Formaldehyde Removal by Expanded Clay Pellets and Biofilm in Hydroponics of a Green Wall System
Abstract
:1. Introduction
2. Materials and Methods
2.1. The Assembly of a Green Wall System
2.2. Enrichment of the Biofilm
2.3. Measurement of the Fluorescein Diacetate Hydrolysis Activity
2.4. Microscopy Study
2.5. Incubation of Expanded Clay Pellets with a Biofilm in Formaldehyde
2.6. Determination of Formaldehyde Concentration in the Liquid Phase
2.7. Screening of Carbon Sources Stimulating a Microbial Growth in the Presence of Formaldehyde in EcoPlates™
2.8. Statistical Analysis
3. Results
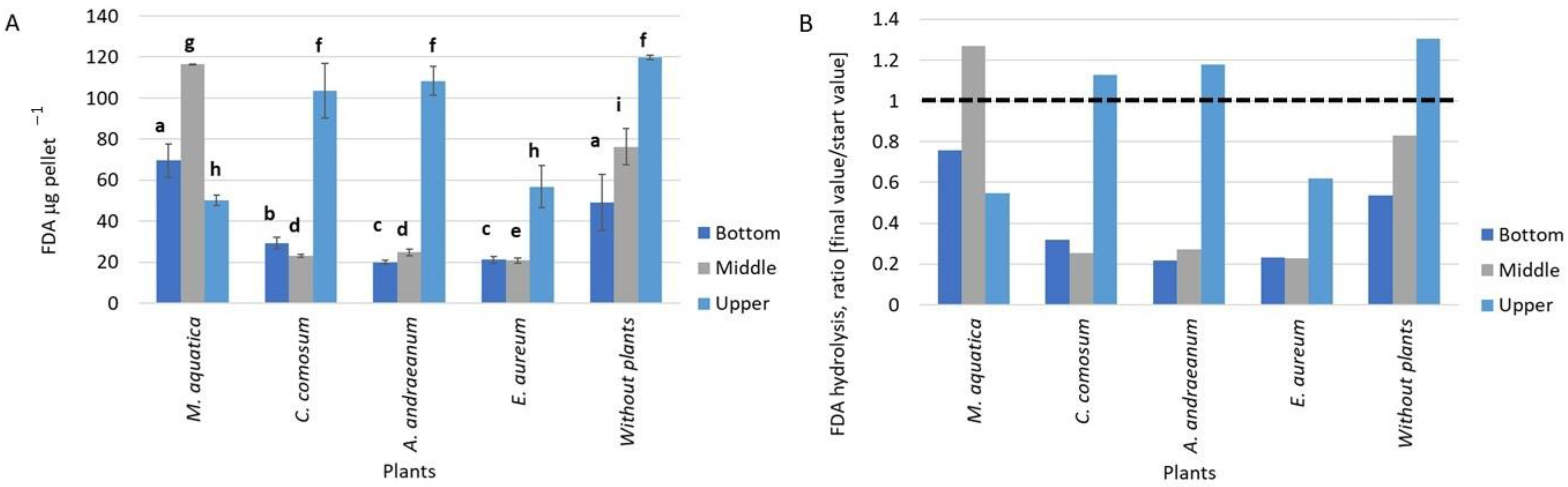
3.1. The Fluorescein Diacetate Hydrolysis Activity in Biofilms on Expanded Clay Pellets Obtained at Different Height Levels of the GWS Modules with Different Plant Species
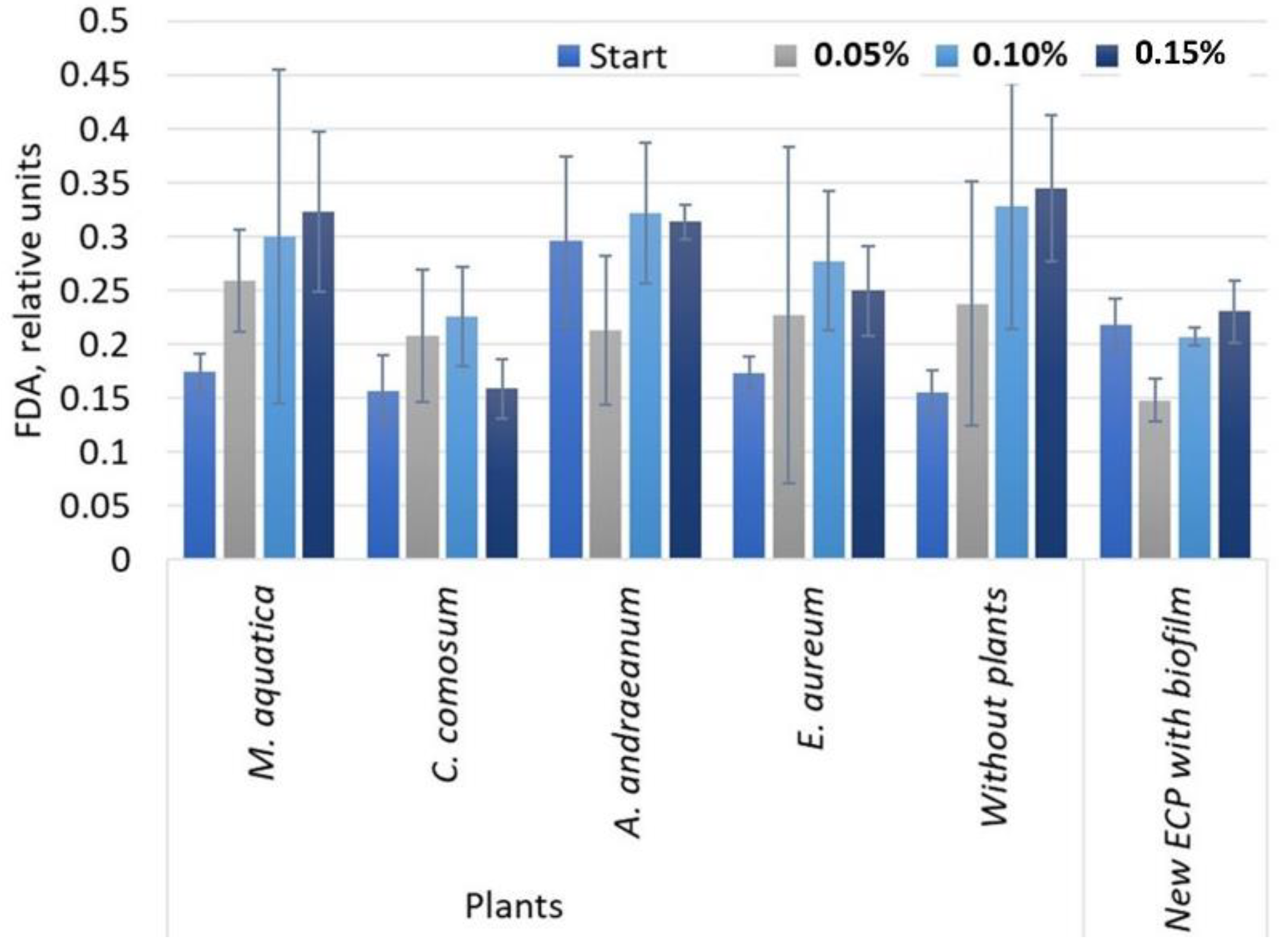
3.2. Biofilm Enrichment in the Synthetic Medium with a Gradual Increase of Carbohydrates
3.3. Determination of Formaldehyde Removal from the Liquid Phase by Expanded Clay Pellets Coated with a Biofilm
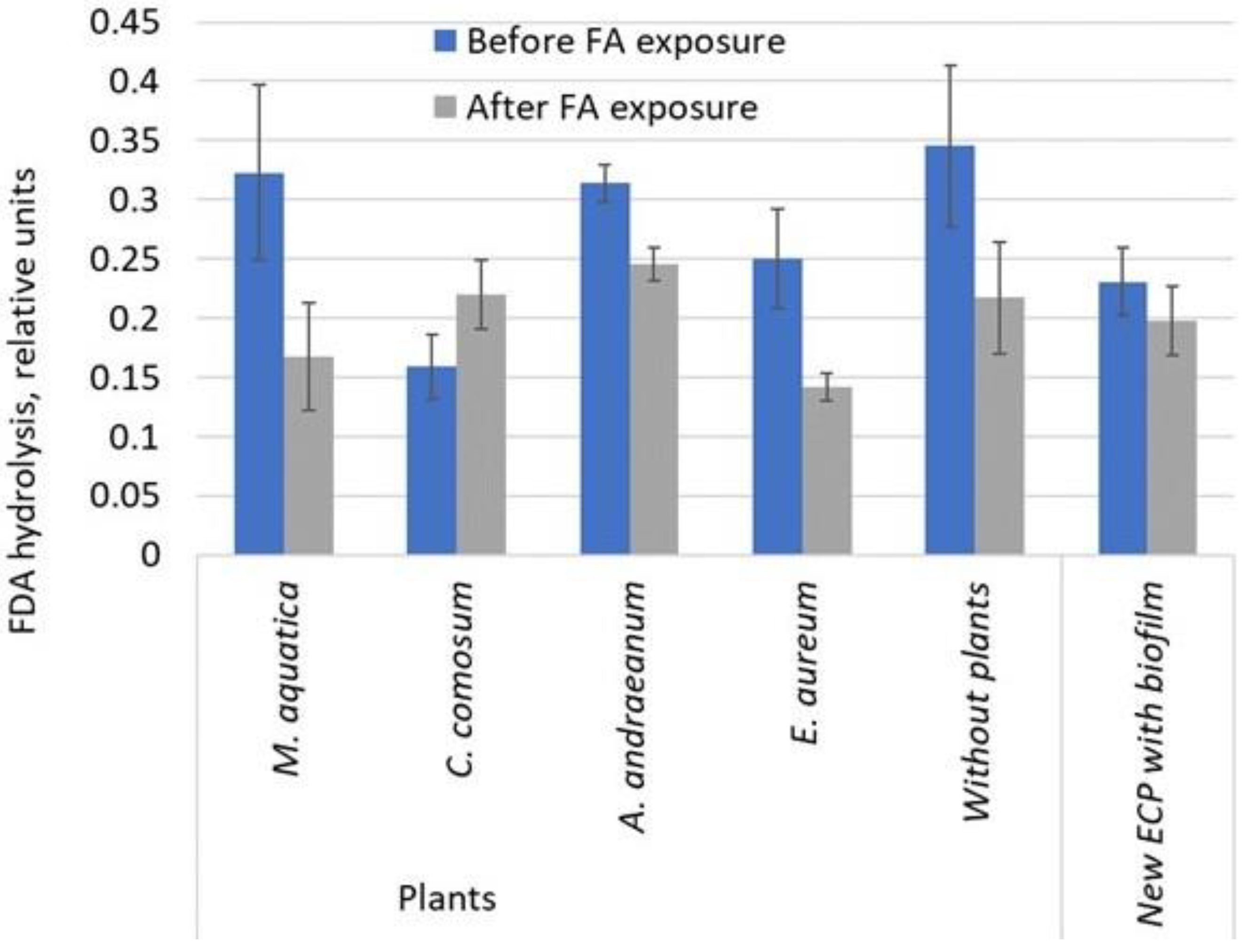
3.4. Determination of the FDA Hydrolysis Activity in a Biofilm on ECP after 24 h Exposure to FA in Liquid Phase
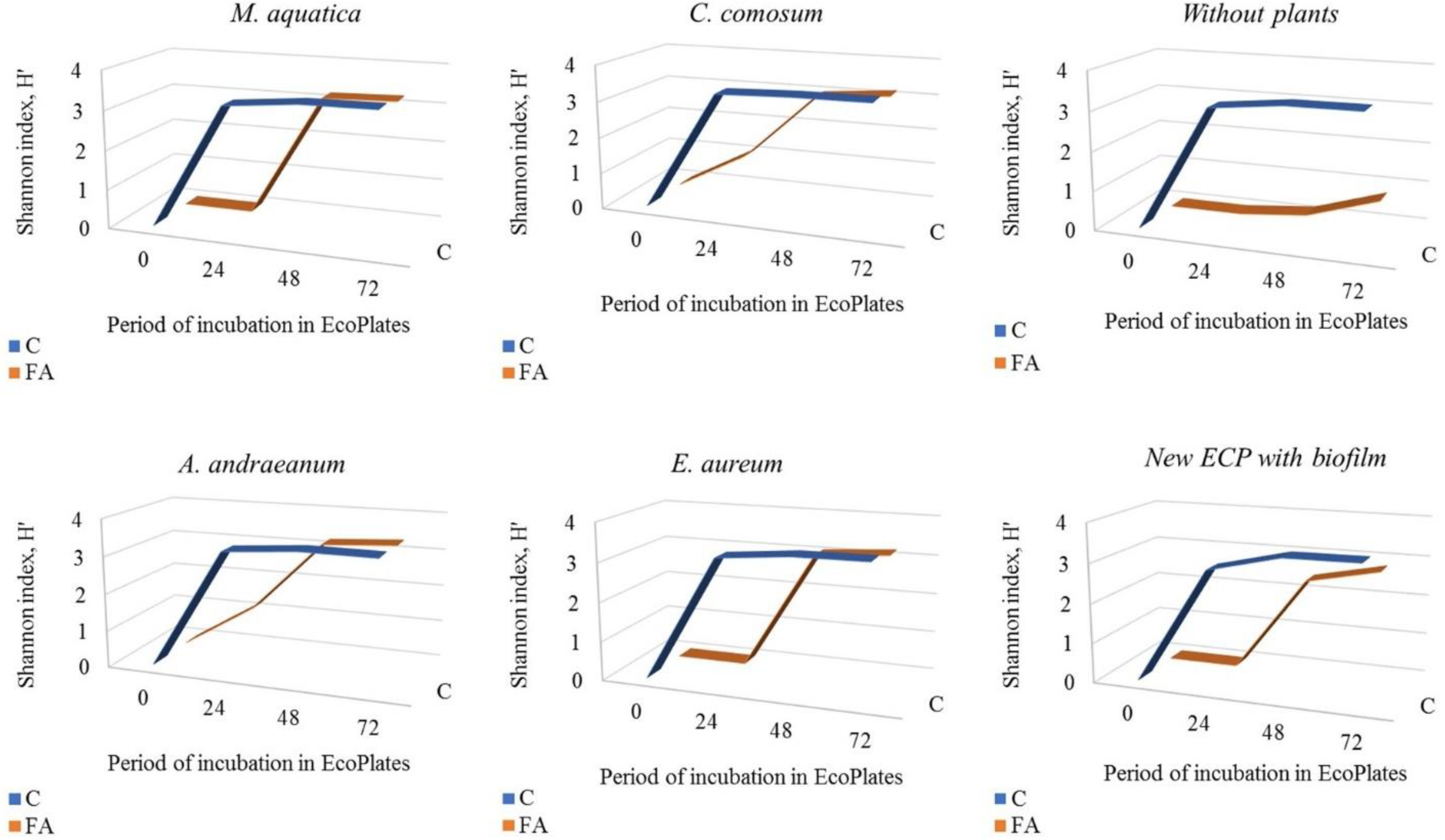
3.5. Screening of Carbon Sources Stimulating Microbial Growth in the Presence of FA in EcoPlates™
4. Discussion
5. Conclusions
- The FDA hydrolysis activity of the biofilm was shown to change depending on the type of ECP treatment. A decrease in enzyme activity after a 21-day pretreatment with FA, as well as after a 24 h incubation of ECP in an FA solution, indicated the inhibition of the metabolic activity of immobilized heterotrophic microorganisms by FA at concentrations up to 100 mg/L. The inhibition effect was highly dependent on the plant species and the sampling location in the GWS.
- The enrichment conditions increased the FDA hydrolysis activity of biofilms in the sets of GWS with M. aquatica, E. aureum, and without plants. Obviously, enrichment conditions were specific to each microbial community, which, in turn, was affected by the selection of plant species.
