Invisible Frost Stress on Introduced Dalbergia odorifera: A Bioassay on Foliar Parameters in Seedlings from Six Provenances
Abstract
:1. Introduction
2. Materials and Methods
2.1. Subsection
2.2. Sampling Dates
- (1)
- At least 5 days remained before leaf sampling was to take place, with an expectation of a dual occurrence of advanced warming and a sudden temperature decline.
- (2)
- The last 1–2 days prior to sampling had to be accompanied by sharp declines in both the lowest and highest daily temperatures, and this had to happen before the occurrence of a frost.
- (3)
- A sampling day had to immediately follow a decline in the highest daily temperature, which was also the day with the lowest daily temperature in the most recent 5 days.
- (4)
- Any day that did not have characteristics of temperature fluctuation following any of rules (1)–(3) could not be chosen for foliar sampling.
2.3. Sampling and Chemical Analysis
2.4. Statistical Analysis
3. Results
3.1. Photosynthetic Pigments
3.2. Antioxidant Activity
3.3. Soluble Sugar, Protein, and EC
3.4. Regression of Foliar Parameters against Meteorological Factors
3.5. Analyses of the Meteorological Driving Forces Generating Frost
4. Discussion
4.1. Characterization of Frost Impairment
4.2. Invisible Effect of Frost on the Leaves of the Southern Seedlings
4.3. Invisible Frost Effect on Leaves of Southern Seedlings
4.4. Limits of This Study
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tao, Z.X.; Xu, Y.J.; Ge, Q.S.; Dai, J.H.; Wang, H.J. Reduced frost hardiness in temperate woody species due to climate warming: A model-based analysis. Clim. Chang. 2021, 165, 35. [Google Scholar] [CrossRef]
- He, Z.; Du, J.; Chen, L.; Zhu, X.; Lin, P.; Zhao, M.; Fang, S. Impacts of recent climate extremes on spring phenology in arid-mountain ecosystems in China. Agric. For. Meteorol. 2018, 260, 31–40. [Google Scholar] [CrossRef]
- Wang, X.; Meng, L.; Wei, H. Biomass, carbohydrate, and leakage conductance in buds of six ornamental tree species subjected to a “false spring” in Northeast China. Ann. For. Res. 2022, 65, 15–30. [Google Scholar] [CrossRef]
- Vitasse, Y.; Schneider, L.; Rixen, C.; Christen, D.; Rebetez, M. Increase in the risk of exposure of forest and fruit trees to spring frosts at higher elevations in Switzerland over the last four decades. Agric. For. Meteorol. 2018, 248, 60–69. [Google Scholar] [CrossRef]
- Vitra, A.; Lenz, A.; Vitasse, Y. Frost hardening and dehardening potential in temperate trees from winter to budburst. New Phytol. 2017, 216, 113–123. [Google Scholar] [CrossRef] [PubMed]
- Marquis, B.; Bergeron, Y.; Houle, D.; Leduc, M.; Rossi, S. Variability in frost occurrence under climate change and consequent risk of damage to trees of western Quebec, Canada. Sci. Rep. 2022, 12, 7220. [Google Scholar] [CrossRef] [PubMed]
- Nole, A.; Rita, A.; Ferrara, A.M.S.; Borghetti, M. Effects of a large-scale late spring frost on a beech (Fagus sylvatica L.) dominated Mediterranean mountain forest derived from the spatio-temporal variations of NDVI. Ann. For. Sci. 2018, 75, 83. [Google Scholar] [CrossRef]
- Hufkens, K.; Friedl, M.A.; Keenan, T.F.; Sonnentag, O.; Bailey, A.; O’Keefe, J.; Richardson, A.D. Ecological impacts of a widespread frost event following early spring leaf-out. Glob. Chang. Biol. 2012, 18, 2365–2377. [Google Scholar] [CrossRef]
