Microplastics in Aquatic and Food Ecosystems: Remediation Coupled with Circular Economy Solutions to Create Resource from Waste
Abstract
:1. Introduction
2. MPs Treatment Strategies and Management
2.1. Physical Technologies
| Sr No. | Method | Basic Used | Targeted MPs | Efficacy (%) | Reference | |
|---|---|---|---|---|---|---|
| MPs polymer types | Size (μm) | |||||
| 1. | Colloidal coagulative gas aprons | Adsorption | Unsurfaced-coated polystyrene and Carboxyl-modified poly-(methyl methacrylate) | 5 | 94 | [39] |
| 2. | Zirconium metal-organic structure-derived foams | Filtration | Varied MPs (nearly all types) | - | 96–1.3 | [40] |
| 3. | Graphene oxide and Chitin sponges | Adsorption | carboxylate-modified polystyrene Polystyrene, and polystyrene modified with amine | - | 90%, for neat polystyrene, 72.3% for carboxylate-based polystyrene and 89% for amine-functionalized polystyrene | [41] |
| 4. | Dissolved air flotation | Both hydrophilic and hydrophobic contact and charge | Polyethylene, Nylon 66/PA66, Polyethylene terephthalate | - | 32–38 | [42] |
| 5. | Disc filter | Based on Retention | varied MPs | >20 | 40–98.3 | [30] |
| 6. | Ionic Liquid Phases based on Magnetic Polyoxometalate | Adsorption | Polystyrene | 1–10 | 90 | [43] |
| 7. | Biochar-derived adsorbents (biochar of spruce bark and pine) | Adsorption | Varied MPs | - | 100% (Polyethylene units) and virtually 100% fleece-based fibers | [38] |
| 8. | Filters consisting of Biochar | Adsorption and filtration | Microbeads of Polystyrene | 10 | above 95% | [37] |
| 9. | Bio-based filter | Gravitational filter | Varied MPs | >100 | 79–89% | [44] |
| 10. | Magnetized nanotubes of carbon | Adsorption | Varied MPs | - | 100% | [45] |
| 11. | Electrocoagulation | Flocculation and settling | Microbeads of Polyethylene | 300–355 | 90–100% | [46] |
2.2. Chemical Technologies
| Method | Based on | Targeted MPs | Efficacy (%) | References | |
|---|---|---|---|---|---|
| MPs polymer types | Particle Size (μm) | ||||
| Coagulation along with sedimentation | Coagulation/settling | All | >10 | >99 | [37] |
| Coagulant of alum and alum along with sand coated with cationic polyamine | Coagulation with flocculation | Polyethylene-based | 10–100 | 70–92.7 | [57] |
| Coagulation/flocculation with polyamine-modified chemicals, iron, aluminum | Coagulation with flocculation | Polystyrene spheres | 1 and 6.3 | 1micron particles removed with 95% efficacy and 6.3 micron with 76% efficacy | [58] |
| Activated carbon granules | Filtration | Nearly all types | 1–5 | 57–61% | [37,51] |
| Inorganic-organic composite gels of silica | Interaction | Polyethylene, polypropylene, Polyethylene terephthalate | - | - | |
| Ozone | Degradation via chemical means | All types | - | 90 | [29] |
| Agglomeration caused by alkoxy-silylation | Agglomeration | Polyethylene, polypropylene | Not dependent on dimension, kind and quantity | - | [53] |
| Impact of branched and linear alkyl trichlorosilane | Adsorption + agglomeration + filtration | polyethylene, (both High-density and low density) and Polypropylene-derived MPs | 1–1000 | 97.8 | [54] |
| Photocatalysis | Heterogeneous photocatalysis induced via visible light and triggered by nanorods of zinc oxide | Polyethylene (Low-density) | - | 30 | [59] |
| Photocatalysis | Protein-based porous N-TiO2 semiconductor resulting in green photocatalysis degradation | Polyethylene (high density) | - | - | [60] |
| Coagulation/flocculation by salts of aluminum and iron | Coagulation with flocculation | Polyethylene | - | - | [49] |
| Coagulation/flocculation using polyamine, iron, and aluminum-derived compounds | Coagulation with flocculation | Polystyrene beads | 1 and 6.3 | 95.2% for 1 μm MPs; 75.5% for 6.3 μm MPs | [58] |
2.3. Biological Technologies
2.3.1. Bioremediation Strategies to Mitigate Microplastic Pollution
Microbial Enzymes-Mediated Bioremediation
Innovative Biotechnological Methods to Augment Enzyme Activities against Microplastics
| Sr No. | Strain | Source/Type of Sample | Recognized Enzyme | Molecular Mass (kDa) | Mps Polymer Types | Dimension (μm) | Ref. |
|---|---|---|---|---|---|---|---|
| 1. | Bacillus subtilis | Soil | Polyurethanase | 28 | Impranil (PU) | 0.002 | [94] |
| 2. | Pseudomonas aestusnigri VGXO14 | marine sand polluted with Crude oil | Hydrolase | 32 | Impranil (PE-PU) | 0.1 | [81] |
| 3. | Thielavia terrestris CAU709 | Soil | Cutinase TtcutA | 25.3 | PET in film form | 5 | [95] |
| 4. | Synechococcus sp. PCC 7002 | Culture | Esterase Hydrolase | - | Nanosphere of PE | 0.0002–0.0099 | [96] |
| 5. | Thermobifida fusca KW3 (DSM 6013) | Culture | Hydrolase TfCut2 | - | nanoparticles of PET | 0.1–0.16 | [97] |
| 6. | Ideonella sakaiensis 201-F6 | recycling site of PET bottle | PETase | 24 | film of PET | 6 | [66] |
| 7. | Aspergillus flavus PEDX3– | Wax moth gut | multicopper oxidases (similar to laccases) | - | LDPE | <0.2 | [27] |
| 8. | Amycolatopsis orientalis ssp. orientalis | Culture | PLAase III | 18 | microfilm and powder of PLA | 0.3–0.5 | [98] |
| PLAase II | 19.4 | ||||||
| PLAase I | 24 | ||||||
| 9. | Humicola insolens | Novozym© 51,032 (a commercial product) | Cutinase | 32 | Particles of PET | 5 | [68] |
2.3.2. Biofilms-Mediated MPs Remediation
2.3.3. Membrane Bioreactor
2.3.4. Nanobioremediation: Bionanomaterials-Based MP Degradation
3. Circular Economy Solution to Plastic Waste and Challenges in Its Implementation
3.1. Plastic Production from Alternate Feedstocks
3.2. Use of Plastic Waste as a Resource
3.3. Redesign Plastics Manufacturing Processes and Products
3.4. Collaboration between Companies and Customers
3.5. Circular Economy Roadblocks in the Plastic Sector
4. Policies Regarding MPs Management
5. Conclusions and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sridhar, A.; Kannan, D.; Kapoor, A.; Prabhakar, S. Extraction and detection methods of microplastics in food and marine systems: A critical review. Chemosphere 2021, 286, 131653. [Google Scholar] [CrossRef] [PubMed]
