Recent Advances in Seaweed Biorefineries and Assessment of Their Potential for Carbon Capture and Storage
Abstract
:1. Introduction
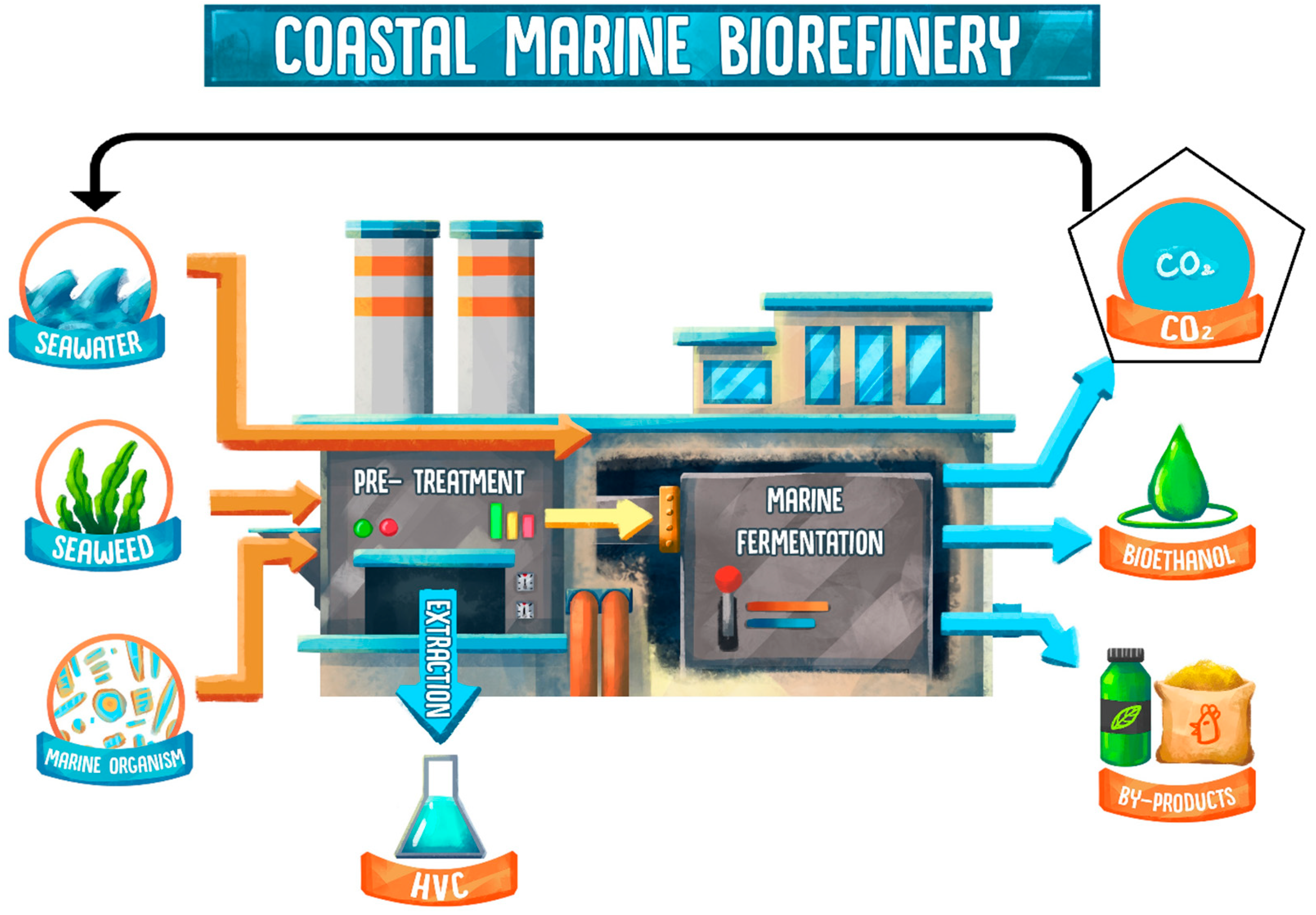
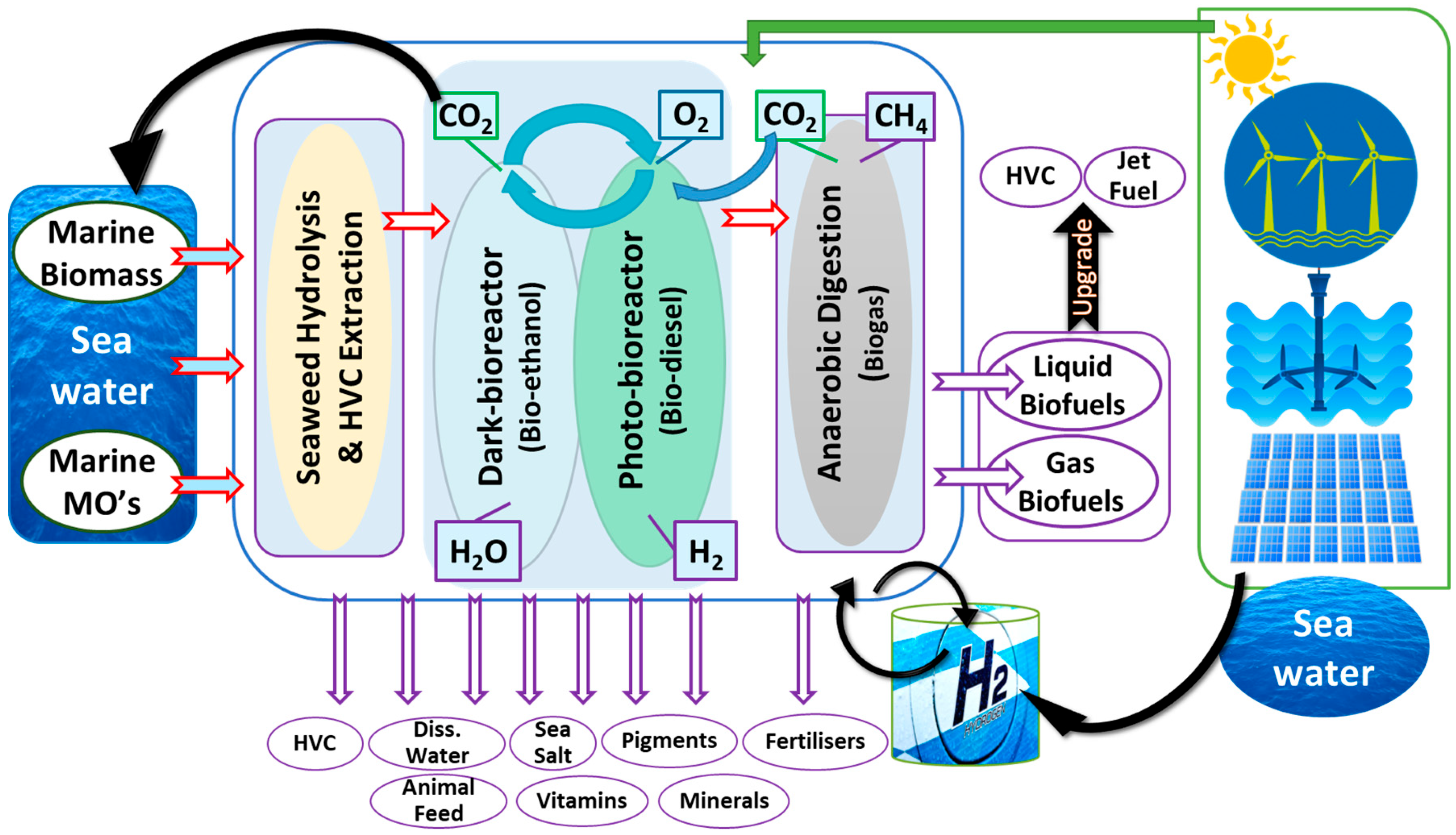
2. Coastal Marine Biorefinery Systems
3. Seaweed
3.1. Seaweed Macro Chemical Composition
3.2. High-Value Chemicals (HVC)
| HVC | Compound | Seaweed Class | Applications | Market Price (USD/kg) | Ref. |
|---|---|---|---|---|---|
| Carrageenan | Sulfated polysaccharide | Red | Stabilizer/gelling agent/texture modifier/thickener | 10.5 | [60,77] * |
| Furcellaran | Sulfated polysaccharide | Red | Gelling agent/food preservative/bacterial growth media | 1 | [78,79] * |
| Ulvan | Sulfated polysaccharide | Green | Animal feed/anticoagulant/immune modulator/drug delivery | 4 | [80] * |
| Fucoidan | Sulfated polysaccharide | Brown | Bioactive agent in food, cosmetics, pharmaceuticals | 11 | [60,81] * |
| Agar | Polysaccharide | Red | Stabilizer/thickener/culture media/moisturising agent/packing material | 18 | [77,82] * |
| Alginate | Polysaccharide | Brown | Thickener, gelling agent, stabilizer, emulsifiers | 12 | [60,77] * |
| Laminarin | Polysaccharide | Brown | Ethanol production/Biomedical agent | 0.42–0.94 | [60] * |
| Mannitol | Sugar alcohol | Brown | Diabetic sweetener/dehydrating agent | 7.3 | [61,83] * |
| Starch | Polysaccharide | All | Biofuel/bioplastic/thickener/stabilizers | _ | [60] |
| Cellulose | Polysaccharide | All | Bioethanol production/nano filter/drug carrier/paper making | _ | [60] |
| R-phyco-erythrin | Phycobiliprotein | Red | Pigment dye/fluorescent label | 180–250 M | [69] * |
| Lectin | Protein | Red and Green | Anti-viral/cancer biomarkers | _ | [84] |
| Taurine | Sulphonic β-amino acid | All | Nutritional and medical dietary supplement | 3 | [60,66,85] * |
| Squalene | Lipid | Green | Antioxidant and moisturising agent/drug carrier | 18 | [35,86] |
| Fucoxanthin | β-carotene | Brown | Medical and nutritional supplement | 200 | [87,88] * |
| Phloro-tannin | Polyphenol | Brown | Animal feed antimicrobial agent/anti-vasoconstriction medication | 70 | [75,89] ** |
4. Extraction Methods for Seaweed HVC
4.1. Conventional Pre-Treatment and Extraction Technologies
| Type | Pre-Treatment | Advantages | Disadvantages | Sust. | Ref. |
|---|---|---|---|---|---|
| Physical | Milling and extrusion | Maintains biochemical activity | High cost and energy | No | [95,102] * |
| Microwave | High yields/Fast/ Low solvent use | High energy/Not suitable for heat-sensitive metabolites | Yes | [90,110,111] [102] * | |
| Ultrasonication | Cheap/Fast/High yields/Low solvent/ Suitable for labile compounds | High energy/Wave attenuation | Yes | [90,104,112,113] [114] * | |
| Pulsed electric field | Fast/Selectivity/Low solvent and energy use | Cost/Incomplete breakdown | Yes | [115,116,117,118] * | |
| Physico-chemical | Hydrothermal | Less equipment/Low inhibitors (at low temp.) | High energy/Low yields at high temperature | No | [102,119,120] * |
| Supercritical fluids | Fast, efficient, and eco-friendly/Pure final product | High costs | Yes | [90,102,104,121] * | |
| Pressurised liquids | Fast reaction/Increased solubility and transfer rate/Low solvent | Unsuitable for unstable metabolites/Not selective | Yes | [90,102,113] * | |
| Chemical | Dilute acid/alkali | Cheap/Simple/Efficient | Fermentation inhibitors/Eco-toxicity | No | [101,102] * |
| Ionic liquid | Efficient/Mild/Low energy/Fewer inhibitory compounds | High cost/Eco-toxicity/Inhibited by water | Yes | [102,122] * | |
| Deep eutectic solvents | Green/Cheap/Tailorable/Low- to non-toxic | Little research | Yes | [102,123] * | |
| Biological | Enzymatic | High yield/Selective/Mild | High cost/Long extraction time | Yes | [102,124,125,126] * |
| Fungal | Eco-friendly/Low chemical needs/Feed co-product | Slow/High space requirements/Constant growth monitoring | Yes | [127,128,129] | |
| Bacterial | Eco-friendly/Ideal environment for enzyme activity | Slow/Few optimised processes/Used in combination with other conventional methods | Yes | [130,131,132,133] |
4.2. Emerging Pre-Treatment and Extraction Technologies
4.2.1. Microwave-Assisted Extraction (MAE)
4.2.2. Ultrasound-assisted Extraction (UAE)
4.2.3. Pulsed Electric Field (PEF)
4.2.4. Supercritical Fluid Extraction (SFE)
4.2.5. Pressurised Liquid Extraction (PLE)
4.2.6. Ionic Liquids (ILs)
4.2.7. Deep Eutectic Solvents (DESs)
| Extraction Method | Compound | Seaweed Species | Conditions | Concentration | Ref. |
|---|---|---|---|---|---|
| Microwave-assisted Extraction | Polyphenols | A. nodosum | 1:10 seaweed:methanol, 110 °C for 15 min at 2.45 GHz | 3.738 mg/g | [135] |
| Ulvan | U. pertusa | 3:40 (seaweed:ethanol), 43.63 min, 600 W, pH 6.57 | 12.573 mg/g | [139] | |
| Carotenoids | C. glomerata | 4 g dw algae per 100 mL of solvent, 60 min, 40 °C, 800 W | 3 mg/mL | [136] | |
| Ultrasound-assisted Extraction | Polysaccharides | S. henslowianum | 40 min, 330 W, solid-to-liquid ratio 1:36 g/mL | 126.3 mg/g | [171] |
| Polyphenols | S. henslowianum | 1 g extract, 102 min, 377 W | 114 mg/g | [171] | |
| R-phycoerythrin | G. turuturu | 20% seaweed: 80% water, 300–340 W, 6 h | 3.25 mg/g | [172] | |
| Polyphenols | F. vesiculosus | 35 kHz, 30 min, 50% ethanol | 572.3 mg/g | [173] | |
| Phlorotannins | F. vesiculosus | 35 kHz, 30 min, 50% ethanol | 476.3 mg/g | [173] | |
| Flavonoids | F. vesiculosus | 35 kHz, 30 min, 50% ethanol | 281 mg/g | [173] | |
| Pulsed Electric Field | Proteins | U. rigida and U. ohno mix | 140 g, 50 pulses of 50 kV, 70.3 mm | 1.92 mg/mL | [174] |
| Starch | U. ohnoi | 200 pulses, field strength of 1 kV cm−1, pulse:50 μs, 3 Hz | 1.54 g/g | [145] | |
| Supercritical Fluid Extraction | Aliphatic hydrocarbons | U. pinnatifida | 0.5 g sample, 50 min, 1 mL min−1 CO2, density 0.55 g mL−1 | 13.6–21.7 μg/g | [175] |
| Fucoxanthin | U. pinnatifida | SC-CO2, 200 bar, 323 K | 7.53 μg/g | [176] | |
| Polyphenol | U. pinnatifida | 250 bar, 333 K | 780 mg/g | [176] | |
| Lipids | S. hemiphyllum | SC-CO2, 1 mL/min, 37.9 MPa/323.15 K, | 55.8 mg/g | [177] | |
| Pressurised Liquid Extraction | Polyphenols | L. ochroleuca | 1 g, 20 mL ethanol:water (1:1), 160 °C, 100 bars, 10 min | 173.65 mg/g | [178] |
| Fatty Acids | F. vesiculous | 1 g, 10 min, 120 °C, 100 bar, ethyl acetate 10 mL | 693.20 mg/g | [151] | |
| Phenols | A. nodosum | 5 g, 50 °C, ethanol, 1500 psi, 5 min | 50.2 mg/g | [179] | |
| Carotenoids | A. nodosum | 5 g, 50 °C, ethanol, 1500 psi, 5 min | 85 μg/g | [179] | |
| Ionic Liquid | Phycobiliproteins | Gracilaria sp. | 0.7 fw/solvent, 20 min, 5.9 pH, 1 M [Ch]Cl | 0.40 mg/g | [180] |
| Agarose | G. dura | 0.5 g, 10 g [Emim] [OAc], 2 h, 100 °C, | 175 mg/g | [181] | |
| Iodine compounds | Laminaria sp. | IL ([EPy]Br) 200 mM, 30 min, 6.5 pH | 3754 μg/g | [182] | |
| Deep Eutectic Solvents | κ-carrageenan | K. alvarezii | 500 mg, 10 g 10% Hydrated choline chloride–glycerol 1:2, 1 h | 301 mg/g | [166] |
5. Bioethanol Production from Seaweeds
5.1. Hydrolysis
5.2. Fermentation Using Seawater-Based Media and Yeast
5.3. Co-Products of Marine Fermentation
6. Evaluation of CO2 Removal and CCS by Seaweeds
6.1. Estimation of Biomass Production and CO2 Sequestration
6.2. Estimation of Theoretical Bioethanol and HVC Production
| Data | Value | Ref. |
|---|---|---|
| Seaweed Biomass Estimation | ||
| 2100 CO2 removal goal | 100 Gt | [3] |
| CO2 to carbon conversion factor | 3.67 | [3] |
| Inshore coastal surface area (Scenario A) | 5.7 million km2 | [231] |
| Total theoretical ocean surface area for Ulva seaweed farms (Scenario B) | 100 million km2 | [235] |
| Ecologically available ocean area for seaweed farms (Scenario C) | 48 million km2 | [236] |