- The FA removal activity of ECP was 76.5 μg ECP−1 after a 24 h incubation in the presence of 100 mg/L FA, while the removal activity of the biofilm varied, with the highest value (59.2 μg ECP−1) observed in the set with M. aquatica.
- The screening of 31 carbon substrates available in EcoPlates™ revealed substrates which stimulated the growth of particular microbial communities obtained from ECP. For example, itaconic acid stimulated microbial growth in the presence of 50 mg/L FA in the sets with C. comosum, A. andraeanum, and E. aureum up to 21%, 37%, and 49%, respectively, as compared to the respective controls.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhang, L.; Routsong, R.; Strand, S.E. Greatly Enhanced Removal of Volatile Organic Carcinogens by a Genetically Modified Houseplant, Pothos Ivy (Epipremnum aureum) Expressing the Mammalian Cytochrome P450 2e1 Gene. Environ. Sci. Technol. 2019, 53, 325–331. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L.; Shen, D.; Luo, K.H. A critical review on VOCs adsorption by different porous materials: Species, mechanisms and modification methods. J. Hazard. Mater. 2020, 389, 122102. [Google Scholar] [PubMed]
- Sahu, L.K.; Tripathi, N.; Yadav, R. Contribution of biogenic and photochemical sources to ambient VOCs during winter to summer transition at a semi-arid urban site in India. Environ. Pollut. 2017, 229, 595–606. [Google Scholar] [CrossRef] [PubMed]
- Peng, W.X.; Yue, X.; Chen, H.; Ma, N.L.; Quan, Z.; Yu, Q.; Wei, Z.; Guan, R.; Lam, S.S.; Rinklebe, J.; et al. A review of plants formaldehyde metabolism: Implications for hazardous emissions and phytoremediation. J. Hazard. Mater. 2022, 436, 129304. [Google Scholar] [PubMed]
- Matheson, S.; Fleck, R.; Irga, P.J.; Torpy, F.R. Phytoremediation for the indoor environment: A state-of-the-art review. Rev. Environ. Sci. Biotechnol. 2023, 22, 249–280. [Google Scholar]
