The Influence of N-Butanol Addition in Gasoline on the Combustion in the Spark Ignition Engine
Abstract
:1. Introduction
1.1. Studies
1.2. Aim of Research
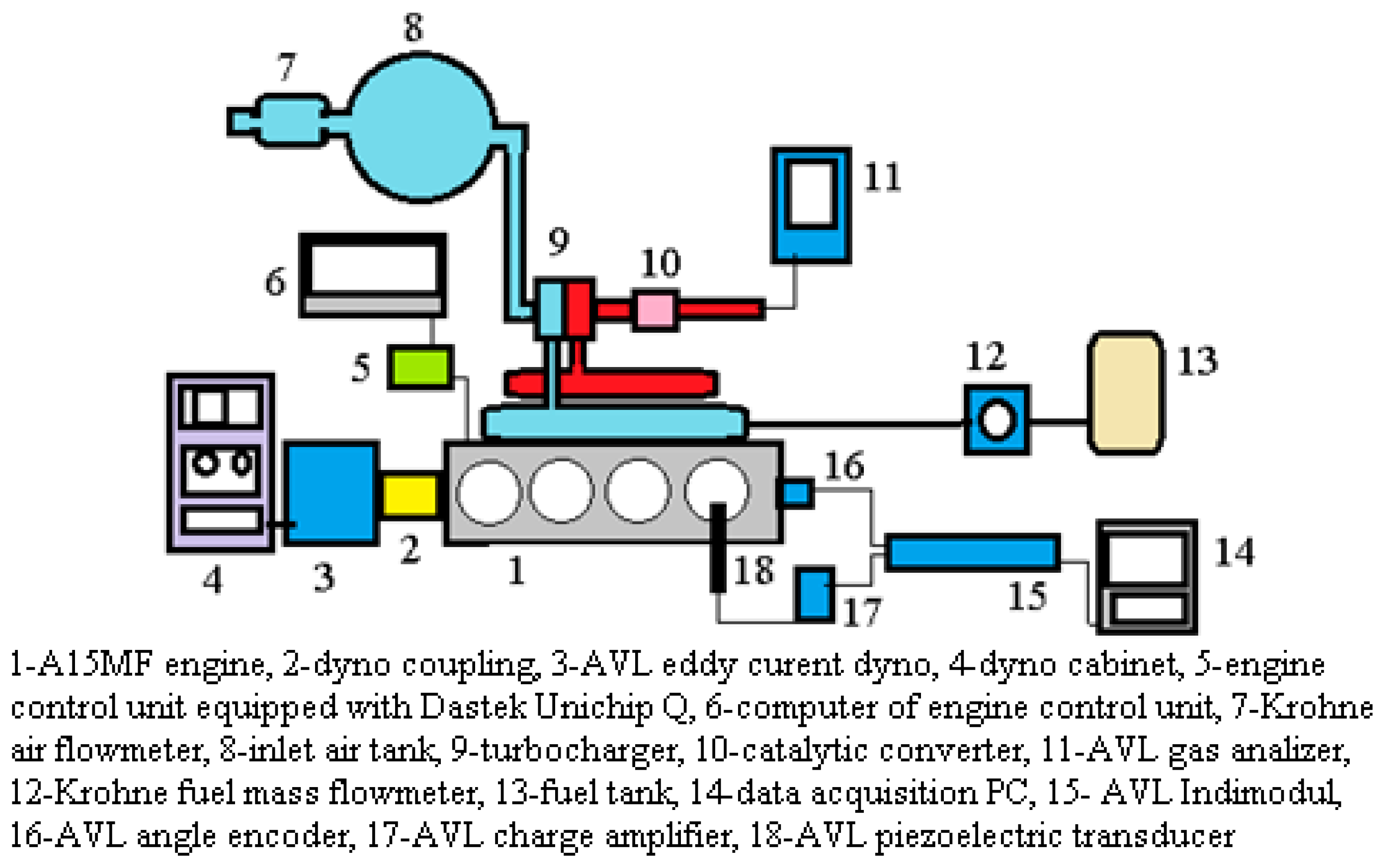
2. Experimental and Theoretical Investigation Design
3. Results and Discussion
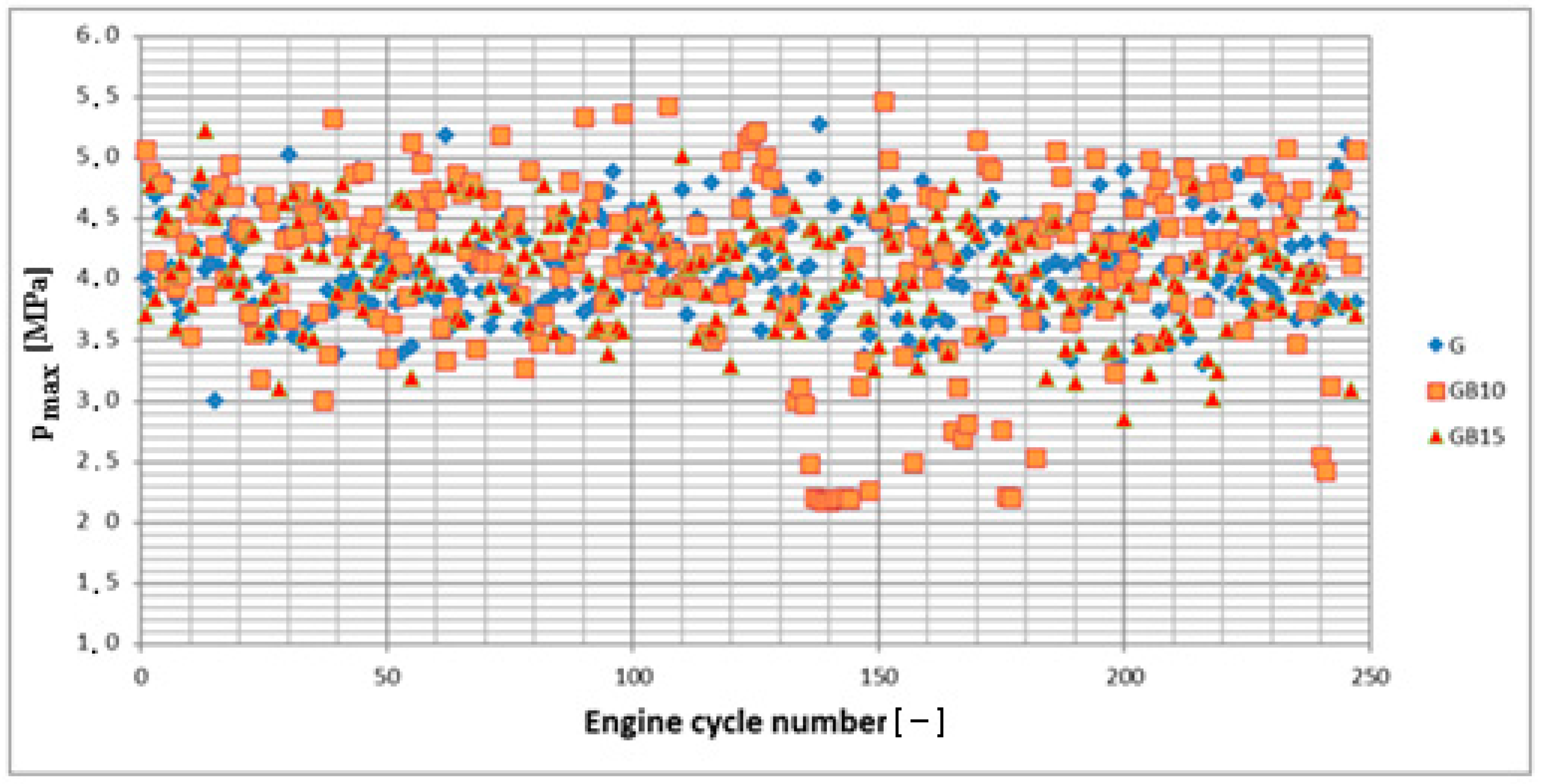
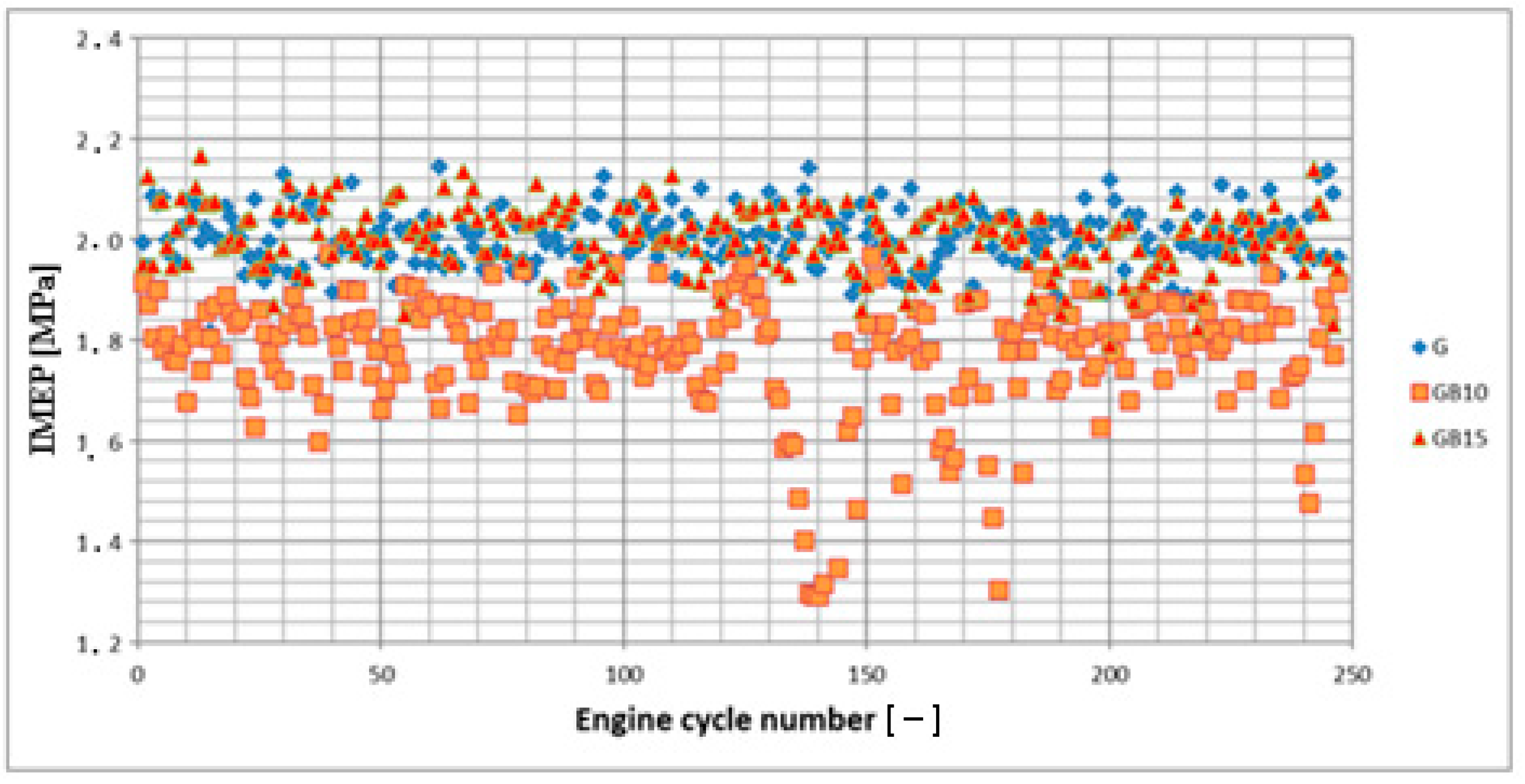
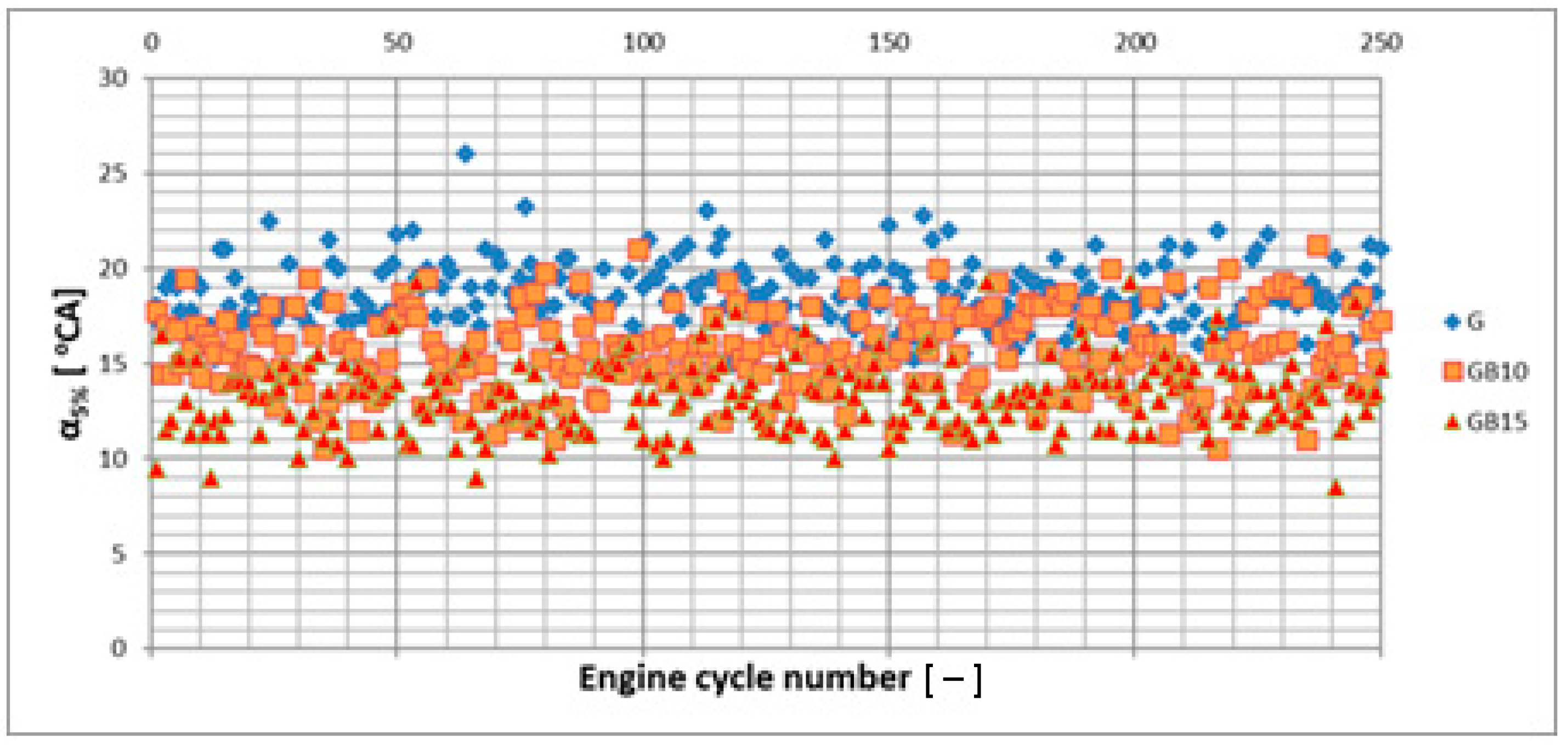
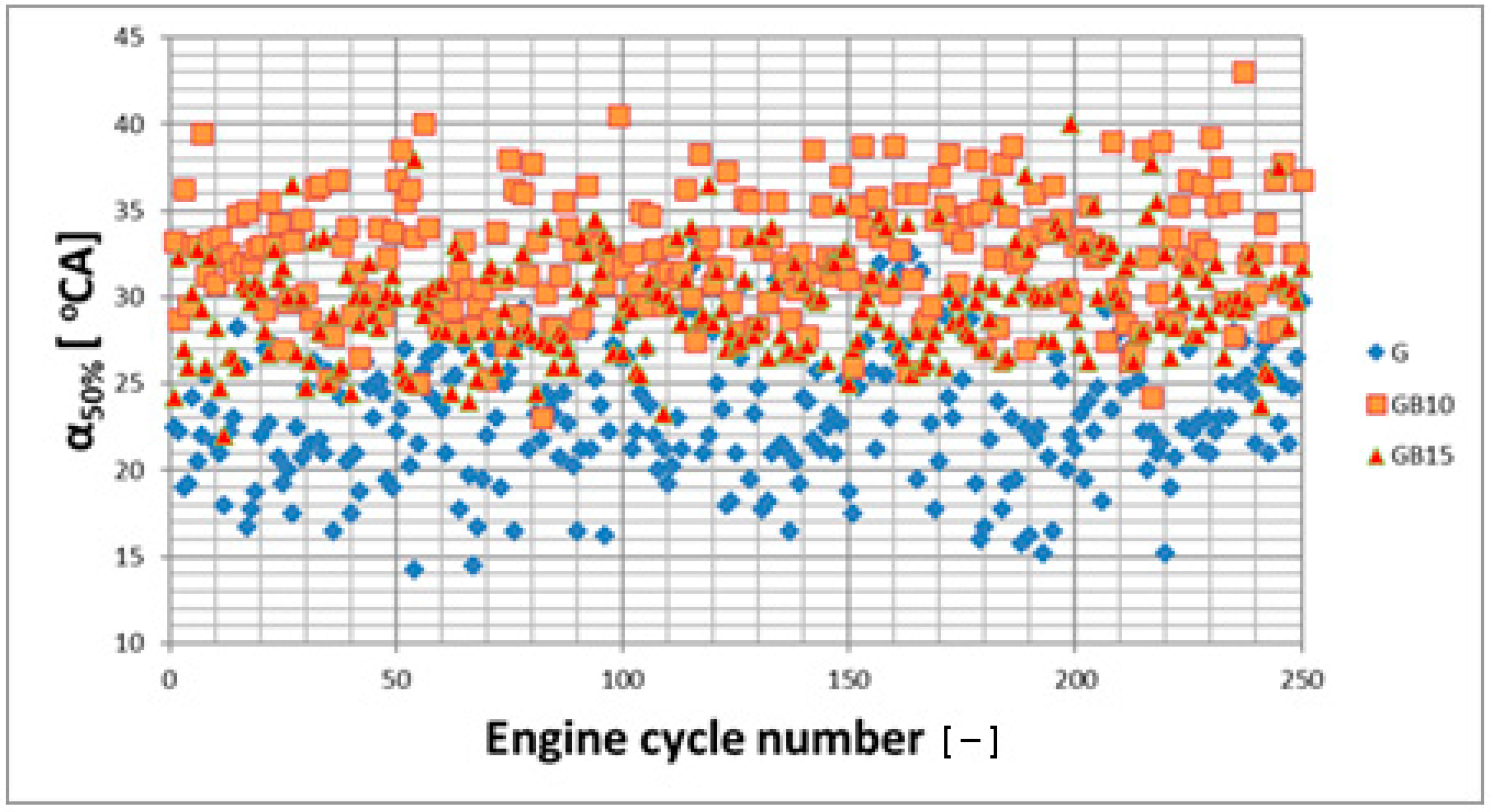
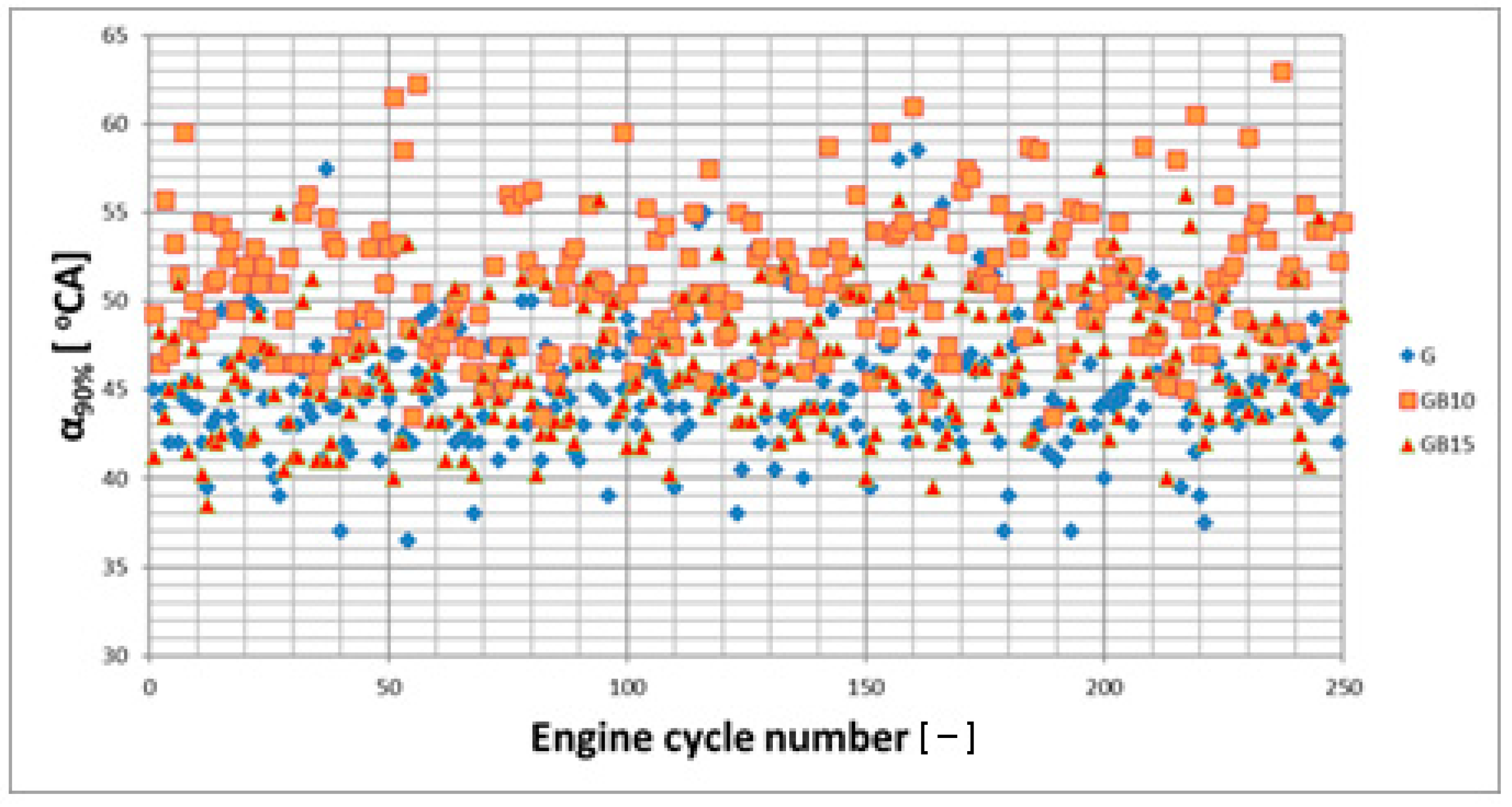
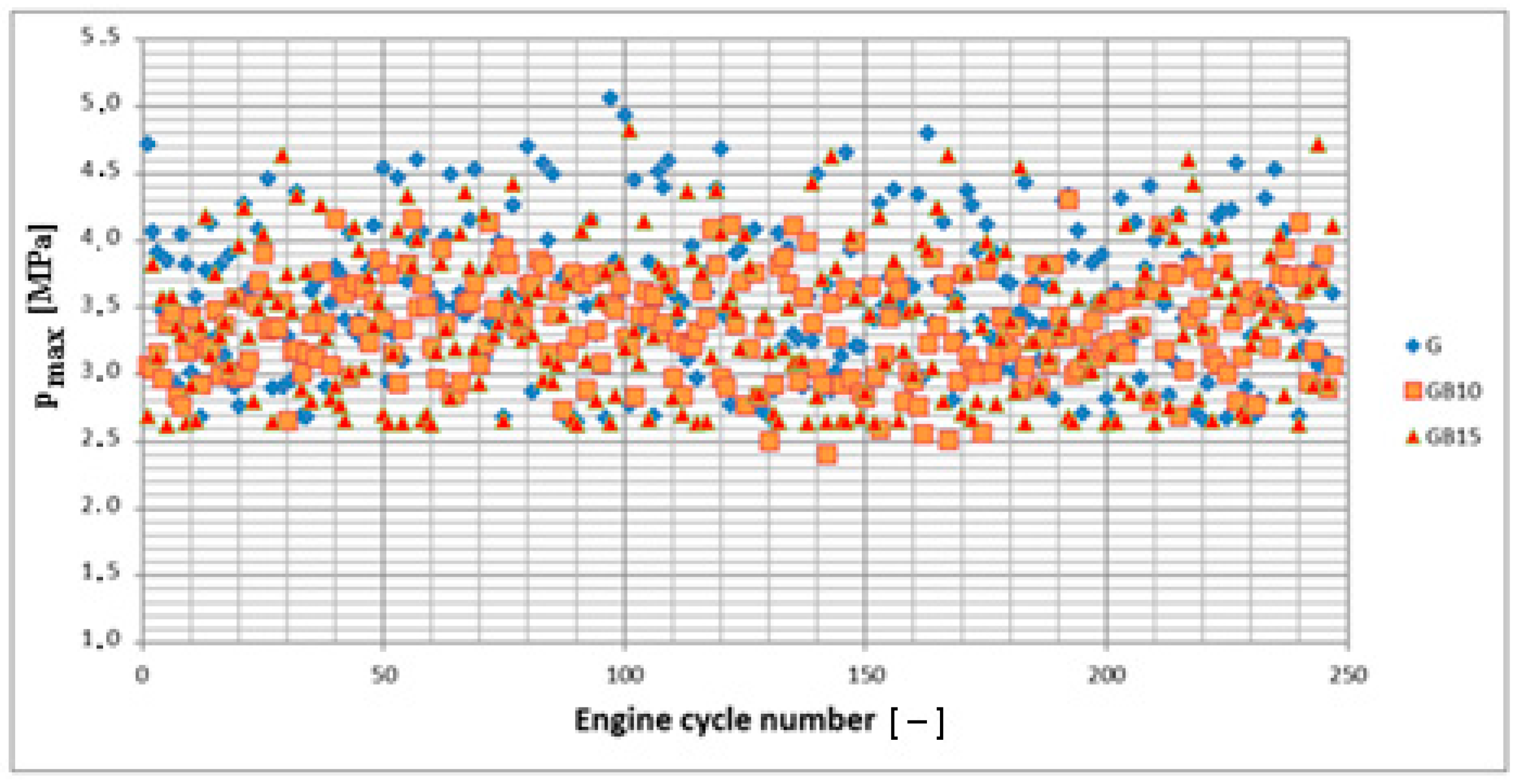
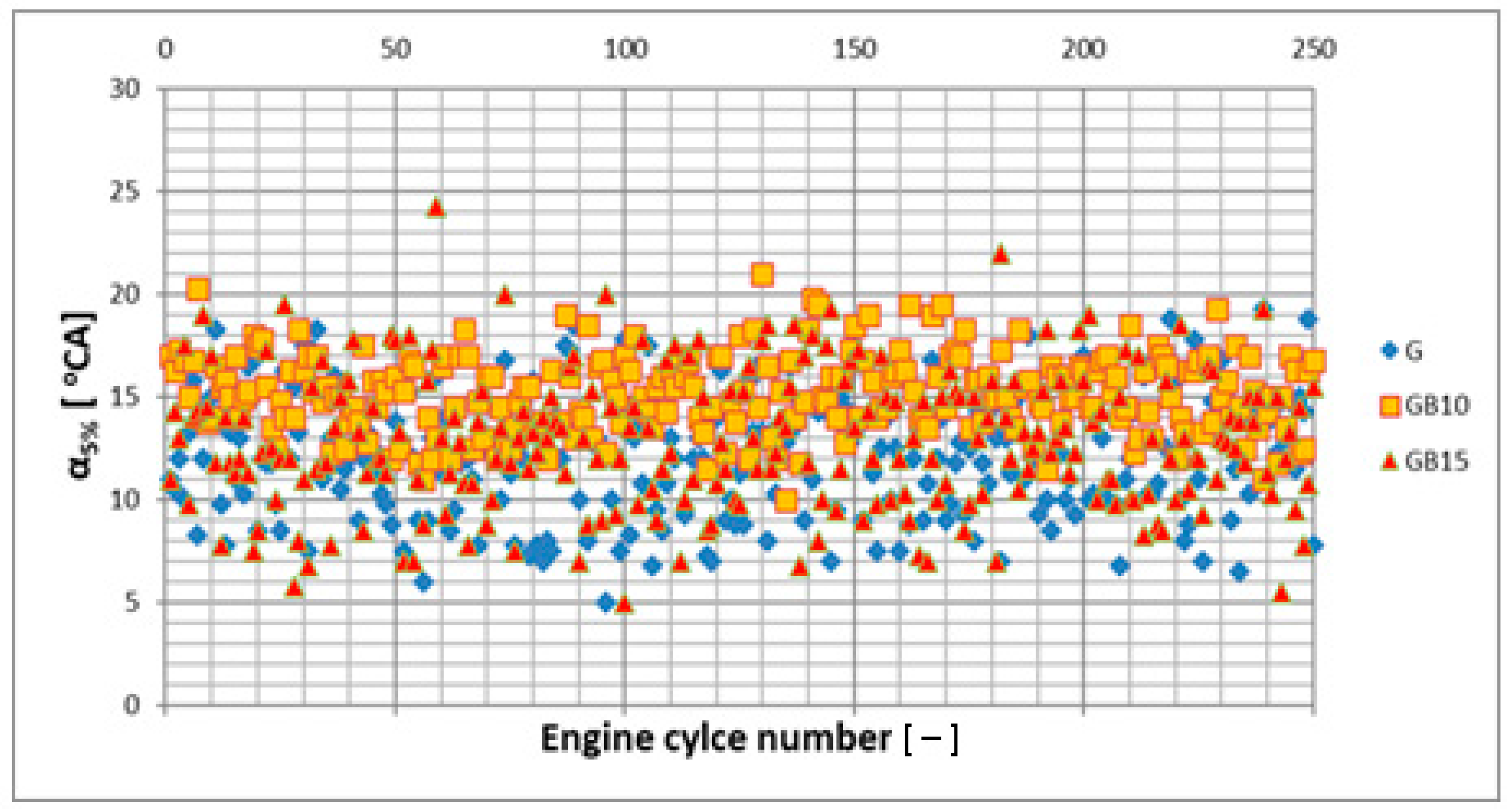
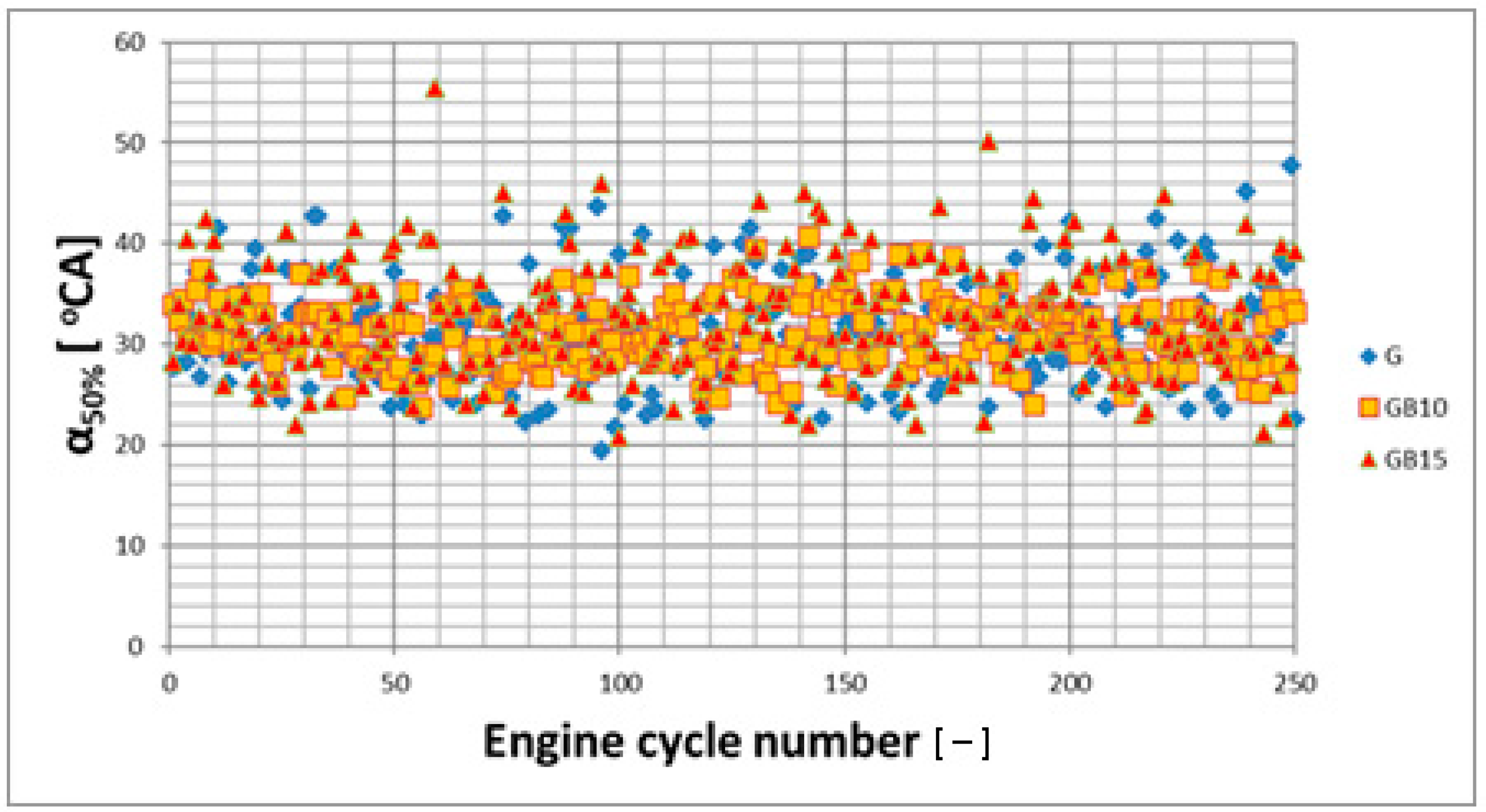
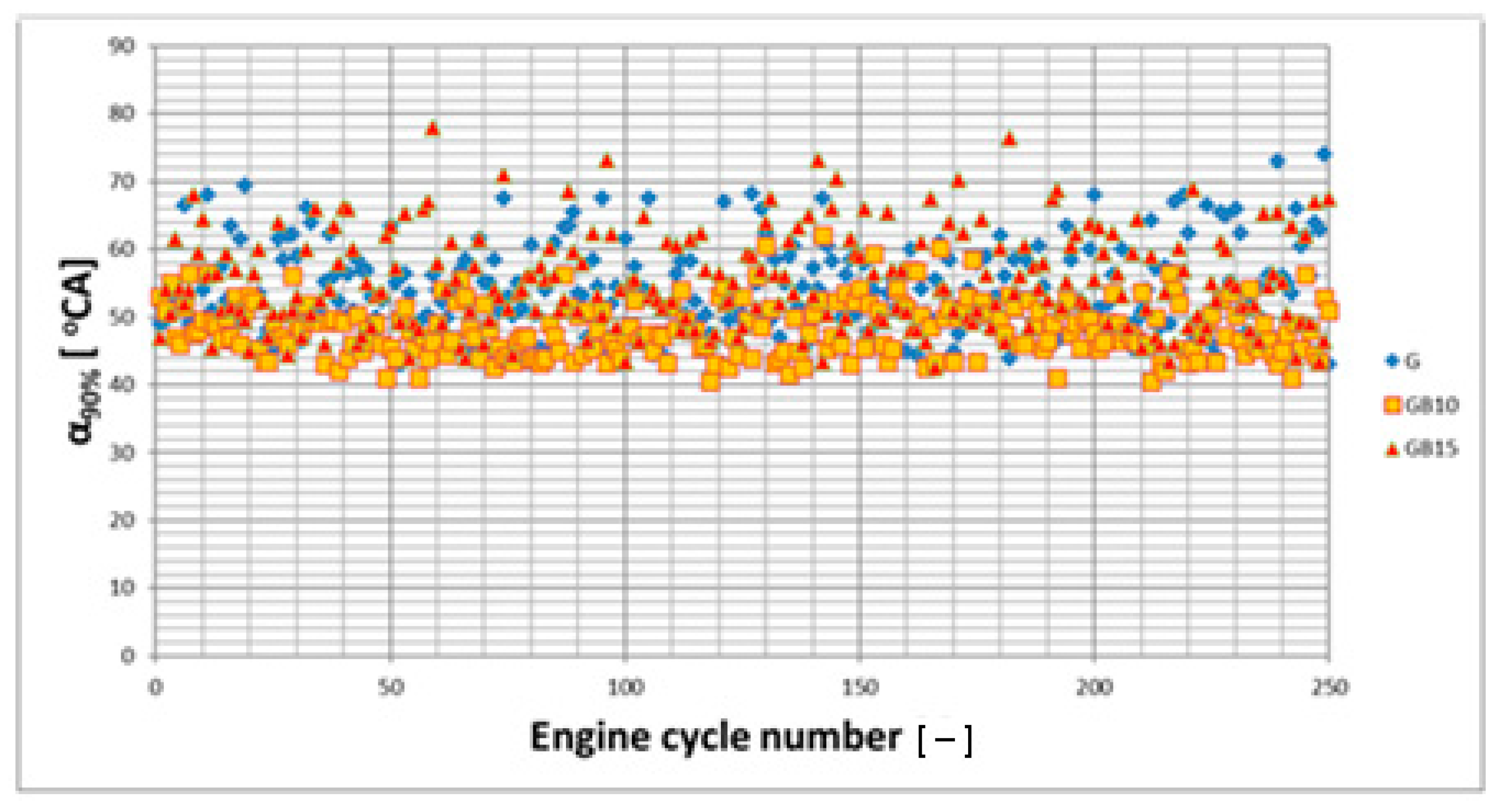
