Adsorption of Zn(II), Pb(II), and Cu(II) by Residual Soil-Derived Zeolite in Single-Component and Competitive Systems
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Reagents
2.2. Preparation of RSDZ
2.3. Characterisation of RSDZ
2.4. Adsorption Test
2.4.1. Adsorption Isotherms
2.4.2. Effect of Initial pH
2.4.3. Adsorption-Desorption Experiment
2.5. Mechanistic Studies
3. Results and Discussion
3.1. Characterisation of RSDZ
3.2. Adsorption Properties in Single-Component Systems
3.2.1. Adsorption Isotherms
3.2.2. Effect of Initial Solution pH
3.3. Adsorption Performance under Multi-Component Competition
3.3.1. Competitive Adsorption in Binary Systems
3.3.2. Competitive Adsorption in the Ternary System
3.4. Reusability
3.5. Adsorption Mechanisms
3.5.1. XPS Characterisation
3.5.2. FT-IR Characterisation
3.5.3. Proposed Adsorption Mechanism
3.6. Economic Feasibility of RSDZ
3.7. Limitation and Outlook
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wu, Y.; Li, X.; Yu, L.; Wang, T.; Wang, J.; Liu, T. Review of soil heavy metal pollution in China: Spatial distribution, primary sources, and remediation alternatives. Resour. Conserv. Recycl. 2022, 181, 106261. [Google Scholar] [CrossRef]
- Li, Q.; Liu, G.; Qi, L.; Wang, H.; Ye, Z.; Zhao, Q. Heavy metal-contained wastewater in China: Discharge, management and treatment. Sci. Total Environ. 2022, 808, 152091. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; He, J.; Sun, J.; Pei, Z.; Wu, Q.; Yu, R. Capacity and mechanisms of Pb(II) and Cd(II) sorption on five plant-based biochars. Sustainability 2023, 15, 7627. [Google Scholar] [CrossRef]
- Fei, Y.; Hu, Y. Recent progress in removal of heavy metals from wastewater: A comprehensive review. Chemosphere 2023, 335, 139077. [Google Scholar] [CrossRef]
- Chen, X.; Hossain, M.F.; Duan, C.; Liu, J.; Tsang, Y.F.; Islam, M.S.; Zhou, Y. Isotherm models for adsorption of heavy metals from water—A review. Chemosphere 2022, 307, 135545. [Google Scholar] [CrossRef]