- Reyhani, M.; Santolini, E.; Tassinari, P.; Torreggiani, D. Environmental assessment of design choices of green walls based for materials combination and plants. Int. J. Life Cycle Assess. 2023, 28, 1078–1091. [Google Scholar] [CrossRef]
- Riley, B. The state of the art of living walls: Lessons learned. Build. Environ. 2017, 114, 219–232. [Google Scholar]
- Goli, A.; Talaiekhozani, A.; Eshtiaghi, N.; Chisti, Y.; Aramesh, R.; Aramesh, R.; Shamiri, A. Biotreatment of formaldehyde-contaminated air in a trickle bed bioreactor. Desalin. Water Treat. 2017, 93, 83–92. [Google Scholar] [CrossRef]
- Pettit, T.; Irga, P.J.; Torpy, F.R. Functional green wall development for increasing air pollutant phytoremediation: Substrate development with coconut coir and activated carbon. J. Hazard. Mater. 2018, 360, 594–603. [Google Scholar] [CrossRef]
- Jamshidi, A.; Hajizadeh, Y.; Amin, M.M.; Kiani, G.; Haidari, R.; Falahi-Nejad, K.; Parseh, I. Biofiltration of formaldehyde, acetaldehyde, and acrolein from polluted airstreams using a biofilter. J. Chem. Technol. Biotechnol. 2018, 93, 1328–1337. [Google Scholar] [CrossRef]
- Suárez-Cáceres, G.P.; Fernández-Cañero, R.; Fernández-Espinosa, A.J.; Rossini-Oliva, S.; Franco-Salas, A.; Pérez-Urrestarazu, L. Volatile organic compounds removal by means of a felt-based living wall to improve indoor air quality. Atmos. Pollut. Res. 2021, 12, 224–229. [Google Scholar] [CrossRef]
- Bandehali, S.; Miri, T.; Onyeaka, H.; Kumar, P. Current state of indoor air phytoremediation using potted plants and green walls. Atmosphere 2021, 12, 473. [Google Scholar] [CrossRef]
- Soreanu, G.; Dixon, M.; Darlington, A. Botanical biofiltration of indoor gaseous pollutants—A mini-review. Chem. Eng. J. 2013, 229, 585–594. [Google Scholar]
- Xu, Z.; Qin, N.; Wang, J.; Tong, H. Formaldehyde Biofiltration as Affected by Spider Plant. Bioresour. Technol. 2010, 101, 6930–6934. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Zhong, J.; Liu, Q.; Yang, H.; Wang, Z.; Li, Y.; Zhang, W.; Agranovski, I. Indoor Formaldehyde Removal by Three Species of Chlorophytum comosum under Dynamic Fumigation System: Part 2—Plant Recovery. Environ. Sci. Pollut. Res. 2021, 28, 8453–8465. [Google Scholar] [CrossRef]