| Wild seaweed average net primary productivity | 420 g C m−2 year−1 | [231] |
| M. pyrifera net primary productivity | 1300 g C m−2 year−1 | [232] |
| Ulva sp. net primary productivity | 838 g C g m−2 year−1 | [237] |
| Carbon to biomass dry weight conversion factor | 4 | [231] |
| Biomass dry weight to fresh weight conversion factor | 4 | [238] |
| HVC Extraction | ||
| Biomass (dw) to bioethanol conversion factor | 0.213 kg/kg | [239] |
| Ethanol density | 783 kg/m3 | |
| Bioethanol market price | USD 0.4/L | [240] |
| Biomass (dw) to phlorotannin conversion factor | 0.002005 mg/kg | [241] |
| Phlorotannin market price | USD 70/kg | [89] |
| Biomass (dw) to protein conversion factor | 0.6169 mg/kg | [242] |
| Carbohydrate ratio of M. pyrifera (dw) | 0.648 kg/kg | [242] |
| Carbohydrate extraction efficiency | 89.67% | [242] |
| Alginate fraction of M. pyrifera carbohydrates | 62.54% | [242] |
| Alginate market price | USD 12/kg | [77] |
| Mannitol fraction of M. pyrifera carbohydrates | 8.05% | [242] |
| Mannitol market price | USD 7.3/kg | [61] |
| Single-cell protein price | USD 10.4/kg | [243] |
| Seaweed | NPP (kg C m−2 yr−1) | CO2 Removed (Gt/year) | Biomass Fresh (Gt) | Biomass Dry (Gt) | Time (Year) |
|---|---|---|---|---|---|
| M. pyrifera | 1.3 | 27.17 | 118.56 | 29.6 | 3.64 |
| Ulva sp. | 0.838 | 17.52 | 76.43 | 19.1 | 5.65 |
| Wild seaweed (average) * | 0.42 | 8.78 | 38.30 | 9.58 | 11.28 |
| Seaweed | NPP (Kg C m−2 yr−1) | CO2 Removed (Gt yr−1) | Biomass Fresh (Gt) | Biomass Dry (Gt) | Time Frame (Year) |
|---|---|---|---|---|---|
| Ulva sp. | 0.838 | 307.29 | 1340.80 | 335 | 0.32 |
| Seaweed | NPP (kg C m−2 yr−1) | CO2 Removed (Gt/year) | Biomass Wet (Gt) | Biomass Dry (Gt) | Time Frame (Year) |
|---|---|---|---|---|---|
| M. pyrifera | 1.3 | 228.82 | 998.4 | 249.6 | 0.43 |
| Ulva sp. | 0.838 | 147.50 | 643.584 | 161 | 0.67 |
| Wild seaweed (average) | 0.42 | 73.93 | 322.56 | 80.6 | 1.34 |
| Product | Price (USD/kg) | Scenario A (5.7 M km2) | Scenario C (48 M km2) | ||
|---|---|---|---|---|---|
| Weight (Million kg) | Value (Million USD) | Weight (Million kg) | Value (Million USD) | ||
| Bioethanol | 0.5068 | 6,310,000 | 3,197,908 | 53,200,000 | 26,961,760 |
| Phlorotannin | 70 | 0.0594 | 4 | 0.5 | 35 |
| Alginate | 12 | 10,800,000 | 129,600,000 | 90,000,000 | 1,080,000,000 |
| Mannitol | 7.2 | 1,390,000 | 10,008,000 | 11,700,000 | 84,240,000 |
| Protein | 10.4 | 183 | 1903 | 1540 | 16,016 |
| Total value | 142,807,815.36 | 1,191,217,811 | |||
7. Future Perspectives
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Obergassel, W.; Arens, C.; Hermwille, L.; Kreibich, N.; Mersmann, F.; Ott, H.E.; Wang-Helmreich, H. Phoenix from the ashes: An analysis of the Paris Agreement to the United Nations Framework Convention on Climate Change: Part 1. Geography 2015, 27, 243–262. [Google Scholar]
- United Nations Paris Climate Agreement Moves Closer to Entry into Force in 2016—United Nations Sustainable Development. Available online: https://www.un.org/sustainabledevelopment/blog/2016/09/paris-climate-agreement-moves-closer-to-entry-into-force-in-2016/ (accessed on 1 October 2021).
- Hood, R. Global Warming. In A Companion to Applied Ethics; Blackwell Publishing Ltd.: Oxford, UK, 2018; pp. 674–684. ISBN 9780470996621. [Google Scholar]
- Elsayed, M.; Abomohra, A.; Ai, P.; Jin, K.; Fan, Q.; Zhang, Y. Acetogenesis and methanogenesis liquid digestates for pretreatment of rice straw: A holistic approach for efficient biomethane production and nutrient recycling. Energy Convers. Manag. 2019, 195, 447–456. [Google Scholar] [CrossRef]
- Osman, M.E.H.; Abo-Shady, A.M.; Elshobary, M.E.; Abd El-Ghafar, M.O.; Abomohra, A. Screening of seaweeds for sustainable biofuel recovery through sequential biodiesel and bioethanol production. Environ. Sci. Pollut. Res. 2020, 27, 32481–32493. [Google Scholar] [CrossRef] [PubMed]
- Zaky, A.; Abomohra, A. Marine-Based Biorefinery: A Path Forward to a Sustainable Future. Ferment 2023, 9, 554. [Google Scholar] [CrossRef]
- Faisal, S.; Zaky, A.; Wang, Q.; Huang, J.; Abomohra, A. Integrated Marine Biogas: A Promising Approach towards Sustainability. Ferment 2022, 8, 520. [Google Scholar] [CrossRef]
- Hu, C.; Wang, M.; Lapointe, B.E.; Brewton, R.A.; Hernandez, F.J. On the Atlantic pelagic Sargassum’s role in carbon fixation and sequestration. Sci. Total Environ. 2021, 781, 146801. [Google Scholar] [CrossRef]
- Khan, F.; Jeong, G.J.; Khan, M.S.A.; Tabassum, N.; Kim, Y.M. Seaweed-Derived Phlorotannins: A Review of Multiple Biological Roles and Action Mechanisms. Mar. Drugs 2022, 20, 384. [Google Scholar] [CrossRef]
- Spagnuolo, D.; Di Martino, A.; Zammuto, V.; Armeli Minicante, S.; Spanò, A.; Manghisi, A.; Gugliandolo, C.; Morabito, M.; Genovese, G. Conventional vs. Innovative Protocols for the Extraction of Polysaccharides from Macroalgae. Sustainability 2022, 14, 5750. [Google Scholar] [CrossRef]
- Zaky, A.S.; Kumar, S.; Welfle, A.J. Integrated Approaches and Future Perspectives. In Waste-to-Energy; Springer International Publishing: Berlin/Heidelberg, Germany, 2022; pp. 613–651. [Google Scholar]
- Zaky, A.S. Introducing a Marine Biorefinery System for the integrated production of biofuels, high-value-chemicals and co-products: A path forward to a sustainable future. Processes 2021, 9, 1841. [Google Scholar] [CrossRef]
- Zaky, A.S.; Greetham, D.; Louis, E.J.; Tucker, G.A.; Du, C. A new isolation and evaluation method for marine-derived yeast spp. with potential applications in industrial biotechnology. J. Microbiol. Biotechnol. 2016, 26, 1891–1907. [Google Scholar] [CrossRef]
- Zaky, A.S.; Greetham, D.; Tucker, G.A.; Du, C. The establishment of a marine focused biorefinery for bioethanol production using seawater and a novel marine yeast strain. Sci. Rep. 2018, 8, 12127. [Google Scholar] [CrossRef] [PubMed]
- Gerbens-Leenes, W.; Hoekstra, A.Y.; Van Der Meer, T.H. The water footprint of bioenergy. Proc. Natl. Acad. Sci. USA 2009, 106, 10219–10223. [Google Scholar] [CrossRef]
- Baghel, R.S.; Suthar, P.; Gajaria, T.K.; Bhattacharya, S.; Anil, A.; Reddy, C.R.K. Seaweed biorefinery: A sustainable process for valorising the biomass of brown seaweed. J. Clean. Prod. 2020, 263, 121359. [Google Scholar] [CrossRef]
- Álvarez-Viñas, M.; Flórez-Fernández, N.; Torres, M.D.; Domínguez, H. Successful Approaches for a Red Seaweed Biorefinery. Mar. Drugs 2019, 17, 620. [Google Scholar] [CrossRef] [PubMed]
- Zollmann, M.; Robin, A.; Prabhu, M.; Polikovsky, M.; Gillis, A.; Greiserman, S.; Golberg, A. Green technology in green macroalgal biorefineries. Phycologia 2019, 58, 516–534. [Google Scholar] [CrossRef]
- Balina, K.; Romagnoli, F.; Blumberga, D. Seaweed biorefinery concept for sustainable use of marine resources. Energy Procedia 2017, 128, 504–511. [Google Scholar] [CrossRef]
- Zaky, A.S.; Carter, C.E.; Meng, F.; French, C.E. A Preliminary Life Cycle Analysis of Bioethanol Production Using Seawater in a Coastal Biorefinery Setting. Process 2021, 9, 1399. [Google Scholar] [CrossRef]
- Zaky, A.S.; French, C.E.; Tucker, G.A.; Du, C. Improving the productivity of bioethanol production using marine yeast and seawater-based media. Biomass Bioenergy 2020, 139, 105615. [Google Scholar] [CrossRef]
- Greetham, D.; Zaky, A.S.; Du, C. Exploring the tolerance of marine yeast to inhibitory compounds for improving bioethanol production. Sustain. Energy Fuels 2019, 3, 1545–1553. [Google Scholar] [CrossRef]
- Zaky, A.S. Marine Fermentation, the Sustainable Approach for Bioethanol Production. EC Microbiol. 2017, 25–27. Available online: https://api.semanticscholar.org/CorpusID:212470469 (accessed on 25 July 2023).
