Natural Organic Matter Removal in Surface Water Treatment via Coagulation—Current Issues, Potential Solutions, and New Findings
Abstract
:1. Introduction
- (a)
- What are the implications of the progressing climate change for typical surface water treatment via coagulation?
- (b)
- What are the potential solutions for ensuring the continuous efficiency of coagulation-based water treatment in the context of both current and anticipated challenges?
- (c)
- What are the negative consequences of employing coagulation-based methods, and how can they be mitigated?
2. The Impact of Climate Change on Surface Water Quality and Its Treatability
3. Understanding NOM and Its Role in Water Treatment
3.1. Characteristics of NOM
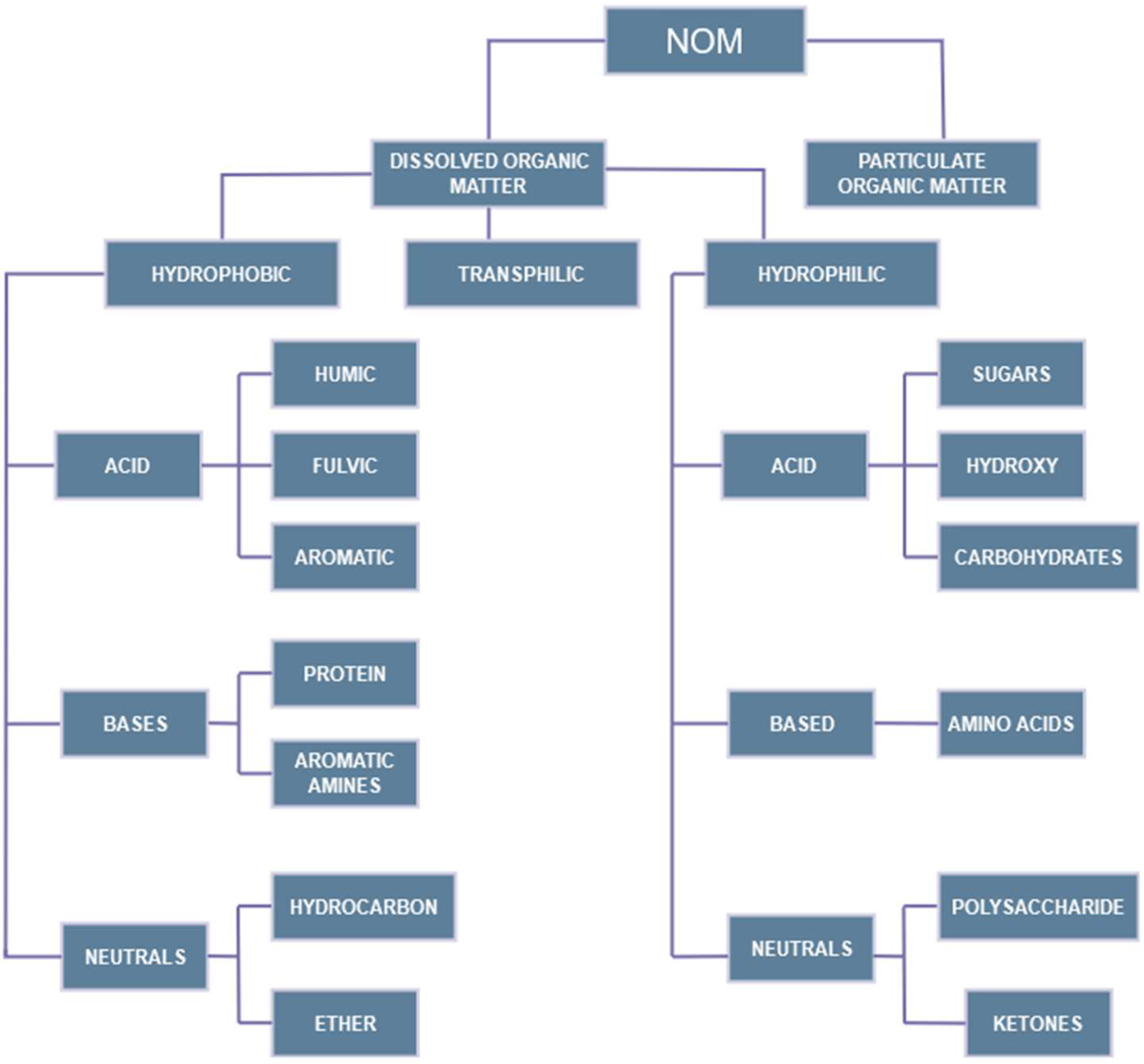
3.2. NOM-Related Problems in Water Treatment
4. Modern-Day Approach to NOM Removal in Drinking Water Treatment
4.1. Coagulation-Based Methods
4.1.1. Metallic Coagulants
4.1.2. Titanium and Zirconium-Based Coagulants
4.1.3. Inorganic Polymeric Coagulants
4.1.4. Biocoagulants
4.1.5. Hybrid Coagulants
4.2. Coagulation-Integrated Processes
4.2.1. Integrated Coagulation–Adsorption Processes as Pre-Treatment to Membrane Filtration
4.2.2. Coagulation Integrated with Biological Processes
4.2.3. Integrated Coagulation–Oxidation Processes as Pre-Treatment to Membrane Filtration
4.2.4. Integrated Coagulation-Ion Exchange Processes
5. Moving Forward
6. Conclusions
- (1)
- The progressing climate change can have a significant impact on the typical surface water treatment system. The predictions of an increase in the proportion of LMW fractions of NOM in the surface water sources allow for speculating on a decrease in the effectiveness of water purification by conventional coagulation using metallic coagulants because it is typically ineffective in removing those fractions of NOM.
