Brown Coal Waste in Agriculture and Environmental Protection: A Review
Abstract
:1. Introduction
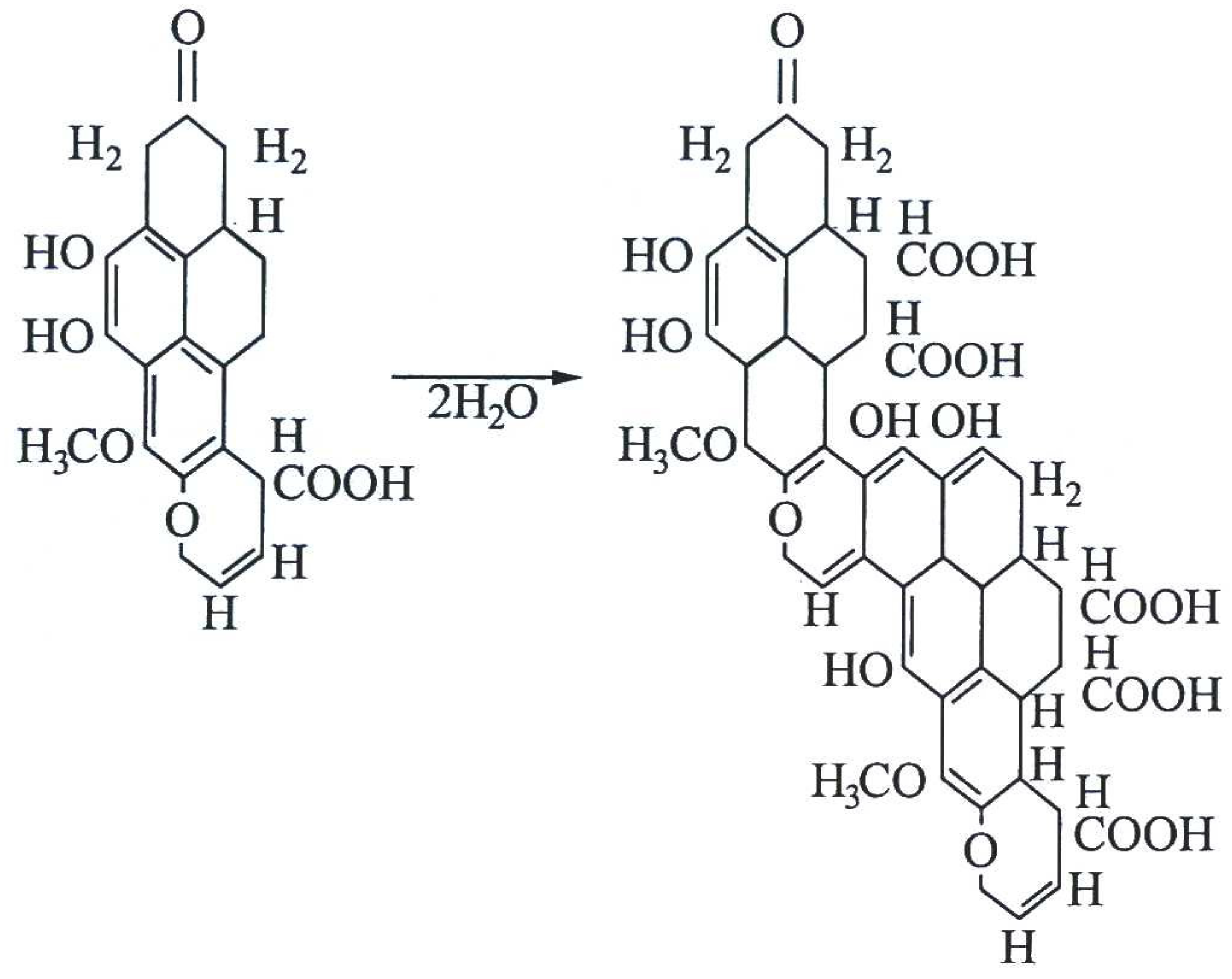
2. Chemical Properties of Brown Coal Waste Components
3. Use of Brown Coal Waste in Agriculture
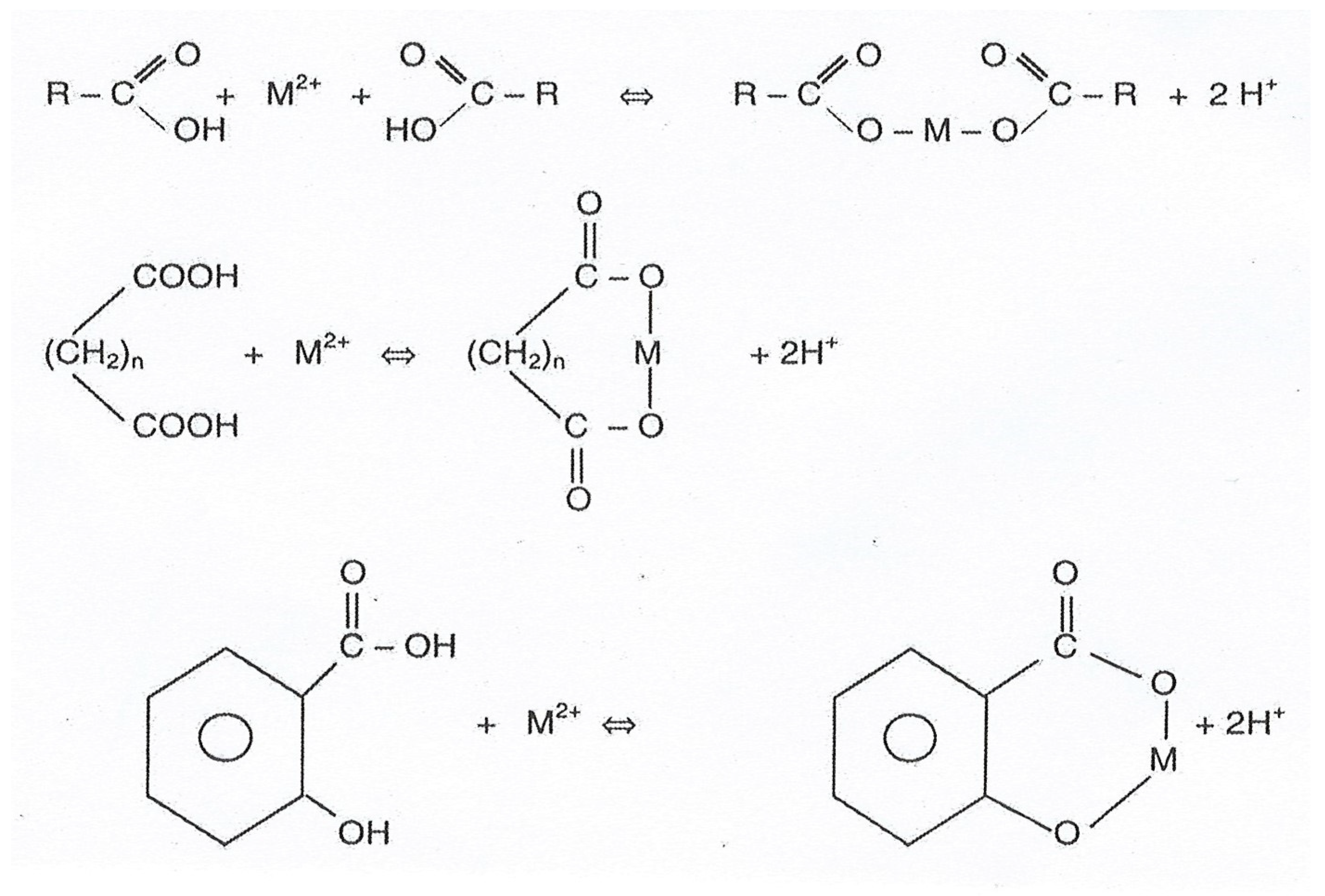
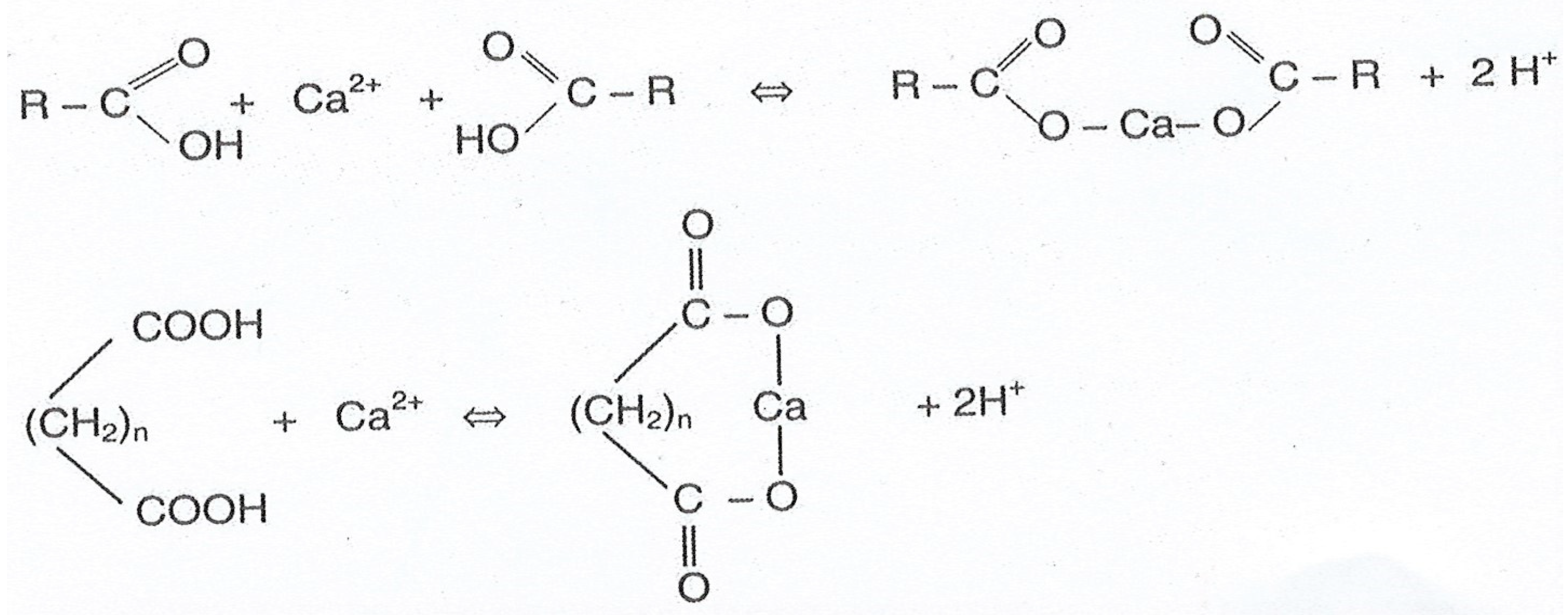
4. Chelation of Heavy Metal Cations by Humic Acids and Fulvic Acids
5. Interaction of Brown Coal Waste and Mineral Fertilization
6. Use of Brown Coal Waste in Cultivation of Vegetables
7. Organomineral Fertilizers Based on Brown Coal Waste
8. Ecological Aspects of Brown Coal Waste Application to Sandy Soils
9. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lehmann, J. Bio-energy in the black. Front. Ecology Environ. 2007, 5, 381–387. [Google Scholar] [CrossRef]
- Polish Brown Coal. (Ed.) Mine Brown Coal “Bełchatów”; KWB: Bełchatów, Poland, 1990; pp. 1–44. (In Polish) [Google Scholar]
- Keiluweit, M.; Nico, P.S.; Johnson, M.G.; Kleber, M. Dynamic molecular structure of plant-derived black carbon (biochar). Environ. Sci. Technol. 2010, 44, 1247–1253. [Google Scholar] [CrossRef] [PubMed]
- O’Keefe, J.M.K.; Bechtel, A.; Christanis, K.; Dai, S.; DiMichele, W.A.; Eble, C.F.; Esterle, J.S.; Mastalerz, M.; Raymond, A.L.; Valentim, B.V.; et al. On the fundamental difference between coal rank and coal type. Intern. J. Coal Geology 2013, 118, 58–87. [Google Scholar] [CrossRef]
- Xu, D.; Li, X.; Chen, J.; Li, J. Research progress of soil and vegetation restoration technology in open-pit coal mine: A review. Agriculture 2023, 13, 226. [Google Scholar] [CrossRef]
- Sourkova, M.; Frouz, J.; Santruckova, H. Accumulation of carbon, nitrogen and phosphorus during soil formation on alder spoil heaps after brown-coal mining, near Sokolov (Czech Republic). Geoderma 2005, 124, 203–214. [Google Scholar] [CrossRef]
- Xu, Z.J.; Zhao, S.M.; Wang, P.Z.; Bi, R.T. Evaluation of the impacts of coal mining on farmland quality in mine-agriculture regions in China. Trans. Chin. Soc. Agric. Eng. 2020, 36, 273–282. [Google Scholar]
- Bekele, A.; Roy, J.L.; Young, M.A. Use of biochar and oxidized lignite for reconstructing functioning agronomic topsoil: Effects on soil properties in a greenhouse study. Canad. J. Soil Sci. 2015, 95, 269–285. [Google Scholar] [CrossRef]
- Chen, Y.; Camps-Arbestain, M.; Shen, Q.; Singh, B.; Cayuela, M.L. The long-term role of organic amendments in building soil nutrient fertility: A meta-analysis and review. Nutrient Cycl. Agroecosys. 2018, 111, 103–125. [Google Scholar] [CrossRef]
- Maciejewska, A. Brown Coal as A Source of Organic Matter and Its Influence on Soil Properties; Warsaw University of Technology: Warsaw, Poland, 1998; pp. 1–74. (In Polish) [Google Scholar]
- Kalembasa, S.; Tengler, S. The Role of Brown Coal in Fertilization and Environment Protection; University of Podlasie in Siedlce: Siedlce, Poland, 2004; pp. 1–175. (In Polish) [Google Scholar]
- Synitsyna, A.O.; Karnozhitsiy, P.V.; Miroshnichenko, D.V.; Bilets, D.Y. The use of brown coal in Ukraine to obtain water-soluble sorbents. Geology 2022, 4, 5–10. [Google Scholar] [CrossRef]