- Geyer, R.; Jambeck, J.R.; Law, K.L. Production, use, and fate of all plastics ever made. Sci. Adv. 2017, 3, e1700782. [Google Scholar] [CrossRef] [PubMed]
- Orlando, M.; Molla, G.; Castellani, P.; Pirillo, V.; Torretta, V.; Ferronato, N. Microbial enzyme biotechnology to reach plastic waste circularity: Current status, problems and perspectives. Int. J. Mol. Sci. 2023, 24, 3877. [Google Scholar] [CrossRef] [PubMed]
- Udovicki, B.; Andjelkovic, M.; Cirkovic-Velickovic, T.; Rajkovic, A. Microplastics in food: Scoping review on health effects, occurrence, and human exposure. Int. J. Food Contam. 2022, 9, 7. [Google Scholar] [CrossRef]
- Andrady, A.L. The plastic in microplastics: A review. Mar. Pollut. Bull. 2017, 119, 12–22. [Google Scholar] [CrossRef]
- Thompson, R.C.; Olsen, Y.; Mitchell, R.P.; Davis, A.; Rowland, S.J.; John, A.W.G.; McGonigle, D.; Russell, A.E. Lost at Sea: Where Is All the Plastic? Science 2004, 304, 838. [Google Scholar] [CrossRef]
- Avio, C.G.; Gorbi, S.; Milan, M.; Benedetti, M.; Fattorini, D.; D’Errico, G.; Pauletto, M.; Bargelloni, L.; Regoli, F. Pollutants bioavailability and toxicological risk from microplastics to marine mussels. Environ. Pollut. 2015, 198, 211–222. [Google Scholar] [CrossRef]
- Smith, M.; Love, D.C.; Rochman, C.M.; Neff, R.A. Microplastics in seafood and the implications for human health. Curr. Environ. Health Rep. 2018, 5, 375–386. [Google Scholar] [CrossRef]
- Andrady, A.L. Microplastics in the marine environment. Mar. Pollut. Bull. 2011, 62, 1596–1605. [Google Scholar] [CrossRef]
- Cole, M. A novel method for preparing microplastic fibers. Sci. Rep. 2016, 6, srep34519. [Google Scholar] [CrossRef]
- Cole, M.; Webb, H.; Lindeque, P.K.; Fileman, E.S.; Halsband, C.; Galloway, T.S. Isolation of microplastics in biota-rich seawater samples and marine organisms. Sci. Rep. 2015, 4, 4528. [Google Scholar] [CrossRef] [PubMed]
- Yee, M.S.-L.; Hii, L.-W.; Looi, C.K.; Lim, W.-M.; Wong, S.-F.; Kok, Y.-Y.; Tan, B.-K.; Wong, C.-Y.; Leong, C.-O. Impact of microplastics and nanoplastics on human health. Nanomaterials 2021, 11, 496. [Google Scholar] [CrossRef] [PubMed]
- King, S.; Locock, K.E. A circular economy framework for plastics: A semi-systematic review. J. Clean. Prod. 2022, 364, 132503. [Google Scholar] [CrossRef]
- Barra, R.; Leonard, S.A.; Whaley, C.; Bierbaum, R. Plastics and the Circular Economy: A STAP Document; Scientific and Technical Advisory Panel (STAP) to the Global Environment Facility (GEF), UN Environment: 2018; Scientific and Technical Advisory Panel: Washington, DC, USA, 2018.
- Shanker, R.; Khan, D.; Hossain, R.; Islam, T.; Locock, K.; Ghose, A.; Sahajwalla, V.; Schandl, H.; Dhodapkar, R. Plastic waste recycling: Existing Indian scenario and future opportunities. Int. J. Environ. Sci. Technol. 2022, 20, 5895–5912. [Google Scholar] [CrossRef]
- Zuccarello, P.; Ferrante, M.; Cristaldi, A.; Copat, C.; Grasso, A.; Sangregorio, D.; Fiore, M.; Conti, G.O. Exposure to microplastics (<10 μm) associated to plastic bottles mineral water consumption: The first quantitative study. Water Res. 2019, 157, 365–371. [Google Scholar] [CrossRef]
- Gündoğdu, S. Contamination of table salts from Turkey with microplastics. Food Addit. Contam. Part A Chem. Anal. Control Expo. Risk Assess. 2018, 35, 1006–1014. [Google Scholar] [CrossRef]
- Barboza, L.G.A.; Vethaak, A.D.; Lavorante, B.R.; Lundebye, A.-K.; Guilhermino, L. Marine microplastic debris: An emerging issue for food security, food safety and human health. Mar. Pollut. Bull. 2018, 133, 336–348. [Google Scholar] [CrossRef]
- Kosuth, M.; Mason, S.A.; Wattenberg, E.V. Anthropogenic contamination of tap water, beer, and sea salt. PLoS ONE 2018, 13, e0194970. [Google Scholar] [CrossRef]
- Catarino, A.I.; Macchia, V.; Sanderson, W.G.; Thompson, R.C.; Henry, T.B. Low levels of microplastics (MP) in wild mussels indicate that MP ingestion by humans is minimal compared to exposure via household fibres fallout during a meal. Environ. Pollut. 2018, 237, 675–684. [Google Scholar] [CrossRef]
- Makhdoumi, P.; Naghshbandi, M.; Ghaderzadeh, K.; Mirzabeigi, M.; Yazdanbakhsh, A.; Hossini, H. Micro-plastic occurrence in bottled vinegar: Qualification, quantification and human risk exposure. Process Saf. Environ. Prot. 2021, 152, 404–413. [Google Scholar] [CrossRef]
- Li, D.; Shi, Y.; Yang, L.; Xiao, L.; Kehoe, D.K.; Gun’ko, Y.K.; Boland, J.J.; Wang, J.J. Microplastic release from the degradation of polypropylene feeding bottles during infant formula preparation. Nat. Food 2020, 1, 746–754. [Google Scholar] [CrossRef] [PubMed]
- Du, F.; Cai, H.; Zhang, Q.; Chen, Q.; Shi, H. Microplastics in take-out food containers. J. Hazard. Mater. 2020, 399, 122969. [Google Scholar] [CrossRef] [PubMed]
- Karami, A.; Golieskardi, A.; Keong Choo, C.; Larat, V.; Galloway, T.S.; Salamatinia, B. The presence of microplastics in commercial salts from different countries. Sci. Rep. 2017, 7, 46173. [Google Scholar] [CrossRef]
- Picó, Y.; Barceló, D. Analysis and prevention of microplastics pollution in water: Current perspectives and future directions. ACS Omega 2019, 4, 6709–6719. [Google Scholar] [CrossRef] [PubMed]
- Parashar, N.; Hait, S. Occurrence and removal of microplastics in a hybrid growth sewage treatment plant from Bihar, India: A preliminary study. J. Clean. Prod. 2022, 376, 134295. [Google Scholar] [CrossRef]
- Zhang, J.; Gao, D.; Li, Q.; Zhao, Y.; Li, L.; Lin, H.; Bi, Q.; Zhao, Y. Biodegradation of polyethylene microplastic particles by the Fungus aspergillus flavus from the guts of wax moth Galleria mellonella. Sci. Total Environ. 2020, 704, 135931. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Xu, E.G.; Li, J.; Chen, Q.; Ma, L.; Zeng, E.Y.; Shi, H. A review of microplastics in table salt, drinking water, and air: Direct human exposure. Environ. Sci. Technol. 2020, 54, 3740–3751. [Google Scholar] [CrossRef]
- Hidayaturrahman, H.; Lee, T.-G. A study on characteristics of microplastic in wastewater of South Korea: Identification, quantification, and fate of microplastics during treatment process. Mar. Pollut. Bull. 2019, 146, 696–702. [Google Scholar] [CrossRef]
