Formulation of a Jet Fuel Surrogate and Its Kinetic Chemical Mechanism by Emulating Physical and Chemical Properties of Real Jet Fuel
Abstract
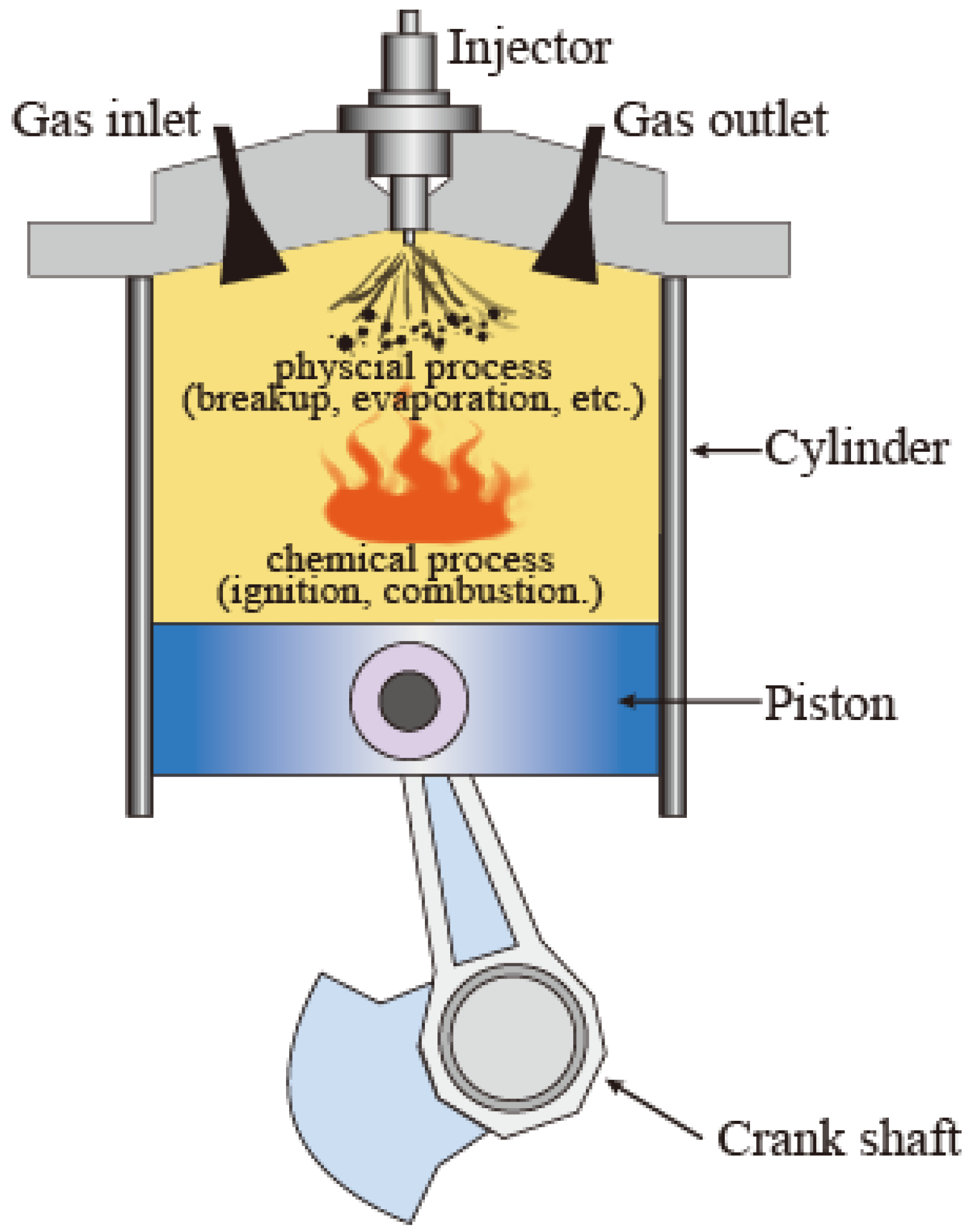
:1. Introduction
2. Formulation of Jet Fuel Surrogate
2.1. Target Fuel and Its Properties
| Target Properties | Jet-A (POSF-4658) |
|---|---|
| CN * | 47.1 |
| MW | 142 kg/kmol |
| H/C | 1.957 |
| LHV | 43.23 MJ/kg |
| TSI | 21.4 |
| Liquid density | Temperature-dependent (See Figure 2) |
| Viscosity | Temperature-dependent (See Figure 2) |
| Surface tension | Temperature-dependent (See Figure 2) |

2.2. Surrogate Fuel Components
2.3. Formation of Jet Fuel Surrogate
| Properties | Estimation Approaches |
|---|---|
| MW | Average of mole fraction: is mole fraction of component i, |
| H/C | is the number of carbon atoms of component i |
| TSI | Average of mole fraction: |
| LHV | Average of mass fraction: |
| CN | Average of volume fraction: is volume fraction of component i |
| Density | Average of volume fraction: |
| MW | Average of mole fraction: |
| H/C | is the number of hydrogen atoms of component i; |
| Viscosity | Grunberg–Nissan equation [24]: is the binary interaction parameter |
| Surface tension | Parachor correlation: is liquid surface tension, P is parachor, is liquid mixture molar density |
3. Kinetic Modelling
3.1. Methodology
- (1)
- The initial stage of the reduction and optimization process involves conducting a reaction pathway analysis to identify the key reactions. Subsequently, unimportant species and reactions are eliminated from the initial kinetic model. Simultaneously, the rate of production (ROP) and sensitivity analyses are performed to evaluate the remaining species and reactions. This allows for a clear understanding of the impact of each reaction on the oxidation process.
- (2)
- Subsequently, the reaction rate constants were optimized to improve the agreement between the simulated and experimental data of fuel ignition delay time (IDT).
- (3)
- Afterward, the concentrations of species and laminar flame speeds predicted by the reduced mechanism were compared to the corresponding measurements. This allowed for references to fine-tune the reaction rate constants further.
- (4)
- Steps 1 to 3 were iteratively repeated until the desired size and accuracy of the mechanism were attained.
3.2. Toluene Sub-Mechanism
3.3. Decalin Sub-Mechanism
3.4. N-Dodecane Sub-Mechanism
3.5. Isocetane Sub-Mechanism
