea × viminalis under various Ca/Mg ratios. Part 2. Effect on organic acid, phenolics and salicylic acid contents. Acta Physiol. Plant. 2014, 36, 903–913. [Google Scholar] [CrossRef]
- Mleczek, M.; Budka, A.; Gąsecka, M.; Budzyńska, S.; Drzewiecka, K.; Magdziak, Z.; Rutkowski, P.; Goliński, P.; Niedzielski, P. Copper, lead and zinc interactions during phytoextraction using Acer platanoides L.—A pot trial. Environ. Sci. Pollut. Res. 2023, 30, 27191–27207. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Drzewiecka, K.; Gawrysiak, P.; Woźniak, M.; Rybak, M. Metal Accumulation and Tolerance of Energy Willow to Copper and Nickel under Simulated Drought Conditions. Sustainability 2023, 15, 13084. https://doi.org/10.3390/su151713084
Drzewiecka K, Gawrysiak P, Woźniak M, Rybak M. Metal Accumulation and Tolerance of Energy Willow to Copper and Nickel under Simulated Drought Conditions. Sustainability. 2023; 15(17):13084. https://doi.org/10.3390/su151713084
Chicago/Turabian StyleDrzewiecka, Kinga, Przemysław Gawrysiak, Magdalena Woźniak, and Michał Rybak. 2023. "Metal Accumulation and Tolerance of Energy Willow to Copper and Nickel under Simulated Drought Conditions" Sustainability 15, no. 17: 13084. https://doi.org/10.3390/su151713084