- Symanowicz, B.; Kalembasa, S.; Jaremko, D.; Niedbała, M. Polish brown coals waste—Potential source of plants nutrients. Ann. Univ. Mariae Curie-Skłodowska. Sect. E Agric. 2013, 68, 21–27. (In Polish) [Google Scholar]
- Zhao, Y.; Naeth, M.A. Application timing optimization of lignite-derived humic substances for three agricultural plant species and soil fertility. J. Environ. Qual. 2022, 51, 1035–1043. [Google Scholar] [CrossRef] [PubMed]
- Votolin, K.S.; Zherebtsov, S.I.; Malyshenko, N.V.; Shpakodraev, K.M.; Ismagilov, Z.R. Structural-group composition and biological activity of brown coal fulvic acids. Solid Fuel Chem. 2022, 56, 418–425. [Google Scholar] [CrossRef]
- Uzinger, N.; Rékási, M.; Draskovits, E.; Anton, A. Stabilization of Cr, Pb, and Zn in soil using lignite. Soil Sedim. Contamin. An Internat. J. 2014, 23, 270–286. [Google Scholar] [CrossRef]
- Li, C.; Xiong, Y.; Zou, J.; Dong, L.; Ren, P.; Huang, G. Impact of biochar and lignite-based amendments on microbial communities and greenhouse gas emissions from agricultural soil. Vadase Zone J. 2021, 20, e20105. [Google Scholar] [CrossRef]
- Clouard, M.; Criquet, S.; Borschneck, D.; Ziarelli, F.; Marzaioli, F.; Balesolent, J.; Keller, C. Impact of lignite on pedogenic processes and microbial functions in Mediterranean soils. Geoderma 2014, 232, 257–269. [Google Scholar] [CrossRef]
- Tran, C.K.T.; Rose, M.T.; Cavagnaro, T.R.; Patti, A.F. Lignite amendment has limited impacts on soil microbial communities and mineral nitrogen availability. App. Soil Ecol. 2015, 95, 140–150. [Google Scholar] [CrossRef]
- Eswaran, S.V. Value-added products from soil, brown coal, and composted city solid waste. Front. Sustain. Food Syst. 2021, 5, 738899. [Google Scholar] [CrossRef]
- Ekin, Z. Integrated use of humic acid and plant growth promoting rhizobacteria to ensure higher potato productivity in sustainable agriculture. Sustainability 2019, 11, 3417. [Google Scholar] [CrossRef]
- Pandit, N.R.; Schmidt, H.P.; Mulder, J.; Hale, S.E.; Husson, O.; Cornelissen, G. Nutrient of effect of various composting methods with and without biochar on soil fertility and maize growth. Arch. Agron. Soil Sci. 2020, 66, 250–265. [Google Scholar] [CrossRef]
- Symanowicz, B.; Becher, M.; Jaremko, D.; Toczko, M.; Toczko, R.; Krasuski, S. The delayed effect of low-energy lignite organic matter on the treatment optimization of Zea mays L. grown for silage. Agriculture 2022, 12, 1639. [Google Scholar] [CrossRef]
- Eyheraguibel, B.; Silvestre, J.; Morard, P. Effects of humic substances derived from organic waste enhancement on the growth and mineral nutrition of maize. Bioresource Technol. 2008, 99, 4206–4212. [Google Scholar] [CrossRef] [PubMed]
- Saha, B.K.; Rose, M.T.; Wong, V.; Cavagnaro, T.R.; Patti, A.F. Brown coal-urea blend for increasing nitrogen use efficiency and biomass yield. In Proceedings of the International Nitrogen Initiative Conference, Solutions to Improve Nitrogen Use Efficiency for the World, Melbourne, Australia, 4–8 December 2016; pp. 4–8. [Google Scholar]
