Architecture Optimization of a Single-Chamber Air-Cathode MFC by Increasing the Number of Cathode Electrodes
Abstract
:1. Introduction
2. Materials and Methods
2.1. MFC Construction and Operation
2.2. Analytical Methods and Calculations
2.3. Linear Sweep Voltammetry (LSV) and Electrochemical Impedance Spectroscopy (EIS) for the Electrochemical Characterization of the MFC Performance
3. Results
3.1. MFC Operation with 4 and 6 Cathode Electrodes
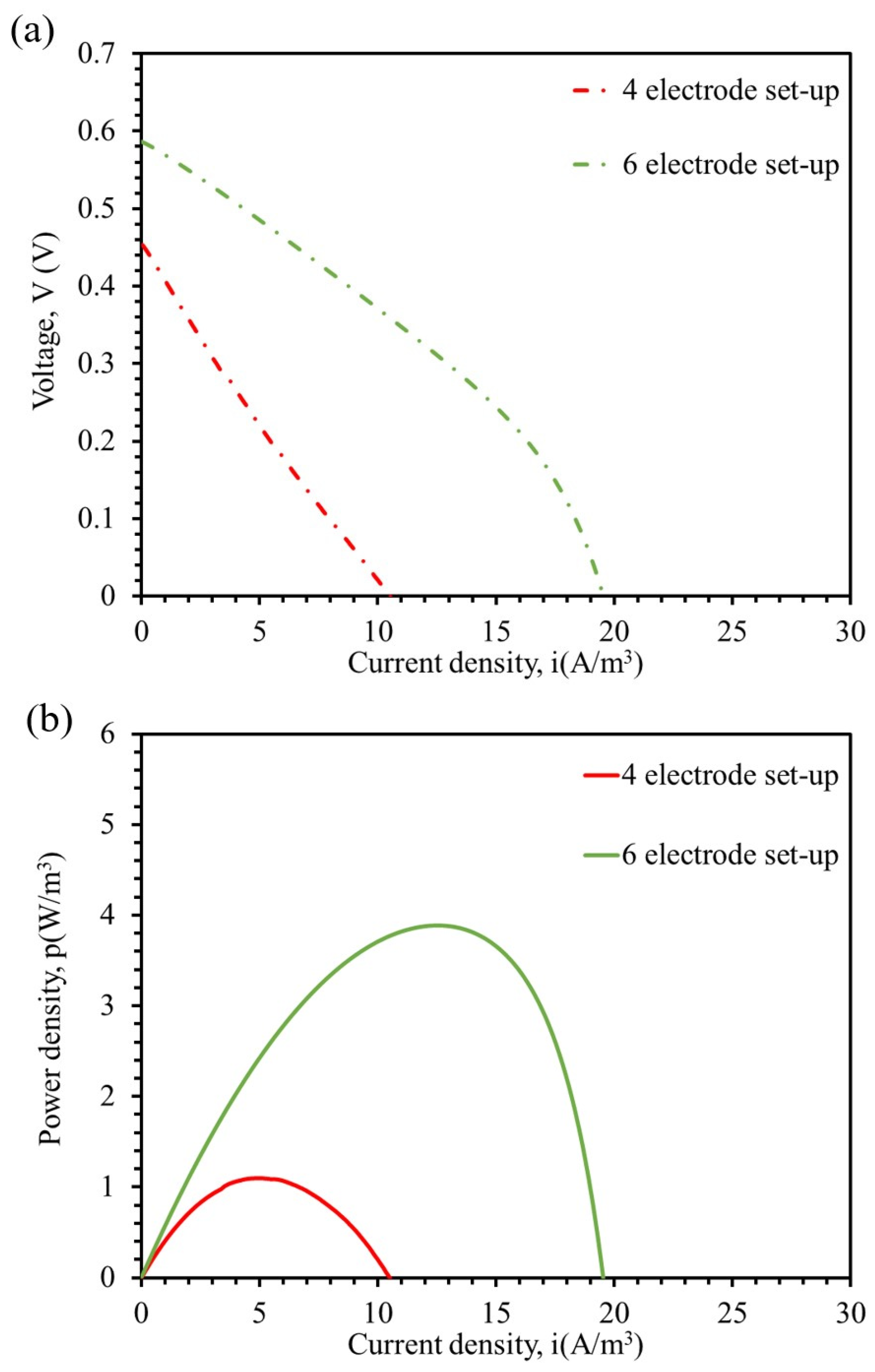
3.2. Effect of the Number of Cathode Electrodes on Polarization and Power Output of the MFC Unit
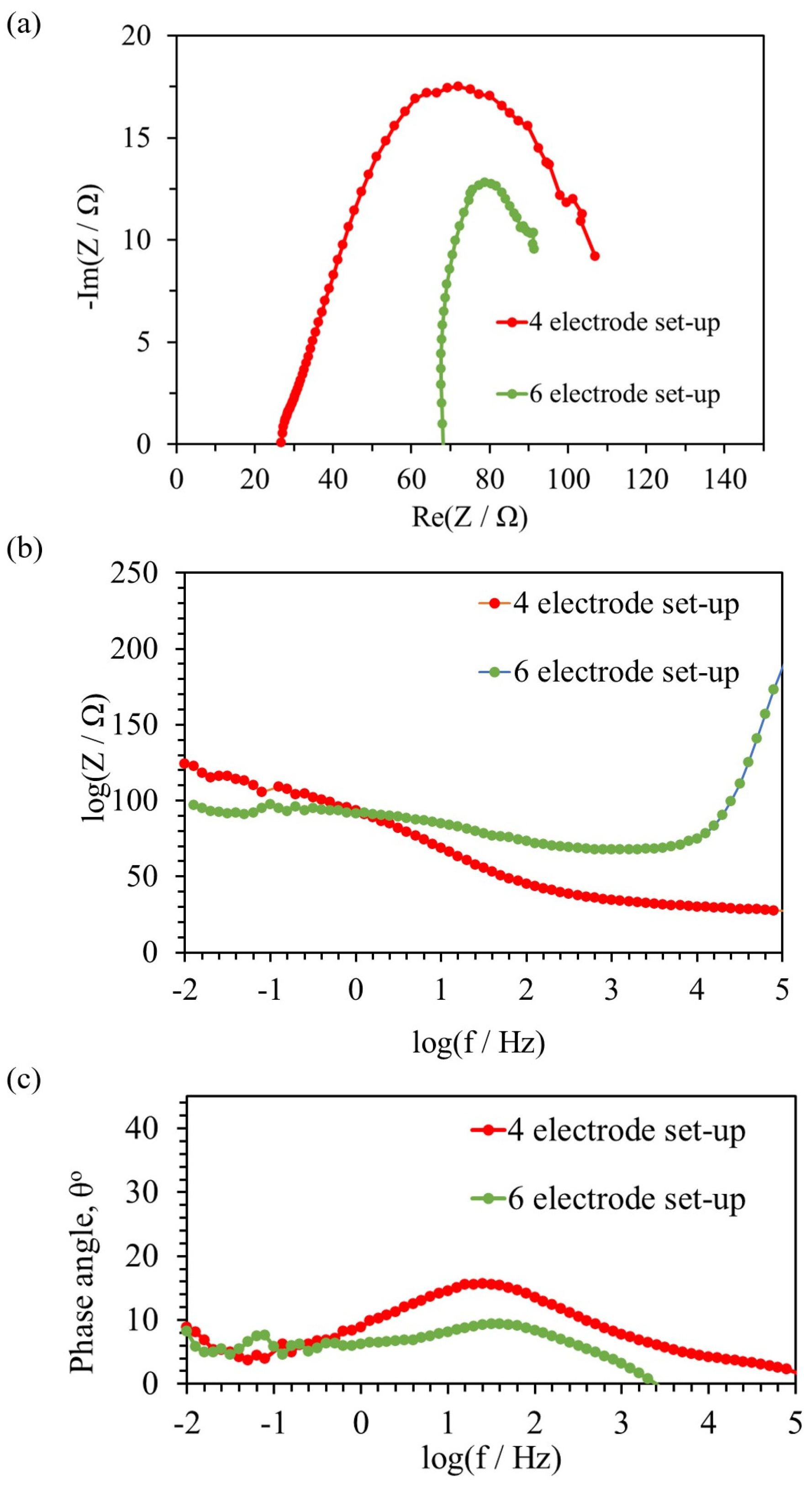
3.3. Electrochemical Impedance Spectroscopy
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Kim, K.-Y.; Rossi, R.; Regan, J.M.; Logan, B.E. Enumeration of exoelectrogens in microbial fuel cell effluents fed acetate or wastewater substrates. Biochem. Eng. J. 2020, 165, 107816. [Google Scholar] [CrossRef]
- Ida, T.K.; Mandal, B. Microbial fuel cell design, application and performance: A review. Mater. Today Proc. 2022, 76, 88–94. [Google Scholar] [CrossRef]
- Janicek, A.; Fan, Y.; Liu, H. Design of microbial fuel cells for practical application: A review and analysis of scale-up studies. Biofuels 2014, 5, 79–92. [Google Scholar] [CrossRef]
- Boas, J.V.; Oliveira, V.B.; Simões, M.; Pinto, A.M. Review on microbial fuel cells applications, developments and costs. J. Environ. Manag. 2022, 307, 114525. [Google Scholar] [CrossRef]
- Sarma, R.; Tamuly, A.; Kakati, B.K. Recent developments in electricity generation by Microbial Fuel Cell using different substrates. Mater. Today Proc. 2021, 49, 457–463. [Google Scholar] [CrossRef]
- Bird, H.; Heidrich, E.S.; Leicester, D.D.; Theodosiou, P. Pilot-scale Microbial Fuel Cells (MFCs): A meta-analysis study to inform full-scale design principles for optimum wastewater treatment. J. Clean. Prod. 2022, 346, 131227. [Google Scholar] [CrossRef]
- Mateo, S.; Cañizares, P.; Rodrigo, M.A.; Fernández-Morales, F.J. Reproducibility and robustness of microbial fuel cells technology. J. Power Sources 2019, 412, 640–647. [Google Scholar] [CrossRef]