- Talvitie, J.; Mikola, A.; Koistinen, A.; Setälä, O. Solutions to microplastic pollution—Removal of microplastics from wastewater effluent with advanced wastewater treatment technologies. Water Res. 2017, 123, 401–407. [Google Scholar] [CrossRef]
- Sembiring, E.; Fajar, M.; Handajani, M. Performance of rapid sand filter—Single media to remove microplastics. Water Supply 2021, 21, 2273–2284. [Google Scholar] [CrossRef]
- Soyer, E.; Akgiray, Ö.; Eldem, N.Ö.; Saatçı, A.M. On the use of crushed recycled glass instead of silica sand in dual-media filters. CLEAN—Soil Air Water 2013, 41, 325–332. [Google Scholar] [CrossRef]
- Poerio, T.; Piacentini, E.; Mazzei, R. Membrane processes for microplastic removal. Molecules 2019, 24, 4148. [Google Scholar] [CrossRef] [PubMed]
- Sol, D.; Laca, A.; Laca, A.; Diaz, M. Approaching the environmental problem of microplastics: Importance of WWTP treatments. Sci. Total Environ. 2020, 740, 140016. [Google Scholar] [CrossRef] [PubMed]
- Andrady, A.L. (Ed.) Plastics and the Ocean: Origin, Characterization, Fate, and Impacts; Wiley: Hoboken, NJ, USA, 2022. [Google Scholar]
- Michielssen, M.R.; Michielssen, E.R.; Ni, J.; Duhaime, M.B. Fate of microplastics and other small anthropogenic litter (SAL) in wastewater treatment plants depends on unit processes employed. Environ. Sci. Water Res. Technol. 2016, 2, 1064–1073. [Google Scholar] [CrossRef]
- Wang, Z.; Sedighi, M.; Lea-Langton, A. Filtration of microplastic spheres by biochar: Removal efficiency and immobilisation mechanisms. Water Res. 2020, 184, 116165. [Google Scholar] [CrossRef]
- Siipola, V.; Pflugmacher, S.; Romar, H.; Wendling, L.; Koukkari, P. Low-cost biochar adsorbents for water purification including microplastics removal. Appl. Sci. 2020, 10, 788. [Google Scholar] [CrossRef]
- Zhang, K.; Hamidian, A.H.; Tubić, A.; Zhang, Y.; Fang, J.K.; Wu, C.; Lam, P.K. Understanding plastic degradation and microplastic formation in the environment: A review. Environ. Pollut. 2021, 274, 116554. [Google Scholar] [CrossRef]
- Chen, X.; Chen, X.; Zhao, Y.; Zhou, H.; Xiong, X.; Wu, C. Effects of microplastic biofilms on nutrient cycling in simulated freshwater systems. Sci. Total Environ. 2020, 719, 137276. [Google Scholar] [CrossRef]
- Sun, C.; Wang, Z.; Chen, L.; Li, F. Fabrication of robust and compressive chitin and graphene oxide sponges for removal of microplastics with different functional groups. Chem. Eng. J. 2020, 393, 124796. [Google Scholar] [CrossRef]
- Wang, J.; Guo, X.; Xue, J. Biofilm-developed microplastics as vectors of pollutants in aquatic environments. Environ. Sci. Technol. 2021, 55, 12780–12790. [Google Scholar] [CrossRef]
- Misra, A. Design and Environmental Applications of Polyoxometalate–Ionic Liquid (POM–IL)—Based Molecular and Composite Materials. Ph.D. Thesis, Universität Ulm, Ulm, Germany, 2021. [Google Scholar]
- Liu, F.; Nord, N.B.; Bester, K.; Vollertsen, J. Microplastics removal from treated wastewater by a biofilter. Water 2020, 12, 1085. [Google Scholar] [CrossRef]
- Tang, Y.; Zhang, S.; Su, Y.; Wu, D.; Zhao, Y.; Xie, B. Removal of microplastics from aqueous solutions by magnetic carbon nanotubes. Chem. Eng. J. 2020, 406, 126804. [Google Scholar] [CrossRef]
- Perren, W.; Wojtasik, A.; Cai, Q. Removal of microbeads from wastewater using electrocoagulation. ACS Omega 2018, 3, 3357–3364. [Google Scholar] [CrossRef] [PubMed]
- Padervand, M.; Lichtfouse, E.; Robert, D.; Wang, C. Removal of microplastics from the environment. A review. Environ. Chem. Lett. 2020, 18, 807–828. [Google Scholar] [CrossRef]
- Alramthi, S.M.; Ali, G.H.; Elthagafi, A.M.; Eldosari, S.H.; Zhu, B.-K.; Safaa, H.M. Oxidation Ditches for Recycling and Reusing Wastewater Are Critical for Long-Term Sustainability—A Case Study. Sustainability 2022, 14, 16737. [Google Scholar] [CrossRef]
- Ma, B.; Xue, W.; Hu, C.; Liu, H.; Qu, J.; Li, L. Characteristics of microplastic removal via coagulation and ultrafiltration during drinking water treatment. Chem. Eng. J. 2018, 359, 159–167. [Google Scholar] [CrossRef]
- Kolya, H.; Kang, C.W. Bio-Based Polymeric Flocculants and Adsorbents for Wastewater Treatment. Sustainability 2023, 15, 9844. [Google Scholar] [CrossRef]
- Herbort, A.F.; Schuhen, K. A concept for the removal of microplastics from the marine environment with innovative host-guest relationships. Environ. Sci. Pollut. Res. 2016, 24, 11061–11065. [Google Scholar] [CrossRef]
- Herbort, A.F.; Sturm, M.T.; Schuhen, K. A new approach for the agglomeration and subsequent removal of polyethylene, polypropylene, and mixtures of both from freshwater systems—A case study. Environ. Sci. Pollut. Res. 2018, 25, 15226–15234. [Google Scholar] [CrossRef]
- Herbort, A.F.; Sturm, M.T.; Fiedler, S.; Abkai, G.; Schuhen, K. Alkoxy-silyl induced agglomeration: A new approach for the sustainable removal of microplastic from aquatic systems. J. Polym. Environ. 2018, 26, 4258–4270. [Google Scholar] [CrossRef]
- Sturm, M.T.; Herbort, A.F.; Horn, H.; Schuhen, K. Comparative study of the influence of linear and branched alkyltrichlorosilanes on the removal efficiency of polyethylene and polypropylene-based microplastic particles from water. Environ. Sci. Pollut. Res. 2020, 27, 10888–10898. [Google Scholar] [CrossRef] [PubMed]
- Brinker, C.J.; Scherer, G.W. Sol–Gel Science: The Physics and Chemistry of Sol–Gel Processing; Academic Press: Cambridge, MA, USA, 1990. [Google Scholar]
- Schuhen, K.; Sturm, M.T.; Herbort, A.F. Technological Approaches for the Reduction of Microplastic Pollution in Seawater Desalination Plants and for Sea Salt Extraction. In Plastics in the Environment; Gomiero, A., Ed.; IntechOpen: London, UK, 2019; ISBN 978-1-83880-492-3. [Google Scholar]
- Shahi, N.K.; Maeng, M.; Kim, D.; Dockko, S. Removal behavior of microplastics using alum coagulant and its enhancement using polyamine-coated sand. Process Saf. Environ. Prot. 2020, 141, 9–17. [Google Scholar] [CrossRef]