- Singh, S.; Melanie, A.; Mayes, M.A.; Shekaofa, A.; Stephanie, N.; Kivlin, S.N.; Sangeeta Bansal, S.; Sindhu Jagadamma, S. Soil organic carbon cycling in response to simulated soil moisture variation under field condition. Sci. Rep. 2021, 11, 10841. [Google Scholar] [CrossRef] [PubMed]
- De Barros, J.A.; de Medeiros, E.; da Costa, D.P.; Duda, G.P.; de Sousa Lima, J.R.; dos Santos, U.J.; Antonino, A.C.; Hammecker, C. Human disturbance affects enzyme activity, microbial biomass and organic carbon in tropical dry sub-humid pasture and forest soils. Arch. Agron. Soil Sci. 2019, 66, 458–471. [Google Scholar] [CrossRef]
- Symanowicz, B.; Toczko, R.; Toczko, M. Enzymatic activity of soil after applying mineral fertilizers and waste lignite to maize grown for silage. Agriculture 2022, 12, 2146. [Google Scholar] [CrossRef]
- Widmer, F.; Rasche, F.; Hartmann, M.; Fliessbach, A. Community structures and substrate utilization of bacteria in soils from organic and conventional farming systems of the DOK long-term field experiment. App. Soil Ecol. 2006, 33, 294–307. [Google Scholar] [CrossRef]
- Xie, W.; Zhou, J.; Wang, H.; Chen, X.N.; Lu, Z.; Yu, J.; Chen, X.G. Short-term effects of copper, cadmium and cypermethrin on dehydrogenase activity and microbial functional diversity in soils after long-term mineral or organic fertilization. Agricult. Ecosyst. Environ. 2009, 129, 450–454. [Google Scholar] [CrossRef]
- Becher, M.; Kalembasa, D.; Kalembasa, S.; Symanowicz, B.; Jaremko, D.; Matyszczak, A. A new method for sequential fractionation of nitrogen in drained organic (Peat) soil. Int. J. Environ. Res. Public Health 2023, 20, 2367. [Google Scholar] [CrossRef]
- Ng, E.L.; Liang, X.; Lam, S.K.; Chen, D.; Weatherly, A.J. What are the social costs and benefits of lignite application to reduce ammonia emissions in intensive feedlot? J. Environ. Manag. 2020, 269, 110821. [Google Scholar] [CrossRef]
- Imbufe, A.U.; Patti, A.F.; Surapeneni, A.; Jakson, R.; Webb, A.J. Effects of brown coal derived materials on pH and electrical conductivity of an acidic vineyard soil. Super Soil 2004. In Proceedings of the 3rd Australian New Zealand Soils Conference, Sydney, Australia, 5–9 December 2004; University of Sydney Australia: Camperdown, Australia. [Google Scholar]
- Brar, B.S.; Singh, J.; Singh, G.; Kaur, G. Effects of long-term application of inorganic and organic fertilizers on soil organic carbon and physical properties in maize-wheat rotation. Agronomy 2015, 5, 220–238. [Google Scholar] [CrossRef]
- Skodras, G.; Kokorootsikos, P.; Serafidou, M. Cation exchange capability and reactivity of low rank coal and chars. Central Eur. J. Chem. 2014, 12, 33–43. [Google Scholar] [CrossRef]
- Hulston, J.; Chaffee, A.L.; Bergins, C.; Strauß, K. Comparison of physico-chemical properties of various lignites treated by mechanical thermal expression. Coal Prep. 2005, 25, 269–293. [Google Scholar] [CrossRef]
