Exploring the Psychophysiological Effects of Viewing Urban Nature through Virtual Reality Using Electroencephalography and Perceived Restorativeness Scale Measures
Abstract
:1. Introduction
1.1. Theoretical Frameworks
1.2. Study Purpose
1.3. Perceived Restorativeness and Environment
1.4. Brain Activity and Environment
1.4.1. Indoor Immersion
1.4.2. Virtual Reality
1.4.3. Frontal Alpha Asymmetry
1.5. Research Questions and Hypotheses
2. Materials and Methods
2.1. Within-Subject Experimental Design
2.2. Participant Recruitment
2.3. Experimental Images
2.4. EEG Data Processing
2.5. Statistical Analysis
3. Results
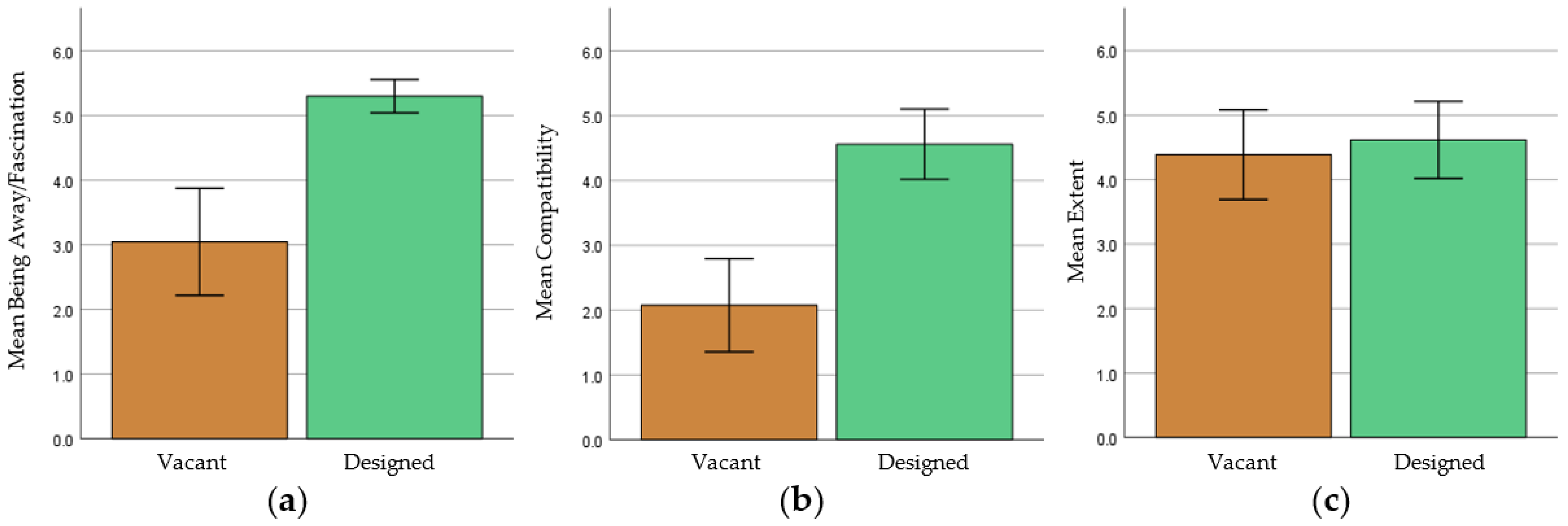
3.1. Perceived Restorativeness
3.1.1. Factor Analysis
3.1.2. Within-Subject Paired t-Test
3.2. EEG Data
3.2.1. Pairwise t-Tests
3.2.2. Within-Subject Paired t-Test and Repeated-Measures ANOVA
3.3. Frontal Alpha Asymmetry
Paired t-Test
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kaplan, R.; Kaplan, S. The Experience of Nature: A Psychological Perspective; Cambridge University Press: Cambridge, UK, 1989. [Google Scholar]
- Ulrich, R. Aesthetic and Affective Response to Natural Environment. In Behavior and the Natural Environment; Altman, I., Wohlwill, J., Eds.; Springer: New York, NY, USA, 1983. [Google Scholar]
- Kaplan, S. The restorative benefits of nature: Toward an integrative framework. J. Environ. Psychol. 1995, 15, 169–182. [Google Scholar] [CrossRef]
- James, W. Psychology; Henry Holt and Company: New York, NY, USA, 1892. [Google Scholar]
- Hartig, T.; Mang, M.; Evans, G.W. Restorative Effects of Natural Environment Experiences. Environ. Behav. 1991, 23, 3–26. [Google Scholar] [CrossRef]
- Hernández, B.; Hidalgo, M. Effect of Urban Vegetation on Psychological Restorativeness. Psychol. Rep. 2005, 96, 1025–1028. [Google Scholar] [CrossRef] [PubMed]
- Hartig, T.; Korpela, K.; Evans, G.; Gärling, T. A Measure of restorative quality in environments. Hous. Theory Soc. 1997, 14, 175–194. [Google Scholar] [CrossRef]
- Hartig, T.; Korpela, K.; Evans, G. Validation of a measure of perceived environmental restorativeness. Psychol. Rep. 1996, 26, 10025970228. [Google Scholar]
- Berto, R. Exposure to restorative environments helps restore attentional capacity. J. Environ. Psychol. 2005, 25, 249–259. [Google Scholar] [CrossRef]
- Berto, R. The Role of Nature in Coping with Psycho-Physiological Stress: A Literature Review on Restorativeness. Behav. Sci. 2014, 4, 394–409. [Google Scholar] [CrossRef] [PubMed]
- Hartig, T.; Kaiser, F.; Bowler, P. Further Development of a Measure of Perceived Environmental Restorativeness; Working Paper #5; Institutet för Bostads-och Urbanforskning: Gävle, Sweden, 1997. [Google Scholar]
- Pasini, M.; Berto, R.; Brondino, M.; Hall, R.; Ortner, C. How to Measure the Restorative Quality of Environments: The PRS-11. Procedia Soc. 2014, 159, 293–297. [Google Scholar] [CrossRef]