- Muhammad, A.N.; Tayyaba, N.; Khurram, S.; Muhammad, A.W.; Tajamal, H.; Muhammad, K.T.; Syed SA, S.; Azizur, R. Heterointerface engineering of water stable ZIF-8@ZIF-67: Adsorption of rhodamine B from water. Surf. Interfaces 2022, 34, 102324. [Google Scholar]
- Muhammad, A.N.; Tayyaba, N.; Shazia, J.; Muhammad, A.W.; Muhammad, S.B.; Syed SA, S.; Azizur, R. Facile synthesis of Tri-metallic layered double hydroxides (NiZnAl-LDHs): Adsorption of Rhodamine-B and methyl orange from water. Inorg. Chem. Commun. 2022, 145, 110008. [Google Scholar]
- Velarde, L.; Nabavi, M.S.; Escalera, E.; Antti, M.; Ahktar, F. Adsorption of heavy metals on natural zeolites: A review. Chemosphere 2023, 328, 138508. [Google Scholar] [CrossRef]
- Erdem, E.; Karapinar, N.; Donat, R. The removal of heavy metal cations by natural zeolites. J. Colloid Interface Sci. 2004, 280, 309–314. [Google Scholar] [CrossRef]
- Hernández-Montoya, V.; Pérez-Cruz, M.A.; Mendoza-CastilloSD, I.; Moreno-Virgen, M.R.; Bonilla-Petriciolet, A. Competitive adsorption of dyes and heavy metals on zeolitic structures. J. Environ. Manag. 2013, 116, 213–221. [Google Scholar] [CrossRef]
- Bai, M.; Xiao, J.; Gao, Q.; Shen, J. Utilization of construction spoil and recycled powder in fired bricks. Case Stud. Constr. Mater. 2023, 18, e02024. [Google Scholar] [CrossRef]
- Santha Kumar, G.; Saine, P.; Deoliya, R.; Mishra, A.K.; Negi, S.K. Characterization of laterite soil and its use in construction applications: A review. Resour. Conserv. Recycl. Adv. 2022, 16, 200120. [Google Scholar] [CrossRef]
- Lou, Y.; Gao, Z.; Sun, G.; Wu, T.; Zhou, F.; Ai, J.; Cen, Y.; Xie, J. Runoff scouring experimental study of rill erosion of spoil tips. Catena 2022, 214, 106249. [Google Scholar] [CrossRef]
- Wu, H.; Yao, P.; Yang, D.; Wang, C.; Shen, J.; Ma, Z. Upcycling of construction spoil powder as partial cement replacement for sustainable cement-based materials: Properties and modification. J. Clean. Prod. 2022, 369, 133361. [Google Scholar] [CrossRef]