- Eysteinsson, T.; Karlman, L.; Fries, A.; Martinsson, O.; Skulason, B. Variation in spring and autumn frost tolerance among provenances of Russian larches (Larix Mill.). Scand. J. For. Res. 2009, 24, 100–110. [Google Scholar] [CrossRef]
- Sklenar, P.; Jaramillo, R.; Wojtasiak, S.S.; Meneses, R.I.; Muriel, P.; Klimes, A. Thermal tolerance of tropical and temperate alpine plants suggests that ‘mountain passes are not higher in the tropics’. Glob. Ecol. Biogeogr. 2023. [Google Scholar] [CrossRef]
- Bojórquez, A.; Álvarez-Yépiz, J.C.; Búrquez, A.; Martínez-Yrízar, A. Understanding and predicting frost-induced tropical tree mortality patterns. Glob. Chang. Biol. 2019, 25, 3817–3828. [Google Scholar] [CrossRef] [PubMed]
- Miner, K.R.; D’Andrilli, J.; Mackelprang, R.; Edwards, A.; Malaska, M.J.; Waldrop, M.P.; Miller, C.E. Emergent biogeochemical risks from Arctic permafrost degradation. Nat. Clim. Chang. 2021, 11, 809–819. [Google Scholar] [CrossRef]
- Bojórquez, A.; Martínez-Yrízar, A.; Álvarez-Yépiz, J.C. A landscape assessment of frost damage in the northmost Neotropical dry forest. Agric. For. Meteorol. 2021, 308–309, 108562. [Google Scholar] [CrossRef]
- Das, A.A.; Ratnam, J. The thermal niche and phylogenetic assembly of evergreen tree metacommunities in a mid-to-upper tropical montane zone. Proc. R. Soc. B-Biol. Sci. 2022, 289, 20220038. [Google Scholar] [CrossRef] [PubMed]
- Hoffmann, W.A.; Flake, S.W.; Abreu, R.C.R.; Pilon, N.A.L.; Rossatto, D.R.; Durigan, G. Rare frost events reinforce tropical savanna-forest boundaries. J. Ecol. 2019, 107, 468–477. [Google Scholar] [CrossRef]
- Pilon, N.A.L.; Cava, M.G.B.; Hoffmann, W.A.; Abreu, R.C.R.; Rossatto, D.R.; Durigan, G. Effects and response of the Cerrado ground-layer to frost along the canopy cover gradient. Oecologia 2022, 200, 199–207. [Google Scholar] [CrossRef]
- Finckh, M.; Wendefeuer, J.; Meller, P. Frost-driven lower treelines in Angola and their implications for tropical forest-grassland mosaics. J. Veg. Sci. 2021, 32, e13084. [Google Scholar] [CrossRef]
- Duan, Y.D.; Guo, B.T.; Zhang, L.; Li, J.X.; Li, S.; Zhao, W.B.; Yang, G.; Zhou, S.; Zhou, C.W.; Song, P.H.; et al. Interactive climate-soil forces shape the spatial distribution of foliar N:P stoichiometry in Vaccinium uliginosum planted in agroforests of Northeast China. Front. Ecol. Evol. 2022, 10, 1065680. [Google Scholar] [CrossRef]
- Mashizi, A.K.; Sharafatmandrad, M.; Naghavi, S. Ecosystems multiple-use management: An approach based on change in economic, social, and ecological values of plant communities. Int. J. Environ. Sci. Technol. 2022, 19, 3845–3858. [Google Scholar] [CrossRef]
- Liao, S.B.; Cai, H.; Tian, P.J.; Zhang, B.B.; Li, Y.P. Combined impacts of the abnormal and urban heat island effect in Guiyang, a typical Karst Mountain City in China. Urban Clim. 2022, 41, 101014. [Google Scholar] [CrossRef]
- Xue, J.S.; Zong, L.; Yang, Y.J.; Bi, X.Y.; Zhang, Y.H.; Zhao, M.H. Diurnal and interannual variations of canopy urban heat island (CUHI) effects over a mountain-valley city with a semi-arid climate. Urban Clim. 2023, 48, 101425. [Google Scholar] [CrossRef]
- Li, Y.; Sun, Y.W.; Li, J.L.; Gao, C. Socioeconomic drivers of urban heat island effect: Empirical evidence from major Chinese cities. Sustain. Cites Soc. 2020, 63, 102425. [Google Scholar] [CrossRef]