3.1. Operation Stability
3.1.1. Operation Stability for Rich Mixtures (Coefficient of Air Excess λ = 0.9)
- —heat release rate;
- —mechanical work variation;
- —pressure variation;
- k—adiabatic exponent;
- V—volume;
- p—pressure;
- α—crankshaft angular position.
3.1.2. Operation Stability for Lean Mixtures (λ = 1.3)
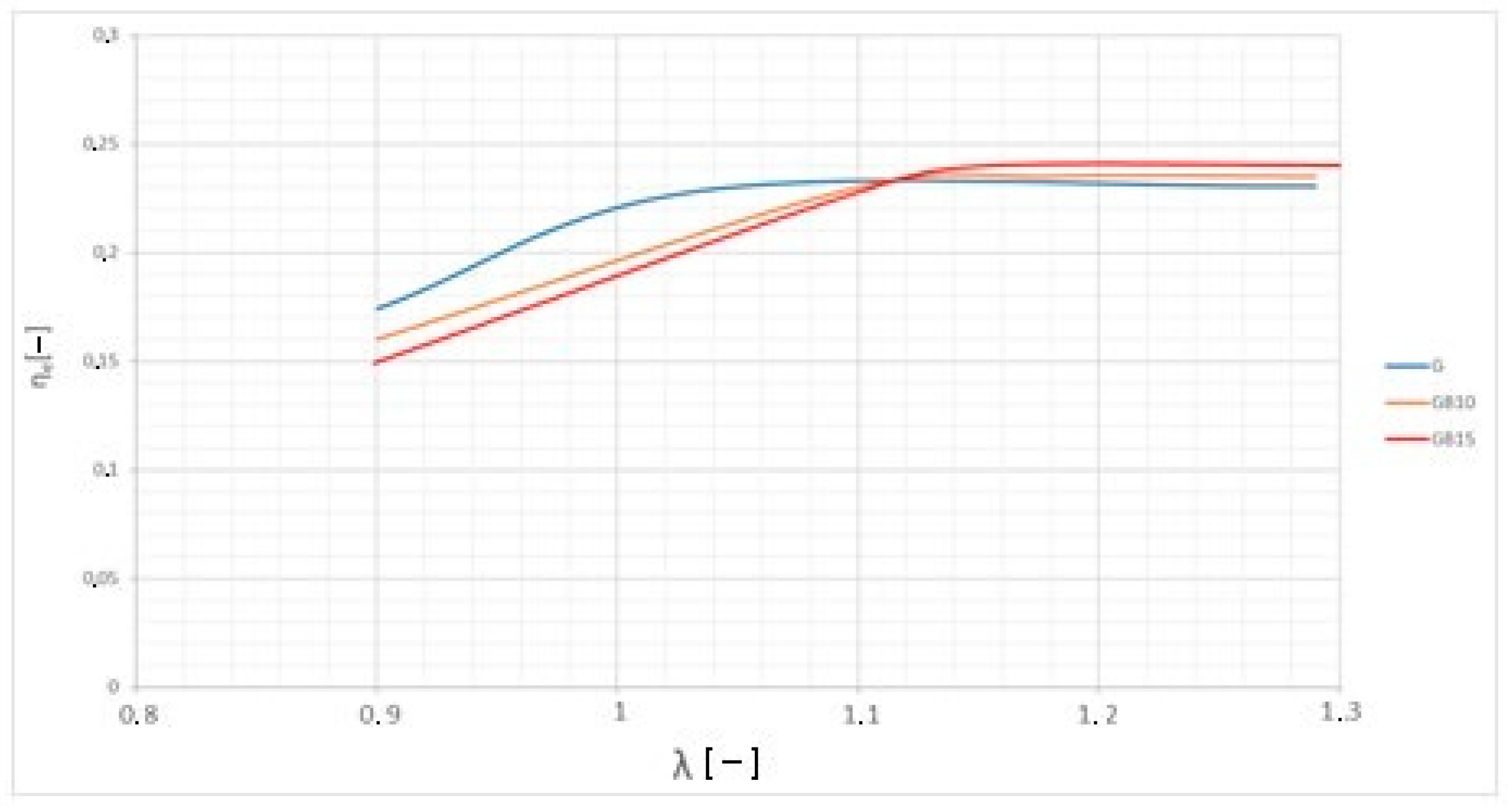
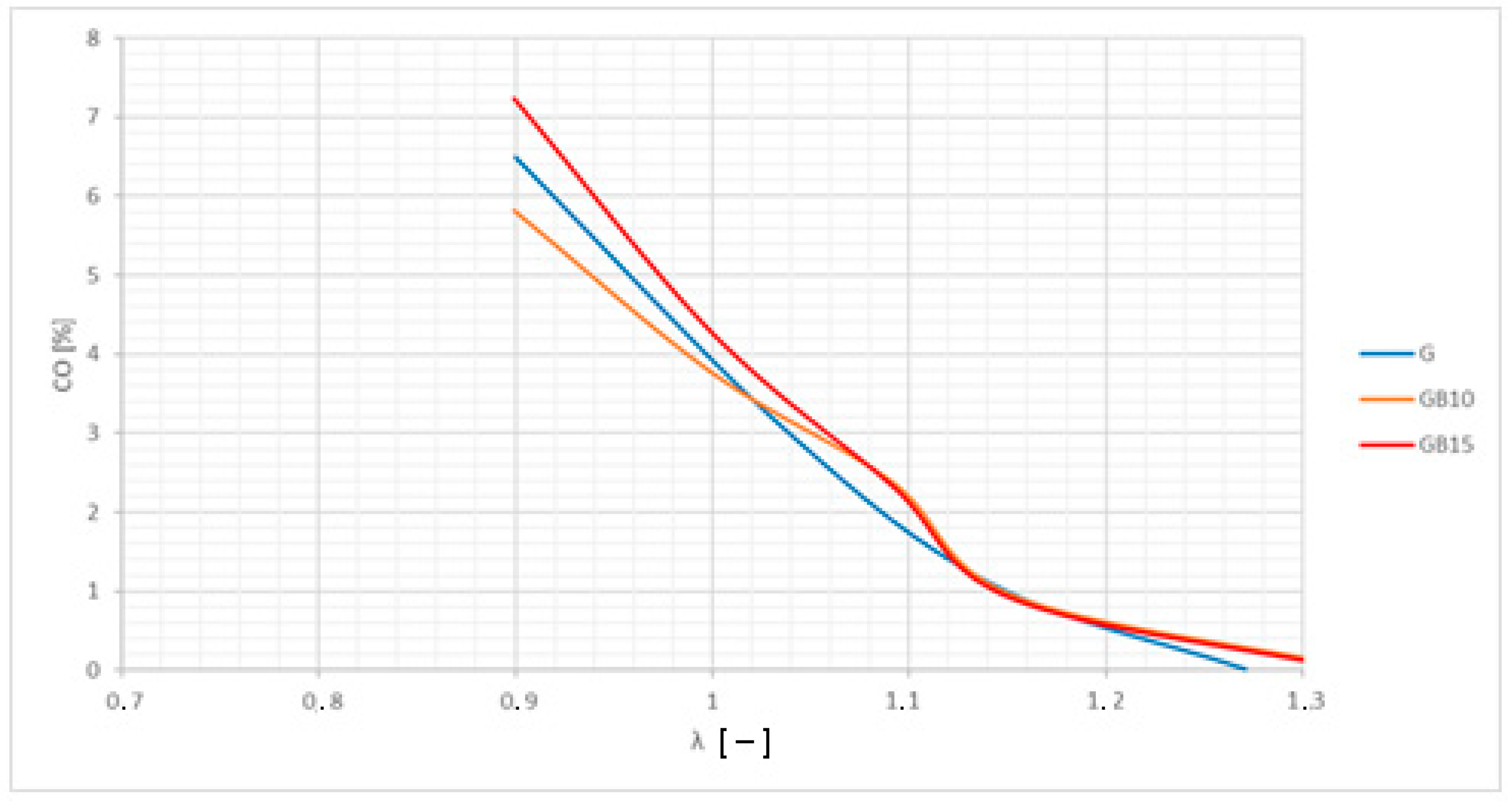
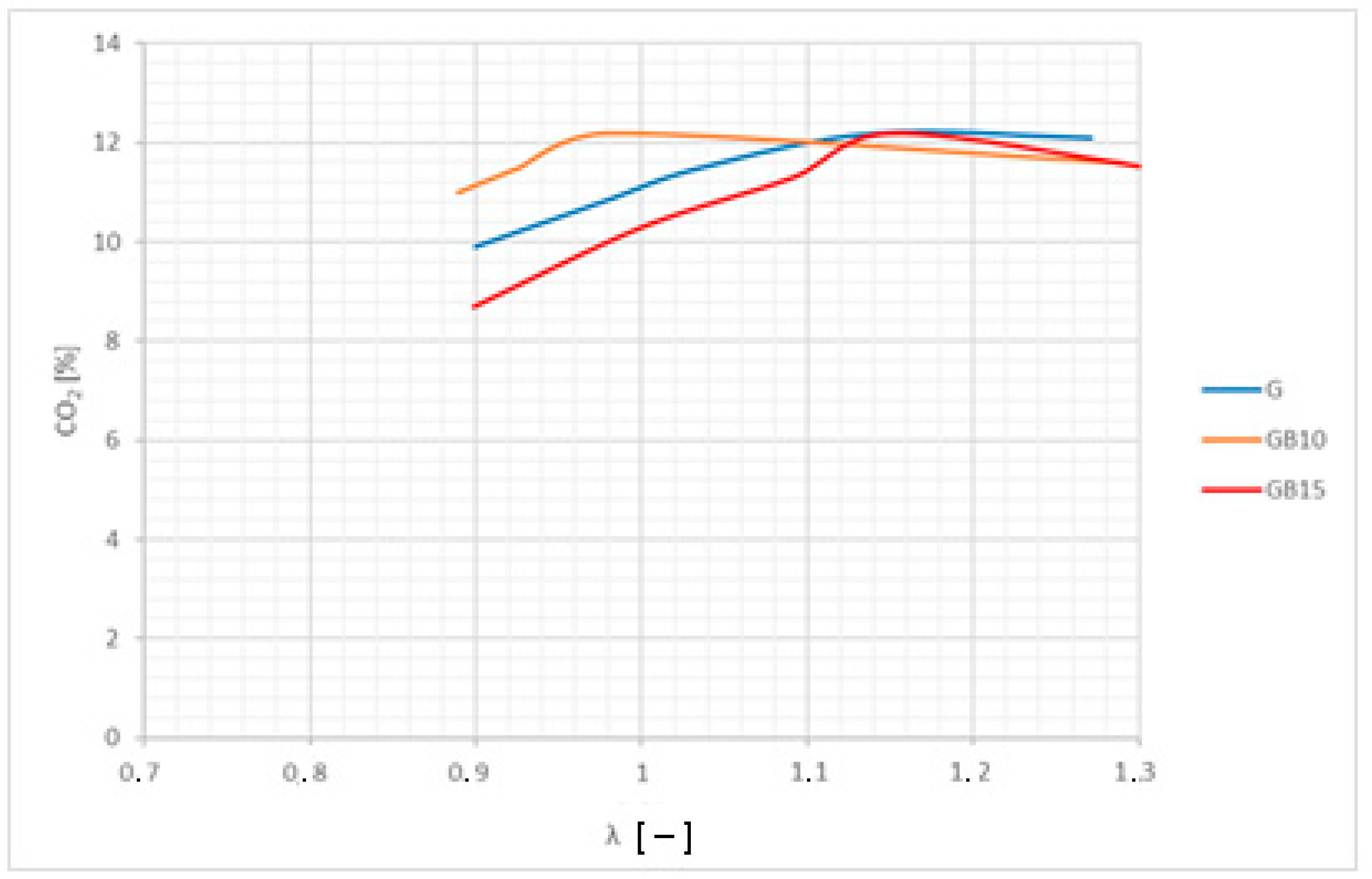
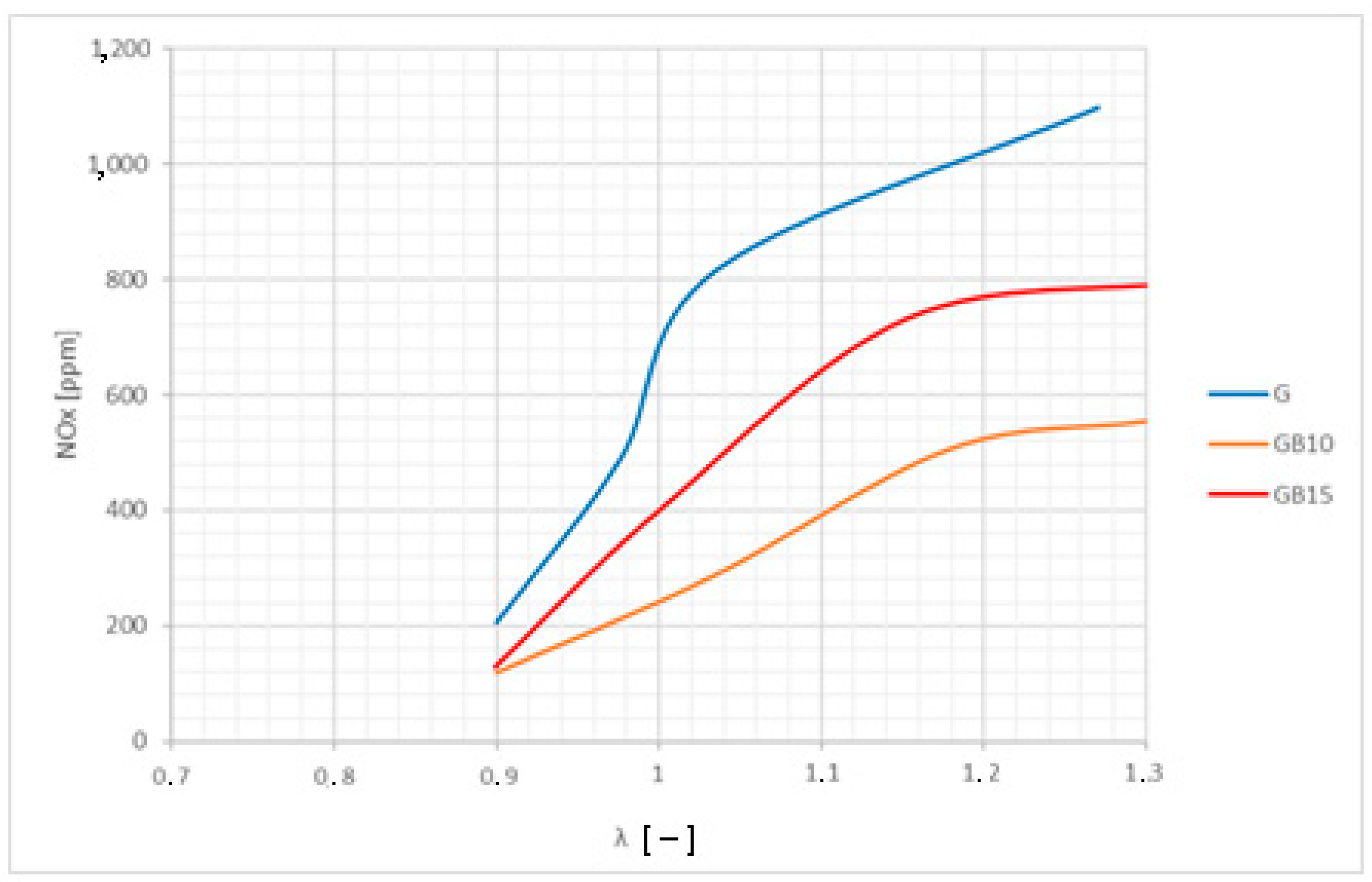
3.2. Brake-Specific Energy Consumption and Emissions