- Santoro, C.; Flores-Cadengo, C.; Soavi, F.; Kodali, M.; Merino-Jimenez, I.; Gajda, I.; Greenman, J.; Ieropoulos, I.; Atanassov, P. Ceramic Microbial Fuel Cells Stack: Power generation in standard and supercapacitive mode. Sci. Rep. 2018, 8, 3281. [Google Scholar] [CrossRef] [PubMed]
- Tremouli, A.; Greenman, J.; Ieropoulos, I. Investigation of ceramic MFC stacks for urine energy extraction. Bioelectrochemistry 2018, 123, 19–25. [Google Scholar] [CrossRef]
- Kim, B.; Choi, S.; Jang, J.K.; Chang, I.S. Self-recoverable voltage reversal in stacked microbial fuel cells due to biofilm capacitance. Bioresour. Technol. 2017, 245, 1286–1289. [Google Scholar] [CrossRef]
- Ledezma, P.; Greenman, J.; Ieropoulos, I. MFC-cascade stacks maximise COD reduction and avoid voltage reversal under adverse conditions. Bioresour. Technol. 2013, 134, 158–165. [Google Scholar] [CrossRef]
- Walter, X.A.; You, J.; Winfield, J.; Bajarunas, U.; Greenman, J.; Ieropoulos, I.A. From the lab to the field: Self-stratifying microbial fuel cells stacks directly powering lights. Appl. Energy 2020, 277, 115514. [Google Scholar] [CrossRef]
- Mehravanfar, H.; Mahdavi, M.A.; Gheshlaghi, R. Economic optimization of stacked microbial fuel cells to maximize power generation and treatment of wastewater with minimal operating costs. Int. J. Hydrogen Energy 2019, 44, 20355–20367. [Google Scholar] [CrossRef]
- Prasad, J.; Tripathi, R.K. Review on improving microbial fuel cell power management systems for consumer applications. Energy Rep. 2022, 8, 10418–10433. [Google Scholar] [CrossRef]
- Kim, T.; An, J.; Jang, J.K.; Chang, I.S. Coupling of anaerobic digester and microbial fuel cell for COD removal and ammonia recovery. Bioresour. Technol. 2015, 195, 217–222. [Google Scholar] [CrossRef] [PubMed]
- Tremouli, A.; Kamperidis, T.; Pandis, P.K.; Argirusis, C.; Lyberatos, G. Exploitation of Digestate from Thermophilic and Mesophilic Anaerobic Digesters Fed with Fermentable Food Waste Using the MFC Technology. Waste Biomass Valoriz. 2021, 12, 5361–5370. [Google Scholar] [CrossRef]