- Hou, Y.; Xiao, J.; Shen, J.; Gao, Q. Effects of distiller’s grains and seawater on properties of sintered brick made from construction spoil. J. Build. Eng. 2022, 62, 105391. [Google Scholar] [CrossRef]
- Jian, S.; Cheng, C.; Lv, Y.; Wang, C.; Tan, H.; Li, B. Preparation and evaluation of high-fluid backfill materials from construction spoil. Constr. Build. Mater. 2022, 345, 128370. [Google Scholar] [CrossRef]
- Rydgren, K.; Halvorsen, R.; Odlang, A.; Skjerdal, G. Restoration of alpine spoil heaps: Successional rates predict vegetation recovery in 50 years. Ecol. Eng. 2011, 37, 294–301. [Google Scholar] [CrossRef]
- Chu, D.; Amar, M.; Benzerzour, M.; Kleib, J.; Abriak, N. Flash-calcined sediments for zinc adsorption. Sustainability 2023, 15, 10230. [Google Scholar] [CrossRef]
- Wang, C.; Zhou, J.; Wang, Y.; Yang, M.; Li, Y.; Meng, C. Synthesis of zeolite X from low-grade bauxite. J. Chem. Technol. Biotechnol. 2013, 88, 1350–1357. [Google Scholar] [CrossRef]
- Garcia, G.; Cardenas, E.; Cabrera, S.; Hedlund, J.; Mouzon, J. Synthesis of zeolite Y from diatomite as silica source. Microporous Mesoporous Mater. 2016, 219, 29–37. [Google Scholar] [CrossRef]
- Wu, Z.; Chen, T.; Liu, H.; Wang, C.; Cheng, P.; Chen, D.; Xie, J. An insight into the comprehensive application of opal-palygorskite clay: Synthesis of 4A zeolite and uptake of Hg2+. Appl. Clay Sci. 2018, 165, 103–111. [Google Scholar] [CrossRef]
- Yang, J.; Liu, H.; Diao, H.; Li, B.; Yue, Y.; Bao, X. A Quasi-solid-phase approach to activate natural minerals for zeolite synthesis. Acs Sustain. Chem. Eng. 2017, 5, 3233–3242. [Google Scholar] [CrossRef]
- Liu, H.; Shen, T.; Li, T.; Yuan, P.; Shi, G.; Bao, X. Green synthesis of zeolites from a natural aluminosilicate mineral rectorite: Effects of thermal treatment temperature. Appl. Clay Sci. 2014, 90, 53–60. [Google Scholar] [CrossRef]
- Kazak, E.; Kazak, A. Experimental features of cation exchange capacity determination in organic-rich mudstones. J. Nat. Gas Sci. Eng. 2020, 83, 103456. [Google Scholar] [CrossRef]