- Purcell, T.; Peron, E.; Berto, R. Why do Preferences Differ between Scene Types? Environ. Behav. 2001, 33, 93–106. [Google Scholar] [CrossRef]
- Lee, K.E.; Williams, K.J.H.; Sargent, L.D.; Williams, N.S.G.; Johnson, K.A. 40-second green roof views sustain attention: The role of micro-breaks in attention restoration. J. Environ. Psychol. 2015, 42, 182–189. [Google Scholar] [CrossRef]
- Wang, X.; Rodiek, S.; Wu, C.; Chen, Y.; Li, Y. Stress recovery and restorative effects of viewing different urban park scenes in Shanghai, China. Urban For. Urban Green. 2016, 15, 112–122. [Google Scholar] [CrossRef]
- Mahamane, S.; Wan, N.; Porter, A.; Hancock, A.S.; Campbell, J.; Lyon, T.E.; Jordan, K.E. Natural Categorization: Electrophysiological Responses to Viewing Natural Versus Built Environments. Front. Psychol. 2020, 11, 990. [Google Scholar] [PubMed]
- Stigsdotter, U.K.; Corazon, S.S.; Sidenius, U.; Kristiansen, J.; Grahn, P. It is not all bad for the grey city—A crossover study on physiological and psychological restoration in a forest and an urban environment. Health Place 2017, 46, 145–154. [Google Scholar] [CrossRef] [PubMed]
- Korpela, K.M. Perceived Restorativeness of Urban and Natural Scenes—Photographic Illustrations. J. Archit. Plan. 2013, 30, 23–38. [Google Scholar]
- Wilkie, S.; Clouston, L. Environment preference and environment type congruence: Effects on perceived restoration potential and restoration outcomes. Urban For. Urban Green. 2015, 14, 368–376. [Google Scholar] [CrossRef]
- Schutte, N.S.; Bhullar, N.; Stilinović, E.J.; Richardson, K. The Impact of Virtual Environments on Restorativeness and Affect. Ecopsychology 2017, 9, 1–7. [Google Scholar] [CrossRef]
- Browning, M.H.E.M.; Mimnaugh, K.J.; van Riper, C.J.; Laurent, H.K.; LaValle, S.M. Can Simulated Nature Support Mental Health? Comparing Short, Single-Doses of 360-Degree Nature Videos in Virtual Reality with the Outdoors. Front. Psychol. 2020, 10, 2667. [Google Scholar] [CrossRef]
- Abhang, P.A.; Gawali, B.W.; Mehrotra, S.C. Chapter 2—Technological Basics of EEG Recording and Operation of Apparatus. In Introduction to EEG- and Speech-Based Emotion Recognition; Abhang, P.A., Gawali, B.W., Mehrotra, S.C., Eds.; Academic Press: Cambridge, MA, USA, 2016; pp. 19–50. [Google Scholar]
- Klimesch, W. EEG alpha and theta oscillations reflect cognitive and memory performance: A review and analysis. Brain Res. Rev. 1999, 29, 169–195. [Google Scholar] [CrossRef]
- Gevins, A.; Smith, M.E. Neurophysiological measures of cognitive workload during human-computer interaction. Theor. Issues Ergon. Sci. 2003, 4, 113–131. [Google Scholar] [CrossRef]
- Sauseng, P.; Hoppe, J.; Klimesch, W.; Gerloff, C.; Hummel, F.C. Dissociation of sustained attention from central executive functions: Local activity and interregional connectivity in the theta range. Eur. J. Neurosci. 2007, 25, 587–593. [Google Scholar] [CrossRef]