- Bundhoo, Z.M.A. Coupling dark fermentation with biochemical or bioelectrochemical systems for enhanced bio-energy production: A review. Int. J. Hydrogen Energy 2017, 42, 26667–26686. [Google Scholar] [CrossRef]
- Zonfa, T.; Kamperidis, T.; Falzarano, M.; Lyberatos, G.; Polettini, A.; Pomi, R.; Rossi, A.; Tremouli, A. Two-Stage Process for Energy Valorization of Cheese Whey through Bio-Electrochemical Hydrogen Production Coupled with Microbial Fuel Cell. Fermentation 2023, 9, 306. [Google Scholar] [CrossRef]
- Ramanavicius, S.; Ramanavicius, A. Charge Transfer and Biocompatibility Aspects in Conducting Polymer-Based Enzymatic Biosensors and Biofuel Cells. Nanomaterials 2021, 11, 371. [Google Scholar] [CrossRef]
- Jimenez, I.M.; Greenman, J.; Ieropoulos, I. Electricity and catholyte production from ceramic MFCs treating urine. Int. J. Hydrogen Energy 2017, 42, 1791–1799. [Google Scholar] [CrossRef]
- Jimenez, I.M.; Brinson, P.; Greenman, J.; Ieropoulos, I. Electronic faucet powered by low cost ceramic microbial fuel cells treating urine. J. Power Sources 2021, 506, 230004. [Google Scholar] [CrossRef]
- Me, M.H.; Abu Bakar, M. Tubular ceramic performance as separator for microbial fuel cell: A review. Int. J. Hydrogen Energy 2020, 45, 22340–22348. [Google Scholar] [CrossRef]
- Kim, T.; Kang, S.; Sung, J.H.; Kang, Y.K.; Kim, Y.H.; Jang, J.K. Characterization of Polyester Cloth as an Alternative Separator to Nafion Membrane in Microbial Fuel Cells for Bioelectricity Generation Using Swine Wastewater. J. Microbiol. Biotechnol. 2016, 26, 2171–2178. [Google Scholar] [CrossRef]
- Zhang, X.; Liang, P.; Shi, J.; Wei, J.; Huang, X. Using a glass fiber separator in a single-chamber air-cathode microbial fuel cell shortens start-up time and improves anode performance at ambient and mesophilic temperatures. Bioresour. Technol. 2013, 130, 529–535. [Google Scholar] [CrossRef] [PubMed]