- Zuo, L.; Wu, D.; Yu, L.; Yuan, Y. Phytoremediation of Formaldehyde by the Stems of Epipremnum aureum and Rohdea japonica. Environ. Sci. Pollut. Res. 2022, 29, 11445–11454. [Google Scholar] [CrossRef]
- Aydogan, A.; Montoya, L.D. Formaldehyde removal by common indoor plant species and various growing media. Atmos. Environ. 2011, 45, 2675–2682. [Google Scholar] [CrossRef]
- Liu, Y.J.; Mu, Y.J.; Zhu, Y.G.; Ding, H.; Crystal Arens, N. Which ornamental plant species effectively remove benzene from indoor air? Atmos. Environ. 2007, 41, 650–654. [Google Scholar] [CrossRef]
- Su, Y.; Liang, Y. Foliar uptake and translocation of formaldehyde with Bracket plants (Chlorophytum comosum). J. Hazard. Mater. 2015, 291, 120–128. [Google Scholar] [CrossRef]
- Teiri, H.; Pourzamani, H.; Hajizadeh, Y. Phytoremediation of VOCs from indoor air by ornamental potted plants: A pilot study using a palm species under the controlled environment. Chemosphere 2018, 197, 375–381. [Google Scholar] [CrossRef]
- Xu, Z.; Wang, L.; Hou, H. Formaldehyde removal by potted plant-soil systems. J. Hazard. Mater. 2011, 192, 314–318. [Google Scholar] [CrossRef] [PubMed]
- He, X.; Li, D.; Ablikim, A.; Yang, Y.; Su, Y. A rapid method to assess the formaldehyde dehydrogenase activity in plants for the remediation of formaldehyde. Environ. Sci. Pollut. Res. 2021, 28, 8782–8790. [Google Scholar] [CrossRef]
- Sharma, S.; Bakht, A.; Jahanzaib, M.; Lee, H.; Park, D. Evaluation of the Effectiveness of Common Indoor Plants in Improving the Indoor Air Quality of Studio Apartments. Atmosphere 2022, 13, 1863. [Google Scholar] [CrossRef]
- Zhao, S.; Su, Y.; Liang, H. Efficiency and mechanism of formaldehyde removal from air by two wild plants; Plantago asiatica L. and Taraxacum mongolicum Hand.-Mazz. J. Environ. Health Sci. Eng. 2019, 17, 141–150. [Google Scholar] [CrossRef]