- Rajala, K.; Grönfors, O.; Hesampour, M.; Mikola, A. Removal of microplastics from secondary wastewater treatment plant effluent by coagulation/flocculation with iron, aluminum and polyamine-based chemicals. Water Res. 2020, 183, 116045. [Google Scholar] [CrossRef] [PubMed]
- Tofa, T.S.; Kunjali, K.L.; Paul, S.; Dutta, J. Visible light photocatalytic degradation of microplastic residues with zinc oxide nanorods. Environ. Chem. Lett. 2019, 17, 1341–1346. [Google Scholar] [CrossRef]
- Ariza–Tarazona, M.C.; Villarreal-Chiu, J.F.; Barbieri, V.; Siligardi, C.; Cedillo-González, E.I. New strategy for microplastic degradation: Green photocatalysis using a protein-based porous NTiO2 semiconductor. Ceram. Int. 2019, 45, 9618–9624. [Google Scholar] [CrossRef]
- Gambarini, V.; Pantos, O.; Kingsbury, J.M.; Weaver, L.; Handley, K.M.; Lear, G. PlasticDB: A database of microorganisms and proteins linked to plastic biodegradation. Database 2022, 2022, baac008. [Google Scholar] [CrossRef] [PubMed]
- Waring, B.G.; Averill, C.; Hawkes, C.V. Differences in fungal and bacterial physiology alter soil carbon and nitrogen cycling: Insights from meta-analysis and theoretical models. Ecol. Lett. 2013, 16, 887–894. [Google Scholar] [CrossRef]
- Nikhil, B.; Adhyaru, D.; Thakor, P. Production of xylanase by Aspergillus flavus FPDN1 on pearl millet bran: Optimization of culture conditions and application in bioethanol production. Int. J. Res. Chem. Environ. 2012, 2, 204–210. [Google Scholar]
- Zhu, N.; Liu, J.; Yang, J.; Lin, Y.; Yang, Y.; Ji, L.; Li, M.; Yuan, H. Comparative analysis of the secretomes of Schizophyllum commune and other wood-decay basidiomycetes during solid-state fermentation reveals its unique lignocellulose-degrading enzyme system. Biotechnol. Biofuels 2016, 9, 42. [Google Scholar] [CrossRef]
- Bornscheuer, U.T. Feeding on plastic. Science 2016, 351, 1154–1155. [Google Scholar] [CrossRef]
- Yoshida, S.; Hiraga, K.; Takehana, T.; Taniguchi, I.; Yamaji, H.; Maeda, Y.; Toyohara, K.; Miyamoto, K.; Kimura, Y.; Oda, K. A bacterium that degrades and assimilates poly(ethylene terephthalate). Science 2016, 351, 1196–1199. [Google Scholar] [CrossRef] [PubMed]
- Joo, S.; Cho, I.J.; Seo, H.; Son, H.F.; Sagong, H.-H.; Shin, T.J.; Choi, S.Y.; Lee, S.Y.; Kim, K.-J. Structural insight into molecular mechanism of poly(ethylene terephthalate) degradation. Nat. Commun. 2018, 9, 382. [Google Scholar] [CrossRef] [PubMed]
- Carniel, A.; Valoni, E.; Nicomedes, J.; da Conceição Gomes, A.; de Castro, A.M. Lipase from Candida antarctica (CALB) and cutinase from Humicola insolens act synergistically for PET hydrolysis to terephthalic acid. Process Biochem. 2017, 59, 84–90. [Google Scholar] [CrossRef]
- Knott, B.C.; Erickson, E.; Allen, M.D.; Gado, J.E.; Graham, R.; Kearns, F.L.; Pardo, I.; Topuzlu, E.; Anderson, J.J.; Austin, H.P.; et al. Characterization and engineering of a two-enzyme system for plastics depolymerization. Proc. Natl. Acad. Sci. USA 2020, 117, 25476–25485. [Google Scholar] [CrossRef]
- da Costa, C.H.S.; dos Santos, A.M.; Alves, C.N.; Martí, S.; Moliner, V.; Santana, K.; Lameira, J. Assessment of the PETase conformational changes induced by poly(ethylene terephthalate) binding. Proteins: Struct. Funct. Bioinform. 2021, 89, 1340–1352. [Google Scholar] [CrossRef]
- Chen, S.; Su, L.; Billig, S.; Zimmermann, W.; Chen, J.; Wu, J. Biochemical characterization of the cutinases from Thermobifida fusca. J. Mol. Catal. B Enzym. 2010, 63, 121–127. [Google Scholar] [CrossRef]
- Stavila, E.; Arsyi, R.; Petrovic, D.; Loos, K. Fusarium solani pisi cutinase-catalyzed synthesis of polyamides. Eur. Polym. J. 2013, 49, 834–842. [Google Scholar] [CrossRef]
- Chen, S.; Tong, X.; Woodard, R.W.; Du, G.; Wu, J.; Chen, J. Identification and characterization of bacterial cutinase. J. Biol. Chem. J. Biol. Chem. 2008, 283, 25854–25862. [Google Scholar] [CrossRef]
- Danso, D.; Chow, J.; Streit, W.R. Plastics: Environmental and biotechnological perspectives on microbial degradation. Appl. Environ. Microbiol. 2019, 85, e01095-19. [Google Scholar] [CrossRef]
- Kawai, F.; Oda, M.; Tamashiro, T.; Waku, T.; Tanaka, N.; Yamamoto, M.; Mizushima, H.; Miyakawa, T.; Tanokura, M. A novel Ca2+-activated, thermostabilized polyesterase capable of hydrolyzing polyethylene terephthalate from Saccharomonospora viridis AHK190. Appl. Microbiol. Biotechnol. 2014, 98, 10053–10064. [Google Scholar] [CrossRef]
- Ru, J.; Huo, Y.; Yang, Y. Microbial degradation and valorization of plastic wastes. Front. Microbiol. 2020, 11, 442. [Google Scholar] [CrossRef] [PubMed]
- Bhatt, P.; Pathak, V.M.; Bagheri, A.R.; Bilal, M. Microplastic contaminants in the aqueous environment, fate, toxicity consequences, and remediation strategies. Environ. Res. 2021, 200, 111762. [Google Scholar] [CrossRef] [PubMed]
- Taniguchi, I.; Yoshida, S.; Hiraga, K.; Miyamoto, K.; Kimura, Y.; Oda, K. Biodegradation of PET: Current status and application aspects. ACS Catal. 2019, 9, 4089–4105. [Google Scholar] [CrossRef]
- Danso, D.; Schmeisser, C.; Chow, J.; Zimmermann, W.; Wei, R.; Leggewie, C.; Li, X.; Hazen, T.; Streit, W.R. New insights into the function and global distribution of polyethylene terephthalate (PET)—Degrading bacteria and enzymes in marine and terrestrial metagenomes. Appl. Environ. Microbiol. 2018, 84, e02773-17. [Google Scholar] [CrossRef]
- De Jesus, R.; Alkendi, R. A minireview on the bioremediative potential of microbial enzymes as solution to emerging microplastic pollution. Front. Microbiol. 2023, 13, 1066133. [Google Scholar] [CrossRef]
- Bollinger, A.; Thies, S.; Knieps-Grünhagen, E.; Gertzen, C.; Kobus, S.; Höppner, A.; Ferrer, M.; Gohlke, H.; Smits, S.H.J.; Jaeger, K.-E. A novel polyester hydrolase from the marine bacterium Pseudomonas aestusnigri—Structural and functional insights. Front. Microbiol. 2020, 11, 114. [Google Scholar] [CrossRef]
- Müller, R.-J.; Schrader, H.; Profe, J.; Dresler, K.; Deckwer, W.-D. Enzymatic degradation of poly(ethylene terephthalate): Rapid hydrolyse using a hydrolase from T. fusca. Macromol. Rapid Commun. 2005, 26, 1400–1405. [Google Scholar] [CrossRef]
- Ronkvist, A.M.; Xie, W.; Lu, W.; Gross, R.A. Cutinase-catalyzed hydrolysis of poly(ethylene terephthalate). Macromolecules 2009, 42, 5128–5138. [Google Scholar] [CrossRef]