- Park, J.; Ok, Y.; Kim, S.; Cho, J.; Heo, J.; Delaune, R.D.; Seo, D. Competitive adsorption of heavy metals onto sesame straw biochar in aqueous solutions. Chemosphere 2016, 142, 77–83. [Google Scholar] [CrossRef]
- Belviso, C. State-of-the-art applications of fly ash from coal and biomass: A focus on zeolite synthesis processes and issues. Prog. Energy Combust. Sci. 2018, 65, 109–135. [Google Scholar] [CrossRef]
- Cheng, Y.; Xu, L.; Liu, C. NaP1 zeolite synthesized via effective extraction of Si and Al from red mud for methylene blue adsorption. Adv. Powder Technol. 2021, 32, 3904–3914. [Google Scholar] [CrossRef]
- Wang, Z.; Fan, Y.; Li, Y.; Qu, F.; Wu, D.; Kong, H. Synthesis of zeolite/hydrous lanthanum oxide composite from coal fly ash for efficient phosphate removal from lake water. Microporous Mesoporous Mater. 2016, 222, 226–234. [Google Scholar] [CrossRef]
- Wu, D.; Lu, Y.; Kong, H.; Ye, C.; Jin, X. Synthesis of zeolite from thermally treated sediment. Ind. Eng. Chem. Res. 2008, 47, 295–302. [Google Scholar] [CrossRef]
- Chen, Q.; Zhao, Y.; Qiu, Q.; Long, L.; Liu, X.; Lin, S.; Jiang, X. Zeolite NaP1 synthesized from municipal solid waste incineration fly ash for photocatalytic degradation of methylene blue. Environ. Res. 2023, 218, 114873. [Google Scholar] [CrossRef]
- Tanaka, H.; Furusawa, S.; Hino, R. Synthesis, characterization, and formation process of Na-X zeolite from coal fly ash. J. Mater. Synth. Process. 2002, 10, 143–148. [Google Scholar] [CrossRef]
- Tanaka, H.; Miyagawa, A.; Eguchi, H.; Hino, R. Synthesis of a single-phase Na-A zeolite from coal fly ash by dialysis. Ind. Eng. Chem. Res. 2004, 43, 6090–6094. [Google Scholar] [CrossRef]
- Zhao, H.; Deng, Y.; Harsh, J.; Flury, H.M.; Boyle, J.S. Alteration of kaolinite to cancrinite and sodalite by simulated Hanford tank waste and its impact on cesium retention. Clays Clay Miner. 2004, 52, 1–13. [Google Scholar] [CrossRef]