- (2)
- Potential solutions for ensuring continuous efficiency of coagulation-based treatment need to provide removal of NOM fractions currently difficult to reduce by conventional coagulation. Such solutions include integrating the coagulation process with other processes, e.g., adsorption, membrane filtration, and ion exchange.
- (3)
- Moving forward, the negative consequences of using conventional coagulation need to be eliminated. Disadvantages associated with the use of traditional coagulants can be resolved, or at least mitigated by using inorganic polymers with no damage to the process’ performance in NOM removal.
- (4)
- Moreover, more environmentally friendly solutions that potentially allow for implementing the concepts of circular economy should be further investigated in terms of the possibility of their implementation on a larger scale. Such solutions include the use of hybrid coagulants and biocoagulants. Future research in that area should take into account not only the technological, economic, and environmental aspects but also the potential impact of climate change-related surface water quality changes on the process efficiency.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- European Environment Agency. Use of Freshwater Resources in Europe. 2019. Available online: https://www.eea.europa.eu/ims/use-of-freshwater-resources-in-europe-1 (accessed on 9 January 2023).
- Center for Sustainable Systems, University of Michigan. U.S Water Supply and Distribution Factsheet. 2022. Available online: https://css.umich.edu/publications/factsheets/water/us-water-supply-and-distribution-factsheet (accessed on 9 January 2023).
- Statistics Canada. Table 38-10-0992-01 Potable Water Volumes Processed by Drinking Water plants, by Source Water Type. 2021. Available online: https://www150.statcan.gc.ca/t1/tbl1/en/tv.action?pid=3810009201 (accessed on 13 August 2023). [CrossRef]
- Sillanpää, M.; Ncibi, M.C.; Matilainen, A. Advanced oxidation processes for the removal of natural organic matter from drinking water sources: A comprehensive review. J. Environ. Manag. 2018, 208, 56–76. [Google Scholar] [CrossRef]
- Sillanpää, M.; Ncibi, M.C.; Matilainen, A.; Vepsäläinen, M. Removal of natural organic matter in drinking water treatment by coagulation: A comprehensive review. Chemosphere 2018, 190, 54–71. [Google Scholar] [CrossRef]
- Ghernaout, D. Water treatment coagulation: Dares and trends. Open Access Libr. J. 2020, 7, 1–18. [Google Scholar] [CrossRef]
- Hadadi, A.; Imessaoudene, A.; Bollinger, J.C.; Assadi, A.A.; Amrane, A.; Mouni, L. Comparison of Four Plant-Based Bio-Coagulants Performances against Alum and Ferric Chloride in the Turbidity Improvement of Bentonite Synthetic Water. Water 2022, 14, 3324. [Google Scholar] [CrossRef]
- Chen, S.; Yuan, Z.; Hanigan, D.; Westerhoff, P.; Zhao, H.; Ni, J. Coagulation behaviors of new covalently bound hybrid coagulants (CBHyC) in surface water treatment. Sep. Purif. Technol. 2017, 192, 322–328. [Google Scholar] [CrossRef]
- Marais, S.; Ncube, E.J.; Msagati, T.A.M.; Mamba, B.B.; Nkambule, T. Comparison of natural organic matter removal by ultrafiltration, granular activated carbon filtration and full scale conventional water treatment. J. Environ. Chem. Eng. 2018, 6, 6282–6289. [Google Scholar] [CrossRef]
- Anderson, L.; DeMont, I.; Dunnington, D.; Bjorndahl, P.; Redden, D.; Brophy, M.; Gagnon, G. A review of long-term change in surface water natural organic matter concentration in the northern hemisphere and the implications for drinking water treatment. Sci. Total Environ. 2023, 858, 159699. [Google Scholar] [CrossRef]
- Slavik, I.; Kostrowski, D.; Uhl, W. Effect of solar radiation on natural organic matter composition in surface waters and resulting impacts on drinking water treatment. Environ. Technol. 2023, 44, 1549–1565. [Google Scholar] [CrossRef] [PubMed]
- Loganathan, P.; Gradzielski, M.; Bustamante, H.; Vigneswaran, S. Progress, challenges, and opportunities in enhancing NOM flocculation using chemically modified chitosan: A review towards future development. Environ. Sci. Water Res. Technol. 2019, 6, 45–61. [Google Scholar] [CrossRef]