- Sulaiman, S.; Yamato, S.; Kanaya, E.; Kim, J.-J.; Koga, Y.; Takano, K.; Kanaya, S. Isolation of a novel cutinase homolog with polyethylene terephthalate-degrading activity from leaf-branch compost by using a metagenomic approach. Appl. Environ. Microbiol. 2012, 78, 1556–1562. [Google Scholar] [CrossRef]
- Wei, R.; Breite, D.; Song, C.; Gräsing, D.; Ploss, T.; Hille, P.; Schwerdtfeger, R.; Matysik, J.; Schulze, A.; Zimmermann, W. Biocatalytic degradation efficiency of postconsumer polyethylene terephthalate packaging determined by their polymer microstructures. Adv. Sci. 2019, 6, 1900491. [Google Scholar] [CrossRef]
- Darby, R.T.; Kaplan, A.M. Fungal susceptibility of polyurethanes. Appl. Microbiol. 1968, 16, 900–905. [Google Scholar] [CrossRef] [PubMed]
- Son, H.F.; Joo, S.; Seo, H.; Sagong, H.Y.; Lee, S.H.; Hong, H.; Kim, K.J. Structural bioinformatics-based protein engineering of thermos-stable PETase from Ideonella sakaiensis. Enzym. Microb. Technol. 2020, 141, 109656. [Google Scholar] [CrossRef] [PubMed]
- Meng, X.; Yang, L.; Liu, H.; Li, Q.; Xu, G.; Zhang, Y.; Guan, F.; Zhang, Y.; Zhang, W.; Wu, N.; et al. Protein engineering of stable IsPETase for PET plastic degradation by Premuse. Int. J. Biol. Macromol. 2021, 180, 667–676. [Google Scholar] [CrossRef] [PubMed]
- Furukawa, M.; Kawakami, N.; Tomizawa, A.; Miyamoto, K. Efficient degradation of poly(ethylene terephthalate) with Thermobifida fusca cutinase exhibiting improved catalytic activity generated using mutagenesis and additive-based approaches. Sci. Rep. 2019, 9, 16038. [Google Scholar] [CrossRef]
- Kim, J.W.; Park, S.-B.; Tran, Q.-G.; Cho, D.-H.; Choi, D.-Y.; Lee, Y.J.; Kim, H.-S. Functional expression of polyethylene terephthalate-degrading enzyme (PETase) in green microalgae. Microb. Cell Factories 2020, 19, 97. [Google Scholar] [CrossRef]
- Moog, D.; Schmitt, J.; Senger, J.; Zarzycki, J.; Rexer, K.-H.; Linne, U.; Erb, T.; Maier, U.G. Using a marine microalga as a chassis for polyethylene terephthalate (PET) degradation. Microb. Cell Factories 2019, 18, 171. [Google Scholar] [CrossRef]
- Mohanan, N.; Wong, C.H.; Budisa, N.; Levin, D.B. Characterization of Polymer Degrading Lipases, LIP1 and LIP2 From Pseudomonas chlororaphis PA23. Front. Bioeng. Biotechnol. 2022, 10, 854298. [Google Scholar] [CrossRef]
- Schwaminger, S.P.; Fehn, S.; Steegmüller, T.; Rauwolf, S.; Löwe, H.; Pflüger-Grau, K.; Berensmeier, S. Immobilization of PETase enzymes on magnetic iron oxide nanoparticles for the decomposition of microplastic PET. Nanoscale Adv. 2021, 3, 4395–4399. [Google Scholar] [CrossRef]
- Rowe, L.; Howard, G.T. Growth of Bacillus subtilis on polyurethane and the purification and characterization of a polyurethanase-lipase enzyme. Int. Biodeterior. Biodegrad. 2002, 50, 33–40. [Google Scholar] [CrossRef]
- Yang, S.; Xu, H.; Yan, Q.; Liu, Y.; Zhou, P.; Jiang, Z. A low molecular mass cutinase of Thielavia terrestris efficiently hydrolyzes poly(esters). J. Ind. Microbiol. Biotechnol. 2013, 40, 217–226. [Google Scholar] [CrossRef]
- Machado, M.; Vimbela, G.; Silva-Oliveira, T.; Bose, A.; Tripathi, A. The response of Synechococcus sp. PCC 7002 to micro−/nano polyethylene particles—Investigation of a key anthropogenic stressor. PLoS ONE 2020, 15, e0232745. [Google Scholar] [CrossRef] [PubMed]
- Barth, M.; Oeser, T.; Wei, R.; Then, J.; Schmidt, J.; Zimmermann, W. Effect of hydrolysis products on the enzymatic degradation of polyethylene terephthalate nanoparticles by a polyester hydrolase from Thermobifida fusca. Biochem. Eng. J. 2015, 93, 222–228. [Google Scholar] [CrossRef]
- Li, F.; Wang, S.; Liu, W.; Chen, G. Purification and characterization of poly(L-lactic acid)-degrading enzymes from Amycolatopsis orientalis ssp. orientalis. FEMS Microbiol. Lett. 2008, 282, 52–58. [Google Scholar] [CrossRef] [PubMed]
- Harrison, J.P.; Sapp, M.; Schratzberger, M.; Osborn, A.M. Interactions between microorganisms and marine microplastics: A call for research. Mar. Technol. Soc. J. 2011, 45, 12–20. [Google Scholar] [CrossRef]
- Rummel, C.D.; Jahnke, A.; Gorokhova, E.; Kühnel, D.; Schmitt-Jansen, M. Impacts of biofilm formation on the fate and potential effects of microplastic in the aquatic environment. Environ. Sci. Technol. Lett. 2017, 4, 258–267. [Google Scholar] [CrossRef]
- Wu, R.-T.; Cai, Y.-F.; Chen, Y.-X.; Yang, Y.-W.; Xing, S.-C.; Liao, X.-D. Occurrence of microplastic in livestock and poultry manure in South China. Environ. Pollut. 2021, 277, 116790. [Google Scholar] [CrossRef]
- Hadad, D.; Geresh, S.; Sivan, A. Biodegradation of polyethylene by the thermophilic bacterium Brevibacillus borstelensis. J. Appl. Microbiol. 2005, 98, 1093–1096. [Google Scholar] [CrossRef] [PubMed]
- Ganesan, S.; Ruendee, T.; Kimura, S.Y.; Chawengkijwanich, C.; Janjaroen, D. Effect of biofilm formation on different types of plastic shopping bags: Structural and physicochemical properties. Environ. Res. 2021, 206, 112542. [Google Scholar] [CrossRef]
- Faheem, M.; Shabbir, S.; Zhao, J.; Kerr, P.G.; Ali, S.; Sultana, N.; Jia, Z. Multifunctional periphytic biofilms: Polyethylene degradation and Cd2+ and Pb2+ bioremediation under high methane scenario. Int. J. Mol. Sci. 2020, 21, 5331. [Google Scholar] [CrossRef]
- Richard, H.; Carpenter, E.J.; Komada, T.; Palmer, P.T.; Rochman, C.M. Biofilm facilitates metal accumulation onto microplastics in estuarine waters. Sci. Total Environ. 2019, 683, 600–608. [Google Scholar] [CrossRef]
- Stabnikova, O.; Stabnikov, V.; Marinin, A.; Klavins, M.; Vaseashta, A. The role of microplastics biofilm in accumulation of trace metals in aquatic environments. World J. Microbiol. Biotechnol. 2022, 38, 117. [Google Scholar] [CrossRef] [PubMed]
- Shabbir, S.; Faheem, M.; Ali, N.; Kerr, P.G.; Wang, L.-F.; Kuppusamy, S.; Li, Y. Periphytic biofilm: An innovative approach for biodegradation of microplastics. Sci. Total Environ. 2020, 717, 137064. [Google Scholar] [CrossRef] [PubMed]