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AVL | Anstalt für Verbrennungskraftmaschinen List GmbH- automotive research institute which fabricates the test bed equipment |
| BSEC | brake-specific energetic consumption |
| °C | Celsius degree |
| °CA | crank angle degree |
| CO | carbon monoxide |
| CO2 | carbon dioxide |
| COV | coefficient of variation |
| NOx | nitrogen oxides |
| G | gasoline |
| GB | gasoline–butanol blend |
| ECU | electronic control unit |
| EU | European Union |
| GHG | greenhouse gas |
| HC | unburned hydrocarbon |
| HCCI | homogeneous charge compression ignition |
| ICE | internal combustion engine |
| LHV | lower heating value |
| MFB | mass fraction burned |
| rpm | revolutions per minute |
| TDC | top dead center |
| α | crankshaft angular position |
| Δ5-90 | combustion duration |
| λ | coefficient of air excess |
References
- Wang, X.; Sun, H.; Wang, Y.; Wang, F.; Zhu, W.; Wu, C.; Wang, Q.; Gao, M. Feasibility of Efficient, Direct, Butanol Production from Food Waste without Nutrient Supplement by Clostridium saccharoperbutylacetonicum N1-4. Sustainability 2023, 15, 6061. [Google Scholar] [CrossRef]
- Meramo, S.; Gonzalez-Quiroga, A.; Gonzalez-Delgado, A. Technical, Environmental and Process Safety Assessment of Acetone–Butanol-Ethanol Fermentation of Cassava Residues. Sustainability 2022, 14, 16185. [Google Scholar] [CrossRef]