- Huang, Y.; Rolfe, A.; Rezvani, S.; Herrador, J.M.H.; Franco, F.; Pinto, F.; Snape, C.; Hewitt, N. Converting brown coal to synthetic liquid fuels through direct coal liquefaction technology: Techno-economic evaluation. Int. J. Energy Res. 2020, 44, 11827–11839. [Google Scholar] [CrossRef]
- Amoah-Antwi, C.; Kwiatkowska-Malina, J.; Thornton, S.F.; Fenton, O.; Malina, G.; Szara, E. Restoration of soil quality using biochar and brown coal waste: A review. Sci. Total Environ. 2020, 722, 137852. [Google Scholar] [CrossRef] [PubMed]
- Kalembasa, S.; Symanowicz, B. The changes of molybdenum and cobalt contents in biomass of goat’s rue (Galega orientalis Lam.). Fres. Environ. Bull. 2009, 18, 1150–1153. [Google Scholar]
- Symanowicz, B.; Kalembasa, S. Effect of iron, molybdenum and cobalt on the amount of nitrogen, biologically reduced by Rhizobium galegae. Ecol. Chem. Eng. 2012, 19, 1311–1320. [Google Scholar] [CrossRef]
- Maciejewska, A. Study of properties and fertility of sandy soil after application of an unconventional fertilizer obtained from lignite. Habilitation thesis. Acta Acad. Agricult. Tech. Olst. Agricultura Sup. D. 1994, 56, 1–67. [Google Scholar]
- Amoah-Antwi, C.; Kwiatkowska-Malina, J.; Fenton, O.; Thornton, S.F.; Malina, G. Holistic assessment of biochar and brown coal waste as organic amendments in sustainable environmental and agricultural applications. Water Air Soil Poll. 2021, 232, 106. [Google Scholar] [CrossRef]
- Robles, J.; Bustos, E.; Lakatos, J. Adsorption study of mercury on lignite in the presence of different anions. J. Environ. Manag. 2017, 186, 285–292. [Google Scholar] [CrossRef]
- Manoharan, V.; Porchelvan, P.; Sekar, S.K. Removal of heavy metals from brown coal lignite using electro kinetic method. Int. J. Earth Sci. Eng. 2013, 6, 1631–1636. [Google Scholar]
- Mercik, S.; Kubik, J. Chelation of heavy metals by humic acids and the effect of peat on the uptake of Zn, Pb, and Cd by plants. Advan. Agricult. Sci. Probl. 1995, 422, 19–31. [Google Scholar]
- Yang, X.; Liu, J.; McGrouther, K.; Huang, H.; Lu, K.; Guo, X.; He, L.; Lin, X.; Che, L.; Ye, Z.; et al. Effect of biochar on the extractability of heavy metals (Cd, Cu, Pb, and Zn) and enzyme activity in soil. Environ. Sci. Poll. Res. 2016, 23, 974–984. [Google Scholar] [CrossRef] [PubMed]
- Olaniran, A.O.; Balgobind, A.; Pillay, B. Bioavailability of heavy metals in soil: Impact on microbial biodegradation of organic compounds and possible improvement strategies. Inter. J. Mol. Sci. 2013, 14, 10197–10228. [Google Scholar] [CrossRef] [PubMed]
- Sabir, M.; Ali, A.; Zia-ur-Rehman, M.; Hakeem, K.R. Contrasting effects of farmyard manure (FYM) and compost for remediation of metal contaminated soil. Inter. J. Phytorem. 2014, 17, 613–621. [Google Scholar] [CrossRef]
- Tsetsegmaa, G.; Akhmadi, K.; Cho, W.; Lee, S.; Chandra, R.; Jeong, C.E.; Chia, R.W.; Kang, H. Effects of oxidized brown coal humic acid fertilizer on the relative height growth rate of three species. Forests 2018, 9, 360. [Google Scholar] [CrossRef]
