WNMS: A New Basaltic Simulant of Mars Regolith
Abstract
:1. Introduction
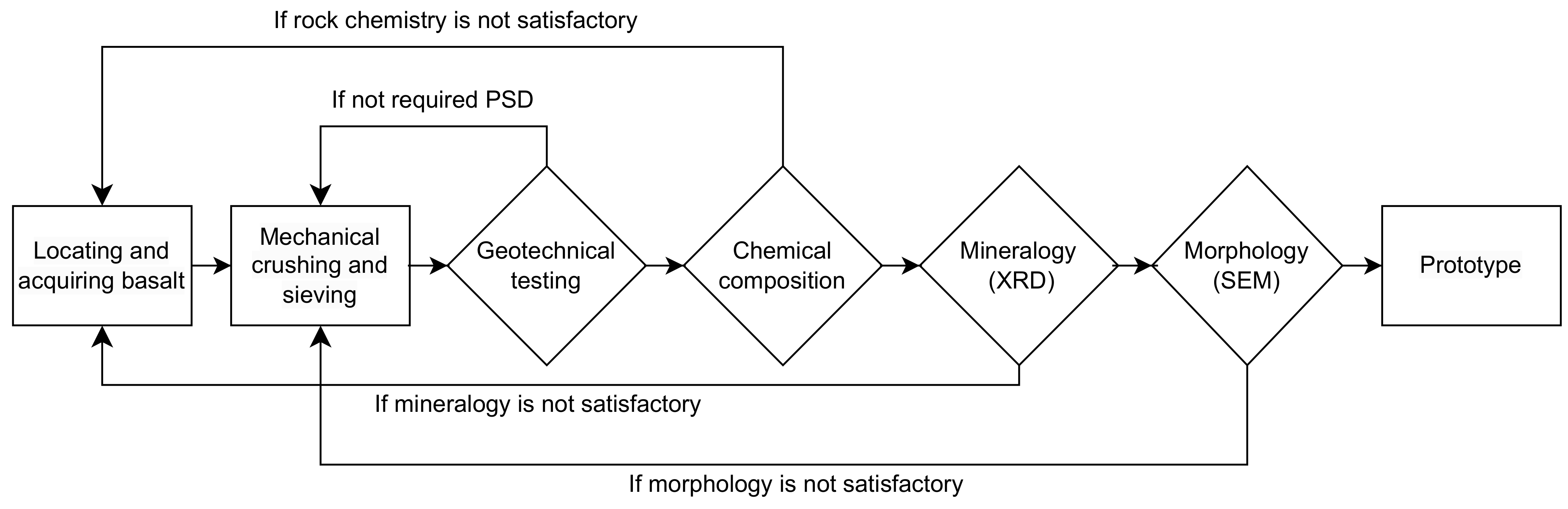
2. Design Approach
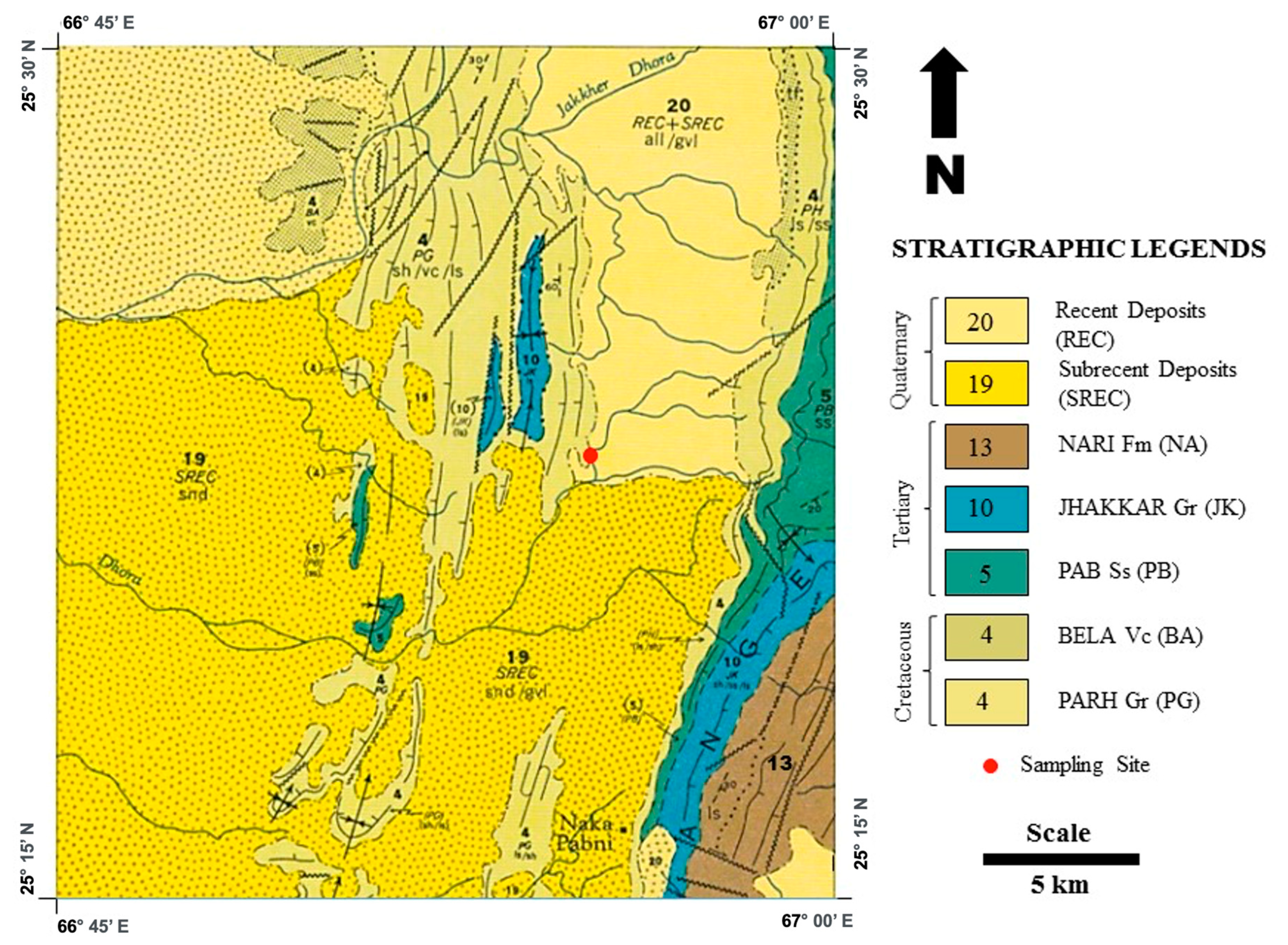
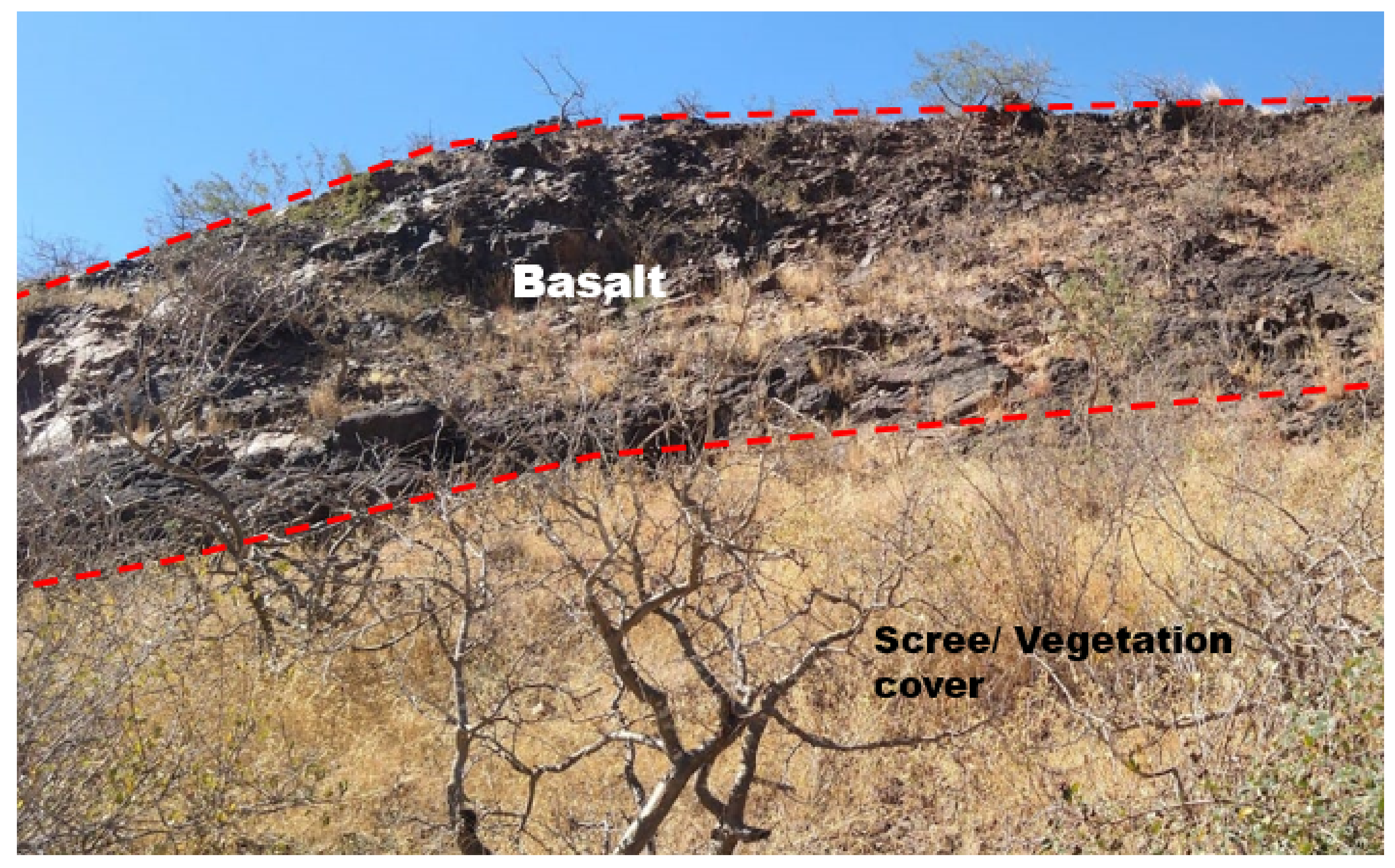
3. Basalt Source and Sampling
4. Production of Rock Dust
5. Test Methods
5.1. Geotechnical Tests
5.2. Energy Dispersive X-ray (EDX) Spectroscopy
5.3. X-ray Diffraction (XRD)
5.4. Scanning Electron Microscopy (SEM)
5.5. Magnetic and Volatile Content
6. Result and Discussion
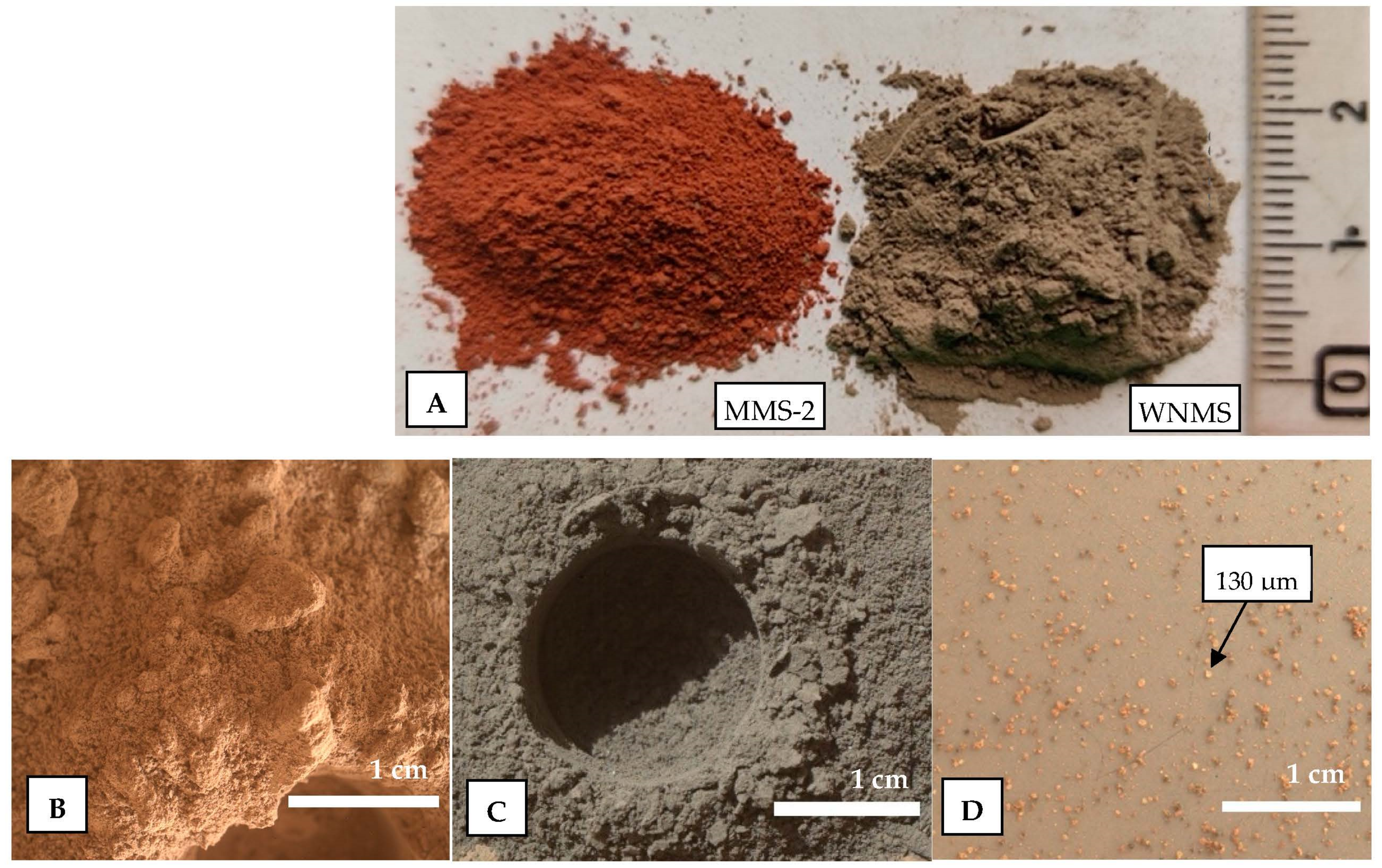
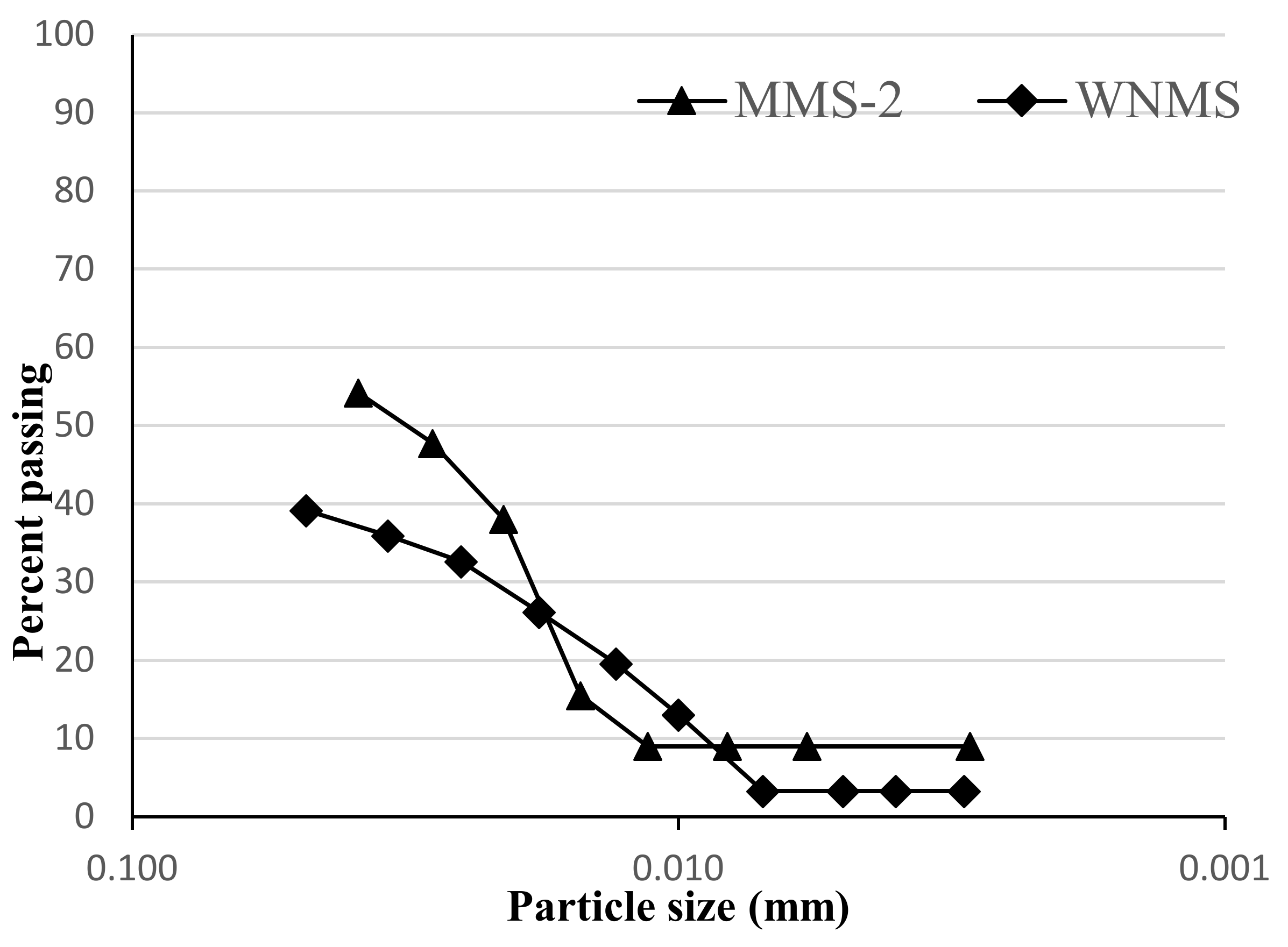
6.1. Density and Particle Size Distribution
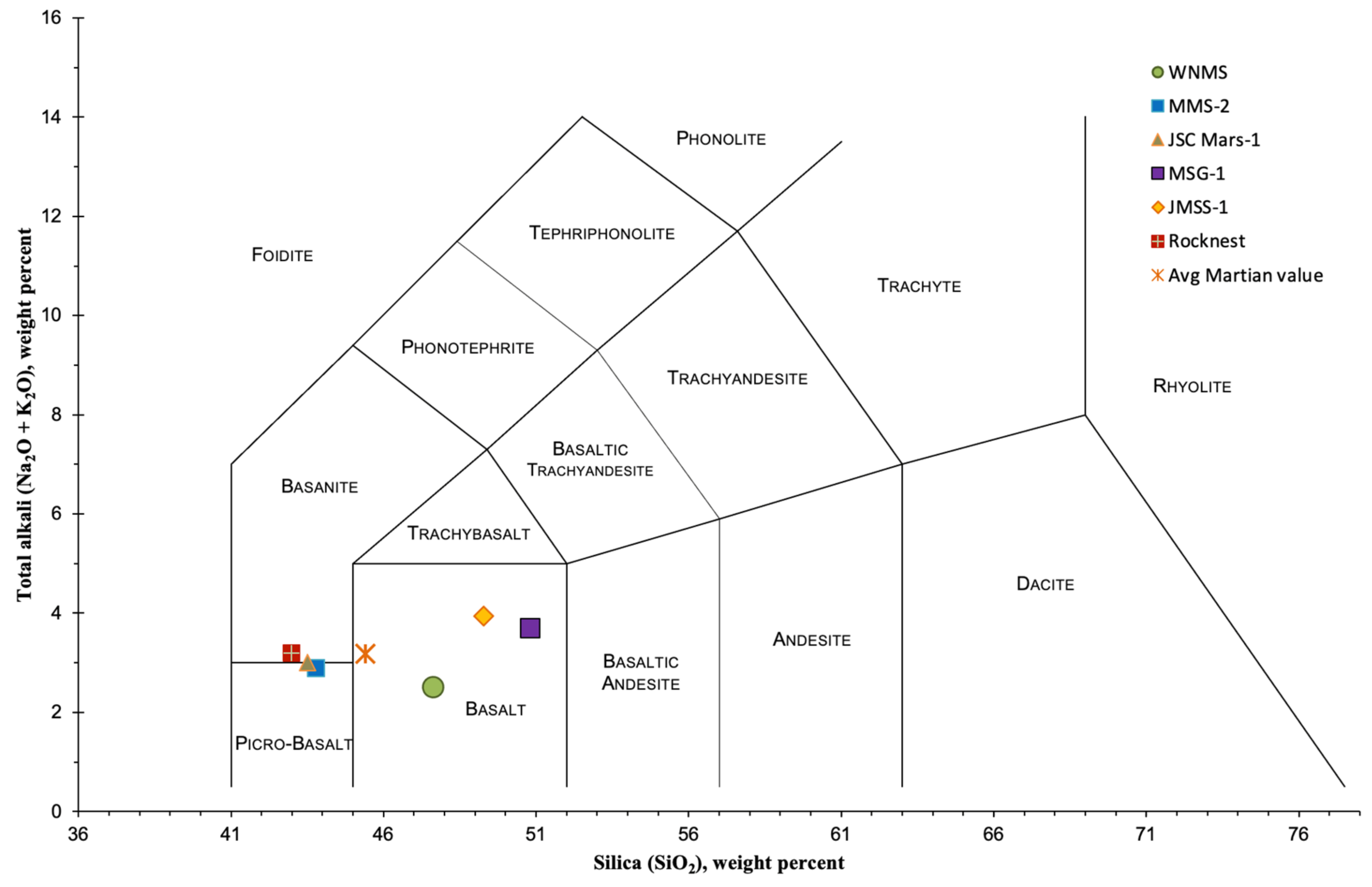
6.2. Chemical Composition