4. Results and Discussion
4.1. Verifications of Toluene
4.1.1. IDT
4.1.2. Species Concentration
4.1.3. Laminar Flame Speed
4.2. Verifications of Decalin
4.2.1. IDT
4.2.2. Species Concentration
4.2.3. Laminar Flame Speed
4.3. Verifications of n-Dodecane
4.3.1. IDT
4.3.2. Species Concentration
4.3.3. Laminar Flame Speed
4.4. Verifications of Isocetane
4.4.1. IDT
4.4.2. Species Concentration
4.5. Verifications of Jet Fuel
4.5.1. IDT
4.5.2. Species Concentration
4.5.3. Laminar Flame Speed
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wolfowitz, P. DoD Management Policy for Energy Commodities and Related Services; Department of Defense: Washington, DC, USA, 2004; p. 4140.25.
- Sarathy, S.; Farooq, A.; Kalghatgi, G.T. Recent progress in gasoline surrogate fuels. Prog. Energy Combust. Sci. 2018, 65, 67–108. [Google Scholar] [CrossRef]
- Zhen, X.; Yang, W.; Daming, L. An overview of the chemical reaction mechanisms for gasoline surrogate fuels. Appl. Therm. Eng. 2017, 124, 1257–1268. [Google Scholar] [CrossRef]
- Kim, D.; Violi, A. Hydrocarbons for the next generation of jet fuel surrogates. Fuel 2018, 228, 438–444. [Google Scholar] [CrossRef]
- Yu, W.; Zhao, F.; Yang, W.; Tay, K.; Xu, H. Development of an optimization methodology for formulating both jet fuel and diesel fuel surrogates and their associated skeletal oxidation mechanisms. Fuel 2018, 231, 361–372. [Google Scholar] [CrossRef]
- Dooley, S.; Won, S.H.; Dryer, F.L. Chapter 10—Surrogate fuels. In Computer Aided Chemical Engineering; Faravelli, T., Manenti, F., Ranzi, E., Eds.; Elsevier: Amsterdam, The Netherlands, 2019; pp. 513–602. [Google Scholar]
- Violi, A.; Yan, S.; Eddings, E.G.; Sarofim, A.F.; Granata, S.; Faravelli, T.; Ranzi, E. Experimental formulation and kinetic model for JP-8 surrogate mixtures. Combust. Sci. Technol. 2002, 174, 399–417. [Google Scholar] [CrossRef]
- Vasu, S.S.; Davidson, D.F.; Hanson, R.K. Jet fuel ignition delay times: Shock tube experiments over wide conditions and surrogate model predictions. Combust. Flame 2008, 152, 125–143. [Google Scholar] [CrossRef]
- Dagaut, P.; El Bakali, A.; Ristori, A. The combustion of kerosene: Experimental results and kinetic modelling using 1- to 3-component surrogate model fuels. Fuel 2006, 85, 944–956. [Google Scholar] [CrossRef]
- Gokulakrishnan, P.; Gaines, G.; Currano, J.; Klassen, M.S.; Roby, R.J. Experimental and Kinetic Modeling of Kerosene-Type Fuels at Gas Turbine Operating Conditions. J. Eng. Gas Turbines Power 2006, 129, 655–663. [Google Scholar] [CrossRef]