- Gilan, I.; Hadar, Y.; Sivan, A. Colonization, biofilm formation and biodegradation of polyethylene by a strain of Rhodococcus ruber. Appl. Microbiol. Biotechnol. 2004, 65, 97–104. [Google Scholar] [CrossRef]
- Paço, A.; Duarte, K.; da Costa, J.P.; Santos, P.S.; Pereira, R.; Pereira, M.; Freitas, A.C.; Duarte, A.C.; Rocha-Santos, T.A. Biodegradation of polyethylene microplastics by the marine fungus Zalerion maritimum. Sci. Total Environ. 2017, 586, 10–15. [Google Scholar] [CrossRef] [PubMed]
- Howard, G.T.; Norton, W.N.; Burks, T. Growth of Acinetobacter gerneri P7 on polyurethane and the purification and characterization of a polyurethanase enzyme. Biodegradation 2012, 23, 561–573. [Google Scholar] [CrossRef]
- Narciso-Ortiz, L.; Coreño-Alonso, A.; Mendoza-Olivares, D.; Lucho-Constantino, C.A.; Lizardi-Jiménez, M.A. Baseline for plastic and hydrocarbon pollution of rivers, reefs, and sediment on beaches in Veracruz State, México, and a proposal for bioremediation. Environ. Sci. Pollut. Res. 2020, 27, 23035–23047. [Google Scholar] [CrossRef]
- Osman, M.; Satti, S.M.; Luqman, A.; Hasan, F.; Shah, Z.; Shah, A.A. Degradation of polyester polyurethane by Aspergillus sp. strain S45 isolated from soil. J. Polym. Environ. 2017, 26, 301–310. [Google Scholar] [CrossRef]
- Magnin, A.; Hoornaert, L.; Pollet, E.; Laurichesse, S.; Phalip, V.; Avérous, L. Isolation and characterization of different promising fungi for biological waste management of polyurethanes. Microb. Biotechnol. 2019, 12, 544–555. [Google Scholar] [CrossRef]
- Vimala, P.P.; Mathew, L. Biodegradation of polyethylene using Bacillus subtilis. Procedia Technol. 2016, 24, 232–239. [Google Scholar] [CrossRef]
- Kırbaş, Z.; Keskin, N.E.V.I.N.; Güner, A. Biodegradation of polyvinylchloride (PVC) by white rot fungi. Bull. Environ. Contam. Toxicol. 1999, 63, 335–342. [Google Scholar] [CrossRef]
- Nakamiya, K.; Hashimoto, S.; Ito, H.; Edmonds, J.S.; Yasuhara, A.; Morita, M. Microbial treatment of bis (2-ethylhexyl) phthalate in polyvinyl chloride with isolated bacteria. J. Biosci. Bioeng. 2005, 99, 115–119. [Google Scholar] [CrossRef] [PubMed]
- Atiq, N.; Ahmed, S.; Ali, M.I.; Ahmad, B.; Robson, G. Isolation and identification of polystyrene biodegrading bacteria from soil. Afr. J. Microbiol. Res. 2010, 4, 1537–1541. [Google Scholar]
- Auta, H.; Emenike, C.; Fauziah, S. Distribution and importance of microplastics in the marine environment: A review of the sources, fate, effects, and potential solutions. Environ. Int. 2017, 102, 165–176. [Google Scholar] [CrossRef] [PubMed]
- Jeon, H.J.; Kim, M.N. Isolation of mesophilic bacterium for biodegradation of polypropylene. Int. Biodeterior. Biodegrad. 2016, 115, 244–249. [Google Scholar] [CrossRef]
- McGivney, E.; Cederholm, L.; Barth, A.; Hakkarainen, M.; Hamacher-Barth, E.; Ogonowski, M.; Gorokhova, E. Rapid physicochemical changes in microplastic induced by biofilm formation. Front. Bioeng. Biotechnol. 2020, 8, 205. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.-L.; Xiang, H.; Xiong, H.-Q.; Fang, Y.-C.; Wang, Y. Bioremediation of microplastics in freshwater environments: A systematic review of biofilm culture, degradation mechanisms, and analytical methods. Sci. Total Environ. 2023, 863, 160953. [Google Scholar] [CrossRef]
- Carr, S.A.; Liu, J.; Tesoro, A.G. Transport and fate of microplastic particles in wastewater treatment plants. Water Res. 2016, 91, 174–182. [Google Scholar] [CrossRef]
- Baresel, C.; Ek, M.; Ejhed, H.; Allard, A.-S.; Magnér, J.; Dahlgren, L.; Westling, K.; Wahlberg, C.; Fortkamp, U.; Söhr, S.; et al. Sustainable treatment systems for removal of pharmaceutical residues and other priority persistent substances. Water Sci. Technol. 2019, 79, 537–543. [Google Scholar] [CrossRef]
- Chamas, A.; Moon, H.; Zheng, J.; Qiu, Y.; Tabassum, T.; Jang, J.H.; Abu-Omar, M.; Scott, S.L.; Suh, S. Degradation rates of plastics in the environment. ACS Sustain. Chem. Eng. 2020, 8, 3494–3511. [Google Scholar] [CrossRef]
- Mishra, A.; Kumar, J.; Melo, J.S. Silica based bio-hybrid materials and their relevance to bionanotechnology. Austin J. Plant Biol. 2020, 6, 1024. [Google Scholar]
- Vázquez-Núñez, E.; Molina-Guerrero, C.E.; Peña-Castro, J.M.; Fernández-Luqueño, F.; De La Rosa-Álvarez, M.G. Use of Nanotechnology for the Bioremediation of Contaminants: A Review. Processes 2020, 8, 826. [Google Scholar] [CrossRef]
- Mueller, N.C.; Nowack, B. Nanoparticles for remediation: Solving big problems with little particles. Elements 2010, 6, 395–400. [Google Scholar] [CrossRef]
- Zhu, Y.; Liu, X.; Hu, Y.; Wang, R.; Chen, M.; Wu, J.; Wang, Y.; Kang, S.; Sun, Y.; Zhu, M. Behavior, remediation effect and toxicity of nanomaterials in water environments. Environ. Res. 2019, 174, 54–60. [Google Scholar] [CrossRef] [PubMed]
- Awasthi, A.; Jadhao, P.; Kumari, K. Clay nano-adsorbent: Structures, applications and mechanism for water treatment. SN Appl. Sci. 2019, 1, 1076. [Google Scholar] [CrossRef]
- Jain, K.; Patel, A.S.; Pardhi, V.P.; Flora, S.J.S. Nanotechnology in wastewater management: A new paradigm towards wastewater treatment. Molecules 2021, 26, 1797. [Google Scholar] [CrossRef]
- Tofa, T.S. Degradation of Microplastic Residuals in Water by Visible Light Photocatalysis. Master’s Thesis, KTH Royal Institute of Technology, Stockholm, Sweden, 2018. [Google Scholar]
- Kiriyanthan, R.M.; Maharajan, T.; Radha, A.; Pandikumar, P. A review on the role of nanotechnology in enhancing environmental sustainability. Chem. Biol. Interface 2021, 11, 13–33. [Google Scholar]
- Baruah, S.; Mahmood, M.A.; Myint, M.T.Z.; Bora, T.; Dutta, J. Enhanced visible light photocatalysis through fast crystallization of zinc oxide nanorods. Beilstein J. Nanotechnol. 2010, 1, 14–20. [Google Scholar] [CrossRef]
- Bolto, B.; Gregory, J. Organic polyelectrolytes in water treatment. Water Res. 2007, 41, 2301–2324. [Google Scholar] [CrossRef]
- Maćczak, P.; Kaczmarek, H.; Ziegler-Borowska, M. Recent Achievements in Polymer Bio-Based Flocculants for Water Treatment. Materials 2020, 13, 3951. [Google Scholar] [CrossRef]