- Qiu, F.; Lv, H.; Zhao, X.; Zhao, D. Impact of an extreme winter storm event on the coagulation/flocculation processes in a prototype surface water treatment plant: Causes and mitigating measures. Int. J. Environ. Res. Public Health 2019, 16, 2808. [Google Scholar] [CrossRef]
- Ghernaout, D. Enhanced coagulation: Promising findings and challenges. Open Access Libr. J. 2020, 7, 1–19. [Google Scholar] [CrossRef]
- Adusei-Gyamfi, J.; Ouddane, B.; Rietveld, L.; Cornard, J.P.; Criquet, J. Natural organic matter-cations complexation and its impact on water treatment: A critical review. Water Res. 2019, 160, 130–147. [Google Scholar] [CrossRef] [PubMed]
- Sciscenko, I.; Arques, A.; Micó, P.; Mora, M.; García Ballesteros, S. Emerging applications of EEM-PARAFAC for water treatment: A concise review. Chem. Eng. J. Adv. 2022, 10, 100286. [Google Scholar] [CrossRef]
- Ibrahim, N.; Aziz, H. Trends on Natural Organic Matter in Drinking Water Sources and its Treatment. Int. J. Sci. Res. Environ. Sci. 2014, 2, 94–106. [Google Scholar] [CrossRef]
- He, H.; Li, T.; He, C.; Chen, J.; Chu, H.; Dong, B. Removal of natural organic matter in full-scale conventional and advanced water treatment plants: Assimilable organic carbon and its precursors. Chem. Eng. J. Adv. 2021, 8, 100183. [Google Scholar] [CrossRef]
- Kumari, M.; Gupta, S.K. Cumulative human health risk analysis of trihalomethanes exposure in drinking water systems. J. Environ. Manag. 2022, 321, 115949. [Google Scholar] [CrossRef]
- Beauchamp, N.; Bouchard, C.; Dorea, C.; Rodriguez, M. Ultraviolet absorbance monitoring for removal of DBP-precursor in waters with variable quality: Enhanced coagulation revisited. Sci. Total Environ. 2020, 717, 137225. [Google Scholar] [CrossRef]
- Wang, P.; Ding, S.; Xiao, R.; An, G.; Fang, C.; Chu, W. Enhanced coagulation for mitigation of disinfection by-product precursors: A review. Adv. Colloid Interface Sci. 2021, 296, 102518. [Google Scholar] [CrossRef]
- Diana, M.; Felipe-Sotelo, M.; Bond, T. Disinfection byproducts potentially responsible for the association between chlorinated drinking water and bladder cancer: A review. Water Res. 2019, 162, 492–504. [Google Scholar] [CrossRef] [PubMed]
- Evlampidou, I.; Font-Ribera, L.; Rojas-Rueda, D.; Gracia-Lavedan, E.; Costet, N.; Pearce, N.; Vineis, P.; Jaakkola, J.J.K.; Delloye, F.; Makris, K.C.; et al. Trihalomethanes in drinking water and bladder cancer burden in the European Union. Environ. Health Perspect. 2020, 128, 17001. [Google Scholar] [CrossRef]
- Cool, G.; Delpla, I.; Gagnon, P.; Lebel, A.; Sadiq, R.; Rodriguez, M.J. Climate change and drinking water quality: Predicting high trihalomethane occurrence in water utilities supplied by surface water. Environ. Model. Softw. 2019, 120, 104479. [Google Scholar] [CrossRef]
- Mitiku, A. Water pollution: Causes and prevention. Int. J. Pharm. Sci. Rev. Res. 2020, 60, 94–101. [Google Scholar]
- Cui, H.; Huang, X.; Yu, Z.; Chen, P.; Cao, X. Application progress of enhanced coagulation in water treatment. RSC Adv. 2020, 10, 20231–20244. [Google Scholar] [CrossRef]
- Dayarathne, H.N.P.; Angove, M.J.; Aryal, R.; Abuel-Naga, H.; Mainali, B. Removal of natural organic matter from source water: Review on coagulants, dual coagulation, alternative coagulants, and mechanisms. J. Water Process Eng. 2021, 40, 101820. [Google Scholar] [CrossRef]
- Matilainen, A.; Vepsäläinen, M.; Sillanpää, M. Natural organic matter removal by coagulation during drinking water treatment: A review. Adv. Colloid Interface Sci. 2010, 159, 189–197. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Zhou, S.; Chiang, P.C.; Shah, K. Evaluation and optimization of enhanced coagulation process: Water and energy nexus. Water-Energy Nexus 2020, 2, 25–36. [Google Scholar] [CrossRef]
- Hussain, S.; Awad, J.; Sarkar, B.; Chow, C.W.K.; Duan, J.; Leeuwen, J. Coagulation of dissolved organic matter in surface water by novel titanium (III) chloride: Mechanistic surface chemical and spectroscopic characterisation. Sep. Purif. Technol. 2019, 213, 213–223. [Google Scholar] [CrossRef]