- Zohner, C.M.; Mo, L.D.; Renner, S.S.; Svenning, J.C.; Vitasse, Y.; Benito, B.M.; Ordonez, A.; Baumgarten, F.; Bastin, J.F.; Sebald, V.; et al. Late-spring frost risk between 1959 and 2017 decreased in North America but increased in Europe and Asia. Proc. Natl. Acad. Sci. USA 2020, 117, 12192–12200. [Google Scholar] [CrossRef]
- Tessier, J.T. Early spring warming may hasten leaf emergence in Erythronium americanum. Am. J. Bot. 2019, 106, 1392–1396. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.F.; Case, B.; Rossi, S.; Dawadi, B.; Liang, E.Y.; Ellison, A.M. Frost controls spring phenology of juvenile Smith fir along elevational gradients on the southeastern Tibetan Plateau. Int. J. Biometeorol. 2019, 63, 963–972. [Google Scholar] [CrossRef] [PubMed]
- Bascietto, M.; Bajocco, S.; Mazzenga, F.; Matteucci, G. Assessing spring frost effects on beech forests in Central Apennines from remotely-sensed data. Agric. For. Meteorol. 2018, 248, 240–250. [Google Scholar] [CrossRef]
- Menzel, A.; Helm, R.; Zang, C. Patterns of late spring frost leaf damage and recovery in a European beech (Fagus sylvatica L.) stand in south-eastern Germany based on repeated digital photographs. Front. Plant Sci. 2015, 6, 110. [Google Scholar] [CrossRef]
- Meiners, S.J.; Presley, G.N. Differential damage of a late frost to Ozark tree species. J. Torrey Bot. Soc. 2015, 142, 12–17. [Google Scholar] [CrossRef]
- Principe, A.; van der Maaten, E.; van der Maaten-Theunissen, M.; Struwe, T.; Wilmking, M.; Kreyling, J. Low resistance but high resilience in growth of a major deciduous forest tree (Fagus sylvatica L.) in response to late spring frost in southern Germany. Tree Struct. Func. 2017, 31, 743–751. [Google Scholar] [CrossRef]
- Hadad, M.; Tardif, J.C.; Conciatori, F.; Waito, J.; Westwood, A. Climate and atmospheric circulation related to frost-ring formation in Picea mariana trees from the Boreal Plains, interior North America. Weather Clim. Extremes 2020, 29, 100264. [Google Scholar] [CrossRef]
- Puchalka, R.; Koprowski, M.; Przybylak, J.; Przybylak, R.; Dabrowski, H.P. Did the late spring frost in 2007 and 2011 affect tree-ring width and earlywood vessel size in Pedunculate oak (Quercus robur) in northern Poland? Int. J. Biometeorol. 2016, 60, 1143–1150. [Google Scholar] [CrossRef] [PubMed]
- Vitasse, Y.; Bottero, A.; Cailleret, M.; Bigler, C.; Fonti, P.; Gessler, A.; Levesque, M.; Rohner, B.; Weber, P.; Rigling, A.; et al. Contrasting resistance and resilience to extreme drought and late spring frost in five major European tree species. Glob. Chang. Biol. 2019, 25, 3781–3792. [Google Scholar] [CrossRef] [PubMed]
- D’Andrea, E.; Scartazza, A.; Battistelli, A.; Collalti, A.; Proiett, S.; Rezaie, N.; Matteucci, G.; Moscatello, S. Unravelling resilience mechanisms in forests: Role of non-structural carbohydrates in responding to extreme weather events. Tree Physiol. 2021, 41, 1808–1818. [Google Scholar] [CrossRef] [PubMed]
- Hauer, R.J.; Wei, H.X.; Koeser, A.K.; Dawson, J.O. Gas Exchange, Water Use Efficiency, and Biomass Partitioning among Geographic Sources of Acer saccharum Subsp. saccharum and Subsp. nigrum Seedlings in Response to Water Stress. Plants 2021, 10, 742. [Google Scholar] [CrossRef]
- USDA. “Dalbergia odorifera”. Germplasm Resources Information Network (GRIN). Available online: https://encyclopedia.thefreedictionary.com/Dalbergia+odorifera#cite_note-Furniture-5 (accessed on 11 April 2017).