- Mouhtaris, T.; Charistos, D.; Kantiranis, N.; Filippidis, A.; Kassoli-Fournaraki, A.; Tsirambidis, A. GIS-type zeolite synthesis from Greek lignite sulphocalcic fly ashes promoted by NaOH solutions. Microporous Mesoporous Mater. 2003, 61, 57–67. [Google Scholar] [CrossRef]
- Ojha, K.; Pradhan, N.; Samanta, A. Zeolite from fly ash: Synthesis and characterization. Bull. Mater. Sci. 2004, 27, 555–564. [Google Scholar] [CrossRef]
- Puccia, V.; Avena, M. On the use of the Dubinin-Radushkevich equation to distinguish between physical and chemical adsorption at the solid-water interface. Colloid Interface Sci. Commun. 2021, 41, 100376. [Google Scholar] [CrossRef]
- Wang, Z.; Li, W.; Zhu, J.; Wang, D.; Meng, H.; Wang, H.; Li, J. Simultaneous adsorption of phosphate and zinc by lanthanum modified zeolite. Environ. Technol. Innov. 2021, 24, 101906. [Google Scholar] [CrossRef]
- Merrikhpour, H.; Jalali, M. Comparative and competitive adsorption of cadmium, copper, nickel, and lead ions by Iranian natural zeolite. Clean Technol. Environ. Policy 2013, 15, 303–316. [Google Scholar] [CrossRef]
- Nguyen, T.; Loganathan, P.; Nguyen, T.; Vigneswara, S.; Kandasamy, J.; Naidu, R. Simultaneous adsorption of Cd, Cr, Cu, Pb, and Zn by an iron-coated Australian zeolite in batch and fixed-bed column studies. Chem. Eng. J. 2015, 270, 393–404. [Google Scholar] [CrossRef]
- Lv, B.; Deng, X.; Jiao, F.; Dong, B.; Fang, C.; Xing, B. Removal of Pb2+ in aqueous solutions using Na-type zeolite synthesized from coal gasification slag in a fluidized bed: Hydrodynamic and adsorption. Process Saf. Environ. Prot. 2023, 174, 869–881. [Google Scholar] [CrossRef]
- Wan Ngah, W.; Teong, L.; Toh, R.; Hanafiah, M.A.K.M. Comparative study on adsorption and desorption of Cu(II) ions by three types of chitosan–zeolite composites. Chem. Eng. J. 2013, 223, 231–238. [Google Scholar] [CrossRef]