- Organisation for Economic Co-operation and Development. Meeting Policy Challenges for a Sustainable Bioeconomy; OECD iLibrary: Paris, France, 2018; ISBN 9789264292338. [Google Scholar]
- Abomohra, A.; Almutairi, A.W. A close-loop integrated approach for microalgae cultivation and efficient utilization of agar-free seaweed residues for enhanced biofuel recovery. Bioresour. Technol. 2020, 317, 124027. [Google Scholar] [CrossRef] [PubMed]
- El-Hefnawy, M.E.; Alhayyani, S.; Ismail, A.; El-Sherbiny, M.; Al-Harbi, M.; Abomohra, A.; Sakran, M.; Zidan, N. Integrated approach for enhanced crude bio-oil yield from microalgae cultivated on the aqueous phase of hydrothermal co-liquefaction with agar-free seaweed residues. J. Clean. Prod. 2023, 392, 136286. [Google Scholar] [CrossRef]
- Mehariya, S.; Goswami, R.K.; Karthikeysan, O.P.; Verma, P. Microalgae for high-value products: A way towards green nutraceutical and pharmaceutical compounds. Chemosphere 2021, 280, 130553. [Google Scholar] [CrossRef] [PubMed]
- Beyer, A.S.; Meier, J.; Jiménez-Muñoz, M.; Meixner, R.; Ende, S.S.W.; Abomohra, A.; Henjes, J. New microalgae media formulated with completely recycled phosphorus originating from agricultural sidestreams. J. Appl. Phycol. 2023, 1, 1–16. [Google Scholar] [CrossRef]
- Milledge, J.J.; Harvey, P.J. Potential process ‘hurdles’ in the use of macroalgae as feedstock for biofuel production in the British Isles. J. Chem. Technol. Biotechnol. 2016, 91, 2221–2234. [Google Scholar] [CrossRef] [PubMed]
- Michalak, I. The application of seaweeds in environmental biotechnology. Adv. Bot. Res. 2020, 95, 85–111. [Google Scholar] [CrossRef]
- Henriques, B.; Rocha, L.S.; Lopes, C.B.; Figueira, P.; Duarte, A.C.; Vale, C.; Pardal, M.A.; Pereira, E. A macroalgae-based biotechnology for water remediation: Simultaneous removal of Cd, Pb and Hg by living Ulva lactuca. J. Environ. Manag. 2017, 191, 275–289. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Z.; Liu, J.; Li, S.; Chen, Y.; Du, P.; Zhu, Y.; Liao, Y.; Chen, Q.; Shou, L.; Yan, X.; et al. Kelp cultivation effectively improves water quality and regulates phytoplankton community in a turbid, highly eutrophic bay. Sci. Total Environ. 2020, 707, 135561. [Google Scholar] [CrossRef]
- Xiao, X.; Agusti, S.; Lin, F.; Li, K.; Pan, Y.; Yu, Y.; Zheng, Y.; Wu, J.; Duarte, C.M. Nutrient removal from Chinese coastal waters by large-scale seaweed aquaculture. Sci. Rep. 2017, 7, 46613. [Google Scholar] [CrossRef]
- Abomohra, A.; El-Hefnawy, M.E.; Wang, Q.; Huang, J.; Li, L.; Tang, J.; Mohammed, S. Sequential bioethanol and biogas production coupled with heavy metal removal using dry seaweeds: Towards enhanced economic feasibility. J. Clean. Prod. 2021, 316, 128341. [Google Scholar] [CrossRef]
- Salehi, B.; Sharifi-Rad, J.; Seca, A.M.L.; Pinto, D.C.G.A.; Michalak, I.; Trincone, A.; Mishra, A.P.; Nigam, M.; Zam, W.; Martins, N. Current trends on seaweeds: Looking at chemical composition, phytopharmacology, and cosmetic applications. Molecules 2019, 24, 4182. [Google Scholar] [CrossRef] [PubMed]
- Nunes, N.; Ferraz, S.; Valente, S.; Barreto, M.C.; Pinheiro de Carvalho, M.A.A. Biochemical composition, nutritional value, and antioxidant properties of seven seaweed species from the Madeira Archipelago. J. Appl. Phycol. 2017, 29, 2427–2437. [Google Scholar] [CrossRef]
- El-Said, G.F.; El-Sikaily, A. Chemical composition of some seaweed from Mediterranean Sea coast, Egypt. Environ. Monit. Assess. 2013, 185, 6089–6099. [Google Scholar] [CrossRef]
- Parthiban, C.; Saranya, C.; Girija, K.; Hemalatha, A.; Suresh, M.; Anantharaman, P. Biochemical composition of some selected seaweeds from Tuticorin coast. Pelagia Res. Libr. 2013, 4, 362–366. [Google Scholar]
- Peng, Y.; Hu, J.; Yang, B.; Lin, X.P.; Zhou, X.F.; Yang, X.W.; Liu, Y. Chemical composition of seaweeds. In Seaweed Sustainability: Food and Non-Food Applications; Elsevier Inc.: Amsterdam, The Netherlands, 2015; pp. 79–124. ISBN 9780124199583. [Google Scholar]
- Marinho-Soriano, E.; Fonseca, P.C.; Carneiro, M.A.A.; Moreira, W.S.C. Seasonal variation in the chemical composition of two tropical seaweeds. Bioresour. Technol. 2006, 97, 2402–2406. [Google Scholar] [CrossRef]
- Gorham, J.; Lewey, S.A. Seasonal changes in the chemical composition of Sargassum muticum. Mar. Biol. 1984, 80, 103–107. [Google Scholar] [CrossRef]
- Kumar, V.; Kaladharan, P. Seaweeds as source of protein for animal feed. J. Mar. Biol. Assoc. India 2007, 49, 35–40. [Google Scholar]
- Mišurcová, L. Chemical Composition of Seaweeds. In Handbook of Marine Macroalgae: Biotechnology and Applied Phycology; John Wiley and Sons: Hoboken, NJ, USA, 2011; pp. 171–192. ISBN 9780470979181. [Google Scholar]
- Vieira, E.F.; Soares, C.; Machado, S.; Correia, M.; Ramalhosa, M.J.; Oliva-teles, M.T.; Paula Carvalho, A.; Domingues, V.F.; Antunes, F.; Oliveira, T.A.C.; et al. Seaweeds from the Portuguese coast as a source of proteinaceous material: Total and free amino acid composition profile. Food Chem. 2018, 269, 264–275. [Google Scholar] [CrossRef]
- Miyashita, K.; Mikami, N.; Hosokawa, M. Chemical and nutritional characteristics of brown seaweed lipids: A review. J. Funct. Foods 2013, 5, 1507–1517. [Google Scholar] [CrossRef]
- Kendel, M.; Wielgosz-Collin, G.; Bertrand, S.; Roussakis, C.; Bourgougnon, N.; Bedoux, G. Lipid composition, fatty acids and sterols in the seaweeds Ulva armoricana, and Solieria chordalis from Brittany (France): An analysis from nutritional, chemotaxonomic, and antiproliferative activity perspectives. Mar. Drugs 2015, 13, 5606–5628. [Google Scholar] [CrossRef]
- Dawczynski, C.; Schubert, R.; Jahreis, G. Amino acids, fatty acids, and dietary fibre in edible seaweed products. Food Chem. 2007, 103, 891–899. [Google Scholar] [CrossRef]
- Newton, I.S. Long chain fatty acids in health and nutrition. J. Food Lipids 1996, 3, 233–249. [Google Scholar] [CrossRef]
- Liu, K. Characterization of ash in algae and other materials by determination of wet acid indigestible ash and microscopic examination. Algal Res. 2017, 25, 307–321. [Google Scholar] [CrossRef]
- Rasyid, A. Evaluation of nutritional composition of the dried seaweed Ulva lactuca from Pameungpeuk waters, Indonesia. Trop. Life Sci. Res. 2017, 28, 119–125. [Google Scholar] [CrossRef] [PubMed]
- Smitha, J.L.; Summers, G.; Wong, R. Nutrient and heavy metal content of edible seaweeds in New Zealand. N. Z. J. Crop Hortic. Sci. 2010, 38, 19–28. [Google Scholar] [CrossRef]
- Krishnaiah, D.; Sarbatly, R.; Prasad, D.M.R.; Engineering Programme, A.B.C. Mineral Content of Some Seaweeds from Sabah’s South China Sea. Asian J. Sci. Res. 2008, 1, 166–170. [Google Scholar] [CrossRef]
- Lunde, G. Analysis of trace elements in seaweed. J. Sci. Food Agric. 1970, 21, 416–418. [Google Scholar] [CrossRef]
- Ryan, S.; McLoughlin, P.; O’Donovan, O. A comprehensive study of metal distribution in three main classes of seaweed. Environ. Pollut. 2012, 167, 171–177. [Google Scholar] [CrossRef]
- Rupérez, P. Mineral content of edible marine seaweeds. Food Chem. 2002, 79, 23–26. [Google Scholar] [CrossRef]
- Harrysson, H.; Hayes, M.; Eimer, F.; Carlsson, N.G.; Toth, G.B.; Undeland, I. Production of protein extracts from Swedish red, green, and brown seaweeds, Porphyra umbilicalis Kützing, Ulva lactuca Linnaeus, and Saccharina latissima (Linnaeus) J. V. Lamouroux using three different methods. J. Appl. Phycol. 2018, 30, 3565–3580. [Google Scholar] [CrossRef]
- Kadam, S.U.; Álvarez, C.; Tiwari, B.K.; O’Donnell, C.P. Extraction and characterization of protein from Irish brown seaweed Ascophyllum nodosum. Food Res. Int. 2017, 99, 1021–1027. [Google Scholar] [CrossRef]
- Angell, A.R.; Mata, L.; de Nys, R.; Paul, N.A. The protein content of seaweeds: A universal nitrogen-to-protein conversion factor of five. J. Appl. Phycol. 2016, 28, 511–524. [Google Scholar] [CrossRef]
- Leandro, A.; Pacheco, D.; Cotas, J.; Marques, J.C.; Pereira, L.; Gonçalves, A.M.M. Seaweed’s bioactive candidate compounds to food industry and global food security. Life 2020, 10, 140. [Google Scholar] [CrossRef] [PubMed]
- Olatunji, O. Aquatic Biopolymers; Springer Series on Polymer and Composite Materials; Springer International Publishing: Cham, Switzerland, 2020; ISBN 978-3-030-34708-6. [Google Scholar]
- Bayu, A.; Handayani, T. High-value chemicals from marine macroalgae: Opportunities and challenges for marine-based bioenergy development. In IOP Conference Series: Earth and Environmental Science, Proceedings of the 3rd International Conference on Biomass: Accelerating the Technical Development and Commercialization for Sustainable Bio-Based Products and Energy, Bogor, Indonesia, 1–2 August 2018; IOP Publishing: Bristol, UK, 2018. [Google Scholar]
- Corino, C.; Di Giancamillo, A.; Modina, S.C.; Rossi, R. Prebiotic effects of seaweed polysaccharides in pigs. Animals 2021, 11, 1573. [Google Scholar] [CrossRef] [PubMed]
- Jiao, G.; Yu, G.; Zhang, J.; Ewart, H.S. Chemical structures and bioactivities of sulfated polysaccharides from marine algae. Mar. Drugs 2011, 9, 196–233. [Google Scholar] [CrossRef] [PubMed]
- Market Data Forecast Alginates Market Growth, Size, Share, Trends and Forecast to 2025. Available online: https://www.marketdataforecast.com/market-reports/alginates-market (accessed on 24 June 2020).