- Elma, M.; Pratiwi, A.E.; Rahma, A.; Rampun, E.L.A.; Mahmud, M.; Abdi, C.; Rosadi, R.; Yanto, D.H.Y.; Bilad, M.R. Combination of coagulation, adsorption, and ultrafiltration processes for organic matter removal from peat water. Sustainability 2022, 14, 370. [Google Scholar] [CrossRef]
- Ratnaweera, H. Meeting tomorrow’s challenges in particle separation with coagulation. Multidiscip. Adv. Effic. Sep. Process. 2020, 7, 207–223. [Google Scholar] [CrossRef]
- Van Dyke, N.; Yenugadhati, N.; Birkett, N.; Lindsay, J.; Turner, M.; Willhite, C.; Krewski, D. Association between aluminum in drinking water and incident Alzheimer’s disease in the Canadian Study of Health and Aging cohort. Neuro Toxicol. 2021, 83, 157–165. [Google Scholar] [CrossRef]
- Krupińska, I. Aluminium drinking water treatment residuals and their toxic impact on human health. Molecules 2020, 25, 641. [Google Scholar] [CrossRef] [PubMed]
- Bondy, S.; Campbell, A. Aluminum and Neurodegenerative Disease. Handb. Neurotox. 2021, 1, 131–156. [Google Scholar] [CrossRef]
- Exley, C. Aluminum Should Now Be Considered a Primary Etiological Factor in Alzheimer’s Disease. J. Alzheimer’s Dis. Rep. 2017, 1, 23–25. [Google Scholar] [CrossRef] [PubMed]
- Muisa-Zikali, N.; Hoko, Z.; Chifamba, P. Impacts of alum residues from Morton Jaffray Water Works on water quality and fish, Harare, Zimbabwe. Phys. Chem. Earth 2011, 36, 853–864. [Google Scholar] [CrossRef]
- Saleem, M.; Bachmann, R. A contemporary review on plant-based coagulants for applications in water treatment. J. Ind. Eng. Chem. 2018, 72, 281–297. [Google Scholar] [CrossRef]
- Karnaningroem, N.; Anggraeni, D.R. Study of Life Cycle Assessment (LCA) on Water Treatment. IOP Conf. Ser. Earth Environ. Sci. 2021, 799, 012036. [Google Scholar] [CrossRef]
- Setareh, P.; Khezri, S.M.; Hossaini, H.; Pirsaheb, M. Coupling effect of ozone/ultrasound with coagulation for improving NOM and turbidity removal from surface water. J. Water Process Eng. 2020, 37, 101340. [Google Scholar] [CrossRef]
- Gan, Y.; Jingbiao, L.; Li, Z.; Wu, B.; Wenguang, H.; Li, H.; Zhang, S. Potential of titanium coagulants for water and wastewater treatment: Current status and future perspectives. Chem. Eng. J. 2021, 406, 126837. [Google Scholar] [CrossRef]
- Go, R.J.; Yang, H.L.; Kan, C.C.; Ong, D.; Garcia-Segura, S.; De Luna, M.D. Natural organic matter removal from raw surface water: Benchmarking performance of chemical coagulants through excitation-emission fluorescence matrix spectroscopy analysis. Water 2021, 13, 146. [Google Scholar] [CrossRef]
- Lapointe, M.; Papineau, I.; Peldszus, S.; Peleato, N.M.; Barbeau, B. Identifying the best coagulant for simultaneous water treatment objectives: Interactions of mononuclear and polynuclear aluminum species with different natural organic matter fractions. J. Water Process Eng. 2021, 40, 101829. [Google Scholar] [CrossRef]
- Yue, Y.; An, G.; Lin, L.; Demissie, H.; Yang, X.; Jiao, R.; Wang, D. Design and coagulation mechanism of a new functional composite coagulant in removing humic acid. Sep. Purif. Technol. 2022, 292, 121016. [Google Scholar] [CrossRef]
- Musteret, C.P.; Morosanu, I.; Ciobanu, R.; Plavan, O.; Gherghel, A.; Al-Refai, M.; Ioana, R.; Teodosiu, C. Assessment of coagulation-flocculation process efficiency for the natural organic matter removal in drinking water treatment. Water 2021, 13, 3073. [Google Scholar] [CrossRef]
- Zhang, Z.; Jing, R.; He, S.; Qian, J.; Zhang, K.; Ma, G.; Chang, X.; Zhang, M.; Li, Y. Coagulation of low temperature and low turbidity water: Adjusting basicity of polyaluminum chloride (PAC) and using chitosan as coagulant aid. Sep. Purif. Technol. 2018, 206, 131–139. [Google Scholar] [CrossRef]
- Pandey, P.; Khan, F. Moringa oleifera Plant as potent alternate to chemical coagulant in water purification. Braz. J. Pharm. Sci. 2023, 58, 201158e. [Google Scholar] [CrossRef]
- Yang, Z.; Li, H.; Yan, H.; Wu, H.; Yang, H.; Wu, Q.; Li, H.; Li, A.; Cheng, R. Evaluation of a novel chitosan-based flocculant with high flocculation performance, low toxicity and good floc properties. J. Hazard. Mater. 2014, 276, 480–488. [Google Scholar] [CrossRef] [PubMed]