| Oxides | WNMS (%) | MMS-2 * | JSC Mars-1 [14] | MSG-1 [6] | JMSS-1 [17] | Rocknest [21] | Avg Martian Value [48] |
|---|---|---|---|---|---|---|---|
| SiO2 | 47.62 | 43.80 | 43.50 | 50.80 | 49.28 | 42.97 | 45.41 |
| Al2O3 | 12.15 | 13.07 | 23.30 | 8.90 | 13.64 | 9.37 | 9.71 |
| Fe2O3 | 18.88 | 18.37 | 15.60 | 13.30 | 16.00 | 19.18 | 16.73 |
| CaO | 11.41 | 7.98 | 6.20 | 3.70 | 7.56 | 7.26 | 6.37 |
| TiO2 | 3.31 | 0.83 | 3.80 | 0.30 | 1.78 | 1.19 | 0.90 |
| K2O | 0.37 | 0.37 | 0.60 | 0.30 | 1.02 | 0.49 | 0.44 |
| SO3 | - | 6.11 | - | 2.10 | - | 5.47 | 6.16 |
| MnO | - | 0.13 | 0.30 | 0.10 | 0.14 | 0.42 | 0.33 |
| Cr2O3 | - | 0.04 | - | 0.10 | - | 0.49 | 0.36 |