- Prabhu, M.; Chemodanov, A.; Gottlieb, R.; Kazir, M.; Nahor, O.; Gozin, M.; Israel, A.; Livney, Y.D.; Golberg, A. Starch from the sea: The green macroalga Ulva ohnoi as a potential source for sustainable starch production in the marine biorefinery. Algal Res. 2019, 37, 215–227. [Google Scholar] [CrossRef]
- Harnedy, P.A.; Fitzgerald, R.J. Bioactive proteins, peptides, and amino acids from macroalgae. J. Phycol. 2011, 47, 218–232. [Google Scholar] [CrossRef]
- Okolie, C.L.; Mason, B.; Critchley, A.T. Seaweeds as a Source of Proteins for Use in Pharmaceuticals and High-Value Applications. In Novel Proteins for Food, Pharmaceuticals and Agriculture; John Wiley & Sons, Ltd.: Chichester, UK, 2018; pp. 217–238. [Google Scholar]
- Bleakley, S.; Hayes, M. Algal proteins: Extraction, application, and challenges concerning production. Foods 2017, 6, 33. [Google Scholar] [CrossRef]
- Fleurence, J. R-Phycoerythrin from red macroalgae: Strategies for extraction and potential application in Biotechnology. Appl. Biotechnol. Food Sci. Policy 2003, 1, 63–68. [Google Scholar]
- Saluri, M.; Kaldmäe, M.; Tuvikene, R. Reliable quantification of R-phycoerythrin from red algal crude extracts. J. Appl. Phycol. 2020, 32, 1421–1428. [Google Scholar] [CrossRef]
- Dumay, J.; Morançais, M.; Nguyen, H.P.T.; Fleurence, J. Extraction and purification of r-phycoerythrin from marine red algae. Methods Mol. Biol. 2015, 1308, 109–117. [Google Scholar] [CrossRef] [PubMed]
- Dumay, J.; Clément, N.; Morançais, M.; Fleurence, J. Optimization of hydrolysis conditions of Palmaria palmata to enhance R-phycoerythrin extraction. Bioresour. Technol. 2013, 131, 21–27. [Google Scholar] [CrossRef] [PubMed]
- Van Chuyen, H.; Eun, J.B. Marine carotenoids: Bioactivities and potential benefits to human health. Crit. Rev. Food Sci. Nutr. 2017, 57, 2600–2610. [Google Scholar] [CrossRef] [PubMed]
- Galasso, C.; Corinaldesi, C.; Sansone, C. Carotenoids from marine organisms: Biological functions and industrial applications. Antioxidants 2017, 6, 96. [Google Scholar] [CrossRef] [PubMed]
- Ford, L.; Stratakos, A.C.; Theodoridou, K.; Dick, J.T.A.; Sheldrake, G.N.; Linton, M.; Corcionivoschi, N.; Walsh, P.J. Polyphenols from Brown Seaweeds as a Potential Antimicrobial Agent in Animal Feeds. ACS Omega 2020, 5, 9093–9103. [Google Scholar] [CrossRef] [PubMed]
- Barot, M.; Kumar JI, N.; Kumar, R.N. Bioactive compounds and antifungal activity of three different seaweed species Ulva lactuca, Sargassum tenerrimum and Laurencia obtusa collected from Okha coast, Western India. J. Coast. Life Med. 2016, 4, 284–289. [Google Scholar] [CrossRef]
- Hernández-Carmona, G.; Freile-Pelegrín, Y.; Hernández-Garibay, E. Conventional and alternative technologies for the extraction of algal polysaccharides. In Functional Ingredients from Algae for Foods and Nutraceuticals; Elsevier Ltd.: Amsterdam, The Netherlands, 2013; pp. 475–516. ISBN 9780857095121. [Google Scholar]
- Book, C. Furcellaran|9000-21-9. Available online: https://www.chemicalbook.com/ChemicalProductProperty_EN_CB6883069.htm (accessed on 4 July 2020).
- Naylor, J. Production, trade and utilization of seaweeds and seaweed products. FAO Fish. Tech. Pap. 1976, 159, 1–73. [Google Scholar]
- Prabhu, M.S.; Israel, A.; Palatnik, R.R.; Zilberman, D.; Golberg, A. Integrated biorefinery process for sustainable fractionation of Ulva ohnoi (Chlorophyta): Process optimization and revenue analysis. J. Appl. Phycol. 2020, 32, 2271–2282. [Google Scholar] [CrossRef]
- Roos, G.; Cheshire, A.; Clarke, M.; Nayar, S. Harnessing Marine Macroalgae for Industrial Purposes in an Australian Context; IGI Global: Philadelphia, PA, USA, 2018. [Google Scholar]
- Pawel, W.; Grzegorz, S.; Izabela, M. Algae Biomass: Characteristics and Applications; Springer International Publishing: Berlin/Heidelberg, Germany, 2018. [Google Scholar]
- Dai, Y.; Meng, Q.; Mu, W.; Zhang, T. Recent advances in the applications and biotechnological production of mannitol. J. Funct. Foods 2017, 36, 404–409. [Google Scholar] [CrossRef]
- Naeem, A.; Saleemuddin, M.; Hasan Khan, R. Glycoprotein Targeting and Other Applications of Lectins in Biotechnology. Curr. Protein Pept. Sci. 2007, 8, 261–271. [Google Scholar] [CrossRef]
- PharmaCompass Taurine Price. Available online: https://www.pharmacompass.com/price/taurine (accessed on 4 July 2020).
- Spanova, M.; Daum, G. Squalene—Biochemistry, molecular biology, process biotechnology, and applications. Eur. J. Lipid Sci. Technol. 2011, 113, 1299–1320. [Google Scholar] [CrossRef]
- Chen, H.; Zhou, D.; Luo, G.; Zhang, S.; Chen, J. Macroalgae for biofuels production: Progress and perspectives. Renew. Sustain. Energy Rev. 2015, 47, 427–437. [Google Scholar] [CrossRef]
- Shannon, E.; Abu-Ghannam, N. Enzymatic extraction of fucoxanthin from brown seaweeds. Int. J. Food Sci. Technol. 2018, 53, 2195–2204. [Google Scholar] [CrossRef]
- Alibaba.com. Phlorotannin-Phlorotannin Manufacturers, Suppliers and Exporters on Alibaba.com. Available online: https://www.alibaba.com/trade/search?fsb=y&IndexArea=product_en&CatId=&SearchText=phlorotannin (accessed on 23 July 2020).
- Michalak, I.; Chojnacka, K. Algal extracts: Technology and advances. Eng. Life Sci. 2014, 14, 581–591. [Google Scholar] [CrossRef]
- Kim, H.M.; Wi, S.G.; Jung, S.; Song, Y.; Bae, H.J. Efficient approach for bioethanol production from red seaweed Gelidium amansii. Bioresour. Technol. 2015, 175, 128–134. [Google Scholar] [CrossRef] [PubMed]
- Adams, J.M.M.; Schmidt, A.; Gallagher, J.A. The impact of sample preparation of the macroalgae Laminaria digitata on the production of the biofuels bioethanol and biomethane. J. Appl. Phycol. 2015, 27, 985–991. [Google Scholar] [CrossRef]
- Adams, J.M.M.; Bleathman, G.; Thomas, D.; Gallagher, J.A. The effect of mechanical pre-processing and different drying methodologies on bioethanol production using the brown macroalga Laminaria digitata (Hudson) JV Lamouroux. J. Appl. Phycol. 2017, 29, 2463–2469. [Google Scholar] [CrossRef]
- Tedesco, S.; Mac Lochlainn, D.; Olabi, A.G. Particle size reduction optimization of Laminaria spp. biomass for enhanced methane production. Energy 2014, 76, 857–862. [Google Scholar] [CrossRef]
- Onumaegbu, C.; Mooney, J.; Alaswad, A.; Olabi, A.G. Pre-treatment methods for production of biofuel from microalgae biomass. Renew. Sustain. Energy Rev. 2018, 93, 16–26. [Google Scholar] [CrossRef]
- Tedesco, S.; Marrero Barroso, T.; Olabi, A.G. Optimization of mechanical pre-treatment of Laminariaceae spp. biomass-derived biogas. Renew. Energy 2014, 62, 527–534. [Google Scholar] [CrossRef]
- Michalak, I.; Chojnacka, K. Production of Seaweed Extracts by Biological and Chemical Methods. In Marine Algae Extracts: Processes, Products, and Applications; Wiley-VCH Verlag GmbH & Co., KGaA: Weinheim, Germany, 2015; Volume 1–2, pp. 121–144. ISBN 9783527679577. [Google Scholar]
- Azizi, N.; Najafpour, G.; Younesi, H. Acid pretreatment and enzymatic saccharification of brown seaweed for polyhydroxybutyrate (PHB) production using Cupriavidus necator. Int. J. Biol. Macromol. 2017, 101, 1029–1040. [Google Scholar] [CrossRef]
- Chen, H. Lignocellulose biorefinery feedstock engineering. In Lignocellulose Biorefinery Engineering; Elsevier: Amsterdam, The Netherlands, 2015; pp. 37–86. [Google Scholar]
- Iwaki, A.; Kawai, T.; Yamamoto, Y.; Izawa, S. Biomass conversion inhibitors furfural and 5-hydroxymethylfurfural induce formation of messenger RNP granules and attenuate translation activity in Saccharomyces cerevisiae. Appl. Environ. Microbiol. 2013, 79, 1661–1667. [Google Scholar] [CrossRef]
- Offei, F.; Mensah, M.; Thygesen, A.; Kemausuor, F. Seaweed bioethanol production: A process selection review on hydrolysis and fermentation. Fermentation 2018, 4, 99. [Google Scholar] [CrossRef]
- del Río, P.G.; Gomes-Dias, J.S.; Rocha, C.M.R.; Romaní, A.; Garrote, G.; Domingues, L. Recent trends on seaweed fractionation for liquid biofuels production. Bioresour. Technol. 2020, 299, 122613. [Google Scholar] [CrossRef]
- Vanegas, C.H.; Hernon, A.; Bartlett, J. Enzymatic and organic acid pretreatment of seaweed: Effect on reducing sugars production and on biogas inhibition. Int. J. Ambient Energy 2015, 36, 2–7. [Google Scholar] [CrossRef]
- Kadam, S.U.; Tiwari, B.K.; O’Donnell, C.P. Application of novel extraction technologies for bioactives from marine algae. J. Agric. Food Chem. 2013, 61, 4667–4675. [Google Scholar] [CrossRef] [PubMed]
- Johnson, E. Integrated enzyme production lowers the cost of cellulosic ethanol. Biofuels Bioprod. Biorefining 2016, 10, 164–174. [Google Scholar] [CrossRef]
- Ebaid, R.; Wang, H.; Sha, C.; Abomohra, A.; Shao, W. Recent trends in hyperthermophilic enzymes production and future perspectives for biofuel industry: A critical review. J. Clean. Prod. 2019, 238, 117925. [Google Scholar] [CrossRef]
- Lara, A.; Rodríguez-Jasso, R.M.; Loredo-Treviño, A.; Aguilar, C.N.; Meyer, A.S.; Ruiz, H.A. Enzymes in the third generation biorefinery for macroalgae biomass. In Biomass, Biofuels, Biochemicals; Elsevier: Amsterdam, The Netherlands, 2020; pp. 363–396. [Google Scholar]
- Ramluckan, K.; Moodley, K.G.; Bux, F. An evaluation of the efficacy of using selected solvents for the extraction of lipids from algal biomass by the soxhlet extraction method. Fuel 2014, 116, 103–108. [Google Scholar] [CrossRef]
- Subramanian, R. How to Choose Solvent for Soxhlet Extration? 2014. Available online: researchgate.net (accessed on 25 July 2023).