- He, K.; Chen, Y.; Tang, Z.; Hu, Y. Removal of heavy metal ions from aqueous solution by zeolite synthesized from fly ash. Environ. Sci. Pollut. Res. 2016, 23, 2778–2788. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Bai, P.; Yan, Y.; Yan, W.; Shi, W.; Xu, R. Removal of Zn2+, Pb2+, Cd2+, and Cu2+ from aqueous solution by synthetic clinoptilolite. Microporous Mesoporous Mater. 2019, 273, 203–211. [Google Scholar] [CrossRef]
- Bashir, A.; Manzoor, T.; Malik, L.; Qureashi, A.; Pandith, A.H. Enhanced and selective adsorption of Zn(II), Pb(II), Cd(II), and Hg(II) Ions by a dumbbell- and flower-shaped potato starch phosphate polymer: A combined experimental and DFT calculation study. ACS Omega 2020, 5, 4853–4867. [Google Scholar] [CrossRef] [PubMed]
- Kenawy, I.; Hafez, M.; Ismail, M.; Hashem, M.A. Adsorption of Cu(II), Cd(II), Hg(II), Pb(II) and Zn(II) from aqueous single metal solutions by guanyl-modified cellulose. Int. J. Biol. Macromol. 2018, 107, 1538–1549. [Google Scholar] [CrossRef] [PubMed]
- Ouki, S.; Kavannagh, M. Treatment of metals-contaminated wastewaters by use of natural zeolites. Water Sci. Technol. 1999, 39, 115–122. [Google Scholar] [CrossRef]
- Guan, Q.; Wu, D.; Lin, Y.; Chen, X.; Wang, X.; Li, C.; He, S.; Kong, H. Application of zeolitic material synthesized from thermally treated sediment to the removal of trivalent chromium from wastewater. J. Hazard. Mater. 2009, 167, 244–249. [Google Scholar] [CrossRef]
- Zhan, Y.; Zhang, H.; Lin, J.; Zhang, Z.; Gao, J. Role of zeolite’s exchangeable cations in phosphate adsorption onto zirconium-modified zeolite. J. Mol. Liq. 2017, 243, 624–637. [Google Scholar] [CrossRef]
- Yurekli, Y. Determination of adsorption characteristics of synthetic NaX nanoparticles. J. Hazard. Mater. 2019, 378, 120743. [Google Scholar] [CrossRef]
- Chen, M.; Nong, S.; Zhao, Y.; Riaz, M.S.; Xiao, Y.; Molokeev, M.S.; Haung, F. Renewable P-type zeolite for superior absorption of heavy metals: Isotherms, kinetics, and mechanism. Sci. Total Environ. 2020, 726, 138535. [Google Scholar] [CrossRef]
- Yu, L.; Xu, C.; Zhang, W.; Zhou, Q.; Fu, X.; Liang, Y.; Guo, Z.; Wang, W. Tailoring the pore structure, morphology and acidity of MFI zeolites by the regulation of Si/Al ratio in the synthetic gel. J. Solid State Chem. 2023, 327, 124271. [Google Scholar] [CrossRef]