- Khairul Zaman, N.; Rohani, R.; Yusoff, I.I.; Kamsol, M.A.; Basiron, S.A.; Rashid, A.I.A. Eco-friendly coagulant versus industrially used coagulants: Identification of their coagulation performance, mechanism and optimization in water treatment process. Int. J. Environ. Res. Public Health 2021, 18, 9164. [Google Scholar] [CrossRef] [PubMed]
- Okoro, B.U.; Sharifi, S.; Jesson, M.; Bridgeman, J.; Moruzzi, R. Characterisation and performance of three Kenaf coagulation products under different operating conditions. Water Res. 2021, 188, 116517. [Google Scholar] [CrossRef] [PubMed]
- Okuda, T.; Ali, E.N. Application of Moringa oleifera Plant in Water Treatment. In Water and Wastewater Treatment Technologies. Energy, Environment and Sustainability; Springer: Singapore, 2018. [Google Scholar] [CrossRef]
- Yamaguchi, N.U.; Cusioli, L.; Quesada, H.; Ferreira, M.; Fagundes-Klen, M.; Vieira, A.; Gomes, R.; Vieira, M.; Bergamasco, R. A review of Moringa oleifera seeds in water treatment: Trends and future challenges. Process. Saf. Environ. Prot. 2021, 147, 405–420. [Google Scholar] [CrossRef]
- Teixeira, M.R.; Camacho, F.; Sousa, V.S.; Bergamasco, R. Green technologies for cyanobacteria and natural organic matter water treatment using natural based products. J. Clean. Prod. 2017, 162, 484–490. [Google Scholar] [CrossRef]
- Camacho, F.; Sousa, V.S.; Bergamasco, R.; Teixeira, M. The use of Moringa oleifera as a natural coagulant in surface water treatment. Chem. Eng. J. 2017, 313, 226–237. [Google Scholar] [CrossRef]
- Bu, F.; Gao, B.; Shen, X.; Wang, W.; Yue, Q. The combination of coagulation and ozonation as a pre-treatment of ultrafiltration in water treatment. Chemosphere 2019, 231, 349–356. [Google Scholar] [CrossRef]
- Bu, F.; Gao, B.; Yue, Q.; Liu, C.; Wang, W.; Shen, X. The combination of coagulation and adsorption for controlling ultra-filtration membrane fouling in water treatment. Water 2019, 11, 90. [Google Scholar] [CrossRef]
- Alhweij, H.; Emanuelsson, E.; Shahid, S.; Wenk, J. Organic matter removal and antifouling performance of sulfonated polyaniline nanofiltration (S-PANI NF) membranes. J. Environ. Chem. Eng. 2022, 10, 107906. [Google Scholar] [CrossRef]
- Krzeminski, P.; Vogelsang, C.; Meyn, T.; Köhler, S.J.; Poutanen, H.; de Wit, H.A.; Uhl, W. Natural organic matter fractions and their removal in full-scale drinking water treatment under cold climate conditions in Nordic capitals. J. Environ. Manag. 2019, 241, 427–438. [Google Scholar] [CrossRef]
- Deng, L.; Ngo, H.; Guo, W.; Zhang, H.H. Pre-coagulation coupled with sponge-membrane filtration for organic matter removal and membrane fouling control during drinking water treatment. Water Res. 2019, 157, 155–166. [Google Scholar] [CrossRef] [PubMed]
- Wang, N.; Li, X.; Yang, Y.; Zhou, Z.; Shang, Y.; Zhuang, X. Photocatalysis-coagulation to control ultrafiltration membrane fouling caused by natural organic matter. J. Clean. Prod. 2020, 265, 121790. [Google Scholar] [CrossRef]
- Finkbeiner, P.; Redman, J.; Patriarca, V.; Moore, G.; Jefferson, B.; Jarvis, P. Understanding the potential for selective natural organic matter removal by ion exchange. Water Res. 2018, 146, 256–263. [Google Scholar] [CrossRef]
- Finkbeiner, P.; Moore, G.; Pereira, R.; Jefferson, B.; Jarvis, P. The combined influence of hydrophobicity, charge and molecular weight on natural organic matter removal by ion exchange and coagulation. Chemosphere 2020, 238, 124633. [Google Scholar] [CrossRef]
- Kurniawan, S.B.; Abdullah, S.R.S.; Imron, M.F.; Said, N.S.M.; Ismail, N.I.; Hasan, H.A.; Othman, A.R.; Purwanti, I.F. Challenges and Opportunities of Biocoagulant/Bioflocculant Application for Drinking Water and Wastewater Treatment and Its Potential for Sludge Recovery. Int. J. Environ. Res. Public Health 2020, 17, 9312. [Google Scholar] [CrossRef]
- Sukmana, H.; Bellahsen, N.; Pantoja, F.; Hodur, C. Adsorption and coagulation in wastewater treatment–Review. Prog. Agric. Eng. Sci. 2021, 17, 49–68. [Google Scholar] [CrossRef]
- Sillanpää, M. Natural Organic Matter in Water. In Characterization and Treatment Methods; IWA Publishing: London, UK, 2014. [Google Scholar]