- Gomez, L.; Tiwari, B.; Garcia-Vaquero, M. Emerging extraction techniques: Microwave-assisted extraction. In Sustainable Seaweed Technologies; Elsevier: Amsterdam, The Netherlands, 2020; pp. 207–224. [Google Scholar]
- Esquivel-Hernández, D.A.; Ibarra-Garza, I.P.; Rodríguez-Rodríguez, J.; Cuéllar-Bermúdez, S.P.; Rostro-Alanis, M.d.J.; Alemán-Nava, G.S.; García-Pérez, J.S.; Parra-Saldívar, R. Green extraction technologies for high-value metabolites from algae: A review. Biofuels Bioprod. Biorefining 2017, 11, 215–231. [Google Scholar] [CrossRef]
- Hahn, T.; Lang, S.; Ulber, R.; Muffler, K. Novel procedures for the extraction of fucoidan from brown algae. Process Biochem. 2012, 47, 1691–1698. [Google Scholar] [CrossRef]
- Ibañez, E.; Herrero, M.; Mendiola, J.A.; Castro-Puyana, M. Extraction and characterization of bioactive compounds with health benefits from marine resources: Macro and micro algae, cyanobacteria, and invertebrates. In Marine Bioactive Compounds: Sources, Characterization and Applications; Springer: New York, NY, USA, 2012; Volume 9781461412, pp. 55–98. ISBN 9781461412472. [Google Scholar]
- Tiwari, B.K. Ultrasound: A clean, green extraction technology. TrAC-Trends Anal. Chem. 2015, 71, 100–109. [Google Scholar] [CrossRef]
- Martínez, J.M.; Delso, C.; Álvarez, I.; Raso, J. Pulsed electric field-assisted extraction of valuable compounds from microorganisms. Compr. Rev. Food Sci. Food Saf. 2020, 19, 530–552. [Google Scholar] [CrossRef] [PubMed]
- Buchmann, L.; Mathys, A. Perspective on Pulsed Electric Field Treatment in the Bio-based Industry. Front. Bioeng. Biotechnol. 2019, 7, 265. [Google Scholar] [CrossRef] [PubMed]
- Aryee, A.N.; Agyei, D.; Akanbi, T.O. Recovery and utilization of seaweed pigments in food processing. Curr. Opin. Food Sci. 2018, 19, 113–119. [Google Scholar] [CrossRef]
- Vorobiev, E.; Lebovka, N. Pulsed electric field in green processing and preservation of food products. In Green Food Processing Techniques; Elsevier: Amsterdam, The Netherlands, 2019; pp. 403–430. [Google Scholar]
- Lin, R.; Deng, C.; Ding, L.; Bose, A.; Murphy, J.D. Improving gaseous biofuel production from seaweed Saccharina latissima: The effect of hydrothermal pretreatment on energy efficiency. Energy Convers. Manag. 2019, 196, 1385–1394. [Google Scholar] [CrossRef]
- Yang, B.; Tao, L.; Wyman, C.E. Strengths, challenges, and opportunities for hydrothermal pretreatment in lignocellulosic biorefineries. Biofuels Bioprod. Biorefining 2018, 12, 125–138. [Google Scholar] [CrossRef]
- Ibáñez, E.; Mendiola, J.A.; Castro-Puyana, M. Supercritical Fluid Extraction. In Encyclopedia of Food and Health; Elsevier Inc.: Amsterdam, The Netherlands, 2015; pp. 227–233. ISBN 9780123849533. [Google Scholar]
- Weldemhret, T.G.; Bañares, A.B.; Ramos, K.R.M.; Lee, W.K.; Nisola, G.M.; Valdehuesa, K.N.G.; Chung, W.J. Current advances in ionic liquid-based pre-treatment and depolymerization of macroalgal biomass. Renew. Energy 2020, 152, 283–299. [Google Scholar] [CrossRef]
- Zdanowicz, M.; Wilpiszewska, K.; Spychaj, T. Deep eutectic solvents for polysaccharides processing. A review. Carbohydr. Polym. 2018, 200, 361–380. [Google Scholar] [CrossRef]
- Nadar, S.S.; Rao, P.; Rathod, V.K. Enzyme assisted extraction of biomolecules as an approach to novel extraction technology: A review. Food Res. Int. 2018, 108, 309–330. [Google Scholar] [CrossRef]
- Jeevan Kumar, S.P.; Vijay Kumar, G.; Dash, A.; Scholz, P.; Banerjee, R. Sustainable green solvents and techniques for lipid extraction from microalgae: A review. Algal Res. 2017, 21, 138–147. [Google Scholar] [CrossRef]
- Marathe, S.J.; Jadhav, S.B.; Bankar, S.B.; Singhal, R.S. Enzyme-Assisted Extraction of Bioactives. In Food Bioactives: Extraction and Biotechnology Applications; Springer International Publishing: Berlin/Heidelberg, Germany, 2017; pp. 171–201. ISBN 9783319516394. [Google Scholar]
- Sulfahri; Mushlihah, S.; Husain, D.R.; Langford, A.; Tassakka, A.C.M.A.R. Fungal pretreatment as a sustainable and low cost option for bioethanol production from marine algae. J. Clean. Prod. 2020, 265, 121763. [Google Scholar] [CrossRef]
- Yahmed, N.B.; Carrere, H.; Marzouki, M.N.; Smaali, I. Enhancement of biogas production from Ulva sp. by using solid-state fermentation as biological pretreatment. Algal Res. 2017, 27, 206–214. [Google Scholar] [CrossRef]
- Nadir, N.; Liyana Ismail, N.; Shah Hussain, A. Fungal Pretreatment of Lignocellulosic Materials. In Biomass for Bioenergy—Recent Trends and Future Challenges; IntechOpen: London, UK, 2019. [Google Scholar]
- Singh, S.; Goyal, A.; Moholkar, V.S. Synthesis of bioethanol from invasive weeds: Process design, optimization, and intensification with ultrasound. In Waste Biorefinery: Potential and Perspectives; Elsevier: Amsterdam, The Netherlands, 2018; pp. 445–485. ISBN 9780444639929. [Google Scholar]
- Kim, E.J.; Fathoni, A.; Jeong, G.T.; Do Jeong, H.; Nam, T.J.; Kong, I.S.; Kim, J.K. Microbacterium oxydans, a novel alginate- and laminarin-degrading bacterium for the reutilization of brown-seaweed waste. J. Environ. Manag. 2013, 130, 153–159. [Google Scholar] [CrossRef] [PubMed]
- Kang, S.; Kim, J.K. Reuse of red seaweed waste by a novel bacterium, Bacillus sp. SYR4 isolated from a sandbar. World J. Microbiol. Biotechnol. 2015, 31, 209–217. [Google Scholar] [CrossRef]
- Thompson, T.M.; Young, B.R.; Baroutian, S. Advances in the pretreatment of brown macroalgae for biogas production. Fuel Process. Technol. 2019, 195, 106151. [Google Scholar] [CrossRef]
- Grosso, C.; Valentão, P.; Ferreres, F.; Andrade, P.B. Alternative and efficient extraction methods for marine-derived compounds. Mar. Drugs 2015, 13, 3182–3230. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Y.; Zhang, J.; Fan, J.; Clark, J.; Shen, P.; Li, Y.; Zhang, C. Microwave assisted extraction of phenolic compounds from four economic brown macroalgae species and evaluation of their antioxidant activities and inhibitory effects on α-amylase, α-glucosidase, pancreatic lipase and tyrosinase. Food Res. Int. 2018, 113, 288–297. [Google Scholar] [CrossRef]
- Fabrowska, J.; Messyasz, B.; Szyling, J.; Walkowiak, J.; Łęska, B. Isolation of chlorophylls and carotenoids from freshwater algae using different extraction methods. Phycol. Res. 2018, 66, 52–57. [Google Scholar] [CrossRef]
- Ciko, A.M.; Jokić, S.; Šubarić, D.; Jerković, I. Overview on the application of modern methods for the extraction of bioactive compounds from marine macroalgae. Mar. Drugs 2018, 16, 348. [Google Scholar] [CrossRef]
- Boulho, R.; Marty, C.; Freile-Pelegrín, Y.; Robledo, D.; Bourgougnon, N.; Bedoux, G. Antiherpetic (HSV-1) activity of carrageenans from the red seaweed Solieria chordalis (Rhodophyta, Gigartinales) extracted by microwave-assisted extraction (MAE). J. Appl. Phycol. 2017, 29, 2219–2228. [Google Scholar] [CrossRef]
- Le, B.; Golokhvast, K.S.; Yang, S.H.; Sun, S. Optimization of Microwave-Assisted Extraction of Polysaccharides from Ulva pertusa and Evaluation of Their Antioxidant Activity. Antioxidants 2019, 8, 129. [Google Scholar] [CrossRef] [PubMed]
- Sousa, A.M.M.; Alves, V.D.; Morais, S.; Delerue-Matos, C.; Gonçalves, M.P. Agar extraction from integrated multitrophic aquacultured Gracilaria vermiculophylla: Evaluation of a microwave-assisted process using response surface methodology. Bioresour. Technol. 2010, 101, 3258–3267. [Google Scholar] [CrossRef] [PubMed]
- Wen, C.; Zhang, J.; Zhang, H.; Dzah, C.S.; Zandile, M.; Duan, Y.; Ma, H.; Luo, X. Advances in ultrasound assisted extraction of bioactive compounds from cash crops—A review. Ultrason. Sonochem. 2018, 48, 538–549. [Google Scholar] [CrossRef] [PubMed]
- Sharma, R.; Bhunia, B.; Mondal, A.; Kanti Bandyopadhyay, T.; Devi, I.; Oinam, G.; Prasanna, R.; Abraham, G.; Nath Tiwari, O. Statistical optimization of process parameters for improvement of phycobiliproteins (PBPs) yield using ultrasound-assisted extraction and its kinetic study. Ultrason. Sonochem. 2020, 60, 104762. [Google Scholar] [CrossRef] [PubMed]
- Sengar, A.S.; Rawson, A.; Muthiah, M.; Kalakandan, S.K. Comparison of different ultrasound assisted extraction techniques for pectin from tomato processing waste. Ultrason. Sonochem. 2020, 61, 104812. [Google Scholar] [CrossRef] [PubMed]
- Chemat, F.; Abert Vian, M.; Fabiano-Tixier, A.S.; Nutrizio, M.; Režek Jambrak, A.; Munekata, P.E.S.; Lorenzo, J.M.; Barba, F.J.; Binello, A.; Cravotto, G. A review of sustainable and intensified techniques for extraction of food and natural products. Green Chem. 2020, 22, 2325–2353. [Google Scholar] [CrossRef]
- Prabhu, M.S.; Levkov, K.; Livney, Y.D.; Israel, A.; Golberg, A. High-Voltage Pulsed Electric Field Preprocessing Enhances Extraction of Starch, Proteins, and Ash from Marine Macroalgae Ulva ohnoi. ACS Sustain. Chem. Eng. 2019, 7, 17453–17463. [Google Scholar] [CrossRef]
- European Commission FieldFOOD Has Its Finger on the Pulse of Food Processing: Research and Innovation. Available online: https://ec.europa.eu/research-and-innovation/en/projects/success-stories/all/fieldfood-has-its-finger-pulse-food-processing (accessed on 17 August 2022).
- Herrero, M.; Cifuentes, A.; Ibañez, E. Sub- and supercritical fluid extraction of functional ingredients from different natural sources: Plants, food-by-products, algae and microalgae—A review. Food Chem. 2006, 98, 136–148. [Google Scholar] [CrossRef]
- Knez, Ž.; Pantić, M.; Cör, D.; Novak, Z.; Knez Hrnčič, M. Are supercritical fluids solvents for the future? Chem. Eng. Process.-Process Intensif. 2019, 141, 107532. [Google Scholar] [CrossRef]
- Manjare, S.D.; Dhingra, K. Supercritical fluids in separation and purification: A review. Mater. Sci. Energy Technol. 2019, 2, 463–484. [Google Scholar] [CrossRef]
- Gullón, B.; Gagaoua, M.; Barba, F.J.; Gullón, P.; Zhang, W.; Lorenzo, J.M. Seaweeds as promising resource of bioactive compounds: Overview of novel extraction strategies and design of tailored meat products. Trends Food Sci. Technol. 2020, 100, 1–18. [Google Scholar] [CrossRef]
- Otero, P.; Quintana, S.E.; Reglero, G.; Fornari, T.; García-Risco, M.R. Pressurized Liquid Extraction (PLE) as an innovative green technology for the effective enrichment of galician algae extracts with high quality fatty acids and antimicrobial and antioxidant properties. Mar. Drugs 2018, 16, 156. [Google Scholar] [CrossRef] [PubMed]
- Alvarez-Rivera, G.; Bueno, M.; Ballesteros-Vivas, D.; Mendiola, J.A.; Ibañez, E. Pressurized liquid extraction. In Liquid-Phase Extraction; Elsevier: Amsterdam, The Netherlands, 2019; pp. 375–398. ISBN 9780128169117. [Google Scholar]
- Duarte, K.; Justino, C.I.L.; Gomes, A.M.; Rocha-Santos, T.; Duarte, A.C. Green analytical methodologies for preparation of extracts and analysis of bioactive compounds. In Comprehensive Analytical Chemistry; Elsevier B.V.: Amsterdam, The Netherlands, 2014; Volume 65, pp. 59–78. [Google Scholar]
- Herrero, M.; Mendiola, J.A.; Plaza, M.; Ibañez, E. Screening for bioactive compounds from algae. In Advanced Biofuels and Bioproducts; Springer: New York, NY, USA, 2012; Volume 9781461433, pp. 833–872. ISBN 9781461433484. [Google Scholar]
- Golmakani, M.T.; Mendiola, J.A.; Rezaei, K.; Ibáñez, E. Expanded ethanol with CO2 and pressurized ethyl lactate to obtain fractions enriched in γ-Linolenic Acid from Arthrospira platensis (Spirulina). J. Supercrit. Fluids 2012, 62, 109–115. [Google Scholar] [CrossRef]
- Zhao, Q.; Anderson, J.L. Ionic liquids. In Comprehensive Sampling and Sample Preparation; Elsevier Inc.: Amsterdam, The Netherlands, 2012; Volume 2, pp. 213–242. ISBN 9780123813749. [Google Scholar]
- Vo Dinh, T.; Saravana, P.S.; Woo, H.C.; Chun, B.S. Ionic liquid-assisted subcritical water enhances the extraction of phenolics from brown seaweed and its antioxidant activity. Sep. Purif. Technol. 2018, 196, 287–299. [Google Scholar] [CrossRef]
- Deetlefs, M.; Seddon, K. Ionic Liquids: The discovery most likely to shape the 21st century. Catalyst 2014. Available online: https://www.stem.org.uk/system/files/elibrary-resources/legacy_files_migrated/36428-Catalyst_25_2_602.pdf (accessed on 17 August 2022).
