Performance of Alkali-Activated Slag Concrete Masonry Blocks Subjected to Accelerated Carbonation Curing
Abstract
:1. Introduction
2. Research Significance
3. Materials and Methods
3.1. Materials
3.2. Mix Design
- -
- Binder percentage: 13% of the total mass [4];
- -
- Binder-to-aggregate ratio (B/A): 1:6;
- -
- Alkaline solution-to-binder ratio (AAS/B): 0.6;
- -
- SS-to-SH ratio (SS/SH): 1.5.
3.3. Sample Preparation
3.4. Performance Evaluation
4. Results and Discussion
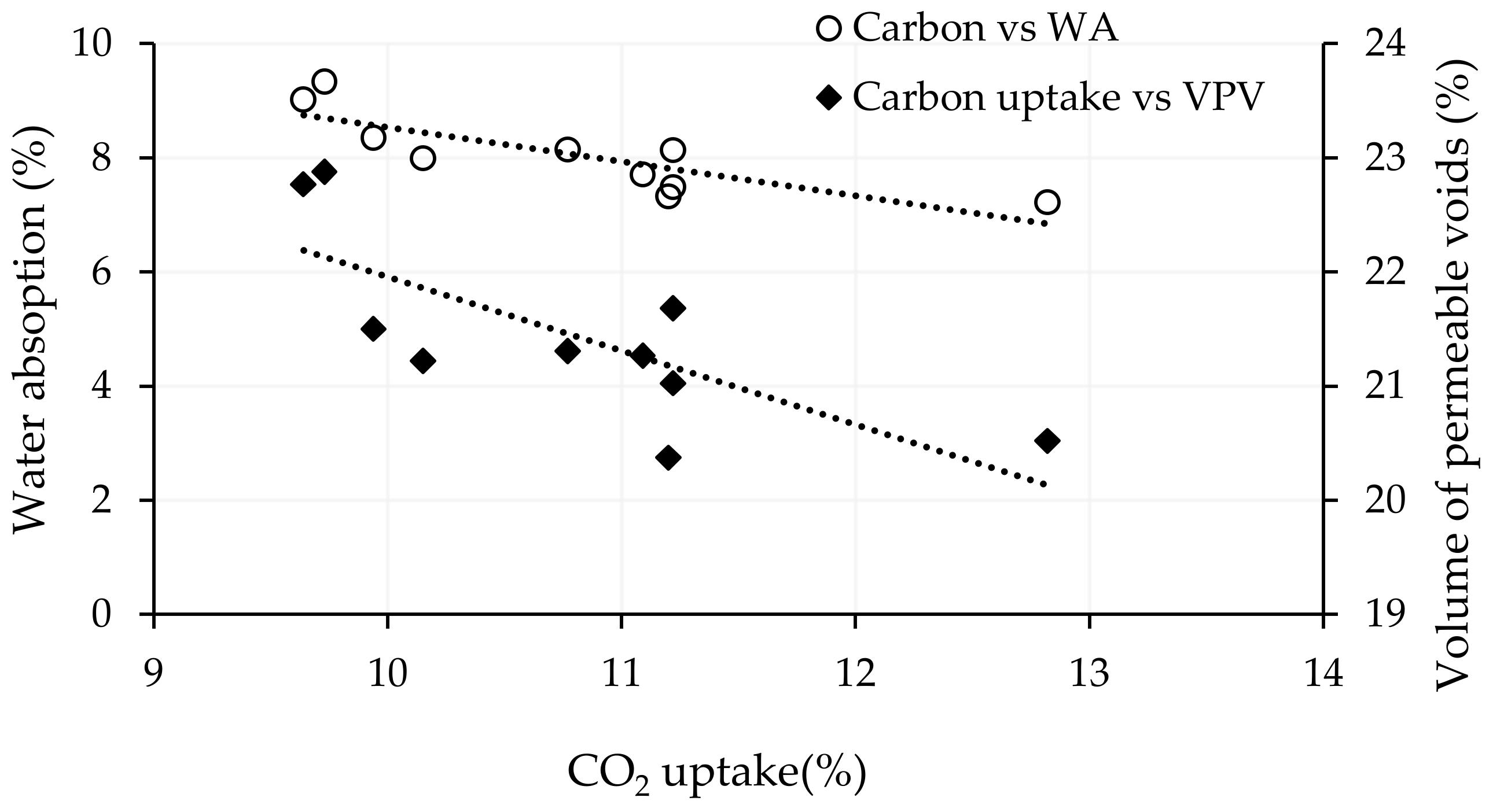
4.1. CO2 Uptake
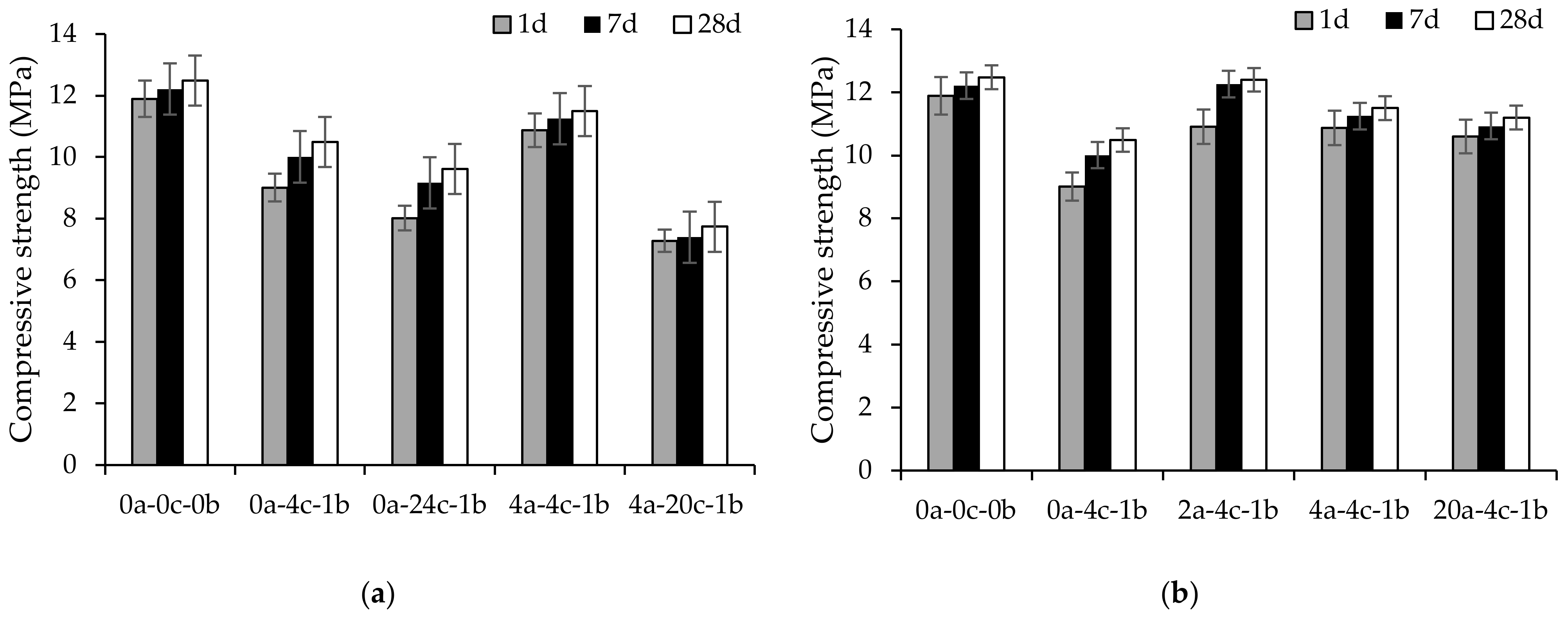
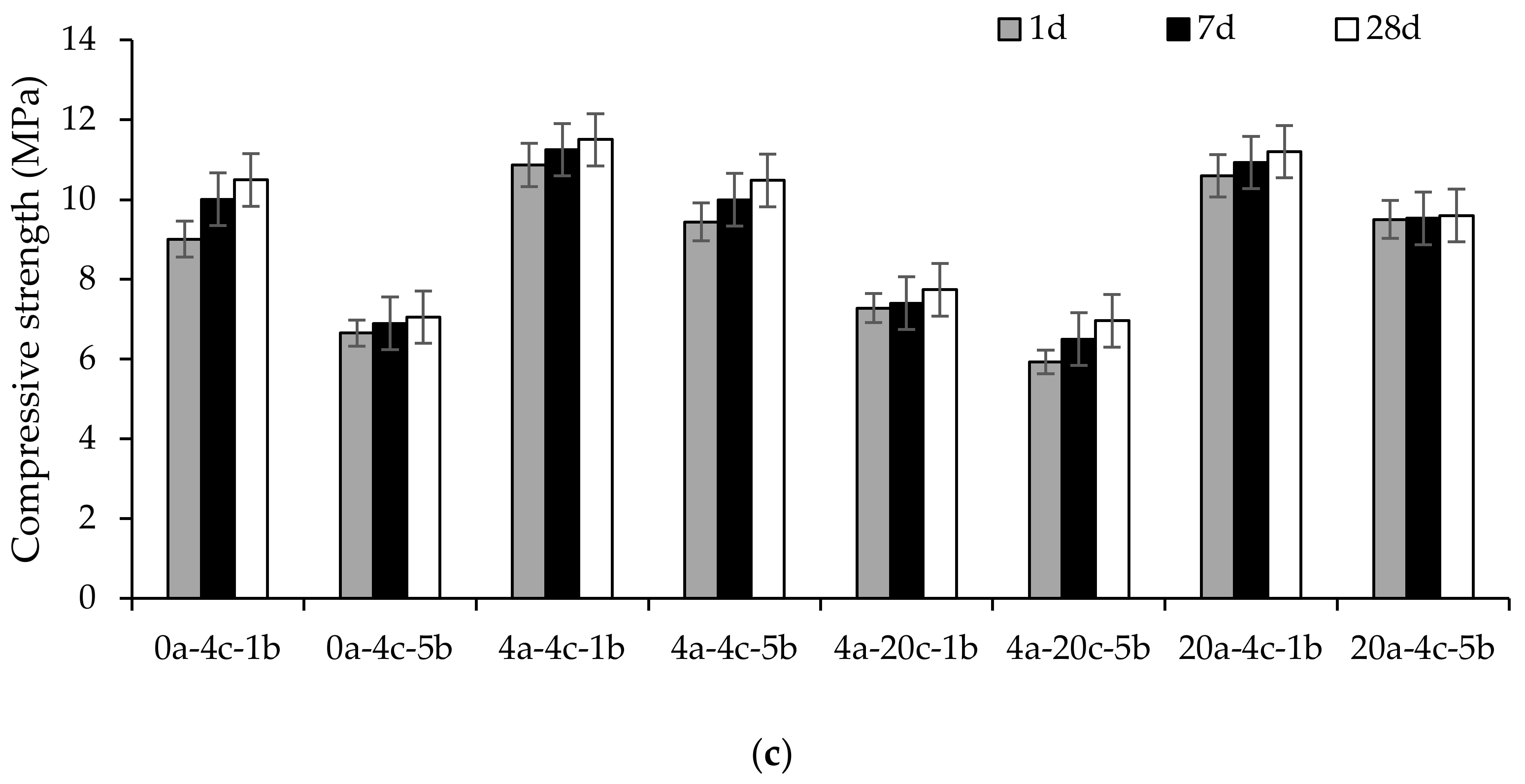
4.2. Compressive Strength
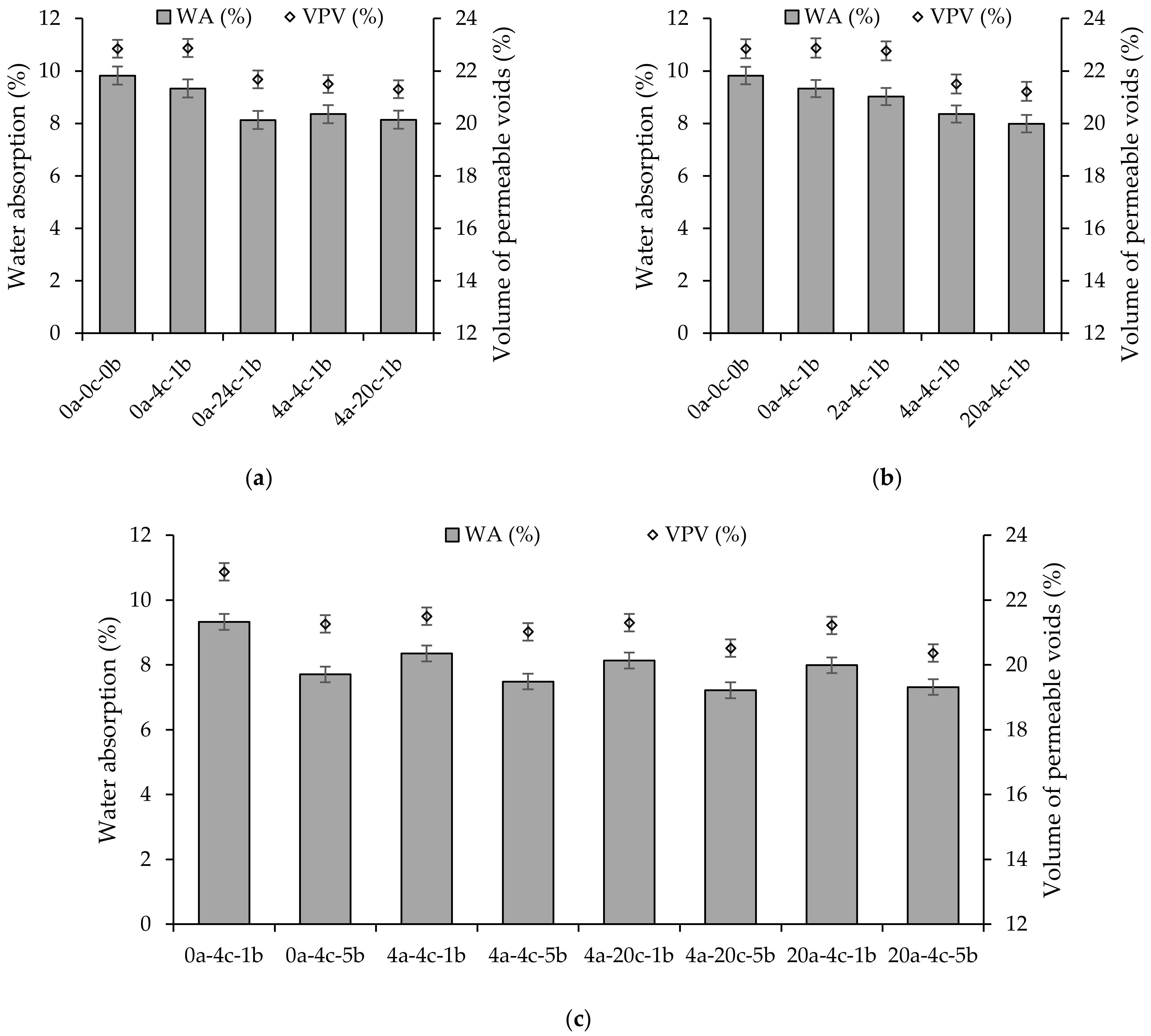
4.3. Water Absorption and Volume of Permeable Voids
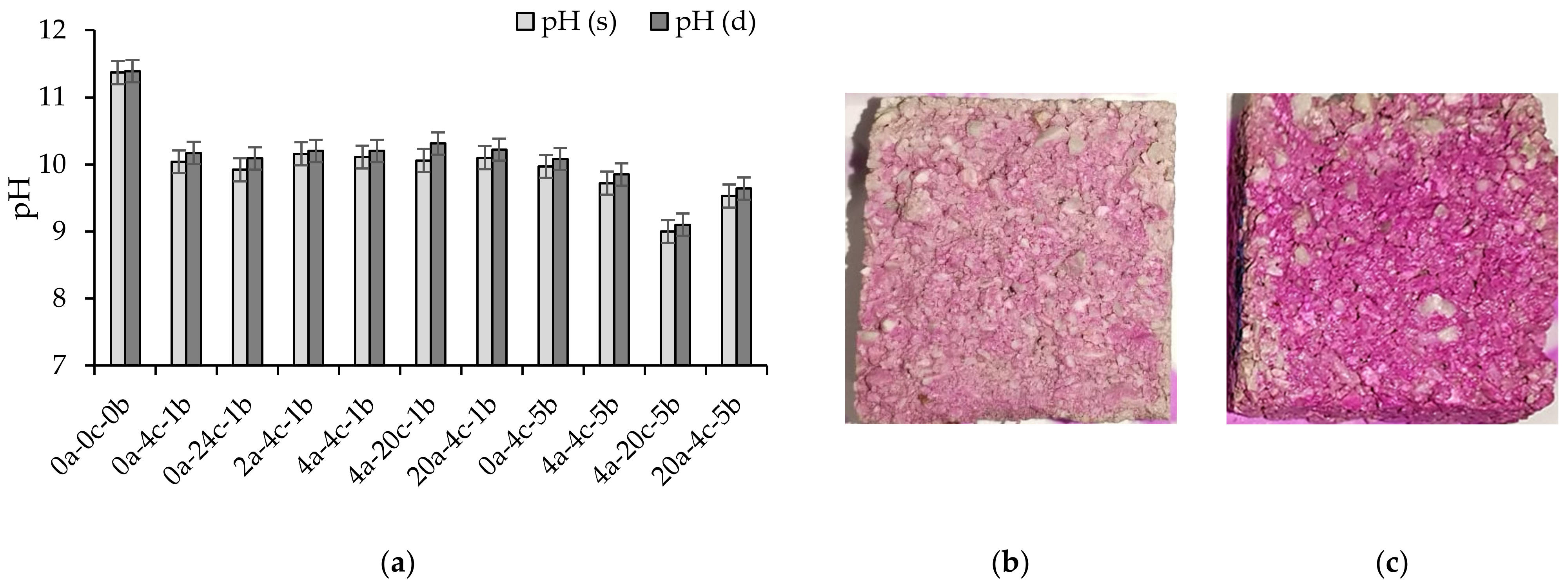
4.4. pH
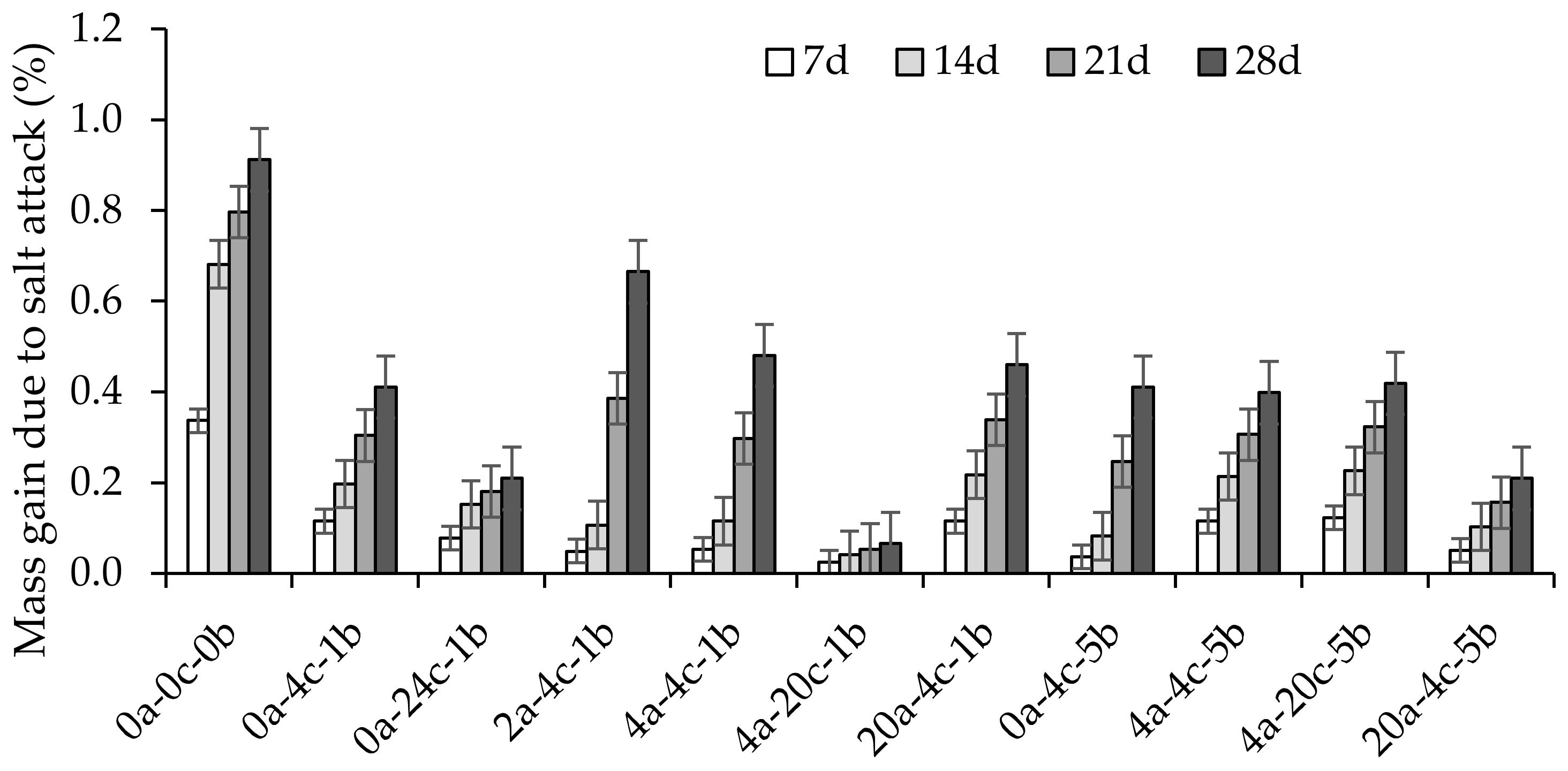
4.5. Salt Attack
4.6. X-ray Diffraction
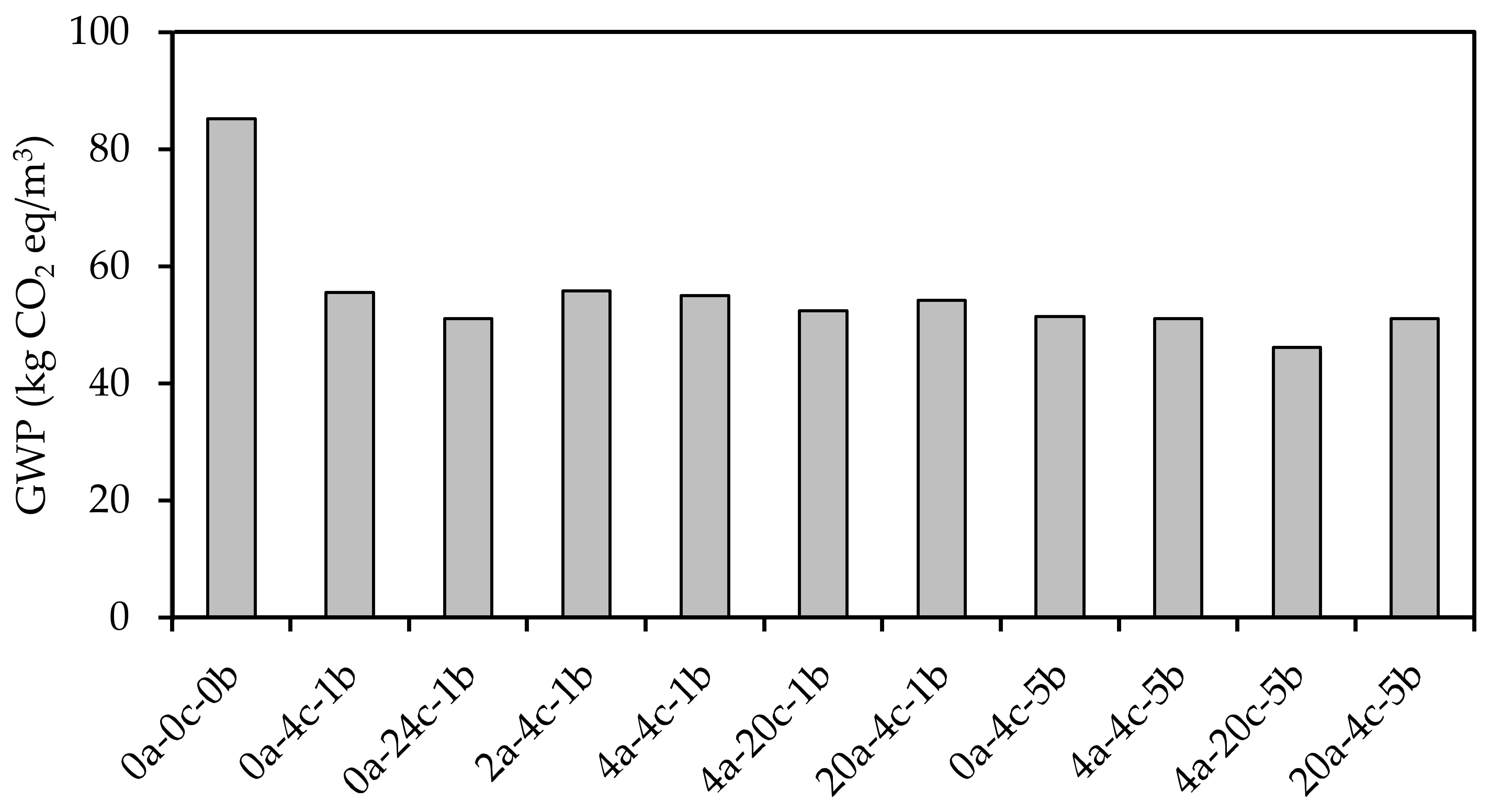
4.7. Environmental Impact