- Atikah, T.D.; Wardani, W.; Surya, D. Population, economic value and the trade of Dalbergia latifolia in Lombok, West Nusa Tenggara, Indonesia. IOP Conf. Ser. Earth Environ. Sci. 2021, 762, 012015. [Google Scholar] [CrossRef]
- Cui, Z.; Li, X.; Xu, D.; Yang, Z. Changes in Non-Structural Carbohydrates, Wood Properties and Essential Oil During Chemically-Induced Heartwood Formation in Dalbergia odorifera. Front. Plant Sci. 2020, 11, 1161. [Google Scholar] [CrossRef]
- WCMC. Dalbergia odorifera. The IUCN Red List of Threatened Species 1998: e.T32398A9698077. Available online: https://www.iucnredlist.org/species/32398/9698077 (accessed on 21 April 2017).
- Li, X.W.; Chen, Q.X.; Lei, H.Q.; Wang, J.W.; Yang, S.; Wei, H.X. Nutrient Uptake and Utilization by Fragrant Rosewood (Dalbergia odorifera) Seedlings Cultured with Oligosaccharide Addition under Different Lighting Spectra. Forests 2018, 9, 29. [Google Scholar] [CrossRef]
- Li, X.W.; Gao, Y.; Wei, H.X.; Xia, H.T.; Chen, Q.X. Growth, biomass accumulation and foliar nutrient status in fragrant rosewood (Dalbergia odorifera TC Chen) seedlings cultured with conventional and exponential fertilizations under different photoperiod regimes. Soil Sci. Plant Nutr. 2017, 63, 153–162. [Google Scholar] [CrossRef]
- Li, X.W.; Xia, H.T.; Wang, J.W.; Chen, Q.X. Nutrient uptake and assimilation in fragrant rosewood (Dalbergia odorifera TC Chen) seedlings in growing media with un-composted spent mushroom residue. PLoS ONE 2021, 16, e0249534. [Google Scholar]
- Gu, L.; Hanson, P.J.; Mac Post, W.; Kaiser, D.P.; Yang, B.; Nemani, R.; Pallardy, S.G.; Meyers, T. The 2007 eastern US spring freezes: Increased cold damage in a warming world? Bioscience 2008, 58, 253–262. [Google Scholar] [CrossRef]
- Augspurger, C.K. Spring 2007 warmth and frost: Phenology, damage and refoliation in a temperate deciduous forest. Funct. Ecol. 2009, 23, 1031–1039. [Google Scholar] [CrossRef]
- Hiscox, J.D.; Israelstam, G.F. A method for the extraction of chlorophyll from leaf tissue without maceration. Can. J. Bot. 1979, 57, 1332–1334. [Google Scholar] [CrossRef]
- Lichenthaler, H.K.; Wellburn, A.R. Determinations of total carotenoids and chlorophylls a and b of leaf extracts in different solvents. Biochem. Soc. Trans. 1983, 11, 591–592. [Google Scholar] [CrossRef]