- Koók, L.; Dörgő, G.; Bakonyi, P.; Rózsenberszki, T.; Nemestóthy, N.; Bélafi-Bakó, K.; Abonyi, J. Directions of membrane separator development for microbial fuel cells: A retrospective analysis using frequent itemset mining and descriptive statistical approach. J. Power Sources 2020, 478, 229014. [Google Scholar] [CrossRef]
- Hirsch, L.O.; Dubrovin, I.A.; Gandu, B.; Emanuel, E.; Kjellerup, B.V.; Ugur, G.E.; Schechter, A.; Cahan, R. Anode amendment with kaolin and activated carbon increases electricity generation in a microbial fuel cell. Bioelectrochemistry 2023, 153, 108486. [Google Scholar] [CrossRef]
- Kamperidis, T.; Pandis, P.K.; Argirusis, C.; Lyberatos, G.; Tremouli, A. Effect of Food Waste Condensate Concentration on the Performance of Microbial Fuel Cells with Different Cathode Assemblies. Sustainability 2022, 14, 2625. [Google Scholar] [CrossRef]
- Yousefi, V.; Mohebbi-Kalhori, D.; Samimi, A. Ceramic-based microbial fuel cells (MFCs): A review. Int. J. Hydrogen Energy 2017, 42, 1672–1690. [Google Scholar] [CrossRef]
- Santoro, C.; Arbizzani, C.; Erable, B.; Ieropoulos, I. Microbial fuel cells: From fundamentals to applications. A review. J. Power Sources 2017, 356, 225–244. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Guo, S.; Wang, J.; Li, C. Fundamental development and research of cathodic compartment in microbial fuel cells: A review. J. Environ. Chem. Eng. 2022, 10, 3. [Google Scholar] [CrossRef]
- Li, X.; Hu, B.; Suib, S.; Lei, Y.; Li, B. Electricity generation in continuous flow microbial fuel cells (MFCs) with manganese dioxide (MnO2) cathodes. Biochem. Eng. J. 2011, 54, 10–15. [Google Scholar] [CrossRef]
- Lv, K.; Zhang, H.; Chen, S. Nitrogen and phosphorus co-doped carbon modified activated carbon as an efficient oxygen reduction catalyst for microbial fuel cells. RSC Adv. 2018, 8, 848–855. [Google Scholar] [CrossRef] [PubMed]
- Cheng, S.; Logan, B.E. Increasing power generation for scaling up single-chamber air cathode microbial fuel cells. Bioresour. Technol. 2011, 102, 4468–4473. [Google Scholar] [CrossRef]