| P2O5 | - | 0.13 | 0.90 | 0.40 | 0.30 | 0.95 | 0.83 |
| MgO | 4.14 | 6.66 | 3.40 | 16.70 | 6.35 | 8.69 | 8.35 |
| Na2O | 2.14 | 2.51 | 2.40 | 3.40 | 2.92 | 2.70 | 2.73 |
| Cl | - | - | - | - | - | 0.69 | 0.68 |
| Total | 100.02 | 100.00 | 100.00 | 100.10 | 98.99 ** | 99.87 | 99.00 |
6.3. Mineralogy
6.4. Particle Morphology
6.5. Magnetic Properties and Volatile Content
7. Conclusions and Recommendations
- The area of Winder Nai in Pakistan is suitable due to its availability and ease of access. The particle density and bulk density of the simulant are 2.58 g/cm3 and 1.16 g/cm3, respectively. The WNMS shows a well-graded particle size distribution. It also shows the presence of angular to subangular fine and granoulous particles with some surface texture.
- The elemental composition of WNMS is within ±5 wt% of the elemental composition at Rocknest and average Martian regolith values with the exception of SO3.
- SiO2, Al2O3, and Fe2O3 for WNMS and MMS-2 have a good match to the Martian regolith values compared to JSC Mars-1, MSG-1, and JMSS-1. The content of CaO and TiO2 in WNMS is higher compared to that of the regolith. However, the values are within acceptable limits. The SO3 content in most of the simulants is lower than the Martian regolith. The difference in MgO and SO3 content would not affect sulfur concrete, generally reported to be the most suitable concrete for Mars.
- Based on the Total Alkali Silica (TAS) diagram, the rock can be classified as basalt.
- XRD spectrum indicates peaks corresponding to basalt, indicating plagioclase (46.18%) and pyroxene (43.20%). The total iron oxide minerals have a total content of 3.78%.
- WNMS shows good magnetic properties with the presence of 3.8 wt% highly paramagnetic magnetic content. In comparison, MMS-2 has a 3.1 wt% magnetic content. The volatile loss of WNMS is 0.25 wt% at 125 °C, 1.73 wt% at 500 °C and 3.05 wt% at 950 °C. These values are higher than the regolith values and lower than those for JSC Mars-1 and MMS-2, JMSS-1, and MSG-1.