- Eddings, E.G.; Yan, S.; Ciro, W.; Sarofim, A.F. Formulation of a surrogate for the simulation of jet fuel pool fires. Combust. Sci. Technol. 2005, 177, 715–739. [Google Scholar] [CrossRef]
- Dooley, S.; Won, S.H.; Chaos, M.; Heyne, J.; Ju, Y.; Dryer, F.L.; Kumar, K.; Sung, C.-J.; Wang, H.; Oehlschlaeger, M.A.; et al. A jet fuel surrogate formulated by real fuel properties. Combust. Flame 2010, 157, 2333–2339. [Google Scholar] [CrossRef]
- Dooley, S.; Won, S.H.; Heyne, J.; Farouk, T.I.; Ju, Y.; Dryer, F.L.; Kumar, K.; Hui, X.; Sung, C.-J.; Wang, H.; et al. The experimental evaluation of a methodology for surrogate fuel formulation to emulate gas phase combustion kinetic phenomena. Combust. Flame 2012, 159, 1444–1466. [Google Scholar] [CrossRef]
- Malewicki, T.; Gudiyella, S.; Brezinsky, K. Experimental and modeling study on the oxidation of Jet A and the n-dodecane/iso-octane/n-propylbenzene/1, 3, 5-trimethylbenzene surrogate fuel. Combust. Flame 2013, 160, 17–30. [Google Scholar] [CrossRef]
- Kim, D.; Martz, J.; Violi, A. A surrogate for emulating the physical and chemical properties of conventional jet fuel. Combust. Flame 2014, 161, 1489–1498. [Google Scholar] [CrossRef]
- Verhelst, S.; Turner, J.W.; Sileghem, L.; Vancoillie, J. Methanol as a fuel for internal combustion engines. Prog. Energy Combust. Sci. 2019, 70, 43–88. [Google Scholar] [CrossRef]
- Bae, C.; Kim, J. Alternative fuels for internal combustion engines. Proc. Combust. Inst. 2017, 36, 3389–3413. [Google Scholar] [CrossRef]
- Yu, W.; Yang, W.; Tay, K.; Zhao, F. An optimization method for formulating model-based jet fuel surrogate by emulating physical, gas phase chemical properties and threshold sooting index (TSI) of real jet fuel under engine relevant conditions. Combust. Flame 2018, 193, 192–217. [Google Scholar] [CrossRef]
- McDaniel, A.; Dickerson, T.; Luning-Prak, D.; Hamilton, L.; Cowart, J. A Technical Evaluation of New Renewable Jet and Diesel Fuels Operated in Neat Form in Multiple Diesel Engines; SAE Technical Paper; SAE International: Warrendale, PA, USA, 2016. [Google Scholar] [CrossRef]
- Murphy, M.J.; Taylor, J.D.; McCormick, R.L. Compendium of Experimental Cetane Number Data; National Renewable Energy Laboratory: Golden, CO, USA, 2004.
- DIPPR Project 801—Full Version; Design Institute for Physical Property Research/AIChE: New York, NY, USA, 2012.