- Maldonado-Diaz, G.; Arriaga, S. Biofiltration of high formaldehyde loads with ozone additions in long-term operation. Appl. Microbiol. Biotechnol. 2015, 99, 43–53. [Google Scholar] [CrossRef]
- Wen, H.; Wang, J.; Yang, C.; Bi, X.; Zou, P. Study of the Performance of a Composite Bioreactor on Removal of High Concentrations of Formaldehyde. Water Air Soil Pollut. 2020, 231, 131. [Google Scholar] [CrossRef]
- Pereira, L.C.; Pereira, C.B.; Correia, L.V.; Matera, T.C.; Dos Santos, R.F.; de Carvalho, C.; Osipi, E.A.F.; Braccini, A.L. Corn responsiveness to azospirillum: Accessing the effect of root exudates on the bacterial growth and its ability to fix nitrogen. Plants 2020, 9, 923. [Google Scholar] [CrossRef]
- Gabor, E.M.; De Vries, E.J.; Janssen, D.B. Efficient recovery of environmental DNA for expression cloning by indirect extraction methods. FEMS Microbiol. Ecol. 2003, 44, 153–163. [Google Scholar] [CrossRef]
- Lichstein, H.C.; Soule, M.H. Studies of the Effect of Sodium Azide on Microbic Growth and Respiration: II. The Action of Sodium Azide on Bacterial Catalase. J. Bacteriol. 1944, 47, 239–251. [Google Scholar]
- Ning, Z.; Kennedy, K.J.; Fernandes, L. Biosorption of 2,4-dichlorophenol by live and chemically inactivated anaerobic granules. Water Res. 1996, 30, 2039–2044. [Google Scholar] [CrossRef]
- Shushunova, N.; Korol, E.; Luzay, E.; Shafieva, D. Impact of the Innovative Green Wall Modular Systems on the Urban Air. Sustainability 2023, 15, 9732. [Google Scholar] [CrossRef]
- Yang, Y.; Hu, K.; Liu, Y.; Wang, Z.; Dong, K.; Lv, P.; Shi, X. Optimisation of Building Green Performances Using Vertical Greening Systems: A Case Study in Changzhou, China. Sustainability 2023, 15, 4494. [Google Scholar] [CrossRef]
- Wu, D.; Yu, L. Effects of Airflow Rate and Plant Species on Formaldehyde Removal by Active Green Walls. Environ. Sci. Pollut. Res. 2022, 29, 88812–88822. [Google Scholar] [CrossRef]