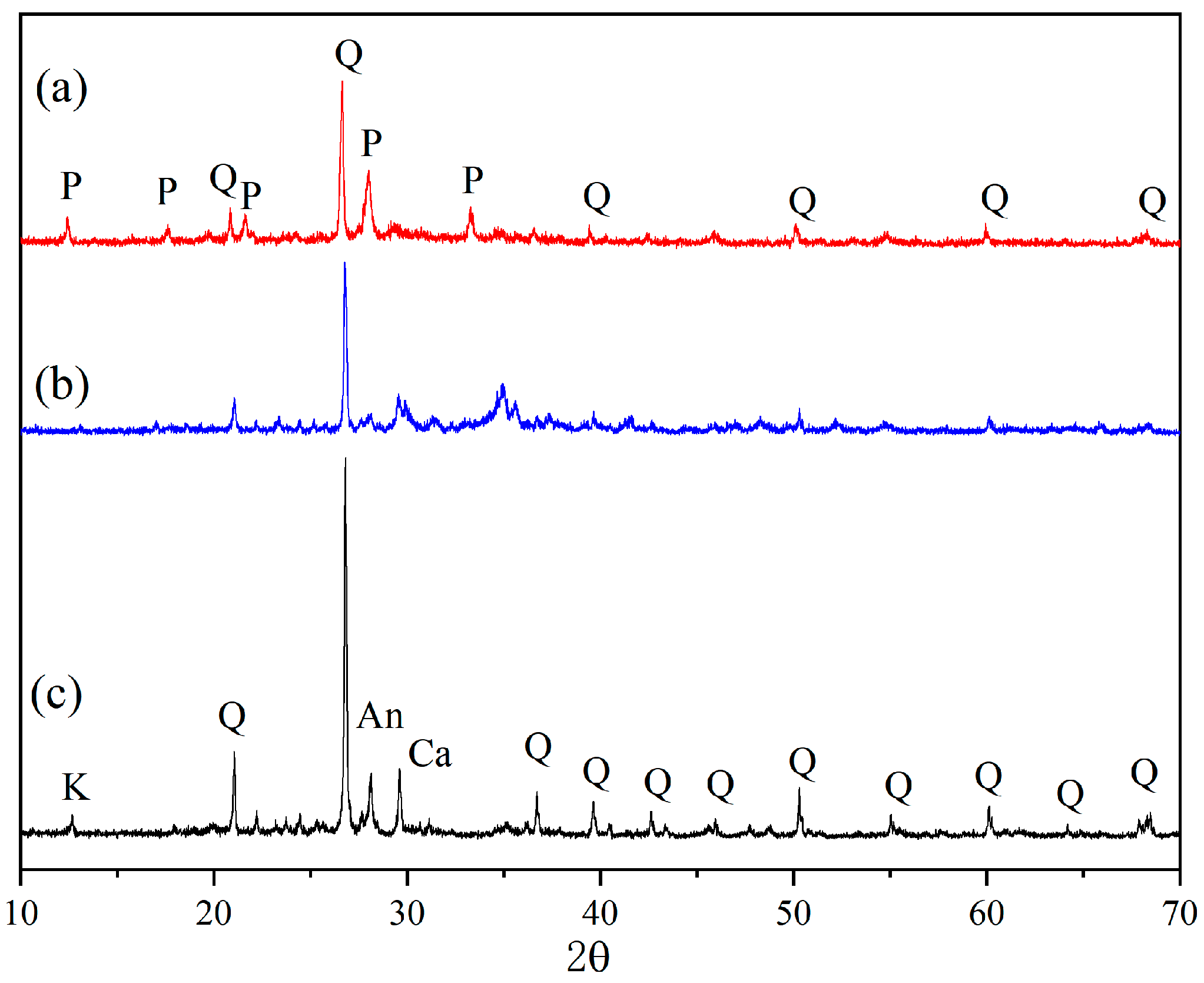

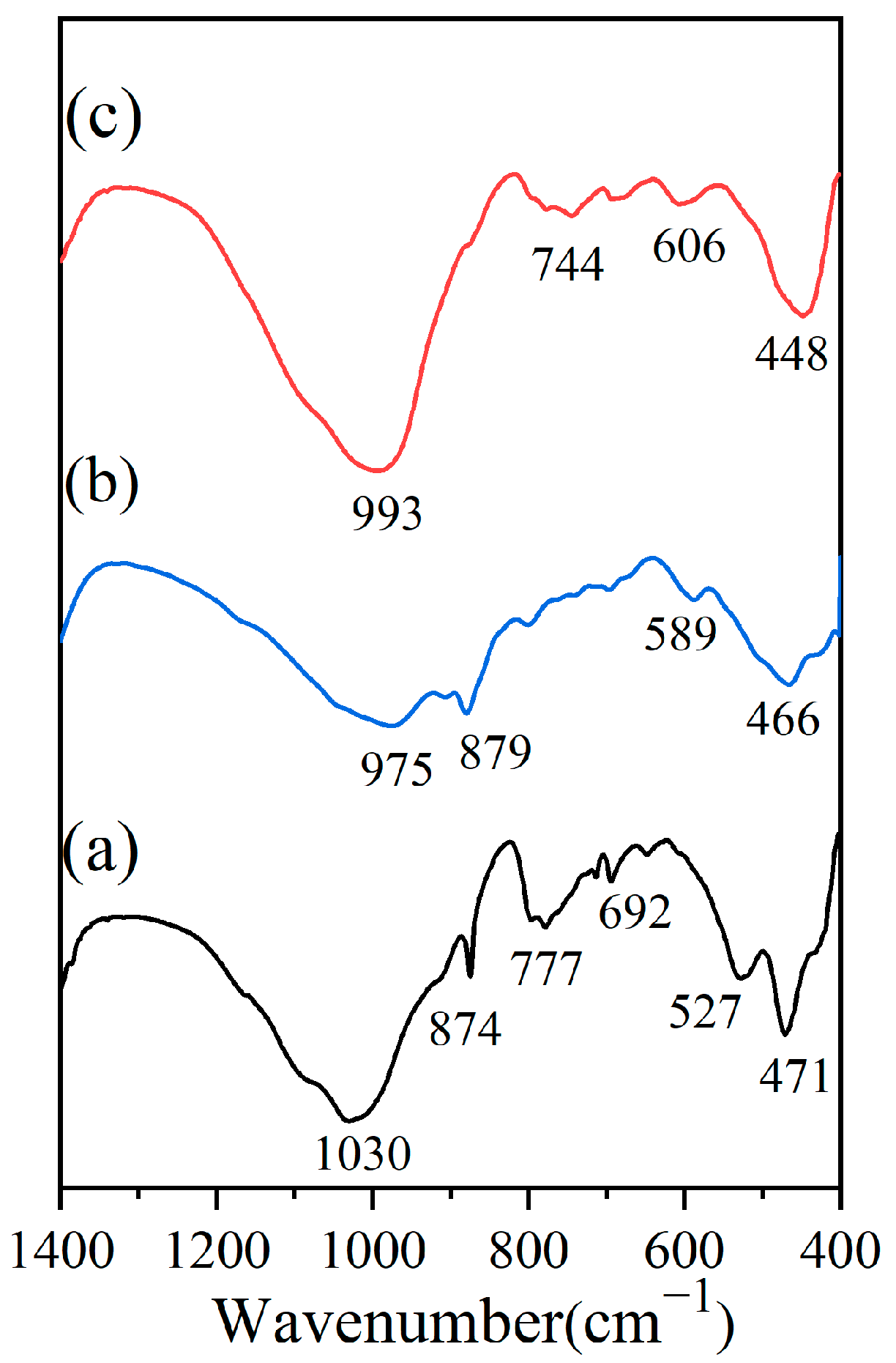

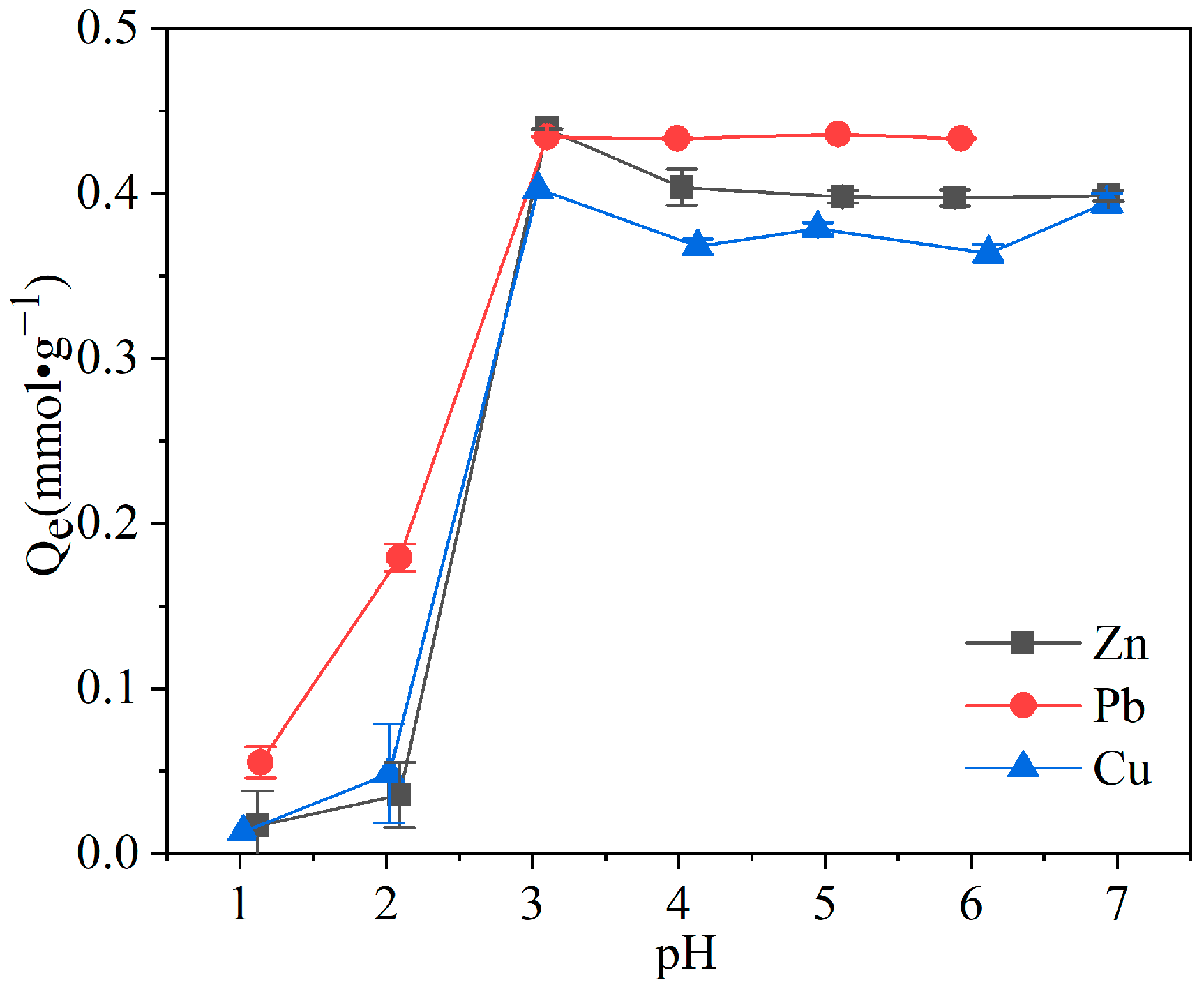
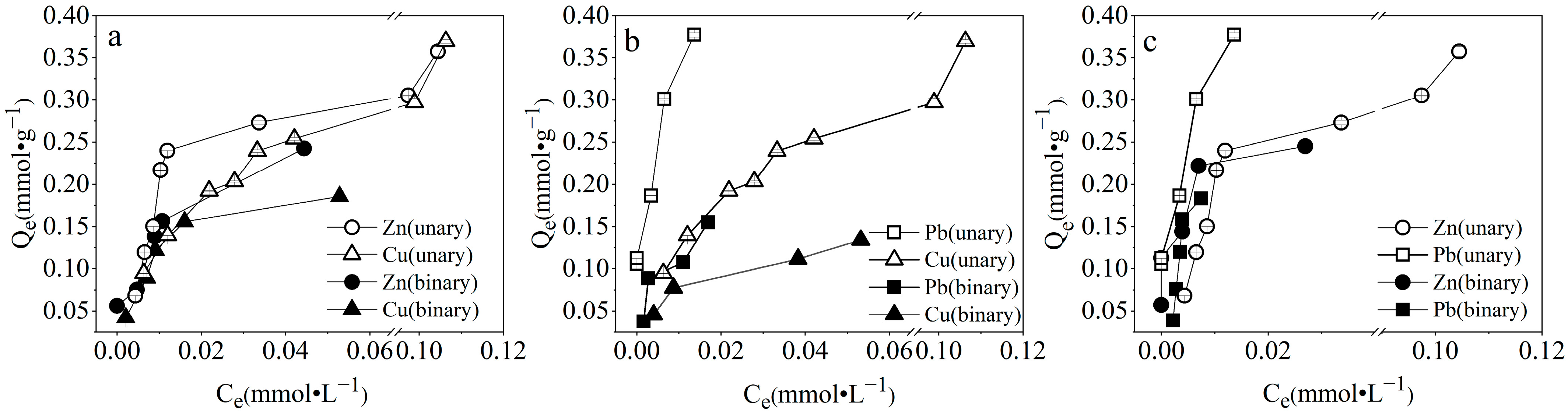




| Sample | SiO2 | Al2O3 | CaO | Fe2O3 | Na2O | K2O | MgO | BET/(m2·g−1) | Average Pore Size/(nm) | CEC/(meq·g−1) |
|---|---|---|---|---|---|---|---|---|---|---|
| RS | 58.21% | 15% | 10.46% | 7.17% | - 1 | 3.43% | 2.65% | 9.03 | 18.85 | 0.12 |
| RSFP | 30.65% | 7.33% | 4.97% | 4.63% | 49.24% | 1.64% | 0.53% | 0.15 | 25.03 | 0.11 |
| RSDZ | 55.41% | 15.8% | 10.1% | 0.22% | 13.7% | 1.78% | 1.6% | 47.77 | 41.60 | 0.61 |
| Heavy Metals | Langmuir Model | Freundlich Model | D-R Model | |||||
|---|---|---|---|---|---|---|---|---|
| KL/(L·mmol−1) | qmax/ (mmol·g−1) | R2 | KF/ (mmol·g−1)·(mmol·L−1)−1/n | 1/n | R2 | E/(KJ·mol−1) | R2 | |
| Zn2+ | 81.93 | 0.37 | 0.979 | 0.92 | 0.390 | 0.742 | 12.91 | 0.769 |
| Pb2+ | 666.54 | 0.38 | 0.910 | 3.76 | 0.524 | 0.947 | 12.91 | 0.956 |
| Cu2+ | 41.05 | 0.40 | 0.965 | 0.96 | 0.437 | 0.9581 | 12.91 | 0.969 |
| Adsorbent | Langmuir Adsorption Maximum (mmol·g−1) | Reference | ||
|---|---|---|---|---|
| Zn2+ | Pb2+ | Cu2+ | ||
| RSDZ | 0.37 | 0.38 | 0.40 | This study |
| Iranian natural zeolite | - | 0.03 | 0.07 | [38] |
| Iron-coated Australian zeolite | 0.10 | 0.05 | 0.15 | [39] |
| Clinoptilolite | 0.10 | 0.21 | - | [10] |
| Erionite | 0.10 | 0.26 | - | [10] |
| Na-type zeolite | - | 0.30 | - | [40] |
| Lanthanum modified zeolite | 0.27 | - | - | [37] |
| Chitosan–zeolite composite | - | - | 0.40 | [41] |
| Synthetic-zeolite | - | 0.32 | 0.88 | [42] |
| Synthetic Na-clinoptilolite | 0.48 | 0.88 | 0.53 | [43] |
| Systems | Heavy Metals | Kd (L∙kg−1) | ||||
|---|---|---|---|---|---|---|
| 0.1 mmol·L−1 | 0.2 mmol·L−1 | 0.3 mmol·L−1 | 0.4 mmol·L−1 | 0.5 mmol·L−1 | ||
| Zn-Cu | Cu2+ | 20,238.10 | 12,637.43 | 13,342.83 | 9726.44 | 3511.93 |