- Papillon, J.; Ondel, O.; Maire, É. Scale up of single-chamber microbial fuel cells with stainless steel 3D anode: Effect of electrode surface areas and electrode spacing. Bioresour. Technol. Rep. 2021, 13, 100632. [Google Scholar] [CrossRef]
- Walter, X.A.; Santoro, C.; Greenman, J.; Ieropoulos, I. Self-stratifying microbial fuel cell: The importance of the cathode electrode immersion height. Int. J. Hydrogen Energy 2019, 44, 4524–4532. [Google Scholar] [CrossRef] [PubMed]
- Kolajo, O.O.; Pandit, C.; Thapa, B.S.; Pandit, S.; Mathuriya, A.S.; Gupta, P.K.; Jadhav, D.A.; Lahiri, D.; Nag, M.; Upadhye, V.J. Impact of cathode biofouling in microbial fuel cells and mitigation techniques. Biocatal. Agric. Biotechnol. 2022, 43, 102408. [Google Scholar] [CrossRef]
- Tremouli, A.; Martinos, M.; Bebelis, S.; Lyberatos, G. Performance assessment of a four-air cathode single-chamber microbial fuel cell under conditions of synthetic and municipal wastewater treatments. J. Appl. Electrochem. 2016, 46, 515–525. [Google Scholar] [CrossRef]
- Logan, B.E. Microbial Fuel Cells; John Wiley & Sons: Hoboken, NJ, USA, 2008. [Google Scholar] [CrossRef]
- APHA/AWWA/WEF. Standard Methods for the Examination of Water and Wastewater; American Public Health Association: Baltimore, MD, USA, 2012; pp. 36–37. ISBN 978-0875532356. [Google Scholar]
- Houghton, J.; Santoro, C.; Soavi, F.; Serov, A.; Ieropoulos, I.; Arbizzani, C.; Atanassov, P. Supercapacitive microbial fuel cell: Characterization and analysis for improved charge storage/delivery performance. Bioresour. Technol. 2016, 218, 552–560. [Google Scholar] [CrossRef]
- Tremouli, A.; Antonopoulou, G.; Bebelis, S.; Lyberatos, G. Operation and characterization of a microbial fuel cell fed with pretreated cheese whey at different organic loads. Bioresour. Technol. 2013, 131, 380–389. [Google Scholar] [CrossRef]
- Sekar, N.; Ramasamy, R.P. Electrochemical Impedance Spectroscopy for Microbial Fuel Cell Characterization. J. Microb. Biochem. Technol. 2013, 6, 1–14. [Google Scholar] [CrossRef]