- Teiri, H.; Hajizadeh, Y.; Azhdarpoor, A. A Review of Different Phytoremediation Methods and Critical Factors for Purification of Common Indoor Air Pollutants: An Approach with Sensitive Analysis. Air Qual. Atmos. Health 2022, 15, 373–391. [Google Scholar] [CrossRef]
- Wrobetz, A.; Matteazzi, M.; Montoya, L.D. Formaldehyde Sorption to Porous Media for Air Quality Applications. In Proceedings of the Healthy Buildings 2015 America: Innovation in a Time of Energy Uncertainty and Climate Adaptation, HB 2015, Boulder, CO, USA, 19–22 July 2015. [Google Scholar]
- Kalniņš, M.; Andersone-Ozola, U.; Gudrā, D.; Sieriņa, A.; Fridmanis, D.; Ievinsh, G.; Muter, O. Effect of Bioaugmentation on the Growth and Rhizosphere Microbiome Assembly of Hydroponic Cultures of Mentha Aquatica. Ecol. Genet. Genom. 2022, 22, 100107. [Google Scholar] [CrossRef]
- Bevilacqua, P.; Bruno, R.; Arcuri, N. Green Roofs in a Mediterranean Climate: Energy Performances Based on in-Situ Experimental Data. Renew Energy 2020, 152, 1414–1430. [Google Scholar] [CrossRef]
- Bevilacqua, P. The Effectiveness of Green Roofs in Reducing Building Energy Consumptions across Different Climates. A Summary of Literature Results. Renew. Sustain. Energy Rev. 2021, 151, 111523. [Google Scholar] [CrossRef]
- Abedi, S.; Yarahmadi, R.; Farshad, A.A.; Najjar, N.; Ebrahimi, H.; Soleimani-Alyar, S. Evaluation of the critical parameters on the removal efficiency of a botanical biofilter system. Build. Environ. 2022, 212, 108811. [Google Scholar] [CrossRef]
- Adam, G.; Duncan, H. Development of a sensitive and rapid method for the measurement of total microbial activity using fluorescein diacetate (FDA) in a range of soils. Soil Biol. Biochem. 2001, 33, 943–951. [Google Scholar] [CrossRef]
- Bandick, A.K.; Dick, R.P. Field management effects on soil enzyme activities. Soil Biol. Biochem. 1999, 31, 1471–1479. [Google Scholar] [CrossRef]
- Muter, O.; Perkons, I.; Svinka, V.; Svinka, R.; Bartkevics, V. Distinguishing the roles of carrier and biofilm in filtering media for the removal of pharmaceutical compounds from wastewater. Process Saf. Environ. Prot. 2017, 111, 462–474. [Google Scholar] [CrossRef]