- Sule, A.; Latiff, Z.A.; Abas, M.A.; Veza, I.; Soudagar, M.E.M.; Harny, I.; Epin, V. Dual Effects of N-Butanol and Magnetite Nanoparticle to Biodiesel-diesel Fuel Blends as Additives on Emission Pattern and Performance of a Diesel Engine with ANN Validation. Sustainability 2023, 15, 1404. [Google Scholar] [CrossRef]
- Rayapureddy, S.M.; Matijosius, J.; Rimkus, A.; Caban, J.; Slowik, T. Comparative study of Combustion, Performance and Emission Characteristics of Hydrotreated Vegetable Oil-Biobutanol Fuel Blends and Diesel Fuel on a CI Engine. Sustainability 2023, 14, 7324. [Google Scholar] [CrossRef]
- Lapuerta, M.; Ballesteros, R.; Barba, J. Strategies to Introduce n-Butanol in Gasoline Blends. Sustainability 2017, 9, 589. [Google Scholar] [CrossRef]
- Shang, W.; Yu, X.; Miao, K.; Guo, Z.; Liu, H.; Xing, X. Research on Combustion and Emission Characteristics of a N-Butanol Combined Injection SI Engine. Sustainability 2023, 15, 9696. [Google Scholar] [CrossRef]
- Lewandrowski, J.; Rosenfeld, J.; Pape, D.; Hendrickson, T.; Kirsten, J.; Moffroid, K. 2020 The greenhouse gas benefits of corn ethanol—Assessing recent evidence. Biofuels 2020, 11, 361–375. [Google Scholar] [CrossRef]
- Tyler, L.; Nathan, P.H. Environmental outcomes of the US Renewable Fuel Standard. Proc. Natl. Acad. Sci. USA 2022, 119, e2101084119. [Google Scholar]
- Douglas, L. US Corn-Based Ethanol Worse for Climate than Gasoline, Study Finds. Thomson Reuters. 2022. Available online: https://www.reuters.com/business/environment/us-corn-based-ethanol-worse-climate-than-gasoline-study-finds-2022-02-14/ (accessed on 20 July 2023).
- US Department of Energy, Alternative Fuel Data Center. Available online: https://afdc.energy.gov/laws/RFS#:~:text=The%20Renewable%20Fuel%20Standard%20(RFS,Act%20of%202007%20(EISA) (accessed on 20 July 2023).