- Khosla, A.; Khandnor, P.; Chand, T. A comparative analysis of signal processing and classification methods for different applications based on EEG signals. Biocybern. Biomed. Eng. 2020, 40, 649–690. [Google Scholar] [CrossRef]
- Schomer, D.L.; Lopes da Silva, F.H. Niedermeyer’s Electroencephalography: Basic Principles, Clinical Applications, and Related Fields, 7th ed.; Oxford University Press: New York, NY, USA, 2018. [Google Scholar]
- Ulrich, R.S. Natural Versus Urban Scenes: Some Psychophysiological Effects. Environ. Behav. 1991, 13, 523–556. [Google Scholar] [CrossRef]
- Chang, C.Y.; Hammitt, W.E.; Chen, P.K.; Machnik, L.; Su, W.C. Psychophysiological responses and restorative values of natural environments in Taiwan. Landsc. Urban Plan. 2008, 85, 79–84. [Google Scholar] [CrossRef]
- Grassini, S.; Segurini, G.V.; Koivisto, M. Watching Nature Videos Promotes Physiological Restoration: Evidence from the Modulation of Alpha Waves in Electroencephalography. Front. Psychol. 2022, 13, 871143. [Google Scholar] [CrossRef]
- Elsadek, M.; Shao, Y.; Liu, B. Benefits of Indirect Contact with Nature on the Physiopsychological Well-Being of Elderly People. HERD Health Environ. Res. Des. J. 2021, 14, 227–241. [Google Scholar] [CrossRef] [PubMed]
- Jiang, M.; Hassan, D.; Chen, Q.; Liu, Y. Effects of different landscape visual stimuli on psychophysiological responses in Chinese students. Indoor Built Environ. 2019, 29, 1006–1016. [Google Scholar] [CrossRef]
- Elsadek, M.; Liu, B.; Xie, J. Window view and relaxation: Viewing green space from a high-rise estate improves urban dwellers’ wellbeing. Urban For. Urban Green. 2020, 55, 126846. [Google Scholar] [CrossRef]
- Grassini, S.; Revonsuo, A.; Castellotti, S.; Petrizzo, I.; Benedetti, V.; Koivisto, M. Processing of natural scenery is associated with lower attentional and cognitive load compared with urban ones. J. Environ. Psychol. 2019, 62, 1–11. [Google Scholar] [CrossRef]
- Wang, Y.; Jiang, M.; Huang, Y.; Sheng, Z.; Huang, X.; Lin, W.; Chen, Q.; Li, X.; Luo, Z.; Lv, B. Physiological and Psychological Effects of Watching Videos of Different Durations Showing Urban Bamboo Forests with Varied Structures. Int. J. Environ. Res. Public Health 2020, 17, 3434. [Google Scholar] [CrossRef]
- Teo, W.P.; Muthalib, M.; Yamin, S.; Hendy, A.M.; Bramstedt, K.; Kotsopoulos, E.; Perrey, S.; Ayaz, H. Does a Combination of Virtual Reality, Neuromodulation and Neuroimaging Provide a Comprehensive Platform for Neurorehabilitation?—A Narrative Review of the Literature. Front. Hum. Neurosci. 2016, 10, 284. [Google Scholar] [CrossRef]
- Wohlgenannt, I.; Simons, A.; Stieglitz, S. Virtual Reality. Bus. Inf. Syst. Eng. 2020, 62, 455–461. [Google Scholar] [CrossRef]