- Neely, W.B. Action of formaldehyde on microorganisms. Iii. Bactericidal action of sublethal concentrations of formaldehyde on aerobacter aerogenes. J. Bacteriol. 1963, 86, 445–448. [Google Scholar] [CrossRef] [PubMed]
- Hohreiter, D.W.; Rigg, D.K. Derivation of Ambient Water Quality Criteria for Formaldehyde. Chemosphere 2001, 45, 471–486. [Google Scholar] [CrossRef] [PubMed]
- Mei, X.; Guo, Z.; Liu, J.; Bi, S.; Li, P.; Wang, Y.; Shen, W.; Yang, Y.; Wang, Y.; Xiao, Y.; et al. Treatment of Formaldehyde Wastewater by a Membrane-Aerated Biofilm Reactor (MABR): The Degradation of Formaldehyde in the Presence of the Cosubstrate Methanol. Chem. Eng. J. 2019, 372, 673–683. [Google Scholar] [CrossRef]
- Ong, S.L.; Sarkar, S.K.; Lee, L.Y.; Hu, J.Y.; Ng, H.Y.; van Loosdrecht, M. Effect of Formaldehyde on Biofilm Activity and Morphology in an Ultracompact Biofilm Reactor for Carbonaceous Wastewater Treatment. Water Environ. Res. 2006, 78, 372–380. [Google Scholar] [CrossRef]
- Qadery, F.; Ayati, B.; Ganjidoust, H. Role of Moving Bed Biofilm Reactor and Sequencing Batch Reactor in Biological Degradation of Formaldehyde Wastewater. Iran. J. Environ. Health Sci. Eng. 2011, 8, 295–306. [Google Scholar]
- Badri, D.V.; Vivanco, J.M. Regulation and Function of Root Exudates. Plant Cell Environ. 2009, 32, 666–681. [Google Scholar] [CrossRef]
- Williams, A.; de Vries, F.T. Plant Root Exudation under Drought: Implications for Ecosystem Functioning. New Phytol. 2020, 225, 1899–1905. [Google Scholar] [CrossRef]
- Stach, J.E.M.; Burns, R.G. Enrichment versus Biofilm Culture: A Functional and Phylogenetic Comparison of Polycyclic Aromatic Hydrocarbon-Degrading Microbial Communities. Environ. Microbiol. 2002, 4, 169–182. [Google Scholar] [CrossRef]
- Flamholz, A.; Dugan, E.; Milo, R.; Savage, D. 100x Trace Elements for M9 Minimal Medium. Bio-Protocol Preprint. Available online: Bio-protocol.org/prep1287 (accessed on 2 September 2023).