| Raw Water Characteristics | Treatment Method | Operating Conditions and Materials | Coagulant Type and Dose | Efficiency | Ref. |
|---|---|---|---|---|---|
| Peat Water, Indonesia pH = 6.3 DOC = 36.4 [mg/L] UV254 = 0.976 [cm−1] KMnO4 Organic Substances = 120.0 [mg KMnO4/L] | Coagulation–Adsorption–Ultrafiltration | Coagulation: pH = 6.0 rapid mixing: 1 min, 100 rpm slow mixing: 20 min, 40 rpm Adsorption: PAC (particle size of 100 mesh; surface area of 800 m2/g, dose of 120 mg/L) mixed at 180 rpm for 3 h UF: the polysulfone membrane, pore size < 0.1 µm, pressure 3 bar | Aluminum sulfate: 175 [mg/L] | After coagulation: pH = 3.65 Removal of KMnO4 organic substances = 78% Reduction in UV254 = 75% After adsorption: Removal of KMnO4 organics = 96% Reduction in UV254 = 92% After UF: Reduction in UV254 = 95% | [31] |
| Synthetic Water pH = 8.3 UV254 = 0.231 [cm−1] DOC = 3.95 [mg/L] | Coagulation–Adsorption | Coagulation: rapid mixing: 1.5 min at 200 rpm slow mixing: 15 min at 40 rpm sedimentation: 30 min Adsorption: PAC (dosage of 50 mg/L) | Inorganic–organic hybrid coagulant PACl-PDMDAAC: 5 [mg/L] | Removal efficiency of UV254 = 86% Removal efficiency of DOC = 46% | [55] |
| Coagulation + Adsorption | Coagulation + Adsorption: PAC (dosage of 50 mg/L to the rapid mixing tank) rapid mixing: 1.5 min at 200 rpm slow mixing: 15 min at 40 rpm sedimentation: 30 min | Removal efficiency of UV254 = 84% Removal efficiency of DOC = 43% | |||
| Synthetic Water pH = 7.25 UV254 = 0.087 [cm−1] DOC = 5.29 [mg/L] Turbidity = 3.32 [NTU] | Membrane–Filtration (CMF) | Ultrafiltration: Polyvinylidene fluoride (PVDF) membrane (pore size of 0.07 mm, effective surface area of 0.2 m2), permeate flux of 10 L/m2h | - | Removal efficiency of UV254 = 14.22% Removal efficiency of DOC = 17.52% Removal efficiency of turbidity = 97.83% | [59] |
| Coagulation–Membrane Filtration (P-MF) | Polyaluminum chloride PACl: 10 mg/L | Removal efficiency of UV254 = 52.16% Removal efficiency of DOC = 47.83% Removal efficiency of turbidity = 98.45% | |||
| Coagulation–Membrane Filtration with sponge biomass carriers (P-SMF) | Polyester-polyurethane porous sponge cubes (10 mm × 10 mm × 10 mm, density of 28–45 kg/m3, cell count of 90 cells/in) previously acclimatized for 15 days for biomass enrichment were added after coagulation, prior to UF. Ultrafiltration: Polyvinylidene fluoride (PVDF) membrane (pore size of 0.07 mm, effective surface area of 0.2 m2), permeate flux of 10 L/m2h | Removal efficiency of UV254 = 74.71% Removal efficiency of DOC = 68.30% The removal efficiency of turbidity = 98.76 | |||
| Ravash Dam Water, Iran pH = 7.85–7.91 UV254 = 0.019–0.051 [cm−1] TOC = 3.19–6.0 [mg/L] Turbidity = 1.08–4.5 [NTU] | Ultrasound/Ozonation | Ultrasound/Ozonation: US frequency 80 kHz, power intensity 200 W/cm2, O3 dosage of 3 mg/L, reaction time 8 min | - | Removal efficiency of UV254 = 84% Removal efficiency of turbidity = 33% | [40] |
| Coagulation | Coagulant aid: anionic polyelectrolyte BASF LT25, dose: 0.1 mg/L | Polyaluminum chloride PACl: 0.81 mg/L | Removal efficiency of UV254 = 65% Removal efficiency of turbidity = 15% | ||
| Ultrasound/Ozonation–Coagulation | Ultrasound/Ozonation: US frequency 80 kHz, power intensity 200 W/cm2, O3 dosage of 3 mg/L, reaction time 8 min Coagulant aid: anionic polyelectrolyte BASF LT25, dose: 0.1 mg/L | Removal efficiency of UV254 = 95% Removal efficiency of turbidity = 50% | |||
| Tonghui River Water, China UV254 = 0.085 [cm−1] DOC = 2.24 [mg/L] | Coagulation | rapid mixing: 1 min at 120 rpm slow mixing: 20 min at 50 rpm sedimentation: 20 min pH = 5 | 0.2 mmol | Removal efficiency of UV254 = 46.89% Removal efficiency of DOC = 17.68% | [60] |
| Photocatalysis | The dose of the composite nano-photocatalyst Bi-Ti/PAC: 2.0 g/L, 300 W xenon light, visible light irradiation 350 u/m2, reaction time: 20 min | - | Removal efficiency of UV254 = 75.46% Removal efficiency of DOC = 48.71% | ||
| Photocatalysis–coagulation | Photocatalysis: the dose of the composite nano-photocatalyst Bi-Ti/PAC: 2.0 g/L, 300 W xenon light, visible light irradiation 350 u/m2, reaction time: 20 min Coagulation: rapid mixing: 1 min at 120 rpm slow mixing: 20 min at 50 rpm sedimentation: 20 min pH = 7 | 0.04 mmol | Removal efficiency of UV254 = 81.2% Removal efficiency of DOC = 57.78% | ||