- Minakshi, M.; Mitchell, D.R.G.; Munnangi, A.R.; Barlow, A.J.; Fichtner, M. New insights into the electrochemistry of magnesium molybdate hierarchical architectures for high performance sodium devices. Nanoscale 2018, 10, 13277–13288. [Google Scholar] [CrossRef] [PubMed]




| Cycle # | Imax (mA) | Δt (h) | COD Removal (%) | CE (%) | Eyield (mJ/g COD/L) |
|---|---|---|---|---|---|
| 1 | 2.0 | 67 | 92% | 13% | 17.7 |
| 2 | 1.9 | 71 | 96% | 15% | 9.50 |
| 3 | 2.6 | 44 | 93% | 14% | 12.2 |
| 4 | 2.6 | 162 | 89% | 28% | 20.4 |
| 5 | 3.2 | 74 | 89% | 15% | 13.2 |
| 6 | 3.2 | 113 | 95% | 18% | 20.0 |
| 7 | 3.0 | 69 | 89% | 13% | 11.7 |
| Electrodes # | Open Circuit Voltage (V) | Volumetric Power Density (W/m3) | Internal Resistance (Ω) |
|---|---|---|---|
| 4 | 0.44 | 1.1 | 124 |
| 6 | 0.59 | 3.9 | 82 |
| Electrodes # | OCV (V) | RS (Ω) | RCT (Ω) | RBF (Ω) | CBF (F) | CCT (F) |
|---|---|---|---|---|---|---|
| 4 | 0.45 | 27 | 94 | 4.0 | 0.3 × 10−4 | 4.8 × 10−3 |
| 6 | 0.59 | 68 | 24 | 3.0 | 0.4 × 10−4 | 5.1 × 10−3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kamperidis, T.; Tremouli, A.; Lyberatos, G. Architecture Optimization of a Single-Chamber Air-Cathode MFC by Increasing the Number of Cathode Electrodes. Sustainability 2023, 15, 13107. https://doi.org/10.3390/su151713107
Kamperidis T, Tremouli A, Lyberatos G. Architecture Optimization of a Single-Chamber Air-Cathode MFC by Increasing the Number of Cathode Electrodes. Sustainability. 2023; 15(17):13107. https://doi.org/10.3390/su151713107
Chicago/Turabian StyleKamperidis, Theofilos, Asimina Tremouli, and Gerasimos Lyberatos. 2023. "Architecture Optimization of a Single-Chamber Air-Cathode MFC by Increasing the Number of Cathode Electrodes" Sustainability 15, no. 17: 13107. https://doi.org/10.3390/su151713107
APA StyleKamperidis, T., Tremouli, A., & Lyberatos, G. (2023). Architecture Optimization of a Single-Chamber Air-Cathode MFC by Increasing the Number of Cathode Electrodes. Sustainability, 15(17), 13107. https://doi.org/10.3390/su151713107