- Gao, T.; Zhang, T.; Zhu, L.; Gao, Y.; Qiu, L. Exploring Psychophysiological Restoration and Individual Preference in the Different Environments Based on Virtual Reality. Int. J. Environ. Res. Public Health 2019, 16, 3102. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.C.; Sit, C.H.P.; Tang, T.W.; Tsai, C.L. Psychological and Physiological Responses in Patients with Generalized Anxiety Disorder: The Use of Acute Exercise and Virtual Reality Environment. Int. J. Environ. Res. Public Health 2020, 17, 4855. [Google Scholar] [CrossRef] [PubMed]
- Zhu, H.; Yang, F.; Bao, Z.; Nan, X. A study on the impact of Visible Green Index and vegetation structures on brain wave change in residential landscape. Urban For. Urban Green. 2021, 64, 127299. [Google Scholar] [CrossRef]
- Hu, M.; Roberts, J. Built Environment Evaluation in Virtual Reality Environments-A Cognitive Neuroscience Approach. Urban Sci. 2020, 4, 48. [Google Scholar] [CrossRef]
- Rounds, J.D.; Cruz-Garza, J.G.; Kalantari, S. Using Posterior EEG Theta Band to Assess the Effects of Architectural Designs on Landmark Recognition in an Urban Setting. Front. Hum. Neurosci. 2020, 14, 584385. [Google Scholar] [CrossRef]
- Allen, J.J.B.; Coan, J.A.; Nazarian, M. Issues and assumptions on the road from raw signals to metrics of frontal EEG asymmetry in emotion. Biol. Psychol. 2004, 67, 183–218. [Google Scholar] [CrossRef]
- Coan, J.A.; Allen, J.J.B. The state and trait nature of frontal EEG asymmetry in emotion. In The Asymmetrical Brain; Hugdahl, K., Davidson, R.J., Eds.; Boston Review: Boston, MA, USA, 2003; pp. 565–615. [Google Scholar]
- Coan, J.A.; Allen, J.J.B. Frontal EEG asymmetry as a moderator and mediator of emotion. Biol. Psychol. 2004, 67, 7–49. [Google Scholar] [CrossRef]
- Davidson, R.J. Cerebral asymmetry and emotion: Conceptual and methodological conundrums. Cogn. Emot. 1993, 7, 115–138. [Google Scholar] [CrossRef]
- Smith, E.E.; Reznik, S.J.; Stewart, J.L.; Allen, J.J.B. Assessing and conceptualizing frontal EEG asymmetry: An updated primer on recording, processing, analyzing, and interpreting frontal alpha asymmetry. Int. J. Psychophysiol. 2017, 111, 98–114. [Google Scholar] [CrossRef]
- Harmon-Jones, E. Clarifying the emotive functions of asymmetrical frontal cortical activity. Psychophysiology 2003, 40, 838–848. [Google Scholar] [CrossRef] [PubMed]
- Olszewska-Guizzo, A.; Paiva, T.; Barbosa, F. Effects of 3D Contemplative Landscape Videos on Brain Activity in a Passive Exposure EEG Experiment. Front. Psychiatry 2018, 9, 317. [Google Scholar] [CrossRef] [PubMed]
- Olszewska-Guizzo, A.; Sia, A.; Fogel, A.; Ho, R. Can Exposure to Certain Urban Green Spaces Trigger Frontal Alpha Asymmetry in the Brain?—Preliminary Findings from a Passive Task EEG Study. Int. J. Environ. Res. Public Health 2020, 17, 394. [Google Scholar] [CrossRef] [PubMed]