- Jalvo, B.; Aguilar-Sanchez, A.; Ruiz-Caldas, M.X.; Mathew, A.P. Water filtration membranes based on non-woven cellulose fabrics: Effect of nanopolysaccharide coatings on selective particle rejection, antifouling, and antibacterial properties. Nanomaterials 2021, 11, 1752. [Google Scholar] [CrossRef]
- Bahi, A.; Shao, J.; Mohseni, M.; Ko, F.K. Membranes based on electrospun lignin-zeolite composite nanofibers. Sep. Purif. Technol. 2017, 187, 207–213. [Google Scholar] [CrossRef]
- Grbic, J.; Nguyen, B.; Guo, E.; You, J.B.; Sinton, D.; Rochman, C.M. Magnetic extraction of microplastics from environmental samples. Environ. Sci. Technol. Lett. 2019, 6, 68–72. [Google Scholar] [CrossRef]
- Abdullah, M.M.S.; Atta, A.M.; Allohedan, H.A.; Alkhathlan, H.Z.; Khan, M.; Ezzat, A.O. Green synthesis of hydrophobic magnetite nanoparticles coated with plant extract and their application as petroleum oil spill collectors. Nanomaterials 2018, 8, 855. [Google Scholar] [CrossRef] [PubMed]
- Elmaci, G. Microwave-assisted rapid synthesis of C@Fe3O4 composite for removal of microplastics from drinking water. Adıyaman Univ. J. Sci. 2020, 10, 207–217. [Google Scholar] [CrossRef]
- Wang, L.; Kaeppler, A.; Fischer, D.; Simmchen, J. Photocatalytic TiO2 micromotors for removal of microplastics and suspended matter. ACS Appl. Mater. Interfaces 2019, 11, 32937–32944. [Google Scholar] [CrossRef]
- Chellasamy, G.; Kiriyanthan, R.M.; Maharajan, T.; Radha, A.; Yun, K. Remediation of microplastics using bionanomaterials: A review. Environ. Res. 2022, 208, 112724. [Google Scholar] [CrossRef]
- Peydayesh, M.; Suta, T.; Usuelli, M.; Handschin, S.; Canelli, G.; Bagnani, M.; Mezzenga, R. Sustainable removal of microplastics and natural organic matter from water by coagulation–flocculation with protein amyloid fibrils. Environ. Sci. Technol. 2021, 55, 8848–8858. [Google Scholar] [CrossRef]
- Dijkstra, H.; van Beukering, P.; Brouwer, R. Business models and sustainable plastic management: A systematic review of the literature. J. Clean. Prod. 2020, 258, 120967. [Google Scholar] [CrossRef]
- Gharfalkar, M.; Court, R.; Campbell, C.; Ali, Z.; Hillier, G. Analysis of waste hierarchy in the European waste directive 2008/98/EC. Waste Manag. 2015, 39, 305–313. [Google Scholar] [CrossRef]
- Johansen, M.R.; Christensen, T.B.; Ramos, T.M.; Syberg, K. A review of the plastic value chain from a circular economy perspective. J. Environ. Manag. 2021, 302, 113975. [Google Scholar] [CrossRef]
- Odegard, I.Y.R.; Nusselder, S.; Lindgreen, E.R.; Bergsma, G.C.; de Graaff, L. Biobased Plastics in a Circular Economy: Policy Suggestions for Biobased and Biobased Biodegradable Plastics; CE Delft: Delft, The Netherlands, 2017. [Google Scholar]
- Solaiman, D.K.Y.; Ashby, R.D.; Foglia, T.A.; Marmer, W.N. Conversion of agricultural feedstock and coproducts into poly(hydroxyalkanoates). Appl. Microbiol. Biotechnol. 2006, 71, 783–789. [Google Scholar] [CrossRef] [PubMed]
- Sheldon, R.A. Green and sustainable manufacture of chemicals from biomass: State of the art. Green Chem. 2014, 16, 950–963. [Google Scholar] [CrossRef]
- Hobbs, C.E. Recent advances in bio-based flame retardant additives for synthetic polymeric materials. Polymers 2019, 11, 224. [Google Scholar] [CrossRef]
- Kwabena, J.; Berko-Boateng, V.N.; Ama, T. Case studies in construction materials use of waste plastic materials for road construction in Ghana. Case Stud. Constr. Mater. 2017, 6, 1–7. [Google Scholar]
- Roy, P.; Mohanty, A.K.; Misra, M. Microplastics in ecosystems: Their implications and mitigation pathways. Environ. Sci. Adv. 2022, 1, 9–29. [Google Scholar] [CrossRef]
- Sun, L.; Li, H.; Dong, L.; Fang, K.; Ren, J.; Geng, Y.; Fujii, M.; Zhang, W.; Zhang, N.; Liu, Z. Eco-benefits assessment on urban industrial symbiosis based on material flows analysis and emergy evaluation approach: A case of Liuzhou city, China. Resour. Conserv. Recycl. 2017, 119, 78–88. [Google Scholar] [CrossRef]
- Zhu, J.-B.; Watson, E.M.; Tang, J.; Chen, E.Y.-X. A synthetic polymer system with repeatable chemical recyclability. Science 2018, 360, 398–403. [Google Scholar] [CrossRef]
- Brentrup, F.; Küsters, J.; Kuhlmann, H.; Lammel, J. Environmental impact assessment of agricultural production systems using the life cycle assessment methodology: I. Theoretical concept of a LCA method tailored to crop production. Eur. J. Agron. 2004, 20, 247–264. [Google Scholar] [CrossRef]
- Brand Hijacks Bottles for Refilling to Drastically Reduce Plastic Waste. Available online: https://www.plasticstoday.com/packaging/brand-hijacks-bottles-refilling-drastically-reduce-plastic-waste (accessed on 22 June 2020).
- Coelho, P.M.; Corona, B.; ten Klooster, R.; Worrell, E. Sustainability of reusable packaging—Current situation and trends. Resour. Conserv. Recycl. X 2020, 6, 100037. [Google Scholar] [CrossRef]
- Briassoulis, D.; Pikasi, A.; Hiskakis, M. End-of-waste life: Inventory of alternative end-of-use recirculation routes of bio-based plastics in the European Union context. Crit. Rev. Environ. Sci. Technol. 2019, 49, 1835–1892. [Google Scholar] [CrossRef]
- Geng, Y.; Tsuyoshi, F.; Chen, X. Evaluation of innovative municipal solid waste management through urban symbiosis: A case study of Kawasaki. J. Clean. Prod. 2010, 18, 993–1000. [Google Scholar] [CrossRef]
- Usman, S.; Razis, A.F.A.; Shaari, K.; Amal, M.N.A.; Saad, M.Z.; Isa, N.M.; Nazarudin, M.F.; Zulkifli, S.Z.; Sutra, J.; Ibrahim, M.A. Microplastics pollution as an invisible potential threat to food safety and security, policy challenges and the way forward. Int. J. Environ. Res. Public Health 2020, 17, 9591. [Google Scholar] [CrossRef] [PubMed]
- Kalantar, Z.N.; Karim, M.R.; Mahrez, A. A review of using waste and virgin polymer in pavement. Constr. Build. Mater. 2012, 33, 55–62. [Google Scholar] [CrossRef]
- Ministry of Environment, Forest and Climate Change. Government Notifies the Plastic Waste Management Amendment Rules, 2021, Prohibiting Identified Single Use Plastic Items by 2022. Available online: https://pib.gov.in/PressReleaseIframePage.aspx?PRID=1745433 (accessed on 3 August 2021).