- Rukeya, S.; Nijat, K.; Abdugheni, A.; Li, H.; Yalkun, A.; Maihemuti, B.; Shi, Q.D. Possibility of optimized indices for the assessment of heavy metal contents in soil around an open pit coal mine area. Int. J. App. Earth Obs. Geoinform. 2018, 240, 457–465. [Google Scholar] [CrossRef]
- Qi, Y.; Hoadley, A.F.A.; Chaffee, A.L.; Garnier, G. Characterisation of lignite as an industrial adsorbent. Fuel 2011, 90, 1567–1574. [Google Scholar] [CrossRef]
- Amoah-Antwi, C.; Kwiatkowska-Malina, J.; Szara, E.; Thornton, S.F.; Fenton, O.; Malina, G. Efficacy of woodchip biochar and brown coal waste as stable sorbents for abatement of bioavailable cadmium, lead and zinc in soil. Water Air Soil Poll. 2020, 231, 515. [Google Scholar] [CrossRef]
- Anemana, T.; Óvári, M.; Szegedi, A.; Uzinger, N.; Rékási, M.; Tatár, E.; Yao, J.; Streli, C.; Záray, G.; Mihucz, V.G. Optimization of lignite particle size for stabilization of trivalent chromium in soils. Soil Sed. Cont. 2020, 29, 272–291. [Google Scholar] [CrossRef]
- Karczewska, A.; Chodak, A.; Kaszubkiewicz, J. The suitability of brown coal a sorbent for heavy metals in polluted soils. App. Geochem. 1996, 11, 343–346. [Google Scholar] [CrossRef]
- Dziadowiec, H. Ecological role of soil humus. Advan. Agricult. Sci. Probl. 1993, 411, 269–282. (In Polish) [Google Scholar]
- Simmler, M.; Ciadamidaro, L.; Schulin, R.; Madejón, P.; Reiser, R.; Clucas, L.; Werber, P.; Robinson, B. Lignite reduces the solubility and plant uptake of cadmium in pasturelands. Environ. Sci. Technol. 2013, 47, 4497–4504. [Google Scholar] [CrossRef] [PubMed]
- Tóth, Š.; Rysak, W.; Symanowicz, B.; Šoltysová, B.; Karahuta, J. The effect of Humac Agro on the yield, sugar content in sugar beet and soil characteristies under conditions of sustainable agricultural management system. Ann. Univ. Mariae Curie-Skłodowska. Sect. E Agric. 2016, 71, 77–86. [Google Scholar]
- Tóth, Š.; Duplák, Š. Effect of a soil-applied humic ameliorative amendment on the yield potential of switchgrass Panicum virgatum L. cultivated under central European continental climate conditions. Agronomy 2023, 13, 1095. [Google Scholar] [CrossRef]
- Arjumend, T.; Abbasi, M.K.; Rafique, E. Effects of lignite-derived humic acid on some selected soil properties, growth and nutrient uptake of wheat (Triticum aestivum L.) grown under greenhouse conditions. Pakistan J. Botany 2015, 47, 2231–2238. [Google Scholar]
- Tahir, M.M.; Khurshid, M.; Khan, M.Z.; Abbasi, M.K.; Kazmi, M.H. Lignite-derived humic acid effect on growth of wheat plants in different soils. Pedosphere 2011, 21, 124–131. [Google Scholar] [CrossRef]
- Qin, K.; Leskovar, D.I. Lignite-derived humic substances modulate pepper and soil biota growth under water deficit stress. J. Plant Nutr. Soil Sci. 2018, 181, 655–663. [Google Scholar] [CrossRef]
- Eprikashvili, L.; Zautashvili, M.; Kordzakhia, T.; Pirtskhalava, N.; Dzagania, M.; Rubashvili, I.; Tsitsishvili, V. Intensification of bioproductivity of agricultural cultures by adding natural zeolites and brown coals into soils. Ann. Agra. Sci. 2016, 14, 67. [Google Scholar] [CrossRef]