5. Conclusions
- The CO2 uptake of alkali-activated slag CMB mixes increased with an increase in the carbonation duration and carbonation pressure. This was owed to the longer contact time between the calcium-rich material of the binder matrix and CO2 and the deeper penetration of CO2 into the samples at higher pressure, respectively. Longer initial curing duration resulted in higher CO2 uptake due to the removal of water from the matrix, facilitating the penetration of CO2.
- All alkali-activated slag CMB mixes achieved a 1-day strength higher than 4.1 MPa, rendering them acceptable for use as non-load-bearing CMBs, as per ASTM C129. This high-strength development could help in the early service of alkali-activated slag CMBs by the construction and building industry.
- The compressive strength decreased as the carbonation curing duration and pressure increased, owing to the decalcification of calcium-rich activation reaction products and the formation of less strength-contributing calcium carbonate polymorphs. Meanwhile, 2 h initial curing had the least impact on the compressive strength, whereas 0-, 4- and 20 h initial curing caused reductions in strength of up to 33%.
- Carbonation curing had a positive impact on the water absorption and volume of permeable voids of alkali-activated slag CMB mixes. Extending the initial and carbonation curing durations and increasing the carbonation pressure reduced the two properties, as the newly formed calcium carbonate, associated with higher CO2 uptake, filled the pores and voids in the alkali-activated matrix.
- The pH values of alkali-activated slag CMB mixes were reduced with carbonation curing to reach a minimal value of 9.1. The drop in pH was evidenced by the formation of a brighter-colored surface and was related to the consumption of alkaline compounds and the formation of calcium carbonate.