- There is a further need to study the petrography through a thin section of rock for the degree of rock alteration and minor phases. It is also recommended that the hygroscopic properties, phase of magnetic particles, and nature of volatile loss at different temperatures be further investigated. Similar endeavors will help researchers in multidisciplinary fields of low-developed and developing countries to play their role in planetary research.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wan, L.; Wendner, R.; Cusatis, G. A novel material for in situ construction on Mars: Experiments and numerical simulations. Constr. Build. Mater. 2016, 120, 222–231. [Google Scholar] [CrossRef]
- Rahim, A.; Gulzar, A.; Khan, A.; Rehman, Z. Mars In Situ Resource Utilization and Sulfur Concrete. In Proceedings of the 17th Biennial International Conference on Engineering, Science, Construction, and Operations in Challenging Environments, Online, 19–23 April 2021; pp. 1231–1241. [Google Scholar] [CrossRef]
- Alexiadis, A.; Alberini, F.; Meyer, M.E. Geopolymers from lunar and Martian soil simulants. Adv. Space Res. 2017, 59, 490–495. [Google Scholar] [CrossRef]
- Franz, H.B.; King, P.L.; Gaillard, F. Chapter 6—Sulfur on Mars from the Atmosphere to the Core. In Volatiles in the Martian Crust; Filiberto, J., Schwenzer, S.P., Eds.; Elsevier: Amsterdam, The Netherlands, 2019; pp. 119–183. ISBN 9780128041918. [Google Scholar]
- Mellon, M.T.; Sizemore, H.G. The history of ground ice at Jezero Crater Mars and other past, present, and future landing sites. Icarus 2022, 371, 114667. [Google Scholar] [CrossRef]
- Cannon, K.M.; Britt, D.T.; Smith, T.M.; Fritsche, R.F.; Batcheldor, D. Mars Global Simulant MGS-1: A Rocknest-Based Open Standard for Basaltic Martian Regolith Simulants. Icarus 2019, 317, 470–478. [Google Scholar] [CrossRef]
- Condie, K.C. 10—Comparative Planetary Evolution. In Earth as an Evolving Planetary System, 2nd ed.; Academic Press: Boston, MA, USA, 2011; pp. 437–492. ISBN 978-0-12-385227-4. [Google Scholar]
- Knauth, L.P.; Burt, D.M.; Wohletz, K.H. Impact Origin of Sediments at the Opportunity Landing Site on Mars. Nature 2005, 438, 1123–1128. [Google Scholar] [CrossRef]
- Gertsch, L.S.; Rostami, J.; Gustafson, R. Review of Lunar Regolith Properties for Design of Low Power Lunar Excavators. In Proceedings of the 6th International Conference on Case Histories in Geotechnical Engineering, Arlington, VA, USA, 11–16 August 2008. [Google Scholar]
- Gaillard, F.; Michalski, J.; Berger, G.; McLennan, S.M.; Scaillet, B. Geochemical Reservoirs and Timing of Sulfur Cycling on Mars. Space Sci. Rev. 2013, 174, 251–300. [Google Scholar] [CrossRef]
- Vaughan, A.; Rice, M.; Horgan, B.; Johnson, J.; Bell, J.; Nunez, J.; Garczynski, B.; Mollerup, J.; Million, C.; St Clair, M. A Mastcam-Z View of Regolith at Jezero Crater: Textural and Spectral Properties. In Proceedings of the AGU Fall Meeting Abstracts, New Orleans, LA, USA, 13–17 December 2021. [Google Scholar]
- Cardarelli, E.L.; Vaughan, A.; Minitti, M.E.; Beegle, L.; Rice, M.; Johnson, J.R.; Horgan, B.; Cousin, A.; Kah, L.C.; Hausrath, E.M.; et al. Regolith at Jezero Crater, Mars: Spectral Diversity, Textures, and Implications for Provenance. In Proceedings of the 53rd Lunar and Planetary Science Conference, The Woodlands, TX, USA, 7–11 March 2022. [Google Scholar]
- Cousin, A.; Meslin, P.Y.; Hausrath, E.M.; Cardarelli, E.; Lasue, J.; Forni, O.; Beyssac, O.; Kah, L.C.; Mandon, L.; Gasnault, O.; et al. Soil Diversity at Mars: Comparison of Dataset from Gale and Jezero Craters. In Proceedings of the 53rd Lunar and Planetary Science Conference, The Woodlands, TX, USA, 7–11 March 2022. [Google Scholar]
- Allen, C.C.; Morris, R.V.; Jager, K.M.; Golden, D.C.; Lindstrom, D.J.; Lindstrom, M.M.; Lockwood, J.P. Martian Regolith Simulant JSC Mars-1. In Proceedings of the Lunar and Planetary Science Conference, Houston, TX, USA, 16–20 March 1998. [Google Scholar]
- Nørnberg, P.; Gunnlaugsson, H.P.; Merrison, J.P.; Vendelboe, A.L. Salten Skov I: A Martian Magnetic Dust Analogue. Planet. Space Sci. 2009, 57, 628–631. [Google Scholar] [CrossRef]
- Li, Y.; Zeng, X.; Agui, J. Developing a Lightweight Martian Soil Simulant for a High-Sinkage Mobility Test. J. Aerosp. Eng. 2015, 28, 4014058. [Google Scholar] [CrossRef]
- Zeng, X.; Li, X.; Wang, S.; Li, S.; Spring, N.; Tang, H.; Li, Y.; Feng, J. JMSS-1: A New Martian Soil Simulant Planetary Science. Earth Planets Space 2015, 67, 1–10. [Google Scholar] [CrossRef]