- Rogers, R.D.; Seddon, K.R. Ionic Liquids—Solvents of the Future? Science 2003, 302, 792–793. [Google Scholar] [CrossRef] [PubMed]
- Alexander, H. Tullo For ionic liquids, the time is now. C&EN Glob. Enterp. 2020, 98, 24–27. [Google Scholar] [CrossRef]
- Asim, A.M.; Uroos, M.; Naz, S.; Sultan, M.; Griffin, G.; Muhammad, N.; Khan, A.S. Acidic ionic liquids: Promising and cost-effective solvents for processing of lignocellulosic biomass. J. Mol. Liq. 2019, 287, 110943. [Google Scholar] [CrossRef]
- George, A.; Brandt, A.; Tran, K.; Zahari, S.M.S.N.S.; Klein-Marcuschamer, D.; Sun, N.; Sathitsuksanoh, N.; Shi, J.; Stavila, V.; Parthasarathi, R.; et al. Design of low-cost ionic liquids for lignocellulosic biomass pretreatment. Green Chem. 2015, 17, 1728–1734. [Google Scholar] [CrossRef]
- Smith, E.L.; Abbott, A.P.; Ryder, K.S. Deep Eutectic Solvents (DESs) and Their Applications. Chem. Rev. 2014, 114, 11060–11082. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; De Oliveira Vigier, K.; Royer, S.; Jérôme, F. Deep eutectic solvents: Syntheses, properties and applications. Chem. Soc. Rev. 2012, 41, 7108–7146. [Google Scholar] [CrossRef] [PubMed]
- Dindarloo Inaloo, I.; Majnooni, S. Deep Eutectic Solvents (DES) as Green and Efficient Solvent/Catalyst Systems for the Synthesis of Carbamates and Ureas from Carbonates. ChemistrySelect 2019, 4, 7811–7817. [Google Scholar] [CrossRef]
- Das, A.K.; Sharma, M.; Mondal, D.; Prasad, K. Deep eutectic solvents as efficient solvent system for the extraction of κ-carrageenan from Kappaphycus alvarezii. Carbohydr. Polym. 2016, 136, 930–935. [Google Scholar] [CrossRef]
- Nie, J.; Chen, D.; Lu, Y. Deep Eutectic Solvents Based Ultrasonic Extraction of Polysaccharides from Edible Brown Seaweed Sargassum horneri. J. Mar. Sci. Eng. 2020, 8, 440. [Google Scholar] [CrossRef]
- Saravana, P.S.; Cho, Y.N.; Woo, H.C.; Chun, B.S. Green and efficient extraction of polysaccharides from brown seaweed by adding deep eutectic solvent in subcritical water hydrolysis. J. Clean. Prod. 2018, 198, 1474–1484. [Google Scholar] [CrossRef]
- Herrero, M.; del Sánchez-Camargo, A.P.; Cifuentes, A.; Ibáñez, E. Plants, seaweeds, microalgae and food by-products as natural sources of functional ingredients obtained using pressurized liquid extraction and supercritical fluid extraction. TrAC-Trends Anal. Chem. 2015, 71, 26–38. [Google Scholar] [CrossRef]
- Yuan, Y.; Macquarrie, D.J. Microwave Assisted Acid Hydrolysis of Brown Seaweed Ascophyllum nodosum for Bioethanol Production and Characterization of Alga Residue. ACS Sustain. Chem. Eng. 2015, 3, 1359–1365. [Google Scholar] [CrossRef]
- Bi, Y.; Lu, Y.; Yu, H.; Luo, L. Optimization of ultrasonic-assisted extraction of bioactive compounds from Sargassum henslowianum using response surface methodology. Pharmacogn. Mag. 2019, 15, 156. [Google Scholar] [CrossRef]
- Le Guillard, C.; Dumay, J.; Donnay-Moreno, C.; Bruzac, S.; Ragon, J.Y.; Fleurence, J.; Bergé, J.P. Ultrasound-assisted extraction of R-phycoerythrin from Grateloupia turuturu with and without enzyme addition. Algal Res. 2015, 12, 522–528. [Google Scholar] [CrossRef]
- Ummat, V.; Tiwari, B.K.; Jaiswal, A.K.; Condon, K.; Garcia-Vaquero, M.; O’Doherty, J.; O’Donnell, C.; Rajauria, G. Optimisation of ultrasound frequency, extraction time and solvent for the recovery of polyphenols, phlorotannins and associated antioxidant activity from brown seaweeds. Mar. Drugs 2020, 18, 250. [Google Scholar] [CrossRef] [PubMed]
- Robin, A.; Kazir, M.; Sack, M.; Israel, A.; Frey, W.; Mueller, G.; Livney, Y.D.; Golberg, A.; Aviv-Yaffo, T. Functional Protein Concentrates Extracted from the Green Marine Macroalga Ulva sp., by High Voltage Pulsed Electric Fields and Mechanical Press. ACS Sustain. Chem. Eng. 2018, 6, 13696–13705. [Google Scholar] [CrossRef]
- Punín Crespo, M.O.; Lage Yusty, M.A. Comparison of supercritical fluid extraction and Soxhlet extraction for the determination of aliphatic hydrocarbons in seaweed samples. Ecotoxicol. Environ. Saf. 2006, 64, 400–405. [Google Scholar] [CrossRef] [PubMed]
- Roh, M.K.; Uddin, M.S.; Chun, B.S. Extraction of fucoxanthin and polyphenol from Undaria pinnatifida using supercritical carbon dioxide with co-solvent. Biotechnol. Bioprocess Eng. 2008, 13, 724–729. [Google Scholar] [CrossRef]
- Cheung, P.C.K.; Leung, A.Y.H.; Ang, P.O. Comparison of Supercritical Carbon Dioxide and Soxhlet Extraction of Lipids from a Brown Seaweed, Sargassum hemiphyllum (Turn.) C. Ag. J. Agric. Food Chem. 1998, 46, 4228–4232. [Google Scholar] [CrossRef]
- Otero, P.; López-Martínez, M.I.; García-Risco, M.R. Application of pressurized liquid extraction (PLE) to obtain bioactive fatty acids and phenols from Laminaria ochroleuca collected in Galicia (NW Spain). J. Pharm. Biomed. Anal. 2019, 164, 86–92. [Google Scholar] [CrossRef] [PubMed]
- Boisvert, C.; Beaulieu, L.; Bonnet, C.; Pelletier, É. Assessment of the Antioxidant and Antibacterial Activities of Three Species of Edible Seaweeds. J. Food Biochem. 2015, 39, 377–387. [Google Scholar] [CrossRef]
- Martins, M.; Vieira, F.A.; Correia, I.; Ferreira, R.A.S.; Abreu, H.; Coutinho, J.A.P.; Ventura, S.P.M. Recovery of phycobiliproteins from the red macroalga: Gracilaria sp. using ionic liquid aqueous solutions. Green Chem. 2016, 18, 4287–4296. [Google Scholar] [CrossRef]
- Trivedi, T.J.; Kumar, A. Efficient Extraction of Agarose from Red Algae Using Ionic Liquids. Green Sustain. Chem. 2014, 04, 190–201. [Google Scholar] [CrossRef]
- Peng, L.Q.; Yu, W.Y.; Xu, J.J.; Cao, J. Pyridinium ionic liquid-based liquid-solid extraction of inorganic and organic iodine from Laminaria. Food Chem. 2018, 239, 1075–1084. [Google Scholar] [CrossRef]
- Patel, A.K.; Dixit, P.; Pandey, A.; Singhania, R.R. Promising enzymes for biomass processing. In Biomass, Biofuels, Biochemicals; Elsevier: Amsterdam, The Netherlands, 2020; pp. 245–271. [Google Scholar]
- Gupta, A.; Prakash, J. Sustainable bio-ethanol production from agro-residues: A review. Renew. Sustain. Energy Rev. 2015, 41, 550–567. [Google Scholar] [CrossRef]
- Agrawal, R.; Semwal, S.; Kumar, R.; Mathur, A.; Gupta, R.P.; Tuli, D.K.; Satlewal, A. Synergistic Enzyme Cocktail to Enhance Hydrolysis of Steam Exploded Wheat Straw at Pilot Scale. Front. Energy Res. 2018, 6, 122. [Google Scholar] [CrossRef]
- Tan, I.S.; Lee, K.T. Solid acid catalysts pretreatment and enzymatic hydrolysis of macroalgae cellulosic residue for the production of bioethanol. Carbohydr. Polym. 2015, 124, 311–321. [Google Scholar] [CrossRef] [PubMed]
- Trivedi, N.; Gupta, V.; Reddy, C.R.K.; Jha, B. Enzymatic hydrolysis and production of bioethanol from common macrophytic green alga Ulva fasciata Delile. Bioresour. Technol. 2013, 150, 106–112. [Google Scholar] [CrossRef] [PubMed]
- Qeshmi, F.I.; Homaei, A.; Fernandes, P.; Hemmati, R.; Dijkstra, B.W.; Khajeh, K. Xylanases from marine microorganisms: A brief overview on scope, sources, features and potential applications. Biochim. Biophys. Acta-Proteins Proteom. 2020, 1868, 140312. [Google Scholar] [CrossRef] [PubMed]
- Parab, P.; Khandeparker, R.; Amberkar, U.; Khodse, V. Enzymatic saccharification of seaweeds into fermentable sugars by xylanase from marine Bacillus sp. strain BT21. 3 Biotech 2017, 7, 296. [Google Scholar] [CrossRef]
- Palavesam, A. Investigation on lignocellulosic saccharification and characterization of haloalkaline solvent tolerant endo-1,4 β-d-xylanase from Halomonas meridiana APCMST-KS4. Biocatal. Agric. Biotechnol. 2015, 4, 761–766. [Google Scholar] [CrossRef]
- Hebbale, D.; Bhargavi, R.; Ramachandra, T.V. Saccharification of macroalgal polysaccharides through prioritized cellulase producing bacteria. Heliyon 2019, 5, e01372. [Google Scholar] [CrossRef]
- Qin, H.M.; Gao, D.; Zhu, M.; Li, C.; Zhu, Z.; Wang, H.; Liu, W.; Tanokura, M.; Lu, F. Biochemical characterization and structural analysis of ulvan lyase from marine Alteromonas sp. reveals the basis for its salt tolerance. Int. J. Biol. Macromol. 2020, 147, 1309–1317. [Google Scholar] [CrossRef]
- Kim, S.W.; Kim, Y.W.; Hong, C.H.; Lyo, I.W.; Lim, H.D.; Kim, G.J.; Shin, H.J. Recombinant agarase increases the production of reducing sugars from HCl-treated Gracilaria verrucosa, a red algae. Algal Res. 2018, 31, 517–524. [Google Scholar] [CrossRef]
- Liu, G.; Wu, S.; Jin, W.; Sun, C. Amy63, a novel type of marine bacterial multifunctional enzyme possessing amylase, agarase and carrageenase activities. Sci. Rep. 2016, 6, 18726. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.L.; Hou, Y.P.; Jin, M.; Zeng, R.Y.; Lin, H.T. Expression and Characterization of a Novel Thermostable and pH-Stable β-Agarase from Deep-Sea Bacterium Flammeovirga Sp. OC4. J. Agric. Food Chem. 2016, 64, 7251–7258. [Google Scholar] [CrossRef] [PubMed]
- Perez, C.M.T.; Pajares, I.G.; Alcantara, V.A.; Simbahan, J.F. Bacterial laminarinase for application in ethanol production from brown algae Sargassum sp. using halotolerant yeast. Biofuel Res. J. 2018, 5, 792–797. [Google Scholar] [CrossRef]
- Kim, D.H.; Kim, D.H.; Lee, S.H.; Kim, K.H. A novel β-glucosidase from Saccharophagus degradans 2-40T for the efficient hydrolysis of laminarin from brown macroalgae. Biotechnol. Biofuels 2018, 11, 64. [Google Scholar] [CrossRef] [PubMed]
- Sadashiv Jagtap, S.; Hehemann, J.-H.; Polz, M.F.; Lee, J.-K.; Zhao, H. Comparative Biochemical Characterization of Three Exolytic Oligoalginate Lyases from Vibrio splendidus Reveals Complementary Substrate Scope, Temperature, and pH Adaptations. Appl. Environ. Microbiol. 2014, 80, 4207–4214. [Google Scholar] [CrossRef] [PubMed]
- Silchenko, A.S.; Kusaykin, M.I.; Kurilenko, V.V.; Zakharenko, A.M.; Isakov, V.V.; Zaporozhets, T.S.; Gazha, A.K.; Zvyagintseva, T.N. Hydrolysis of fucoidan by fucoidanase isolated from the marine bacterium, formosa algae. Mar. Drugs 2013, 11, 2413–2430. [Google Scholar] [CrossRef]
- Torres, M.D.; Kraan, S.; Domínguez, H. Seaweed biorefinery. Rev. Environ. Sci. Biotechnol. 2019, 18, 335–388. [Google Scholar] [CrossRef]