| Synthetic Water UV254 = 0.0231 [cm−1] DOC = 3.975 [mg/L] Turbidity = 15.0 [NTU] | Coagulation–Ozonation | Coagulation: rapid mixing: 1.5 min at 200 rpm slow mixing: 40 min at 15 rpm sedimentation: 30 min Ozonation: The ozone dosage of 2 mg/L | Inorganic–organic hybrid coagulant PACl-PDMDAAC: 7 [mg/L] | Removal efficiency of UV254~85% Removal efficiency of DOC = 38–42% | [55] |
| Ozonation–Coagulation | |||||
| Coagulation–Ozonation–Ultrafiltration | Coagulation: rapid mixing: 1.5 min at 200 rpm slow mixing: 40 min at 15 rpm sedimentation: 30 min Ozonation: The ozone dosage of 2 mg/L Ultrafiltration: Polyvinylidene fluoride membrane with a pore size of 0.03 μm | Removal efficiency of UV254~91% Removal efficiency of DOC = 45–48% | |||
| Ozonation–Coagulation–Ultrafiltration |
| NOM Removal Method | Advantages | Disadvantages | Current Challenges | Future Challenges | Ref. |
|---|---|---|---|---|---|
| Coagulation with metallic salts | Cost-effective High availability Easy handling and storage | Alkalinity reduction Increased corrosivity of water. Residual metal concentration and detrimental human health effect of residual aluminum. Production of high-volume, non-biodegradable sludge. | Mitigating the corrosive properties of post-coagulation water. Difficulty in disposing of post-coagulation sludge. Ineffectiveness of removing low molecular weight NOM. | The effectiveness of NOM removal is expected to decrease with an increase in the proportion of low molecular weight NOM due to an increase in solar radiation. Higher concentrations of LMW fractions of NOM will likely contribute to higher concentrations of DBPs such as THMs. | [4,5,7,11,25,34,36] |
| Coagulation with Ti and Zr-based coagulants | Production of lower amounts of sludge than in the case of metallic salts. Possibility of recycling post-coagulation sludge into an income-generating product. Effective in treating low-alkalinity and high-DOC waters. Effective in removing both LMW and HWM fractions of NOM. | Ti-based coagulants require acidic conditions for effective NOM removal. Very high cost. | Improvement of the economic aspect of coagulation with Ti and Zr-based coagulants. | Further research and analysis are needed to determine the impact of the process on the chemical stability of w, and to determine the exact NOM fractions that can be effectively removed via coagulation with Ti and Zr-based coagulants. | [14,27,30,41] |
| Coagulation with inorganic polymers | Significantly lower concentration of residual metal than metallic salts. Mitigating the harmful effects of residual aluminum due to its lower concentration. Production of lower amounts of sludge than in the case of metallic salts. Better performance in NOM removal than that of conventional coagulants. Little to no alkalinity reduction. | Although the residual metal concentration and the production of post-coagulation chemical sludge are reduced in comparison to the conventional salts, they remain the consequences of the coagulation. | Proper handling of the post-coagulation sludge. | Further research and analysis are needed to determine the exact molecular weight range of NOM that can be effectively removed via coagulation with inorganic polymers, and how the effectiveness of the process may be affected in the future due to the expected NOM composition changes. | [4,5,6,14,34,42,43] |
| Coagulation with biocoagulants | Renewability and biodegradability. A pragmatic option for communities in regions abundant in plants with coagulation-active components. No residual metal concentration. No alkalinity reduction. Lower optimum doses than traditional coagulants. Lower volumes of post-coagulation sludge and lower costs of its disposal than in the case of metallic-based coagulants. | Inconsistent performance and higher variability in effectiveness than chemical coagulants due to the variations in the composition and properties of biocoagulants. Additional steps (e.g., extraction, purification, modification) are often required. Some biocoagulants (e.g., Moringa