- Liu, X.; Lu, X.; Yang, S.; Liu, Y.; Wang, W.Q.; Wei, X.; Ji, H.J.; Zhang, B.; Xin, W.Z.; Wen, J.X.; et al. Role of exogenous abscisic acid in freezing tolerance of mangrove Kandelia obovata under natural frost condition at near 32 degrees N. BMC Plant Biol. 2022, 22, 593. [Google Scholar] [CrossRef] [PubMed]
- Bradford, M.M. Rapid and sensitive method for quantitation of microgram quantitles of protein utilizing principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef] [PubMed]
- Ryu, K.; Dordick, J.S. How do organic solvents affect peroxidase structure and function? Biochemistry 1992, 31, 2588–2598. [Google Scholar] [CrossRef]
- L’Hirondelle, S.J.; Simpson, D.G.; Binder, W.D. Overwinter storability of conifer planting stock: Operational testing of fall frost hardiness. New For. 2006, 32, 307–321. [Google Scholar] [CrossRef]
- Burr, K.E.; Tinus, R.W.; Wallner, S.J.; King, R.M. Comparison of three cold hardiness tests for conifer seedlings. Tree Physiol. 1990, 6, 351–369. [Google Scholar] [CrossRef]
- Wei, H.; Ma, B.; Hauer, R.J.; Liu, C.; Chen, X.; He, X. Relationship between environmental factors and facial expressions of visitors during the urban forest experience. Urban For. Urban Green. 2020, 53, 126699. [Google Scholar] [CrossRef]
- Liu, Y.; Meng, G.Y.; Suo, S.F.; Li, D.; Wang, A.M.; Cheng, X.H.; Yang, J. Spring Failure Analysis of Mining Vibrating Screens: Numerical and Experimental Studies. Appl. Sci. 2019, 9, 3224. [Google Scholar] [CrossRef]
- Jalili, I.; Ebadi, A.; Askari, M.A.; KalatehJari, S.; Aazami, M.A. Foliar application of putrescine, salicylic acid, and ascorbic acid mitigates frost stress damage in Vitis vinifera cv.’Giziluzum’. BMC Plant Biol. 2023, 23, 135. [Google Scholar] [CrossRef] [PubMed]
- Norozi, M.; ValizadehKaji, B.; Karimi, R.; Solgi, M. Potassium and zinc-induced frost tolerance in pistachio flowers is associated with physiological and biochemical changes. Tree Struct. Funct. 2020, 34, 1021–1032. [Google Scholar] [CrossRef]