| Zn2+ | - 1 | 15,891.36 | 15,412.19 | 14,587.10 | 11,467.92 | |
| Cu-Pb | Cu2+ | 11,381.15 | 8847.44 | 2906.36 | 2519.01 | 6093.82 |
| Pb2+ | 22,903.35 | 32,326.21 | 9734.52 | 9171.02 | 18,023.09 | |
| Zn-Pb | Zn2+ | - | - | 36,894.20 | 31,722.99 | 9082.29 |
| Pb2+ | 17,701.93 | 27,519.16 | 34,120.07 | 40,521.55 | 24,523.01 | |
| Heavy Metals | Kd (L·kg−1) | ||||||
|---|---|---|---|---|---|---|---|
| 0.05 mmol·L−1 | 0.1 mmol·L−1 | 0.15 mmol·L−1 | 0.2 mmol·L−1 | 0.25 mmol·L−1 | 0.3 mmol·L−1 | 0.35 mmol·L−1 | |
| Cu2+ | 19,635.29 | 11,987.84 | 10,086.01 | 9525.15 | 7798.27 | 6011.47 | 6066.04 |
| Pb2+ | 16,668.59 | 29,465.30 | 25,666.86 | 21,525.11 | 17,980.01 | 16,721.50 | 22,427.41 |
| Zn2+ | - 1 | - | 1,804,676.92 | 29,383.34 | 13,329.89 | 7354.30 | 6510.40 |
| Cycle Number | Zn2+ | Pb2+ | Cu2+ |
|---|---|---|---|
| 1 | 92.16% | 99.30% | 90.04% |
| 2 | 91.36% | 98.01% | 91.02% |
| 3 | 87.31% | 92.53% | 91.28% |
| 4 | 85.26% | 91.34% | 88.37% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Z.; Li, W.; Wang, L.; Zhang, Y.; Li, J.; Liu, Y. Adsorption of Zn(II), Pb(II), and Cu(II) by Residual Soil-Derived Zeolite in Single-Component and Competitive Systems. Sustainability 2023, 15, 13515. https://doi.org/10.3390/su151813515
Wang Z, Li W, Wang L, Zhang Y, Li J, Liu Y. Adsorption of Zn(II), Pb(II), and Cu(II) by Residual Soil-Derived Zeolite in Single-Component and Competitive Systems. Sustainability. 2023; 15(18):13515. https://doi.org/10.3390/su151813515
Chicago/Turabian StyleWang, Zhe, Wen Li, Liling Wang, Yi Zhang, Jiake Li, and Yuling Liu. 2023. "Adsorption of Zn(II), Pb(II), and Cu(II) by Residual Soil-Derived Zeolite in Single-Component and Competitive Systems" Sustainability 15, no. 18: 13515. https://doi.org/10.3390/su151813515
APA StyleWang, Z., Li, W., Wang, L., Zhang, Y., Li, J., & Liu, Y. (2023). Adsorption of Zn(II), Pb(II), and Cu(II) by Residual Soil-Derived Zeolite in Single-Component and Competitive Systems. Sustainability, 15(18), 13515. https://doi.org/10.3390/su151813515