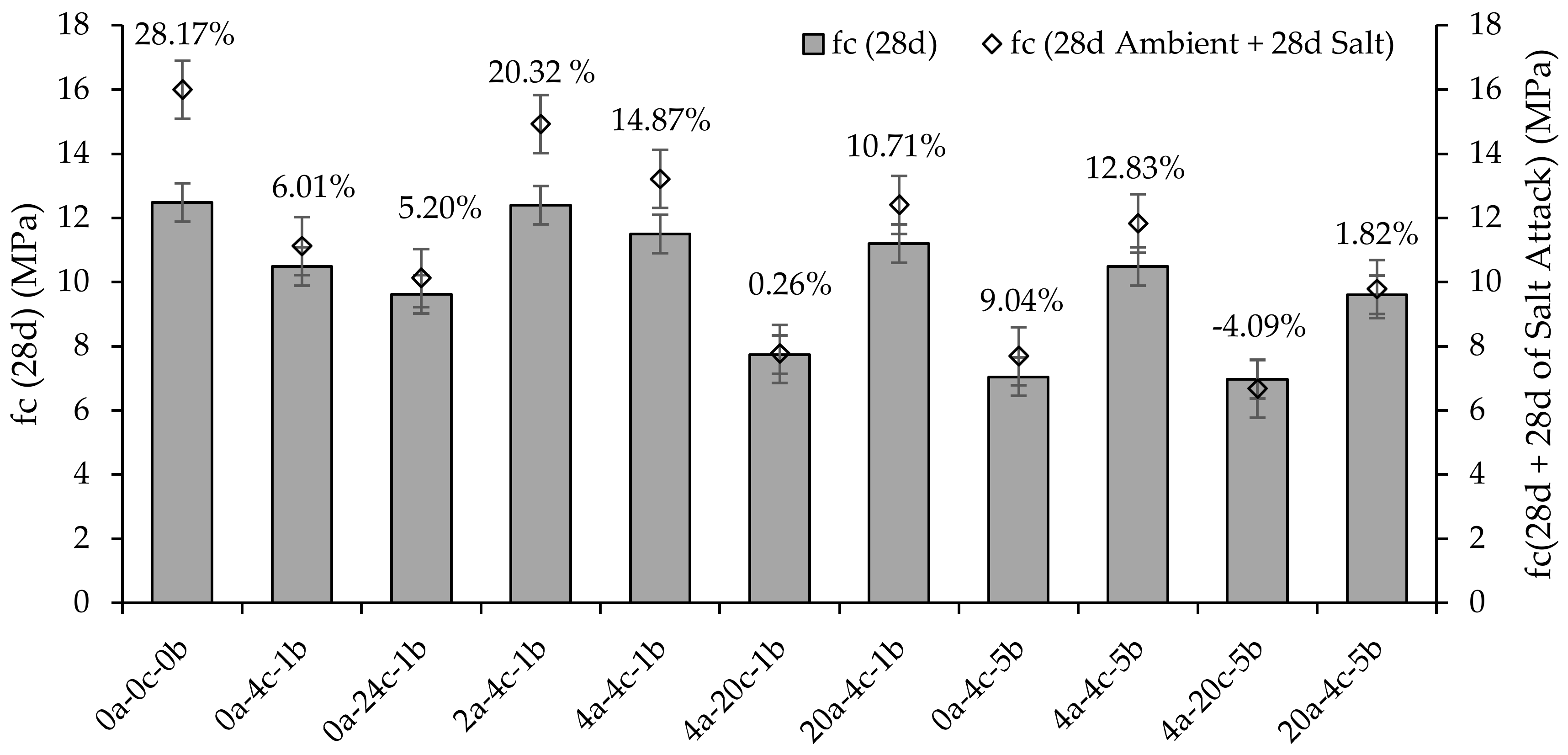
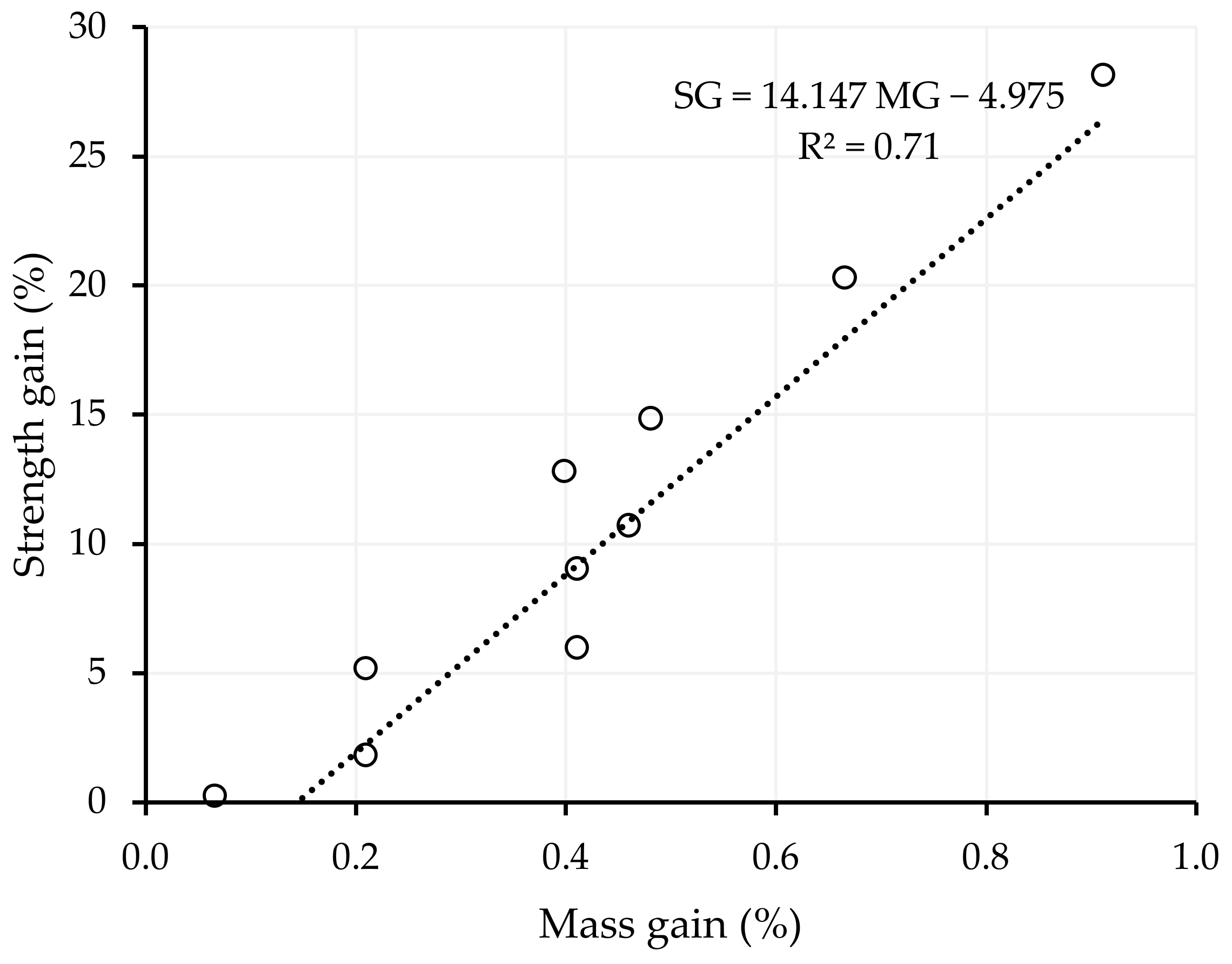
- The masses and strengths of alkali-activated slag CMB mixes increased after 28 days of exposure to salt, except for the sample with the highest CO2 uptake, where its strength was reduced by −4.09%. The mass and strength gains were associated with an increase in the crystalline phases of the CMB matrix. However, as the degree of carbonation increased, lesser activation reaction products were available to react with the NaCl, thereby reducing the mass gain.
- The X-ray diffractograms showed that carbonation curing resulted in the intensification of calcite peaks and reduction in C-S-H peaks. Such an observation explained the drop in strength of alkali-activated slag CMB mixes after carbonation curing. Under salt attack, new chloride-based components, such as Friedle’s salt and Halite, were developed, acting as filler materials, thereby densifying the concrete matrix and increasing its strength.
- The carbonation curing of alkali-activated slag CMB mixes reduced the global warming potential, i.e., carbon footprint, by up to 46%, from 85.2 to 46.1 kg CO2 eq/m3. As all mixes satisfied the strength requirement for non-load-bearing CMBs, the mix subjected to 4 h initial curing followed by 20 h carbonation curing at a pressure of 5 bars (4a-20c-5b) was deemed most suitable among the alkali-activated slag CMB mixes developed herein, as it was associated with the lowest carbon footprint.