- Scott, A.N.; Oze, C.; Tang, Y.; O’Loughlin, A. Development of a Martian Regolith Simulant for In-Situ Resource Utilization Testing. Acta Astronaut. 2017, 131, 45–49. [Google Scholar] [CrossRef]
- Stevens, A.H.; Steer, E.; McDonald, A.; Amador, E.S.; Cockell, C.S. Y-Mars: An Astrobiological Analogue of Martian Mudstone. Earth Space Sci. 2018, 5, 163–174. [Google Scholar] [CrossRef]
- Ramkissoon, N.K.; Pearson, V.K.; Schwenzer, S.P.; Schröder, C.; Kirnbauer, T.; Wood, D.; Seidel, R.G.W.; Miller, M.A.; Olsson-Francis, K. New Simulants for Martian Regolith: Controlling Iron Variability. Planet. Space Sci. 2019, 179, 104722. [Google Scholar] [CrossRef]
- Clark, J.V.; Archer, P.D.; Gruener, J.E.; Ming, D.W.; Tu, V.M.; Niles, P.B.; Mertzman, S.A. JSC-Rocknest: A Large-Scale Mojave Mars Simulant (MMS) Based Soil Simulant for in-Situ Resource Utilization Water-Extraction Studies. Icarus 2020, 351, 113936. [Google Scholar] [CrossRef]
- Guan, J.-Z.; Liu, A.-M.; Xie, K.-Y.; Shi, Z.-N.; Kubikova, B. Preparation and Characterization of Martian Soil Simulant NEU Mars-1. Trans. Nonferr. Met. Soc. China 2020, 30, 212–222. [Google Scholar] [CrossRef]
- Zheng, W.; Qiao, G. Mechanical Behavior of the Metal Parts Welded with Extraterrestrial Regolith Simulant by the Solar Concentrator in ISRU & ISRF Application. Adv. Space Res. 2020, 65, 2303–2314. [Google Scholar] [CrossRef]
- Yin, K.; Liu, J.; Lin, J.; Vasilescu, A.-R.; Othmani, K.; di Filippo, E. Interface Direct Shear Tests on JEZ-1 Mars Regolith Simulant. Appl. Sci. 2021, 11, 7052. [Google Scholar] [CrossRef]
- Yu, W.; Zeng, X.; Li, X.; Wei, G.; Fang, J. New Martian Dust Simulant JMDS-1 and Applications to Laboratory Thermal Conductivity Measurements. Earth Space Sci. 2022, 9, e2020EA001347. [Google Scholar] [CrossRef]
- Karl, D.; Cannon, K.M.; Gurlo, A. Review of Space Resources Processing for Mars Missions: Martian Simulants, Regolith Bonding Concepts and Additive Manufacturing. Open Ceram. 2022, 9, 100216. [Google Scholar] [CrossRef]
- Beegle, L.W.; Peters, G.H.; Mungas, G.S.; Bearman, G.H.; Smith, J.A.; Anderson, R.C. Mojave Martian Simulant: A New Martian Soil Simulant. In Proceedings of the 38th Lunar and Planetary Science Conference, (Lunar and Planetary Science XXXVIII), League City, TX, USA, 12–16 March 2007; p. 2005. [Google Scholar]
- Cannon, K.M.; Britt, D.T.; Metgzer, P.T.; Landsman, Z.A.; Covey, S.D.; Schultz, C.; Peppein, M.; Smit, T.M.; Fritsche, R. New High Fidelity Martian and Phobos Regolith Simulants: Enabling Tools for Exploring the Mars System and ISRU Development. In Proceedings of the 49th Lunar and Planetary Science Conference 2018, Houston, TX, USA, 19–23 March 2018. [Google Scholar]
- Gouache, T.P.; Patel, N.; Brunskill, C.; Scott, G.P.; Saaj, C.M.; Matthews, M.; Cui, L. Soil Simulant Sourcing for the ExoMars Rover Testbed. Planet. Space Sci. 2011, 59, 779–787. [Google Scholar] [CrossRef]
- Vredenburg, E.W. Report on the Geology of Srawan Jhalawan, Makran and the State of Las Bela. Indian Geol. Surv. Rec. 1909, 38, 303–338. [Google Scholar]
- DeJong, K.A.; Farah, A. Geodynamics of Pakistan; DeJong, K.A., Farah, A., Eds.; Geological Survey of Pakistan: Quetta, Pakistan, 1979.
- Khan, W.; McCormick, G.R.; Reagan, M.K. Parh Group Basalts of Northeastern Balochistan, Pakistan: Precursors to the Deccan Traps. In Himalaya and Tibet: Mountain Roots to Mountain Tops; Macfarlane, A., Sorkhabi, R.B., Quade, J., Eds.; Geological Society of America: Boulder, CO, USA, 1999; Volume 328, ISBN 9780813723280. [Google Scholar]
- Otsuki, K.; Hoshino, K.; Anwar, M.; Mengal, J.M.; Brohi, I.A.; Fatmi, A.N.; Yuji, O. Breakup of Gondwanaland and Emplacement of Ophiolitic Complex in Muslimbagh Area of Balochistan, Pakistan. Univ. Peshawar Geol. Bull. 1989, 22, 33–57. [Google Scholar]
- HSC (Hunting Survey Corporation). Reconnaissance Geology of Part of West Pakistan (a Colombo Plan Cooperative Project); Hunting Survey Corporation: Ottawa, ON, Canada, 1960. [Google Scholar]
- ASTM D7263-21; Standard Test Methods for Laboratory Determination of Density and Unit Weight of Soil Specimens. ASTM: West Conshohocken, PA, USA, 2021.
- ASTM D854-14; Standard Test Methods for Specific Gravity of Soil Solids by Water Pycnometer. ASTM: West Conshohocken, PA, USA, 2014.
- ASTM D7928-21e1; Standard Test Method for Particle-Size Distribution (Gradation) of Fine-Grained Soils Using the Sedimentation (Hydrometer) Analysis. ASTM: West Conshohocken, PA, USA, 2021.