- Scheidweiler, D.; Peter, H.; Pramateftaki, P.; de Anna, P.; Battin, T.J. Unraveling the biophysical underpinnings to the success of multispecies biofilms in porous environments. ISME J. 2019, 13, 1700–1710. [Google Scholar] [CrossRef]
- Mountcastle, S.E.; Vyas, N.; Villapun, V.M.; Cox, S.C.; Jabbari, S.; Sammons, R.L.; Shelton, R.M.; Walmsley, A.D.; Kuehne, S.A. Biofilm viability checker: An open-source tool for automated biofilm viability analysis from confocal microscopy images. npj Biofilms Microbiomes 2021, 7, 44. [Google Scholar] [CrossRef]
- Luo, T.L.; Eisenberg, M.C.; Hayashi, M.A.L.; Gonzalez-Cabezas, C.; Foxman, B.; Marrs, C.F.; Rickard, A.H. A Sensitive Thresholding Method for Confocal Laser Scanning Microscope Image Stacks of Microbial Biofilms. Sci. Rep. 2018, 8, 13013. [Google Scholar] [CrossRef] [PubMed]
- Hartmann, R.; Jeckel, H.; Jelli, E.; Singh, P.K.; Vaidya, S.; Bayer, M.; Rode, D.K.H.; Vidakovic, L.; Díaz-Pascual, F.; Fong, J.C.N.; et al. Quantitative image analysis of microbial communities with BiofilmQ. Nat. Microbiol. 2021, 6, 151–156. [Google Scholar] [CrossRef] [PubMed]
- Feng, N.X.; Yu, J.; Xiang, L.; Yu, L.Y.; Zhao, H.M.; Mo, C.H.; Li, Y.W.; Cai, Q.Y.; Wong, M.H.; Li, Q.X. Co-metabolic degradation of the antibiotic ciprofloxacin by the enriched bacterial consortium XG and its bacterial community composition. Sci. Total Environ. 2019, 665, 41–51. [Google Scholar] [CrossRef] [PubMed]



| M. aquatica | C. comosum | A. andraeanum | E. aureum | Nonplanted | New ECP with Biofilm | |
|---|---|---|---|---|---|---|
| D-glucosamic acid | −5.16 | −57.67 | −43.01 | −31.71 | 51.25 | 33.22 |
| Itaconic acid | −60.14 | 20.60 | 36.66 | 49.42 | −98.98 | 6.66 |
| L-arginine | 2.69 | −14.59 | −5.14 | 24.27 | −99.75 | 4.09 |
| L-serine | 18.24 | 8.69 | 5.62 | 16.11 | −98.20 | 1.81 |
| Gamma-hydroxy butyric acid | −90.54 | 4.86 | 0.76 | 15.33 | −98.14 | −5.65 |
| 4-Hydroxy benzoic acid | 2.87 | 5.32 | 0.85 | 14.64 | −98.97 | −6.23 |
| D-galacturonic acid | −90.95 | 3.85 | 2.10 | 6.65 | −98.16 | −6.33 |
| Pyruvic acid methyl ester | 8.21 | −10.20 | −19.31 | −5.79 | −98.50 | −41.56 |
| Tween 40 | 38.16 | −31.38 | −9.12 | −18.58 | −100.00 | −44.76 |
| Beta-methyl-D-glucoside | −31.13 | −62.50 | 40.84 | −61.88 | −94.46 | −79.94 |
| D-malic acid | −68.26 | 28.72 | 5.64 | −63.01 | −97.40 | −85.88 |
| N-acetyl-D-glucosamine | 16.02 | −69.34 | 18.82 | 2.16 | −99.70 | −96.96 |
| L-asparagine | 8.18 | 7.75 | 10.95 | 20.91 | −87.34 | −97.63 |
| D-cellobiose | −29.21 | −24.91 | 4.01 | −71.44 | −96.69 | −99.70 |
| Phenethylamine | 11.29 | −97.13 | 25.81 | −46.32 | −99.36 | −99.75 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Žorža, L.; Ceļmalniece, K.; Sieriņa, A.; Andersone-Ozola, U.; Selga, T.; Ievinsh, G.; Bērziņa, B.; Bartkevičs, V.; Muter, O. Formaldehyde Removal by Expanded Clay Pellets and Biofilm in Hydroponics of a Green Wall System. Sustainability 2023, 15, 13303. https://doi.org/10.3390/su151813303
Žorža L, Ceļmalniece K, Sieriņa A, Andersone-Ozola U, Selga T, Ievinsh G, Bērziņa B, Bartkevičs V, Muter O. Formaldehyde Removal by Expanded Clay Pellets and Biofilm in Hydroponics of a Green Wall System. Sustainability. 2023; 15(18):13303. https://doi.org/10.3390/su151813303
Chicago/Turabian StyleŽorža, Laura, Kristīne Ceļmalniece, Alise Sieriņa, Una Andersone-Ozola, Tūrs Selga, Gederts Ievinsh, Buka Bērziņa, Vadims Bartkevičs, and Olga Muter. 2023. "Formaldehyde Removal by Expanded Clay Pellets and Biofilm in Hydroponics of a Green Wall System" Sustainability 15, no. 18: 13303. https://doi.org/10.3390/su151813303
APA StyleŽorža, L., Ceļmalniece, K., Sieriņa, A., Andersone-Ozola, U., Selga, T., Ievinsh, G., Bērziņa, B., Bartkevičs, V., & Muter, O. (2023). Formaldehyde Removal by Expanded Clay Pellets and Biofilm in Hydroponics of a Green Wall System. Sustainability, 15(18), 13303. https://doi.org/10.3390/su151813303