- Mensch, A.; Santoro, R.; Litzinger, T.; Lee, S.-Y. Sooting Characteristics of Surrogates for Jet Fuels. Combust. Flame 2010, 157, 1097–1105. [Google Scholar] [CrossRef]
- Gill, R.J.; Olson, D.B. Estimation of Soot Thresholds for Fuel Mixtures. Combust. Sci. Technol. 1984, 40, 307–315. [Google Scholar] [CrossRef]
- Grunberg, L.; Nissan, A.H. Mixture Law for Viscosity. Nature 1949, 164, 799–800. [Google Scholar] [CrossRef]
- Poling, B.E.; Thomson, G.H.; Friend, D.G.; Rowley, R.L.; Wilding, W.V. Perry’s Chemical Engineers’ Handbook; McGraw-Hill Publishing: New York, NY, USA, 2008. [Google Scholar]
- Hugill, J.; Van Welsenes, A.J. Surface tension: A simple correlation for natural gas+ conensate systems. Fluid Phase Equilibria 1986, 29, 383–390. [Google Scholar] [CrossRef]
- Agosta, A.; Cernansky, N.; Miller, D.; Faravelli, T.; Ranzi, E. Reference components of jet fuels: Kinetic modeling and experimental results. Exp. Therm. Fluid Sci. 2004, 28, 701–708. [Google Scholar] [CrossRef]
- Wang, H.; Yao, M.; Yue, Z.; Jia, M.; Reitz, R.D. A reduced toluene reference fuel chemical kinetic mechanism for combustion and polycyclic-aromatic hydrocarbon predictions. Combust. Flame 2015, 162, 2390–2404. [Google Scholar] [CrossRef]
- Dagaut, P.; Ristori, A.; Frassoldati, A.; Faravelli, T.; Dayma, G.; Ranzi, E. Experimental and semi-detailed kinetic modeling study of decalin oxidation and pyrolysis over a wide range of conditions. Proc. Combust. Inst. 2013, 34, 289–296. [Google Scholar] [CrossRef]
- Yu, L.; Wu, Z.; Qiu, Y.; Qian, Y.; Mao, Y.; Lu, X. Ignition delay times of decalin over low-to-intermediate temperature ranges: Rapid compression machine measurement and modeling study. Combust. Flame 2018, 196, 160–173. [Google Scholar] [CrossRef]
- Chang, Y.; Jia, M.; Liu, Y.; Li, Y.; Xie, M.; Yin, H. Application of a Decoupling Methodology for Development of Skeletal Oxidation Mechanisms for Heavy n-Alkanes from n-Octane to n-Hexadecane. Energy Fuels 2013, 27, 3467–3479. [Google Scholar] [CrossRef]
- Fan, W.; Jia, M.; Chang, Y.; Xie, M. Understanding the relationship between cetane number and the ignition delay in shock tubes for different fuels based on a skeletal primary reference fuel (n-hexadecane/iso-cetane) mechanism. Energy Fuels 2015, 29, 3413–3427. [Google Scholar] [CrossRef]
- CHEMKIN-PRO RJI, San Diego, CA. 15112, Reaction Design. 2011. Available online: https://www.ansys.com/products/fluids/ansys-chemkin-pro (accessed on 6 July 2023).
- Wang, L.; Wu, Z.; Ahmed, A.; Badra, J.A.; Sarathy, S.M.; Roberts, W.L.; Fang, T. Autoignition of direct injection spray of light naphtha, primary reference fuels, gasoline and gasoline surrogate. Energy 2019, 170, 375–390. [Google Scholar] [CrossRef]
- Shen, H.-P.S.; Vanderover, J.; Oehlschlaeger, M.A. A shock tube study of the autoignition of toluene/air mixtures at high pressures. Proc. Combust. Inst. 2009, 32, 165–172. [Google Scholar] [CrossRef]
- Kukkadapu, G.; Kang, D.; Wagnon, S.W.; Zhang, K.; Mehl, M.; Monge-Palacios, M.; Wang, H.; Goldsborough, S.S.; Westbrook, C.K.; Pitz, W.J. Kinetic modeling study of surrogate components for gasoline, jet and diesel fuels: C7-C11 methylated aromatics. Proceeding Combust. Inst. 2019, 37, 521–529. [Google Scholar] [CrossRef]