- Gautam, M.; Martin, I.D.W. Combustion characteristics of higher-alcohol/gasoline blends. Proc. IMechE Part A J. Power Energy 2000, 214, 497–511. [Google Scholar] [CrossRef]
- Gautam, M.; Martin, I.D.W.; Carder, D. Emission characteristics of higher-alcohol/gasoline blends. Proc. IMechE Part A J. Power Energy 2000, 214, 165–182. [Google Scholar] [CrossRef]
- Yacoub, Y.; Bata, R.; Gautam, M. The performance and emission characteristics of C1-C5 alcohol-gasoline blends with matched oxygen content in a single-cylinder spark-ignition engine. Proc. IMechE Part A J. Power Energy 1998, 212, 363–379. [Google Scholar] [CrossRef]
- Wu, M.; Wang, M.; Liu, J.; Huo, H. Assessment of potential life-cycle energy and greenhouse gas emission effects 293 from using corn-based butanol as a transportation fuel. Biotechnol. Progr. 2008, 24, 1204–1214. [Google Scholar] [CrossRef] [PubMed]
- Galloni, E.; Fontana, G.; Staccone, S.; Scala, F. Performance analyses of a spark-ignition engine firing with gasoline-butanol blends at partial load operation. Energy Convers. Manag. 2016, 110, 319–326. [Google Scholar] [CrossRef]
- Sarathy, S.M.; Thomson, M.J.; Togbe, C.; Dagaut, P.; Halter, F.; Mounaim-Rousselle, C. An experimental and kinetic modeling study of n-butanol combustion. Combust. Flames 2009, 156, 52–64. [Google Scholar] [CrossRef]
- Szwaja, S.; Naber, J.D. Combustion of n-butanol in a spark-ignition IC engine. Fuel 2010, 89, 73–82. [Google Scholar] [CrossRef]
- Alasfour, F.N. Butanol–A single-cylinder engine study: Availability analysis. Appl. Therm. Eng. 1997, 17, 37–49. [Google Scholar] [CrossRef]
- Wallner, T.; Miers, S.A.; McConnell, S. A comparison of ethanol and butanol as oxygenates using a direct-injection, spark-ignition engine. J. Eng. Gas Turbines Power 2009, 131, 032802. [Google Scholar] [CrossRef]
- Rice, R.W.; Sanyal, A.K.; Elrod, A.C.; Bata, R.M. Exhaust-gas emissions of butanol, ethanol, and methanol-gasoline blends. J. Eng. Gas Turbines Power (Trans. ASME) 1991, 113, 77–81. [Google Scholar] [CrossRef]
- Sandu, C.; Pana, C.; Negurescu, N.; Cernat, A.; Nutu, C.; Georgescu, R. The study of the spark ignition engine operation at fuelling with n-butanol-gasoline-blends. E3S Web Conf. 2020, 180, 01010. [Google Scholar] [CrossRef]
- Alasfour, F.N. NOx Emission from a Spark Ignition Engine Using 30% Iso-Butanol-Gasoline Blend: Part 1: Preheating Inlet Air. Appl. Therm. Eng. 1998, 18, 245–256. [Google Scholar] [CrossRef]
- Alasfour, F.N. NOx emission from a spark-ignition engine using 30% iso-butanol—Gasoline blend: Part 2: Ignition timing. Appl. Therm. Eng. 1998, 18, 609–618. [Google Scholar] [CrossRef]
- Balaji, D.; Venkatesan, J. Influence of isobutanol blend in spark ignition engine performance operated with gasoline and ethanol. Int. J. Eng. Sci. Technol. 2010, 2, 2859–2868. [Google Scholar]
- Dernotte, J.; Mounaim-Rousselle, C.; Halter, F.; Seers, P. Evaluation of butanol-gasoline blends in a port fuel-injection, spark-ignition engine. Oil Gas Sci. Technol. 2010, 65, 45–51. [Google Scholar] [CrossRef]
- Heywood, J.B. Internal Combustion Engine Fundamentals; McGraw Hill Series in Mechanical Engineering; McGraw-Hill: New York, NY, USA, 1998. [Google Scholar]
- Zheng, Z.; Yue, L.; Liu, H.; Zhu, Y.; Zhong, X.; Yao, M. Effect of two-stage injection on combustion and emissions 295 under high EGR rate on a diesel engine by fueling blends of diesel/gasoline, diesel/n-butanol, 296 diesel/gasoline/n-butanol and pure diesel. Energy Convers. Manag. 2015, 90, 1–11. [Google Scholar] [CrossRef]
- Zheng, Z.; Li, C.; Liu, H.; Zhang, Y.; Zhong, X.; Yao, M. Experimental study on diesel conventional and low 298 temperature combustion by fueling four isomers of butanol. Fuel 2015, 141, 109–119. [Google Scholar] [CrossRef]
- Hunter, J.; Schuler, D.; Butt, R.H.; Dibble, R.W. Experimental investigation of butanol isomer combustion in Homogeneous Charge Compression Ignition (HCCI) engines. Appl. Energy 2016, 165, 612–626. [Google Scholar]
- Mohamed, E.M. Burning velocities and flame temperatures of ethanol and butanol-air mixtures. J. Technol. 2011, 24, 124–135. [Google Scholar]
- Yusuf, A.A.; Yahyyah, H.; Farooq, A.A.; Buyondo, K.A.; Olupot, P.W.; Nura, S.S.; Sanni, T.; Hannington, T.; Ukundimana, Z.; Hassan, A.S.; et al. Characteristics of ultrafine particle emission from light-vehicle engine at city transport-speed using after-treatment device fueled with n-butanol-hydrogen blend. Case Stud. Chem. Environ. Eng. 2021, 3, 100085. [Google Scholar] [CrossRef]
- Yusuf, A.A.; Inambao, F.L.; Farooq, A.A. Impact of n-butanol-gasoline-hydrogen blends on combustion reactivity, performance and tailpipe emissions using TGDI engine parameters variation. Sustain. Energy Technol. Assess. 2020, 40, 100773. [Google Scholar] [CrossRef]



| Fuel Property | Butanol | Gasoline | |
|---|---|---|---|
| Chemical formula | C4H9OH | C8H18 | |
| Boiling temperature (at 101,325 Pa), [K] | 390 | 303–463 | |
| Carbon content [% mass] | 64.816 | 85.4 | |
| Hydrogen content [% mass] | 13.59 | 14.2 | |
| Oxygen content [% mass] | 21.6 | 0.4 | |
| Density at normal state (at 273.15 K, 101,325 Pa) [kg/m3] | 810 | 735–760 | |
| Kinematic viscosity (at 293.15 K, 0.1 MPa) [m2/s] | 3.6 × 10−6 | (0.37–0.44) × 10−7 | |
| Reid Vapor Pressure [kPa] | 2.2 | 60–62 | |
| Flame temperature (in air at stoichiometric dosage) [K] | 2195 | 2580 | |
| Laminar speed of flame (in air at stoichiometric dosage at 293.15 K, 101,325 Pa) [cm/s] | 48 | 33–43 | |
| Lower heating value(gas at 273.15 K, 101,325 Pa) | [kJ/dm3] | 26,900–29,200 | 32,400 |
| [kJ/kg] | 33,100–33,630 | 42,690 | |
| Molecular mass, [kg/kmol] | 74.12 | 98.5 | |
| Octane number (research) | 94-96 | 90–98 | |
| Cetane number | 25 | 0–10 | |
| Temperature of autoignition [K] | 616-618 | 257–327 | |
| Heat of vaporization (at 298.15 K) [kJ/kg] | 585 | 351 | |
| Stoichiometric air fuel ratio | 11.21 | 14.7 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sandu, C.; Pana, C.; Negurescu, N.; Lazaroiu, G.; Cernat, A.; Georgescu, R.; Nutu, C. The Influence of N-Butanol Addition in Gasoline on the Combustion in the Spark Ignition Engine. Sustainability 2023, 15, 14009. https://doi.org/10.3390/su151814009
Sandu C, Pana C, Negurescu N, Lazaroiu G, Cernat A, Georgescu R, Nutu C. The Influence of N-Butanol Addition in Gasoline on the Combustion in the Spark Ignition Engine. Sustainability. 2023; 15(18):14009. https://doi.org/10.3390/su151814009
Chicago/Turabian StyleSandu, Cristian, Constantin Pana, Niculae Negurescu, Gheorghe Lazaroiu, Alexandru Cernat, Rares Georgescu, and Cristian Nutu. 2023. "The Influence of N-Butanol Addition in Gasoline on the Combustion in the Spark Ignition Engine" Sustainability 15, no. 18: 14009. https://doi.org/10.3390/su151814009
APA StyleSandu, C., Pana, C., Negurescu, N., Lazaroiu, G., Cernat, A., Georgescu, R., & Nutu, C. (2023). The Influence of N-Butanol Addition in Gasoline on the Combustion in the Spark Ignition Engine. Sustainability, 15(18), 14009. https://doi.org/10.3390/su151814009