- Loffredo, E.; Senesi, N. In vitro and in vivo assessment of the potential of compost and its humic acid fraction to protect ornamental plants from soil-borne pathogenic fungi. Sci. Hortic. 2009, 122, 432–439. [Google Scholar] [CrossRef]
- Rose, M.T.; Perkins, E.L.; Saha, B.K.; Tang, E.C.W.; Cavagnaro, T.R.; Jackson, W.R.; Hapgood, K.P.; Hoadley, A.R.A.; Patti, A.F. A slow release nitrogen fertilizer produced by simultaneous granulation and superheated steam drying of urea with brown coal. Chemical Biolog. Technol. Agricult. 2016, 3, 10. [Google Scholar] [CrossRef]
- Foster, E.J.; Hansen, N.; Wallenstein, M.; Cotrufo, M.F. Biochar and manure amendments impact soil nutrients and microbial enzymatic activities in a semi-arid irrigated maize cropping system. Agric. Ecosyst. Environ. 2016, 233, 404–414. [Google Scholar] [CrossRef]
- Luo, Z.B.; Ma, J.; Chen, F.; Li, X.X.; Zhang, Q.; Yang, Y.J. Adaptive development of soil bacterial communities to ecological processes caused by mining activities in the loess plateau, China. Microorganisms 2020, 245, 477. [Google Scholar] [CrossRef] [PubMed]
- Yu, P.; Liu, S.; Han, K.; Guan, S.; Zhou, D. Conversion of cropland to forage land and grassland increases soil labile carbon and enzyme activities in northeastern China. Agric. Ecosyst. Environ. 2017, 245, 83–91. [Google Scholar] [CrossRef]
- Dyśko, J.; Kaniszewski, S.; Kowalczyk, W. Lignite as a new medium in soilless cultivation of tomato. J. Elem. 2015, 20, 559–569. [Google Scholar] [CrossRef]
- Resendiz-Nava, C.N.; Alonso-Onofre, F.; Silva-Rojas, H.; Rebollar-Alviter, A.; Rivera-Pastrana, D.M.; Stasiewicz, M.J.; Nava, G.M.; Mercado-Silva, E.M. Tomato plant microbiota under conventional and organic fertilization regimes in a soilless culture system. Microorganisms 2023, 11, 1633. [Google Scholar] [CrossRef]
- Nowosielski, O. Brown coal as a substrate, and fertilizer, and a raw material for the production of substrates, and fertilizers. Brown Coal 1995, 4, 24–26. (In Polish) [Google Scholar]
- Richter, R.; Hlusek, J. Effect of substances separated from oxihumolite on selected vegetable species. Advan. Agricult. Sci. Probl. 1995, 422, 99–109. [Google Scholar]
- Wang, R.; Haller, P. Applications of wood ash as a construction material in civil engineering: A review. Biomass Conv. Bioref. 2022, 12, 1–12. [Google Scholar] [CrossRef]
- Ayuso, M.; Hernández, T.; García, C.; Pascual, J.A. Stimulation of barley growth and nutrient absorption by humic substances originating from various organic materials. Bioresour. Technol. 1996, 5, 251–257. [Google Scholar] [CrossRef]
- Maciejewska, A. The ecological value of brown coal in agricultural production on sandy soils. Brown Coal 1995, 4, 27–30. (In Polish) [Google Scholar]
- Kwiatkowska-Malina, J.; Maciejewska, A. The effect of brown coal on the microbial activity in soils contaminated by heavy metals. Soil Sci. Ann. 2012, 63, 39–41. [Google Scholar] [CrossRef]