- Pitz, W.J.; Mueller, C.J. Recent progress in the development of diesel surrogate fuels. Prog. Energy Combust. Sci. 2019, 37, 330–350. [Google Scholar] [CrossRef]
- Li, Y.; Cai, J.; Zhang, L.; Yuan, T.; Zhang, K.; Qi, F. Investigation on chemical structures of premixed toluene flames at low pressure. Proceeding Combust. Inst. 2011, 33, 593–600. [Google Scholar] [CrossRef]
- Yuan, W.; Li, Y.; Dagaut, P.; Yang, J.; Qi, F. Investigation on the pyrolysis and oxidation of toluene over a wide range conditions. I. Flow reactor pyrolysis and jet stirred reactor oxidation. Combust. Flame 2015, 162, 3–21. [Google Scholar] [CrossRef]
- Zhao, F.; Yang, W.; Zhou, D.; Yu, W.; Li, J.; Tay, K.L. Numerical modelling of soot formation and oxidation using phenomenological soot modelling approach in a dual-fueled compression ignition engine. Fuel 2017, 188, 382–389. [Google Scholar] [CrossRef]
- Davis, S.; Wang, H.; Breinsky, K.; Law, C.K. Laminar flame speeds and oxidation kinetics of benene-air and toluene-air flames. Conference Laminar flame speeds and oxidation kinetics of benene-air and toluene-air flames. Symp. (Int.) Combust. 1996, 26, 1025–1033. [Google Scholar] [CrossRef]
- Johnston, R.; Farrell, J. Laminar burning velocities and Markstein lengths of aromatics at elevated temperature and pressure. Proc. Combust. Inst. 2005, 30, 217–224. [Google Scholar] [CrossRef]
- Zhu, Y.; Davidson, D.; Hanson, R. Pyrolysis and oxidation of decalin at elevated pressures: A shock-tube study. Combust. Flame 2014, 161, 371–383. [Google Scholar] [CrossRef]
- Oehlschlaeger, M.A.; Shen, H.-P.S.; Frassoldati, A.; Pierucci, S.; Ranzi, E. Experimental and Kinetic Modeling Study of the Pyrolysis and Oxidation of Decalin. Energy Fuels 2009, 23, 1464–1472. [Google Scholar] [CrossRef]
- Curran, H.; Gaffuri, P.; Pitz, W.; Westbrook, C. A Comprehensive Modeling Study of n-Heptane Oxidation. Combust. Flame 1998, 114, 149–177. [Google Scholar] [CrossRef]
- Zeng, M.; Li, Y.; Yuan, W.; Li, T.; Wang, Y.; Zhou, Z.; Zhang, L.; Qi, F. Experimental and kinetic modeling study of laminar premixed decalin flames. Combust. Flame 2017, 36, 1193–1202. [Google Scholar] [CrossRef]
- Li, B.; Zhang, H.; Egolfopoulos, F.N. Laminar flame propagation of atmospheric iso-cetane/air and decalin/air mixtures. Combust. Flame 2014, 161, 154–161. [Google Scholar] [CrossRef]
- Vasu, S.; Davidson, D.; Hong, Z.; Vasudevan, V.; Hanson, R. n-Dodecane oxidation at high-pressures: Measurements of ignition delay times and OH concentration time-histories. Proc. Combust. Inst. 2009, 32, 173–180. [Google Scholar] [CrossRef]
- Shen, H.-P.S.; Steinberg, J.; Vanderover, J.; Oehlschlaeger, M.A. A Shock Tube Study of the Ignition of n-Heptane, n-Decane, n-Dodecane, and n-Tetradecane at Elevated Pressures. Energy Fuels 2009, 23, 2482–2489. [Google Scholar] [CrossRef]
- Haylett, D.R.; Davidson, D.F.; Hanson, R.K. Ignition delay times of low-vapor-pressure fuels measured using an aerosol shock tube. Combust. Flame 2012, 159, 552–561. [Google Scholar] [CrossRef]
- Kumar, K.; Sung, C.J. Laminar flame speeds and extinction limits of preheated n-decane/O2/N2 and n-dodecane/O2/N2 mix-tures. Combust. Flame 2007, 151, 209–224. [Google Scholar] [CrossRef]