- Prozherina, N.; Freiwald, V.; Rousi, M.; Oksanen, E. Interactive effect of springtime frost and elevated ozone on early growth, foliar injuries and leaf structure of birch (Betula pendula). New Phytol. 2003, 159, 623–636. [Google Scholar] [CrossRef]
- Polle, A.; Kroniger, W.; Rennenberg, H. Seasonal fluctuations of ascorbate-related enzymes: Acute and delayed effects of late frost in spring on antioxidative systems in needles of Norway spruce (Picea abies L). Plant Cell Physiol. 1996, 37, 717–725. [Google Scholar] [CrossRef]
- Yang, Y.J.; Liu, T.; Zhang, S.B.; Huang, W. Photoinhibition of oxygen-evolving complex and photosystem II at chilling stress in the tropical tree species Dalbergia odorifera. Photosynthetica 2020, 58, 245–252. [Google Scholar] [CrossRef]
- Li, Y. Cold-Resistance Analysis and Suitability of Cultivation Areas for Dalbergia Odorifera Seedlings in Southern Zhejiang; Zhejiang A&F University: Lin’an, China, 2021; (In Chinese with English Abstract and Legends). [Google Scholar]
- Li, J. Study on Cold Resistance of Different Geographical Provenance of Dalbergia Odorifera Seedlings; Guangxi University: Nanning, China, 2018; (In Chinese with English Abstract and Legends). [Google Scholar]
- Wei, H.X.; Guo, P. Carbohydrate metabolism during new root growth in transplanted Larix olgensis seedlings: Post-transplant response to nursery-applied inorganic fertilizer and organic amendment. iFor. Biogeosci. For. 2017, 10, 15–22. [Google Scholar] [CrossRef]
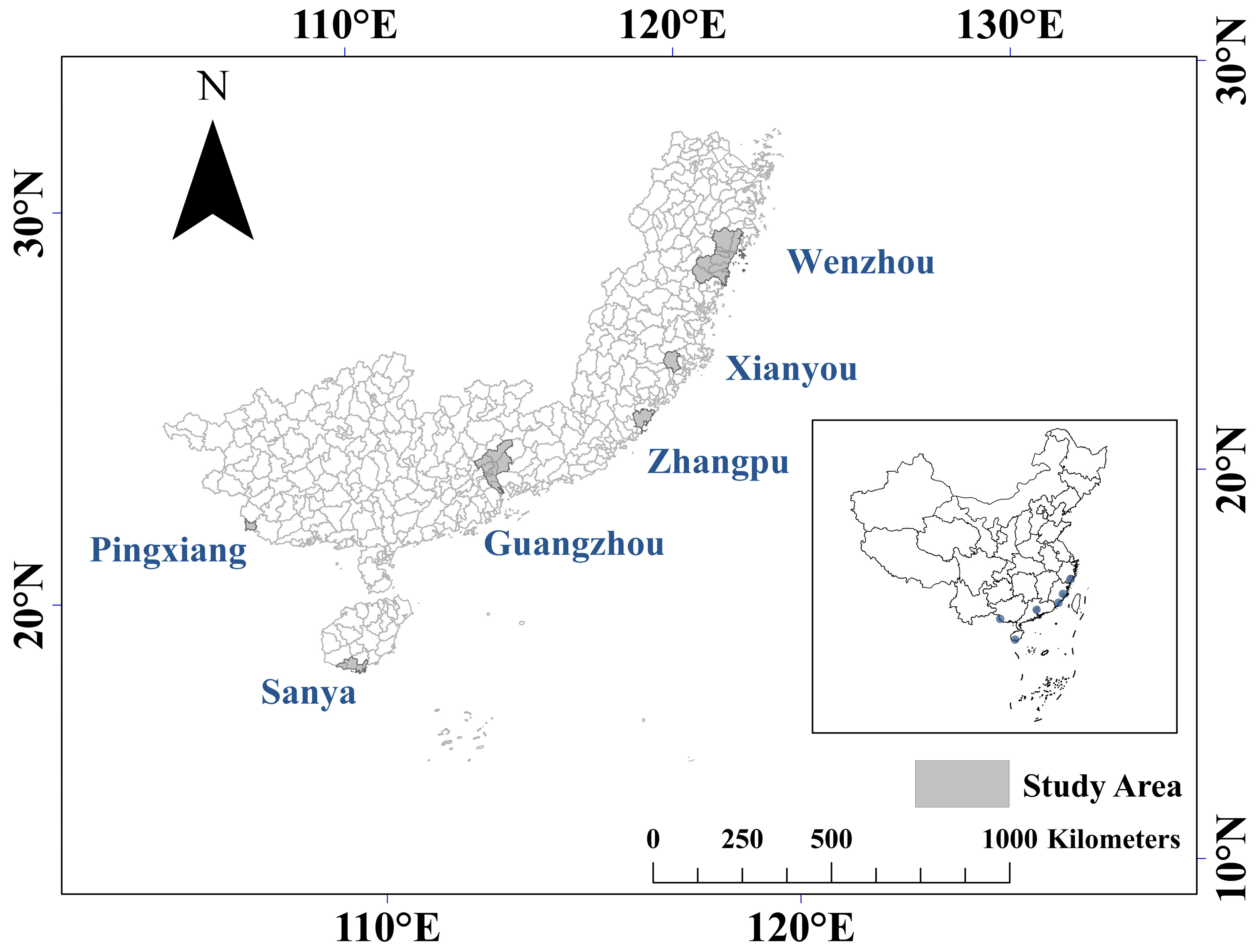







| Plot Order | Province | Municipal | GDP 1 (USD Billion) | Industrial Proportion (%) | Resident Population (Million) | ||
|---|---|---|---|---|---|---|---|
| 1st | 2nd | 3rd | |||||
| 1 | Hainan | Sanya | 43.58 | 13.73 | 20.54 | 65.73 | 0.75 |
| 2 | Guangxi | Pingxiang | 5.69 | 8.77 | 28.84 | 62.39 | 0.12 |
| 3 | Guangdong | Guangzhou | 1810.04 | 1.25 | 31.64 | 67.11 | 13.50 |
| 4 | Fujian | Zhangpu | 276.74 | 13.40 | 48.50 | 38.10 | 5.00 |
| 5 | Fujian | Xianyou | 30.97 | 9.87 | 51.34 | 38.79 | 1.15 |
| 6 | Zhejiang | Wenzhou | 461.81 | 2.80 | 43.80 | 53.40 | 8.11 |
| Source of Variation | ANOVA 1 | 2015 | 2016 | |||||
|---|---|---|---|---|---|---|---|---|
| 15 Sep. | 01 Nov. | 11 Nov. | 27 Nov. | 18 Dec. | 09 Jan. | 25 Jan. | ||
| Chla 2 | F 3 | 11.89 4 | 21.49 | 17.28 | 6.91 | 33.45 | 9.99 | 5.98 |