- Olszewska-Guizzo, A.; Fogel, A.; Escoffier, N.; Ho, R. Effects of COVID-19-related stay-at-home order on neuropsychophysiological response to urban spaces: Beneficial role of exposure to nature? J. Environ. Psychol. 2021, 75, 101590. [Google Scholar] [CrossRef]
- Delorme, A.; Makeig, S. EEGLAB: An open source toolbox for analysis of single-trial EEG dynamics including independent component analysis. J. Neurosci. Methods 2004, 134, 9–21. [Google Scholar] [CrossRef]
- Welch, P. The use of fast Fourier transform for the estimation of power spectra: A method based on time averaging over short, modified periodograms. IEEE Trans. Audio Electroacoust. 1967, 15, 70–73. [Google Scholar] [CrossRef]
- Parke, C.S. Module 5: Identifying and Addressing Outliers. In Essential First Steps to Data Analysis: Scenario Based Examples Using SPSS; Sage Publications, Inc.: Thousand Oaks, CA, USA, 2013; pp. 81–102. [Google Scholar]
- Deng, L.; Li, X.; Luo, H.; Fu, E.K.; Ma, J.; Sun, L.X.; Huang, Z.; Cai, S.Z.; Jia, Y. Empirical study of landscape types, landscape elements and landscape components of the urban park promoting physiological and psychological restoration. Urban For. Urban Green. 2020, 48, 126488. [Google Scholar] [CrossRef]
- Herman, K.; Ciechanowski, L.; Przegalinska, A. Emotional Well-Being in Urban Wilderness: Assessing States of Calmness and Alertness in Informal Green Spaces (IGSs) with Muse-Portable EEG Headband. Sustainability 2021, 13, 2212. [Google Scholar] [CrossRef]
- Reeves, J.P.; Knight, A.T.; Strong, E.A.; Heng, V.; Neale, C.; Cromie, R.; Vercammen, A. The Application of Wearable Technology to Quantify Health and Wellbeing Co-benefits From Urban Wetlands. Front. Psychol. 2019, 10, 1840. [Google Scholar] [CrossRef]
- Neale, C.; Aspinall, P.; Roe, J.; Tilley, S.; Mavros, P.; Cinderby, S.; Coyne, R.; Thin, N.; Ward Thompson, C. The impact of walking in different urban environments on brain activity in older people. Cities Health 2019, 4, 94–106. [Google Scholar] [CrossRef]
- Jiang, B.; Chang, C.Y.; Sullivan, W.C. A dose of nature: Tree cover, stress reduction, and gender differences. Landsc. Urban Plan. 2014, 132, 26–36. [Google Scholar] [CrossRef]
- Sillman, D.; Rigolon, A.; Browning, M.H.E.M.; Yoon, H.; McAnirlin, O. Do sex and gender modify the association between green space and physical health? A systematic review. Environ. Res. 2022, 209, 112869. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Korczykowski, M.; Rao, H.; Fan, Y.; Pluta, J.; Gur, R.C.; McEwen, B.S.; Detre, J.A. Gender difference in neural response to psychological stress. Soc. Cogn. Affect. Neurosci. 2007, 2, 227–239. [Google Scholar] [CrossRef] [PubMed]




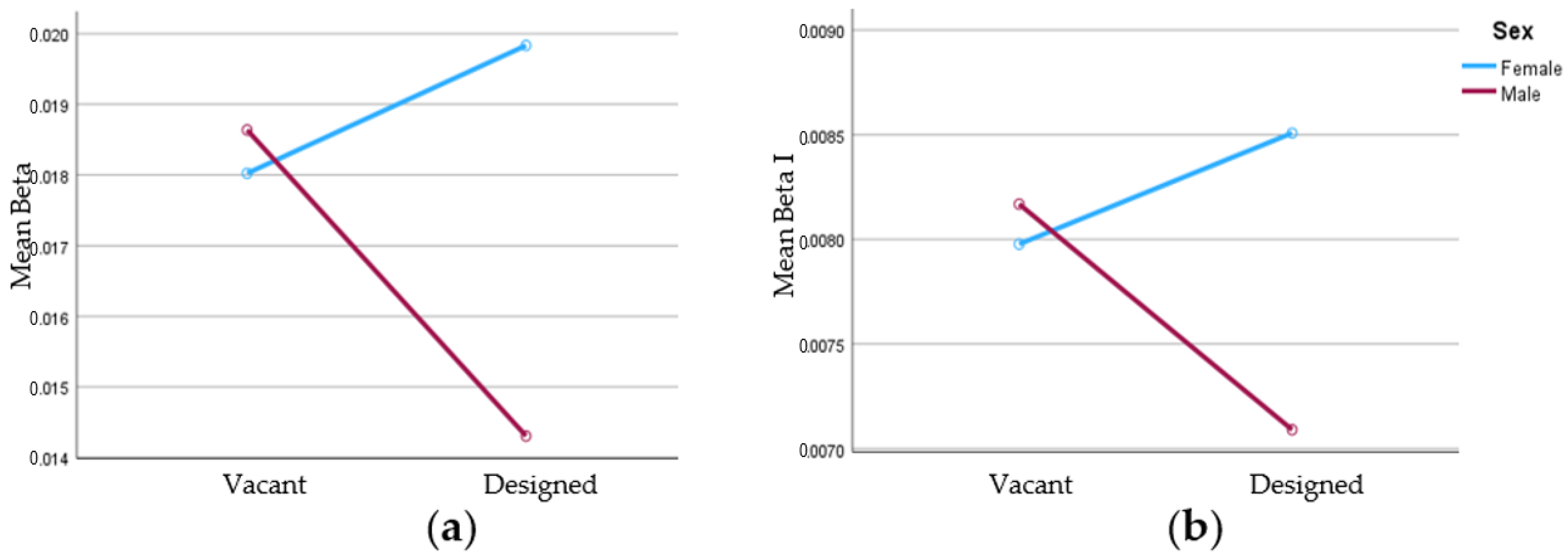

| Frontal | ||||||
| Spectral Power | Source | SS | df | MS | F | Sig. |
| Beta_F7F8 | Stimulus Environment | 0.0038216 | 1 | 0.0038216 | 0.368 | 0.552 |
| Stimulus Environment x Sex | 0.0339466 | 1 | 0.0339466 | 3.265 | 0.088 * | |