- Oehlschlaeger, M.A.; Steinberg, J.; Westbrook, C.K.; Pitz, W.J. The autoignition of iso-cetane at high to moderate temperatures and elevated pressures: Shock tube experiments and kinetic modeling. Combust. Flame 2009, 156, 2165–2172. [Google Scholar] [CrossRef]
- Dagaut, P.; Hadj-Ali, K. Chemical Kinetic Study of the Oxidation of Isocetane (2,2,4,4,6,8,8-Heptamethylnonane) in a Jet-stirred Reactor: Experimental and Modeling. Energy Fuels 2009, 23, 2389–2395. [Google Scholar] [CrossRef]
- Wang, H.; Oehlschlaeger, M.A. Autoignition studies of conventional and Fischer–Tropsch jet fuels. Fuel 2012, 98, 249–258. [Google Scholar] [CrossRef]
- Li, J.; Zhao, Z.; Kazakov, A.; Chaos, M.; Dryer, F.L.; Scire, J.J., Jr. A comprehensive kinetic mechanism for CO, CH2O, and CH3OH combustion. Int. J. Chem. Kinet. 2007, 39, 109–136. [Google Scholar] [CrossRef]






















| Hydrocarbon Class Name | N-Alkane n-Dodecane | Iso-Alkane Isocetane | Cycloalkane Decalin | Aromatic Toluene |
|---|---|---|---|---|
| Formula | C12H26 | C16H34 | C10H18 | C7H8 |
| CN [20] | 82.5 | 15 | 46.5 | 7.4 |
| MW (g/mol) | 170.33 | 226.44 | 138.25 | 92.14 |
| LHV [21] (MJ/kg) | 44.11 | 44.85 | 42.58 | 40.53 |
| TSI [22] | 7.0 | 22 | 22 | 40 |
| H/C | 2.17 | 2.13 | 1.8 | 1.1 |
| Jet Fuel | Surrogate | JFS | UM1 | UM2 | MURI2 | S5 | HEX12 |
|---|---|---|---|---|---|---|---|
| CN 47.1 | Val Dev (%) | 46.93 | 46.8 | 46.7 | 48.5 | 32.1 | 60.5 |
| −0.35 | −0.64 | −0.85 | 2.97 | −31.8 | 28.45 | ||
| H/C 1.957 | Val Dev (%) | 1.94 | 1.967 | 1.881 | 1.950 | 1.807 | 1.856 |
| −0.86 | 0.51 | −3.88 | −0.36 | −7.66 | −5.16 | ||
| MW 142 | Val Dev (%) | 157.23 | 143.5 | 148.6 | 138.7 | 159.2 | 152.2 |
| 10.73 | 1.06 | 4.65 | 2.32 | 12.11 | 7.18 | ||
| LHV | Val Dev (%) | 43.61 | 43.62 | 43.36 | 43.55 | 43.02 | 44.6 |
| 0.87 | 0.90 | 0.30 | 0.74 | −0.49 | 3.17 | ||
| TSI 21.4 | Val Dev (%) | 21.12 | 16.79 | 22.14 | 20.4 | 34.61 | 25.0 |
| −1.29 | −21.52 | 3.45 | −4.67 | 61.72 | 16.84 | ||
| Density | Average dev (%) | 1.84 | −3.4 | 0.6 | −5.518 | 2.392 | 1.423 |
| Viscosity | Average dev (%) | 5.88 | −21.2 | −3.6 | −34.608 | 18.167 | 5.077 |
| Surface tension | Average dev (%) | 8.71 | 9.1 | 15.8 | 3.131 | 19.1 | 18.774 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, G.; Cui, B.; Zhang, C.; Chang, L.; Chen, L. Formulation of a Jet Fuel Surrogate and Its Kinetic Chemical Mechanism by Emulating Physical and Chemical Properties of Real Jet Fuel. Sustainability 2023, 15, 13792. https://doi.org/10.3390/su151813792
Li G, Cui B, Zhang C, Chang L, Chen L. Formulation of a Jet Fuel Surrogate and Its Kinetic Chemical Mechanism by Emulating Physical and Chemical Properties of Real Jet Fuel. Sustainability. 2023; 15(18):13792. https://doi.org/10.3390/su151813792
Chicago/Turabian StyleLi, Guangze, Boxuan Cui, Chenglin Zhang, Liuyong Chang, and Longfei Chen. 2023. "Formulation of a Jet Fuel Surrogate and Its Kinetic Chemical Mechanism by Emulating Physical and Chemical Properties of Real Jet Fuel" Sustainability 15, no. 18: 13792. https://doi.org/10.3390/su151813792
APA StyleLi, G., Cui, B., Zhang, C., Chang, L., & Chen, L. (2023). Formulation of a Jet Fuel Surrogate and Its Kinetic Chemical Mechanism by Emulating Physical and Chemical Properties of Real Jet Fuel. Sustainability, 15(18), 13792. https://doi.org/10.3390/su151813792