- Wu, W.-M.; Yang, J.; Criddle, C.S. Microplastics pollution and reduction strategies. Front. Environ. Sci. Eng. 2017, 11, 6. [Google Scholar] [CrossRef]



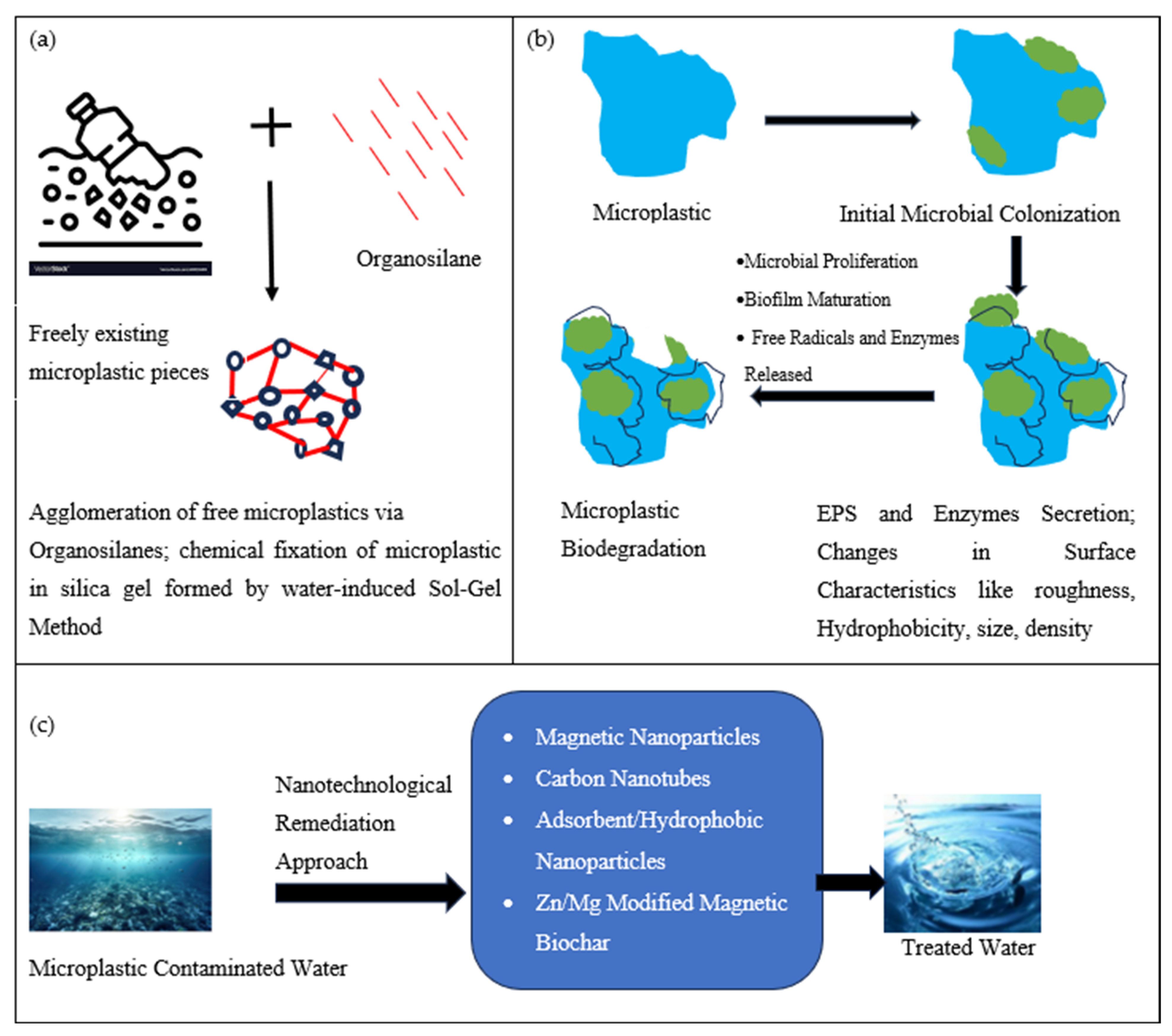

| Exposure Source | Polymer Involved | Level of Exposure | Estimated Consumption | Reference |
|---|---|---|---|---|
| Bottled mineral water | PET | 1,531,525 particles per kg per body weight each day (adults) 3,350,210 particles per kg per body weight per day (children) | 2 L per day (adults) 1 L per day (children) | [16] |
| Salts | PE, PP, PET, PU, PVC, PA | Up to 302 particles/person per year | 4.8–18.01 g/day | [17] |
| Seafood | PE, polyester, semisynthetic cellulose | 518–3078 particles/person per year | 9.6–57 kg/year | [18] |
| Water and beverages | Anthropogenic debris | 4400–5800 particles/person per year | 2.2–3 L/day | [19] |
| Household dust fallout | _ | 13,730–68,414 particles per person per year | Evening meals | [20] |
| Vinegar | PE, butylated hydroxytoluene, Irganox, Erucamide | Up to 3.68 particles/kg/body weight/year (adults) Up to 16.08 particles/kg/body weight per year (children) | 3.1 L per year | [21] |
| Infant feeding bottles | PP | 14,600–4,550,000 particles per capita per day | _ | [22] |
| Mussels | PET, PU | 123–4620 particles/person per year | 0.082–3.08 kg per year | [20] |
| Food contact materials | PP, PS, PE, PET | 12–203 particles/person per week | 4–7 takeout per week | [23] |
| Salts | PE, PP, PET, PS, polyacrylonitrile, PA | 37 particles/person per year | 3.95 g per day | [24] |
| Sr No. | Microorganism Name | Microorganism Category | MPs Polymer Types | Efficacy (%) | References |
|---|---|---|---|---|---|
| 1. | Rhodococcus ruber | Bacteria | PE | 8 | [108] |
| 2. | Zalerion maritimum | Fungus | PE | 43 | [109] |
| 3. | Acinetobacter gerneri | Bacteria | Impranil DLN | _ | [110] |
| 4. | Bacillus muralis | Bacteria | PET | _ | [111] |
| 5. | Aspergillus sp. S45 | Fungus | Polyester PUR film | 15–20 | [112] |
| 6. | Penicillium sp. | Fungus | polyester/polyether film; Impranil DLN | 8.9 | [113] |
| 7. | Bacillus subtilis | Bacteria | PE | 9.26 | [114] |
| 8. | Pleurotus sajor caju PVC film; Poliporus versicolor | Fungus | PVC film | _ | [115] |
| 9. | Mycobacterium sp. NK0301 | Bacteria | PVC film (Plasticized) | _ | [116] |
| 10. | Paenibacillus urinalis NA26; Microbacterium sp. NA23; Pseudomonas aeruginosa NB26; Bacillus sp. NB6 | Bacteria | PS-based film | _ | [117] |
| 11. | Rhodococcus sp. Strain 36; Bacillus sp. Strain 27 | Bacteria | PP MPs | 4–6.4 | [118] |
| 12. | Stenotrophomonas panacihumi | Bacteria | PP film | _ | [119] |
| 13. | Sphingobium, Novosphingobium | Bacteria | PS | _ | [120] |
| 14. | Proteobacteria, Deinococcus-Thermus, Gammaproteobacteria, Alphaproteobacteria, Cyanobacteria | Bacteria | PE | _ | [104,107] |
| Sr No. | Nanomaterials Functionality | Action Mechanism | Nanomaterials Used in MPs Remediation |
|---|---|---|---|
| 1. | Adsorbent | MPs and nano-adsorbent interact to cause adsorption to occur. Hydrophobic, electrostatic, hydrogen-bond, electron conjugate, π-π electron interaction, and complexation are some possible forms of this relationship | composite membranes made of nanofibers of lignin and zeolite [137] |
| Fe nanoparticles (Hydrophobic in nature) [138] | |||
| Magnetized biochar functionalized with Magnesium and Zinc [37] | |||
| Carbon-based magnetic nanotubes [45] | |||
| C@Fe3O4 composite [140] | |||
| 2. | Filter membrane | MPs can be successfully separated from the effluent using nano filters using nanoscale sieves | Cellulose nanocrystals impregnated non-woven [136] |
| TEMPO-oxidized cellulose nanofibers impregnated with unwoven cellulosic fibre | |||
| chitin nanocrystals permeated Non-woven cellulose fabric [136] | |||
| 3. | Flocculant | Charge neutralization is the basis for this technique. The positively charged flocculant balances out the MPs negative charge, causing flocs to form. By using filtering or sedimentation, these flocs can be split | Lysozyme amyloid fibrils [143] |
| 4. | Catalyst/Photocatalyst | When exposed to UV radiation, they form reactive species, which lead to the destruction of MP | Micromotor based on Au@Ni@TiO2 [141] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dhiman, S.; Sharma, C.; Kumar, A.; Pathak, P.; Purohit, S.D. Microplastics in Aquatic and Food Ecosystems: Remediation Coupled with Circular Economy Solutions to Create Resource from Waste. Sustainability 2023, 15, 14184. https://doi.org/10.3390/su151914184
Dhiman S, Sharma C, Kumar A, Pathak P, Purohit SD. Microplastics in Aquatic and Food Ecosystems: Remediation Coupled with Circular Economy Solutions to Create Resource from Waste. Sustainability. 2023; 15(19):14184. https://doi.org/10.3390/su151914184
Chicago/Turabian StyleDhiman, Sunny, Chhavi Sharma, Anu Kumar, Puneet Pathak, and Shiv Dutt Purohit. 2023. "Microplastics in Aquatic and Food Ecosystems: Remediation Coupled with Circular Economy Solutions to Create Resource from Waste" Sustainability 15, no. 19: 14184. https://doi.org/10.3390/su151914184
APA StyleDhiman, S., Sharma, C., Kumar, A., Pathak, P., & Purohit, S. D. (2023). Microplastics in Aquatic and Food Ecosystems: Remediation Coupled with Circular Economy Solutions to Create Resource from Waste. Sustainability, 15(19), 14184. https://doi.org/10.3390/su151914184