- Mikheeva, I.V.; Androkhanov, V.A. Physical properties of technosols at brown coal mine wastes in Eastern Siberia. Soil Tillage Res. 2022, 217, 105264. [Google Scholar] [CrossRef]
- Schnitzer, M. Humic Substances. Chemistry and Reactions. In Soil Organic Matter; Elsevier: Amsterdam, The Netherlands, 1978; pp. 1–64. [Google Scholar]
- Maciejewska, A. Changes in some physical and water properties of very light soil after agro-melioration with Komplet R. Acta Acad. Agricult. Tech. Olst. Agric. 1994, 57, 23–29. (In Polish) [Google Scholar]
- Kalembasa, S.; Symanowicz, B. Sewage sludge processing with brown coal. Advan. Agricult. Sci. Probl. 1995, 422, 75–87. (In Polish) [Google Scholar]
- Domazetis, G.; Raoarun, M.; James, B.D. Low-temperature pyrolysis of brown coal and brown with iron hydroxyl complexes. Energy Fuels 2006, 20, 1997–2007. [Google Scholar] [CrossRef]
- Binner, E.; Facun, J.; Chen, L.; Ninomiya, Y.; Li, C.Z.; Bhattacharya, S. Effect of coal drying on the behavior of inorganic species during Victorian brown coal pyrolysis and combustion. Energy Fuels 2011, 25, 2764–2771. [Google Scholar] [CrossRef]





| Years of Research | Dose Rekulter (t ha−1) | Specific Density (g cm−3) | Bulk Density (g cm−3) | Total Porosity (%) | Air Capacity (%) |
|---|---|---|---|---|---|
| 1 | 0 | 2.60 | 1.55 | 40.22 | 11.00 |
| 40 | 2.51 | 1.49 | 40.62 | 10.78 | |
| 80 | 2.47 | 1.42 | 42.30 | 7.48 | |
| 160 | 2.40 | 1.34 | 43.15 | 5.45 | |
| 5 | 0 | 2.60 | 1.57 | 39.47 | 20.55 |
| 40 | 2.53 | 1.53 | 39.55 | 18.30 | |
| 80 | 2.48 | 1.44 | 41.80 | 11.40 | |
| 160 | 2.42 | 1.38 | 43.18 | 6.05 |
| Years of Research | Dose Rekulter (t ha−1) | Hygroscopic Water (%) | Moisture at the Time of Sampling by Weight (wt.%) | Volumetric Humidity at the Time of Sampling (vol.%) | Capillary Water Capacity by Weight (wt.%) | Volume Capillary Water Capacity (vol.%) | Field Water Capacity (%) |
|---|---|---|---|---|---|---|---|
| 1 | 0 | 0.47 | 9.83 | 15.33 | 18.71 | 29.00 | 10.10 |
| 40 | 0.49 | 10.75 | 16.00 | 20.09 | 29.94 | 10.83 | |
| 80 | 0.62 | 13.05 | 18.57 | 24.41 | 34.77 | 13.15 | |
| 160 | 0.81 | 17.33 | 23.16 | 29.04 | 38.69 | 15.65 | |
| 5 | 0 | 0.47 | 3.43 | 5.43 | 12.12 | 18.93 | 6.55 |
| 40 | 0.52 | 3.68 | 5.61 | 13.94 | 21.24 | 7.50 | |
| 80 | 0.63 | 6.48 | 9.38 | 21.08 | 30.27 | 11.40 | |
| 160 | 0.83 | 10.28 | 14.12 | 26.89 | 37.09 | 14.58 |
| Elements | Seradella (%) | Triticale (%) | Maize (%) | Rape (%) | Spinach (%) |
|---|---|---|---|---|---|
| Zn | 50 | 50 | 50 | 50 | 40 |
| Pb | 50 | 50 | 50 | 30 | 30 |
| Cd | 40 | 30 | 50 | 50 | 30 |
| Treatments | Humac Agro | N | P | K | S |
|---|---|---|---|---|---|
| V1 | 0 | 94.8 | 34.9 | 100.0 | 28.0 |
| V2 | 250 | 94.8 | 34.9 | 100.0 | 28.0 |
| V3 | 500 | 60.3 | 34.9 | 100.0 | 28.0 |
| Fertilization Variant—Humac Agro | Root Yield (Mg ha−1) | Relative to Control (%) | Sugar Content (kg Mg−1) | Sugar Yield (Mg ha−1) | Relative to Control (%) |
|---|---|---|---|---|---|