| P | 0.0003 | <0.0001 | <0.0001 | 0.0030 | <0.0001 | 0.0006 | 0.0053 | |
| Chlb 5 | F | 11.33 | 19.41 | 13.20 | 6.82 | 31.15 | 9.00 | 5.73 |
| P | 0.0003 | <0.0001 | 0.0002 | 0.0031 | <0.0001 | 0.0009 | 0.0063 | |
| Carotenoid | F | 10.24 | 23.30 | 23.40 | 7.15 | 37.23 | 12.53 | 4.01 |
| P | 0.0005 | <0.0001 | <0.0001 | 0.0026 | <0.0001 | 0.0002 | 0.0227 | |
| MDA 6 | F | 18.81 | 3.46 | 4.64 | 1.66 | 3.48 | 1.01 | 6.89 |
| P | <0.0001 | 0.0362 | 0.0138 | 0.2190 | 0.0355 | 0.4509 | 0.0030 | |
| POD 7 | F | 3.96 | 2.76 | 6.82 | 1.93 | 7.33 | 8.29 | 4.82 |
| P | 0.0235 | 0.0694 | 0.0031 | 0.1620 | 0.0023 | 0.0014 | 0.0119 | |
| Sugar | F | 3.75 | 18.48 | 2.68 | 0.15 | 27.91 | 10.85 | 12.72 |
| P | 0.0283 | <0.0001 | 0.0749 | 0.9762 | <0.0001 | 0.0004 | 0.0002 | |
| Protein | F | 4.34 | 12.54 | 5.34 | 1.26 | 37.23 | 4.62 | 6.89 |
| P | 0.0173 | 0.0002 | 0.0082 | 0.3423 | <0.0001 | 0.0140 | 0.0030 | |
| EC 8 | F | 1.52 | 24.46 | 50.12 | 8.34 | 27.95 | 3.56 | 3.93 |
| P | 0.2548 | <0.0001 | <0.0001 | 0.0013 | <0.0001 | 0.0331 | 0.0289 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, X.; Liu, Y.; Yang, S.; Wang, J.; Xia, H.; Liu, X.; Chen, Q. Invisible Frost Stress on Introduced Dalbergia odorifera: A Bioassay on Foliar Parameters in Seedlings from Six Provenances. Sustainability 2023, 15, 14097. https://doi.org/10.3390/su151914097
Li X, Liu Y, Yang S, Wang J, Xia H, Liu X, Chen Q. Invisible Frost Stress on Introduced Dalbergia odorifera: A Bioassay on Foliar Parameters in Seedlings from Six Provenances. Sustainability. 2023; 15(19):14097. https://doi.org/10.3390/su151914097
Chicago/Turabian StyleLi, Xiaowen, Yu Liu, Sheng Yang, Jinwang Wang, Haitao Xia, Xiaojin Liu, and Qiuxia Chen. 2023. "Invisible Frost Stress on Introduced Dalbergia odorifera: A Bioassay on Foliar Parameters in Seedlings from Six Provenances" Sustainability 15, no. 19: 14097. https://doi.org/10.3390/su151914097
APA StyleLi, X., Liu, Y., Yang, S., Wang, J., Xia, H., Liu, X., & Chen, Q. (2023). Invisible Frost Stress on Introduced Dalbergia odorifera: A Bioassay on Foliar Parameters in Seedlings from Six Provenances. Sustainability, 15(19), 14097. https://doi.org/10.3390/su151914097