oleifera seeds) may contribute to an increase in DOC concentration, likely caused by the release of soluble organic alongside the coagulation-active components from the plant seeds. Accessibility may be limited in certain regions or during different seasons. Microorganism-derived biocoagulants may cause microbial contamination. | Averting the issue of soluble organic release by improving the purification methods to obtain pure coagulants. | Further research and analysis are needed to determine the exact molecular weight range of NOM that can be effectively removed via coagulation with biocoagulants, and how their treatability via this method might be influenced by climate change in the future. The effectiveness of removing DBP precursors should be further investigated. | [4,5,12,25,47,50,51,52,54,63] |
| Coagulation with hybrid coagulants | Lower alkalinity reduction. Higher DOC removal at lower doses than in the case of conventional coagulants. Relatively cost-effective. Mitigation of typical drawbacks of coagulation with traditional coagulants. | Additional costs associated with the use of multiple coagulants. Production of post-coagulation sludge. | Logistic challenges associated with the use of multiple coagulants, preparation of hybrid coagulants, and handling the post-coagulation sludge. | Further research and analysis are needed to determine the exact molecular weight range of NOM that can be effectively removed by coagulation with different hybrid coagulants, and how their treatability via this method might be influenced by climate change in the future. The effects of coagulation using hybrid coagulants on the chemical stability of water should also be further explored. The effectiveness of removing individual DBP precursors should be further explored. | [8,44] |
| Coagulation–Adsorption–Ultrafiltration | High NOM-removal efficiency. Removal of the LMW fractions of NOM that cannot be removed by coagulation alone. Better DBP precursor removal. | Adsorbent and coagulant costs. Adsorbent availability may vary depending on the targeted contaminants. Maintenance of the UF membranes. Consequences of coagulation remain the same, although they may be lessened due to the use of coagulant doses lower than in conventional treatment. | Requires regeneration and replacement of adsorbent. Mitigation of membrane fouling. Disposing of post-coagulation sludge. | The potential impact of the climate change implications on NOM in surface waters and its treatability via this method should be further investigated. | [9,31,56,64,65] |
| Coagulation integrated with biological processes | Biological processes may increase overall treatment time. Biological processes are sensitive to conditions. Production of post-coagulation sludge. Alkalinity reduction. | Disposing of post-coagulation sludge. | [18,58,59] | ||
| Coagulation integrated with ion exchange processes | Costs associated with the use of ion exchange resins. Resin fouling. Requires regeneration of the ion exchange resin. Production of post-coagulation sludge. Alkalinity reduction. | [61,62,65] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Knap-Bałdyga, A.; Żubrowska-Sudoł, M. Natural Organic Matter Removal in Surface Water Treatment via Coagulation—Current Issues, Potential Solutions, and New Findings. Sustainability 2023, 15, 13853. https://doi.org/10.3390/su151813853
Knap-Bałdyga A, Żubrowska-Sudoł M. Natural Organic Matter Removal in Surface Water Treatment via Coagulation—Current Issues, Potential Solutions, and New Findings. Sustainability. 2023; 15(18):13853. https://doi.org/10.3390/su151813853
Chicago/Turabian StyleKnap-Bałdyga, Alicja, and Monika Żubrowska-Sudoł. 2023. "Natural Organic Matter Removal in Surface Water Treatment via Coagulation—Current Issues, Potential Solutions, and New Findings" Sustainability 15, no. 18: 13853. https://doi.org/10.3390/su151813853
APA StyleKnap-Bałdyga, A., & Żubrowska-Sudoł, M. (2023). Natural Organic Matter Removal in Surface Water Treatment via Coagulation—Current Issues, Potential Solutions, and New Findings. Sustainability, 15(18), 13853. https://doi.org/10.3390/su151813853