- Limtong, S.; Deejing, S.; Yongmanitchai, W.; Santisopasri, W. Construction of High Ethanol Fermenting Halotolerant Hybrid by Intergeneric Protoplast Fusion of Saccharomyces cerevisiae and Zygosaccharomyces rouxii. Agric. Nat. Resour. 1998, 32, 213–223. [Google Scholar]
- Urano, N.; Hirai, H.; Ishida, M.; Kimura, S. Characterization of Ethanol-Producing Marine Yeasts Isolated from Coastal Water. Fish. Sci. 1998, 64, 633–637. [Google Scholar] [CrossRef]
- Kostas, E.T.; White, D.A.; Du, C.; Cook, D.J. Selection of yeast strains for bioethanol production from UK seaweeds. J. Appl. Phycol. 2016, 28, 1427–1441. [Google Scholar] [CrossRef]
- Ji, S.Q.; Wang, B.; Lu, M.; Li, F.L. Defluviitalea phaphyphila sp. nov., a novel thermophilic bacterium that degrades brown algae. Appl. Environ. Microbiol. 2016, 82, 868–877. [Google Scholar] [CrossRef] [PubMed]
- Ji, S.Q.; Wang, B.; Lu, M.; Li, F.L. Direct bioconversion of brown algae into ethanol by thermophilic bacterium Defluviitalea phaphyphila. Biotechnol. Biofuels 2016, 9, 81. [Google Scholar] [CrossRef]
- Khambhaty, Y.; Upadhyay, D.; Kriplani, Y.; Joshi, N.; Mody, K.; Gandhi, M.R. Bioethanol from Macroalgal Biomass: Utilization of Marine Yeast for Production of the Same. Bioenergy Res. 2013, 6, 188–195. [Google Scholar] [CrossRef]
- Saravanakumar, K.; Senthilraja, P.; Kathiresan, K. Bioethanol production by mangrove-derived marine yeast, Sacchromyces cerevisiae. J. King Saud Univ.-Sci. 2013, 25, 121–127. [Google Scholar] [CrossRef]
- Senthilraja, P.; Kathiresan, K.; Saravanakumar, K. Comparative analysis of bioethanol production by different strains of immobilized marine yeast. J. Yeast Fungal Res. 2011, 2, 113–116. [Google Scholar]
- Senthilraja, K.; Saravanakumar, P. Bio-ethanol production by marine yeasts isolated from coastal mangrove sediment. Int. Multidiscip. Res. J. 2011, 1, 19–24. [Google Scholar]
- Obara, N.; Okai, M.; Ishida, M.; Urano, N. Bioethanol production from mixed biomass (waste of Undaria pinnatifida processing and paper shredding) by fermentation with marine-derived Saccharomyces cerevisiae. Fish. Sci. 2015, 81, 771–776. [Google Scholar] [CrossRef]
- Obara, N.; Ishida, M.; Hamada-Sato, N.; Urano, N. Efficient bioethanol production from paper shredder scrap by a marine derived saccharomyces cerevisiae C-19. Stud. Sci. Technol. 2012, 1, 127–132. [Google Scholar] [CrossRef]
- Sharma, J.; Kumar, S.S.; Kumar, V.; Malyan, S.K.; Mathimani, T.; Bishnoi, N.R.; Pugazhendhi, A. Upgrading of microalgal consortia with CO2 from fermentation of wheat straw for the phycoremediation of domestic wastewater. Bioresour. Technol. 2020, 305, 123063. [Google Scholar] [CrossRef]
- Zhang, Q.; Nurhayati; Cheng, C.L.; Nagarajan, D.; Chang, J.S.; Hu, J.; Lee, D.J. Carbon capture and utilization of fermentation CO2: Integrated ethanol fermentation and succinic acid production as an efficient platform. Appl. Energy 2017, 206, 364–371. [Google Scholar] [CrossRef]
- Schiener, P.; Atack, T.; Wareing, R.A.; Kelly, M.S.; Hughes, A.D. The by-products from marine biofuels as a feed source for the aquaculture industry: A novel example of the biorefinery approach. Biomass Convers. Biorefinery 2016, 6, 281–287. [Google Scholar] [CrossRef]
- Neptune’s Harvest Seaweed Plant Food 0-0-1. Available online: GrowItNaturally.com (accessed on 25 July 2023).
- Øverland, M.; Mydland, L.T.; Skrede, A. Marine macroalgae as sources of protein and bioactive compounds in feed for monogastric animals. J. Sci. Food Agric. 2019, 99, 13–24. [Google Scholar] [CrossRef] [PubMed]
- Bikker, P.; van Krimpen, M.M.; van Wikselaar, P.; Houweling-Tan, B.; Scaccia, N.; van Hal, J.W.; Huijgen, W.J.J.; Cone, J.W.; López-Contreras, A.M. Biorefinery of the green seaweed Ulva lactuca to produce animal feed, chemicals and biofuels. J. Appl. Phycol. 2016, 28, 3511–3525. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Vaquero, M.; Hayes, M. Red and green macroalgae for fish and animal feed and human functional food development. Food Rev. Int. 2016, 32, 15–45. [Google Scholar] [CrossRef]
- Basmal, J. Liquid organic fertilizer from seaweed (Sargassum sp.)and fish waste hydrolysate. Squalen Bull. Mar. Fish. Postharvest Biotechnol. 2010, 5, 59. [Google Scholar] [CrossRef]
- Hwang, H.J.; Kim, S.M.; Chang, J.H.; Lee, S.B. Lactic acid production from seaweed hydrolysate of Enteromorpha prolifera (Chlorophyta). J. Appl. Phycol. 2012, 24, 935–940. [Google Scholar] [CrossRef]
- Lakshmikandan, M.; Murugesan, A.G. Chlorella vulgaris MSU-AGM 14, a fresh water microalgal strain—Growth and photobiological hydrogen production in acid hydrolysate of seaweed Valoniopsis pachynema. Int. J. Hydrogen Energy 2016, 41, 13986–13992. [Google Scholar] [CrossRef]
- Sadhukhan, J.; Gadkari, S.; Martinez-Hernandez, E.; Ng, K.S.; Shemfe, M.; Torres-Garcia, E.; Lynch, J. Novel macroalgae (seaweed) biorefinery systems for integrated chemical, protein, salt, nutrient and mineral extractions and environmental protection by green synthesis and life cycle sustainability assessments. Green Chem. 2019, 21, 2635–2655. [Google Scholar] [CrossRef]
- IPCC. Global Warming of 1.5 °C; IPCC: Geneva, Switzerland, 2019. [Google Scholar]
- Niu, S.; Mai, C.; McKim, K.G.; McCrickard, S. Investigating How YouTubers Participate in a Social Media Campaign. Proc. ACM Human-Comput. Interact. 2021, 5, 1–26. [Google Scholar] [CrossRef]
- Bala, G.; Caldeira, K.; Wickett, M.; Phillips, T.J.; Lobell, D.B.; Delire, C.; Mirin, A. Combined climate and carbon-cycle effects of large-scale deforestation. Proc. Natl. Acad. Sci. USA 2007, 104, 6550–6555. [Google Scholar] [CrossRef]
- Zastrow, M. China’s tree-planting drive could falter in a warming world. Nature 2019, 573, 474–475. [Google Scholar] [CrossRef] [PubMed]
- Hulvey, K.B.; Hobbs, R.J.; Standish, R.J.; Lindenmayer, D.B.; Lach, L.; Perring, M.P. Benefits of tree mixes in carbon plantings. Nat. Clim. Chang. 2013, 3, 869–874. [Google Scholar] [CrossRef]
- Kraan, S. Mass-cultivation of carbohydrate rich macroalgae, a possible solution for sustainable biofuel production. Mitig. Adapt. Strateg. Glob. Chang. 2013, 18, 27–46. [Google Scholar] [CrossRef]
- Mitra, A.; Zaman, S. Blue Carbon Reservoir of the Blue Planet; Springer: New Delhi, India, 2015; ISBN 978-81-322-2106-7. [Google Scholar]
- Nellemann, C.; Corcoran, E.; Duarte, C.M.; Valdés, L.; De Young, C.; Fonseca, L.; Grimsditch, G. Blue Carbon: The Role of Healthy Oceans in Binding Carbon: A Rapid Response Assessment; UNEP/Earthprint: Nairobi, Kenya, 2009; ISBN 9788277010601. [Google Scholar]
- Krause-Jensen, D.; Duarte, C.M. Substantial role of macroalgae in marine carbon sequestration. Nat. Geosci. 2016, 9, 737–742. [Google Scholar] [CrossRef]
- Rassweiler, A.; Reed, D.C.; Harrer, S.L.; Nelson, J.C. Improved estimates of net primary production, growth, and standing crop of Macrocystis pyrifera in Southern California. Ecology 2018, 99, 2132. [Google Scholar] [CrossRef] [PubMed]
- Camus, C.; Infante, J.; Buschmann, A.H. Revisiting the economic profitability of giant kelp Macrocystis pyrifera (Ochrophyta) cultivation in Chile. Aquaculture 2019, 502, 80–86. [Google Scholar] [CrossRef]
- Purcell-Meyerink, D.; Packer, M.A.; Wheeler, T.T.; Hayes, M. Aquaculture production of the brown seaweeds Laminaria digitata and Macrocystis pyrifera: Applications in food and pharmaceuticals. Molecules 2021, 26, 1306. [Google Scholar] [CrossRef]
- Lehahn, Y.; Ingle, K.N.; Golberg, A. Global potential of offshore and shallow waters macroalgal biorefineries to provide for food, chemicals and energy: Feasibility and sustainability. Algal Res. 2016, 17, 150–160. [Google Scholar] [CrossRef]
- Froehlich, H.E.; Afflerbach, J.C.; Frazier, M.; Halpern, B.S. Blue Growth Potential to Mitigate Climate Change through Seaweed Offsetting. Curr. Biol. 2019, 29, 3087–3093.e3. [Google Scholar] [CrossRef]
- Chemodanov, A.; Jinjikhashvily, G.; Habiby, O.; Liberzon, A.; Israel, A.; Yakhini, Z.; Golberg, A. Net primary productivity, biofuel production and CO2 emissions reduction potential of Ulva sp. (Chlorophyta) biomass in a coastal area of the Eastern Mediterranean. Energy Convers. Manag. 2017, 148, 1497–1507. [Google Scholar] [CrossRef]
- Badmus, U.O.; Taggart, M.A.; Boyd, K.G. The effect of different drying methods on certain nutritionally important chemical constituents in edible brown seaweeds. J. Appl. Phycol. 2019, 31, 3883–3897. [Google Scholar] [CrossRef]
- Camus, C.; Ballerino, P.; Delgado, R.; Olivera-Nappa, Á.; Leyton, C.; Buschmann, A.H. Scaling up bioethanol production from the farmed brown macroalga Macrocystis pyrifera in Chile. Biofuels Bioprod. Biorefining 2016, 10, 673–685. [Google Scholar] [CrossRef]
- OECD. Biofuels. In OECD-FAO Agricultural Outlook 2017–2026; OECD Publishing: Paris, France, 2017. [Google Scholar] [CrossRef]
- Leyton, A.; Pezoa-Conte, R.; Barriga, A.; Buschmann, A.H.; Mäki-Arvela, P.; Mikkola, J.P.; Lienqueo, M.E. Identification and efficient extraction method of phlorotannins from the brown seaweed Macrocystis pyrifera using an orthogonal experimental design. Algal Res. 2016, 16, 201–208. [Google Scholar] [CrossRef]
- Leyton, A.; Pezoa-Conte, R.; Mäki-Arvela, P.; Mikkola, J.P.; Lienqueo, M.E. Improvement in carbohydrate and phlorotannin extraction from Macrocystis pyrifera using carbohydrate active enzyme from marine Alternaria sp. as pretreatment. J. Appl. Phycol. 2017, 29, 2039–2048. [Google Scholar] [CrossRef]
- Voutilainen, E.; Pihlajaniemi, V.; Parviainen, T. Economic comparison of food protein production with single-cell organisms from lignocellulose side-streams. Bioresour. Technol. Rep. 2021, 14, 100683. [Google Scholar] [CrossRef]
- Net-Zero Emissions Must Be Met by 2050 or COVID-19 Impact on Global Economies Will Pale Beside Climate Crisis, Secretary-General Tells Finance Summit. Available online: https://press.un.org/en/2020/sgsm20411.doc.htm (accessed on 16 September 2022).