- Yang, B.; Zhu, Z.; Yin, W.; He, J. Effective Flotation Separation of Malachite from Quartz with a Selective Collector: Collection Ability, Separation Performance and Adsorption Mechanism. J. Mol. Liq. 2022, 368, 120658. [Google Scholar] [CrossRef]
- Fu, Y.; Hou, Y.; Wang, R.; Wang, Y.; Yang, X.; Dong, Z.; Liu, J.; Man, X.; Yin, W.; Yang, B.; et al. Detailed Insights into Improved Chlorite Removal during Hematite Reverse Flotation by Sodium Alginate. Miner. Eng. 2021, 173, 107191. [Google Scholar] [CrossRef]
- Doebelin, N.; Kleeberg, R. Profex: A Graphical User Interface for the Rietveld Refinement Program BGMN. J. Appl. Crystallogr. 2015, 48, 1573–1580. [Google Scholar] [CrossRef] [PubMed]
- Powers, M.C. A New Roundness Scale for Sedimentary Particles. J. Sediment. Res. 1953, 23, 117–119. [Google Scholar] [CrossRef]
- Golombek, M.P.; Haldemann, A.F.C.; Simpson, R.A.; Fergason, R.L.; Putzig, N.E.; Arvidson, R.E.; Bell, J.F.; Mellon, M.T. Martian Surface Properties from Joint Analysis of Orbital, Earth-Based, and Surface Observations. In The Martian Surface: Composition, Mineralogy and Physical Properties; Bell, J., Ed.; Cambridge Planetary Science; Cambridge University Press: Cambridge, UK, 2008; pp. 468–498. [Google Scholar] [CrossRef]
- Ming, D.W.; Gellert, R.; Morris, R.V.; Arvidson, R.E.; Brückner, J.; Clark, B.C.; Cohen, B.A.; D’Uston, C.; Economou, T.; Fleischer, I.; et al. Geochemical Properties of Rocks and Soils in Gusev Crater, Mars: Results of the Alpha Particle X-Ray Spectrometer from Cumberland Ridge to Home Plate. J. Geophys. Res. E Planets 2008, 113. [Google Scholar] [CrossRef]
- Achilles, C.N.; Downs, R.T.; Ming, D.W.; Rampe, E.B.; Morris, R.V.; Treiman, A.H.; Morrison, S.M.; Blake, D.F.; Vaniman, D.T.; Ewing, R.C.; et al. Mineralogy of an Active Eolian Sediment from the Namib Dune, Gale Crater, Mars. J. Geophys. Res. Planets 2017, 122, 2344–2361. [Google Scholar] [CrossRef]
- Zayed, A.M.; Brown, K.; Hanhan, A. Effect of Sulfur Trioxide Content on Concrete Structures Using Florida Materials; University of South Florida: Tampa, FL, USA, 2004. [Google Scholar]
- Irvine, T.N.; Baragar, W.R.A. A Guide to the Chemical Classification of the Common Volcanic Rocks. Can. J. Earth Sci. 1971, 8, 523–548. [Google Scholar] [CrossRef]
- Mangold, N.; Thompson, L.; Forni, O.; Williams, A.; Fabre, C.; Le Deit, L.; Wiens, R.; Williams, R.; Anderson, R.; Blaney, D.; et al. Composition of Conglomerates Analyzed by the Curiosity Rover: Implications for Gale Crater Crust and Sediment Sources. J. Geophys. Res. Planets 2016, 121, 353–387. [Google Scholar] [CrossRef]
- Taylor, S.R.; McLennan, S.M. Planetary Crusts: Their Composition, Origin and Evolution; Cambridge Planetary Science: Cambridge, UK, 2009. [Google Scholar]
- Tirsch, D.; Jaumann, R.; Pacifici, A.; Poulet, F. Dark Aeolian Sediments in Martian Craters: Composition and Sources. J. Geophys. Res. Planets 2011, 116. [Google Scholar] [CrossRef]
- Goetz, W.; Pike, W.T.; Hviid, S.F.; Madsen, M.B.; Morris, R.V.; Hecht, M.H.; Staufer, U.; Leer, K.; Sykulska, H.; Hemmig, E.; et al. Microscopy Analysis of Soils at the Phoenix Landing Site, Mars: Classification of Soil Particles and Description of Their Optical and Magnetic Properties. J. Geophys. Res. Planets 2010, 115. [Google Scholar] [CrossRef]
- Gulzar, M.A.; Rahim, A.; Ali, B.; Khan, A.H. An Investigation on Recycling Potential of Sulfur Concrete. J. Build. Eng. 2021, 38, 102175. [Google Scholar] [CrossRef]



| Parameter | Value (%) | Estimated SD |
|---|---|---|
| Plagioclase | 46.18 | 0.009 |
| Pyroxene | 43.20 | 0.009 |
| Calcite | 3.84 | 0.004 |
| Ilmenite | 2.63 | 0.003 |
| Biotite | 2.63 | 0.004 |
| Magnetite | 0.82 | 0.002 |
| Olivine | 0.35 | 0.003 |
| Hematite | 0.33 | 0.001 |
| Statistics | ||
| Rwp | 48.91 | |
| Rexp | 43.45 | |
| X2 | 1.27 | |
| GoF | 1.13 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rahim, A.; Majeed, U.; Zubair, M.I.; Shahzad, M. WNMS: A New Basaltic Simulant of Mars Regolith. Sustainability 2023, 15, 13372. https://doi.org/10.3390/su151813372
Rahim A, Majeed U, Zubair MI, Shahzad M. WNMS: A New Basaltic Simulant of Mars Regolith. Sustainability. 2023; 15(18):13372. https://doi.org/10.3390/su151813372
Chicago/Turabian StyleRahim, Abdur, Umair Majeed, Muhammad Irfan Zubair, and Muhammad Shahzad. 2023. "WNMS: A New Basaltic Simulant of Mars Regolith" Sustainability 15, no. 18: 13372. https://doi.org/10.3390/su151813372