| 0 | 72.81 | 100.0 | 184.3 | 13.42 | 100.0 |
| 250 kg ha−1 | 86.39 | 118.7 | 176.5 | 15.25 | 113.6 |
| 500 kg ha−1 | 95.97 | 131.8 | 181.2 | 17.39 | 129.6 |
| Mean | 85.06 | 125.2 | 180.7 | 15.35 | 121.6 |
| Years of Research | Dose Rekulter (t ha−1) | pHKCl | Hh (cmol(+) kg−1) | Degree of Base Saturation (%) | Ct (g kg−1) | Nt (g kg−1) | Mg2+ (mg kg−1) | Ca2+ (mg kg−1) | K+ (mg kg−1) | H2PO4 (mg kg−1) |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 0 | 4.7 | 4.1 | 39.3 | 7.6 | 0.6 | 4.0 | 86.0 | 8.0 | 2.0 |
| 40 | 6.1 | 2.0 | 80.2 | 10.8 | 0.8 | 37.0 | 257.0 | 17.0 | 12.0 | |
| 80 | 6,8 | 0.9 | 93.9 | 14.5 | 0.9 | 74.0 | 556.0 | 30.0 | 23.0 | |
| 160 | 7.4 | 0.4 | 98.9 | 22.1 | 1.1 | 141.0 | 1504.0 | 42.0 | 47.0 | |
| 5 | 0 | 4.8 | 2.8 | 38.8 | 8.7 | 0.5 | 6.0 | 82.0 | 90.0 | 2.0 |
| 40 | 6.0 | 1.6 | 74.9 | 11.5 | 0.7 | 30.0 | 201.0 | 14.0 | 7.0 | |
| 80 | 6.2 | 1.3 | 88.3 | 15.0 | 0.8 | 36.0 | 386.0 | 22.0 | 14.0 | |
| 160 | 6.6 | 0.4 | 98.2 | 22.7 | 0.9 | 94.0 | 889.0 | 28.0 | 30.0 |
| Years of Research | Dose Complete R (m3 ha−1) | Density of the Solid Phase of the Soil (g cm−3) | Density of the Dried Soil in 105 °C (g cm−3) | Total Porosity (%) | Air Capacity (%) |
|---|---|---|---|---|---|
| 1 | 0 | 2.68 | 1.60 | 39.1 | 9.8 |
| 50 | 2.56 | 1.57 | 38.7 | 9.5 | |
| 100 | 2.48 | 1.48 | 40.4 | 7.6 | |
| 200 | 2.36 | 1.40 | 41.7 | 5.1 | |
| 4 | 0 | 2.67 | 1.60 | 40.5 | 14.0 |
| 50 | 2.57 | 1.56 | 39.1 | 11.3 | |
| 100 | 2.48 | 1.43 | 42.4 | 10.3 | |
| 200 | 2.34 | 1.37 | 41.7 | 6.3 |
| Years of Research | Dose Complete R (m3 ha−1) | Hygroscopic Water (%) | Moisture at the Time of Sampling by Weight (wt.%) | Volumetric Humidity at the Time of Sampling (vol.%) | Capillary Water Capacity by Weight (wt.%) | Volume Capillary Water Capacity (vol.%) | Field Water Capacity (%) |
|---|---|---|---|---|---|---|---|
| 1 | 0 | 0.45 | 8.9 | 15.0 | 18.0 | 29.3 | 9.72 |
| 50 | 0.49 | 9.3 | 15.9 | 18.2 | 29.9 | 9.99 | |
| 100 | 0.63 | 10.2 | 16.7 | 21.2 | 32.7 | 11.45 | |
| 200 | 0.76 | 16.3 | 22.8 | 25.1 | 36.6 | 13.55 | |
| 4 | 0 | 0.45 | 2.5 | 5.2 | 16.3 | 26.2 | 8.8 |
| 50 | 0.47 | 2.9 | 6.2 | 17.3 | 27.9 | 9.3 | |
| 100 | 0.63 | 5.5 | 8.9 | 22.3 | 32.2 | 12.1 | |
| 200 | 0.70 | 7.2 | 10.6 | 24.8 | 34.8 | 13.4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Symanowicz, B.; Toczko, R. Brown Coal Waste in Agriculture and Environmental Protection: A Review. Sustainability 2023, 15, 13371. https://doi.org/10.3390/su151813371
Symanowicz B, Toczko R. Brown Coal Waste in Agriculture and Environmental Protection: A Review. Sustainability. 2023; 15(18):13371. https://doi.org/10.3390/su151813371
Chicago/Turabian StyleSymanowicz, Barbara, and Rafał Toczko. 2023. "Brown Coal Waste in Agriculture and Environmental Protection: A Review" Sustainability 15, no. 18: 13371. https://doi.org/10.3390/su151813371
APA StyleSymanowicz, B., & Toczko, R. (2023). Brown Coal Waste in Agriculture and Environmental Protection: A Review. Sustainability, 15(18), 13371. https://doi.org/10.3390/su151813371