- Van den Hoek, C.; Breeman, A.M.; Stam, W.T. The Geographic Distribution of Seaweed Species in Relation to Temperature: Present and Past. In Expected Effects of Climatic Change on Marine Coastal Ecosystems; Springer: Dordrecht, The Netherlands, 1990; pp. 55–67. [Google Scholar]
- NOAA National Centers for Environmental Information. Monthly Global Climate Report for July 2020. Available online: https://www.ncei.noaa.gov/access/monitoring/monthly-report/global/202007 (accessed on 22 July 2020).
- Rogelj, J.; Shindell, D.; Jiang, K.; Fifita, S.; Forster, P.; Ginzburg, V.; Handa, C.; Kheshgi, H.; Kobayashi, S.; Kriegler, E.; et al. Mitigation Pathways Compatible with 1.5 °C in the Context of Sustainable Development. In Global Warming of 1.5 °C. An IPCC Special Report on the Impacts of Global Warming of 1.5 °C above Pre-Industrial Levels and Related Global Greenhouse Gas Emission Pathways, in the Context of Strengthening the Global Response to the Threat of Climate Change, Sustainable Development, and Efforts to Eradicate Poverty; Cambridge University Press: Cambridge, UK; New York, NY, USA, 2018; pp. 93–174. [Google Scholar] [CrossRef]
- Smale, D.A.; Pessarrodona, A.; King, N.; Burrows, M.T.; Yunnie, A.; Vance, T.; Moore, P. Environmental factors influencing primary productivity of the forest-forming kelp Laminaria hyperborea in the northeast Atlantic. Sci. Rep. 2020, 10, 12161. [Google Scholar] [CrossRef] [PubMed]
- Tait, L.W.; Schiel, D.R. Ecophysiology of Layered Macroalgal Assemblages: Importance of Subcanopy Species Biodiversity in Buffering Primary Production. Front. Mar. Sci. 2018, 5, 444. [Google Scholar] [CrossRef]
- Buschmann, A.H.; Camus, C.; Infante, J.; Neori, A.; Israel, Á.; Hernández-González, M.C.; Pereda, S.V.; Gomez-Pinchetti, J.L.; Golberg, A.; Tadmor-Shalev, N.; et al. Seaweed production: Overview of the global state of exploitation, farming and emerging research activity. Eur. J. Phycol. 2017, 52, 391–406. [Google Scholar] [CrossRef]
- Golberg, A.; Liberzon, A. Modeling of smart mixing regimes to improve marine biorefinery productivity and energy efficiency. Algal Res. 2015, 11, 28–32. [Google Scholar] [CrossRef]
- Duarte, C.M.; Wu, J.; Xiao, X.; Bruhn, A.; Krause-Jensen, D. Can seaweed farming play a role in climate change mitigation and adaptation? Front. Mar. Sci. 2017, 4, 100. [Google Scholar] [CrossRef]
- Campbell, I.; Macleod, A.; Sahlmann, C.; Neves, L.; Funderud, J.; Øverland, M.; Hughes, A.D.; Stanley, M. The environmental risks associated with the development of seaweed farming in Europe—Prioritizing key knowledge gaps. Front. Mar. Sci. 2019, 6, 107. [Google Scholar] [CrossRef]
- Neveux, N.; Bolton, J.J.; Bruhn, A.; Roberts, D.A.; Ras, M. The Bioremediation Potential of Seaweeds: Recycling Nitrogen, Phosphorus, and Other Waste Products. In Blue Biotechnology; Wiley-VCH Verlag GmbH & Co., KgaA: Weinheim, Germany, 2018; pp. 217–239. [Google Scholar]
- BP. BP Statistical Review of World Energy 2019; BP: London, UK, 2019. [Google Scholar]
- FuelsEurope. Refining Products for Our Everyday Life; FuelsEurope: Brussels, Belgium, 2020. [Google Scholar]
- DOE (U.S. Department of Energy). National Algal Biofuels Technology Review; U.S. Department of Energy: Washington, DC, USA, 2016. [Google Scholar]
- U.S. Department of Energy. Energy Efficiency and Renewable Energy Powering the Blue Economy: Exploring Opportunities for Marine Renewable Energy in Maritime Markets; U.S. Department of Energy: Washington, DC, USA, 2019. [Google Scholar]
- Ravanal, M.C.; Sharma, S.; Gimpel, J.; Reveco-Urzua, F.E.; Øverland, M.; Horn, S.J.; Lienqueo, M.E. The role of alginate lyases in the enzymatic saccharification of brown macroalgae, Macrocystis pyrifera and Saccharina latissima. Algal Res. 2017, 26, 287–293. [Google Scholar] [CrossRef]
- Vásquez, V.; Martínez, R.; Bernal, C. Enzyme-assisted extraction of proteins from the seaweeds Macrocystis pyrifera and Chondracanthus chamissoi: Characterization of the extracts and their bioactive potential. J. Appl. Phycol. 2019, 31, 1999–2010. [Google Scholar] [CrossRef]
- Ravanal, M.C.; Pezoa-Conte, R.; von Schoultz, S.; Hemming, J.; Salazar, O.; Anugwom, I.; Jogunola, O.; Mäki-Arvela, P.; Willför, S.; Mikkola, J.P.; et al. Comparison of different types of pretreatment and enzymatic saccharification of Macrocystis pyrifera for the production of biofuel. Algal Res. 2016, 13, 141–147. [Google Scholar] [CrossRef]
- Manandhar, B.; Paudel, P.; Seong, S.H.; Jung, H.A.; Choi, J.S. Characterizing eckol as a therapeutic aid: A systematic review. Mar. Drugs 2019, 17, 361. [Google Scholar] [CrossRef] [PubMed]
- Ford, L.; Theodoridou, K.; Sheldrake, G.N.; Walsh, P.J. A critical review of analytical methods used for the chemical characterisation and quantification of phlorotannin compounds in brown seaweeds. Phytochem. Anal. 2019, 30, 587–599. [Google Scholar] [CrossRef] [PubMed]
- Marketresearchfuture Alginates Market Research Report Information—By Source (Laminaria, Macrocystis, Ascophyllum, and Others), by Function (Thickener, Emulsifier, Stabilizer, Acidity Regulator, Others), by Region—Forecast Till 2027. Available online: https://www.marketresearchfuture.com/reports/alginates-market-1581 (accessed on 25 July 2023).
- Grandviewresearch Mannitol Market Size, Share & Trends Analysis Report by Application (Food Additive, Pharmaceuticals, Industrial, Surfactants), and Segment Forecasts, 2015–2024. Available online: https://www.grandviewresearch.com/industry-analysis/mannitol-market (accessed on 25 July 2023).
- Lorbeer, A.J.; Charoensiddhi, S.; Lahnstein, J.; Lars, C.; Franco, C.M.M.; Bulone, V.; Zhang, W. Sequential extraction and characterization of fucoidans and alginates from Ecklonia radiata, Macrocystis pyrifera, Durvillaea potatorum, and Seirococcus axillaris. J. Appl. Phycol. 2017, 29, 1515–1526. [Google Scholar] [CrossRef]
- Zhang, W.; Oda, T.; Yu, Q.; Jin, J.-O. Fucoidan from Macrocystis pyrifera Has Powerful Immune-Modulatory Effects Compared to Three Other Fucoidans. Mar. Drugs 2015, 13, 1084–1104. [Google Scholar] [CrossRef]
| Enzymes | Producing Organism | Targeted Polysaccharide | Substrate Seaweed sp. | Reducing Sugars Yield | Ref. |
|---|---|---|---|---|---|
| Green Seaweeds | |||||
| Xylanase | Bacillus sp. strain BT21 | Xylan | U. lactuca | 45.84 µg/mg | [189] |
| Xylanase | H. meridiana | Xylan | U. lactuca | 50.03 mg/g | [190] |
| Cellulase | V. parahaemolyticus | Cellulose | U. lactuca | 107.6 mg/g | [191] |
| Cellulase | V. parahaemolyticus | Cellulose | U. intestinalis | 135.9 mg/g | [191] |
| Ulvan lyase | Alteromonas sp. | Ulvan | na | na | [192] |
| Red Seaweeds | |||||
| Xylanase | Bacillus sp. strain BT21 | Xylan | A. plicata | 12.16 µg/mg | [189] |
| Agarase | S. degradans 2–40 | Agar | G. verrucosa | na | [193] |
| Amy63 | V. alginolyticus 63 | Amylose Agarose Carrageenan | na | na | [194] |
| Aga4436 | Flammeovirga sp. OC4 | Agarose | na | na | [195] |
| Brown Seaweeds | |||||
| Laminarinase | Bacillus sp. (8D) | Laminarin | Sargassum sp | 1.97 mg/mL | [196] |
| Bgl1B | S. degradans 2-40T | Laminarin | na | na | [197] |
| Xylanase | Bacillus sp. strain BT21 | Xylan | P. tetrastromatica | 59.56 µg/mg | [189] |
| OLA | V. splendidus 12B01 (12B01) | Alginate | na | 1.6 mg/mL | [198] |
| Fucoidanase | Formosa algae strain KMM 3553 | Fucoidan | F. evanescens F. vesiculosus | na | [199] |
| Marine Yeasts | Source | Fermentation Media (Salt Con.) | Pre-Treatment | Sugar | Max. Produc. (g/L/h) | Max. EtOH (g/L) | Ref. |
|---|---|---|---|---|---|---|---|
| S. cerevisiae YPS128 | Plymouth, UK | C. crispus in fresh water (na) | 5% H2SO4, 121 °C, 15 min | 2.02 g/L | 0.108 | 13 | [203] |
| Defluviitalea. haphyphila Alg1 | Yellow Sea, China | S. japonica in salt water (3%) | Dried, powderised | 5% | 0.14 | 10 | [204,205] |
| Candida sp. | West Coast India | K. alvarezii in salt water (11.25%) | Acid hydrolysis, 100 °C for 1 h | 5.5% | 0.17 | 12.3 | [206] |
| S. cerevisiae JN387604 | Mangrove, Southeast India | Sawdust in 50% Seawater (1.75%) | 0.8% phosphoric acid | 6.84 mg/L | 0.2 | 25.1 | [207] |
| P. salicaria | Mangrove, SE India | Sawdust in freshwater (na) | Dilute phosphoric acid | 2% | 0.37 | 28.5 | [208,209] |
| C. albicans | Mangrove, SE India | Malt broth in 50% Seawater (1.75%) | na | 3 g/L | 0.49 | 47.3 | [208] |
| S. cerevisiae C-19 | Tokyo Bay | U. pinnatifida and paper in freshwater (na) | 3% H2SO4, 121 °C, 1 h and cellulase GC220 and α-amylase | 230 g/L | 0.73 | 87.7 | [210,211] |
| S. cerevisiae AZ65 | Caernarfon, Wales, UK | YPD–seawater medium (3.5%) | na | 200 g/L | 4.15 | 86.72 | [21] |
| Sugarcane molasses–seawater (3.5%) | 91.27 g/L | 2.46 | 50.32 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Johnston, K.G.; Abomohra, A.; French, C.E.; Zaky, A.S. Recent Advances in Seaweed Biorefineries and Assessment of Their Potential for Carbon Capture and Storage. Sustainability 2023, 15, 13193. https://doi.org/10.3390/su151713193
Johnston KG, Abomohra A, French CE, Zaky AS. Recent Advances in Seaweed Biorefineries and Assessment of Their Potential for Carbon Capture and Storage. Sustainability. 2023; 15(17):13193. https://doi.org/10.3390/su151713193
Chicago/Turabian StyleJohnston, Katherine G., Abdelfatah Abomohra, Christopher E. French, and Abdelrahman S. Zaky. 2023. "Recent Advances in Seaweed Biorefineries and Assessment of Their Potential for Carbon Capture and Storage" Sustainability 15, no. 17: 13193. https://doi.org/10.3390/su151713193