| Error (Stimulus Environment) | 0.1767241 | 17 | 0.0103955 | |||
| Beta I_F7F8 | Stimulus Environment | 0.0001592 | 1 | 0.0001592 | 0.308 | 0.586 |
| Stimulus Environment x Sex | 0.0019104 | 1 | 0.0019104 | 3.692 | 0.072 * | |
| Error (Stimulus Environment) | 0.0087975 | 17 | 0.0005175 | |||
| Beta II_F7F8 | Stimulus Environment | 0.0024108 | 1 | 0.0024108 | 0.382 | 0.545 |
| Stimulus Environment x Sex | 0.0193894 | 1 | 0.0193894 | 3.073 | 0.098 * | |
| Error (Stimulus Environment) | 0.1072637 | 17 | 0.0063096 | |||
| Parietal | ||||||
| Spectral Power | Source | SS | df | MS | F | Sig. |
| Beta_P3P4 | Stimulus Environment | 0.000013609 | 1 | 0.000013609 | 0.601 | 0.449 |
| Stimulus Environment x Sex | 0.000080710 | 1 | 0.000080710 | 3.565 | 0.077 * | |
| Error (Stimulus Environment) | 0.000362242 | 16 | 0.000022640 | |||
| Beta I_P3P4 | Stimulus Environment | 0.000000641 | 1 | 0.000000641 | 0.497 | 0.491 |
| Stimulus Environment x Sex | 0.000005537 | 1 | 0.000005537 | 4.289 | 0.055 * | |
| Error (Stimulus Environment) | 0.000020654 | 16 | 0.000001291 | |||
| Beta_P7P8 | Stimulus Environment | 0.000274949 | 1 | 0.000274949 | 0.089 | 0.769 |
| Stimulus Environment x Sex | 0.011404846 | 1 | 0.011404846 | 3.682 | 0.072 * | |
| Error (Stimulus Environment) | 0.052652411 | 17 | 0.003097201 | |||
| Beta I_P7P8 | Stimulus Environment | 0.000006007 | 1 | 0.000006007 | 0.038 | 0.848 |
| Stimulus Environment x Sex | 0.000619247 | 1 | 0.000619247 | 3.886 | 0.065 * | |
| Error (Stimulus Environment) | 0.002709286 | 17 | 0.000159370 | |||
| Beta II_P7P8 | Stimulus Environment | 0.000198630 | 1 | 0.000198630 | 0.105 | 0.750 |
| Stimulus Environment x Sex | 0.006578250 | 1 | 0.006578250 | 3.464 | 0.080 * | |
| Error (Stimulus Environment) | 0.032280665 | 17 | 0.001898863 |
| t | dt | One-Sided p | Two-Sided p | |
|---|---|---|---|---|
| V_AAsym_F7F8 − D_AAsym_F7F8 | 1.5268 | 18 | 0.0721 * | 0.1442 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Seiz, A.; Kweon, B.-S.; Ellis, C.D.; Oh, H.; Pietro, K. Exploring the Psychophysiological Effects of Viewing Urban Nature through Virtual Reality Using Electroencephalography and Perceived Restorativeness Scale Measures. Sustainability 2023, 15, 13090. https://doi.org/10.3390/su151713090
Seiz A, Kweon B-S, Ellis CD, Oh H, Pietro K. Exploring the Psychophysiological Effects of Viewing Urban Nature through Virtual Reality Using Electroencephalography and Perceived Restorativeness Scale Measures. Sustainability. 2023; 15(17):13090. https://doi.org/10.3390/su151713090
Chicago/Turabian StyleSeiz, Audrey, Byoung-Suk Kweon, Christopher D. Ellis, Hyuk Oh, and Kyle Pietro. 2023. "Exploring the Psychophysiological Effects of Viewing Urban Nature through Virtual Reality Using Electroencephalography and Perceived Restorativeness Scale Measures" Sustainability 15, no. 17: 13090. https://doi.org/10.3390/su151713090