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Miller, S.A.; John, V.M.; Pacca, S.A.; Horvath, A. Carbon Dioxide Reduction Potential in the Global Cement Industry by 2050. Cem. Concr. Res. 2018, 114, 115–124. [Google Scholar] [CrossRef]
- Yas, Z.; Jaafer, K. Factors Influencing the Spread of Green Building Projects in the UAE. J. Build. Eng. 2020, 27, 100894. [Google Scholar] [CrossRef]
- Bawab, J.; Khatib, J.; Kenai, S.; Sonebi, M. A Review on Cementitious Materials Including Municipal Solid Waste Incineration Bottom Ash (MSWI-BA) as Aggregates. Buildings 2021, 11, 179. [Google Scholar] [CrossRef]
- El-Hassan, H.; Shao, Y.; Ghouleh, Z. Effect of Initial Curing on Carbonation of Lightweight Concrete Masonry Units. ACI Mater. J. 2013, 110, 441–450. [Google Scholar]
- Liu, Z.; Meng, W. Fundamental Understanding of Carbonation Curing and Durability of Carbonation-Cured Cement-Based Composites: A Review. J. CO2 Util. 2021, 44, 101428. [Google Scholar] [CrossRef]
- Zhang, D.; Ghouleh, Z.; Shao, Y. Review on Carbonation Curing of Cement-Based Materials. J. CO2 Util. 2017, 21, 119–131. [Google Scholar] [CrossRef]
- El-Hassan, H.; Shao, Y. Early Carbonation Curing of Concrete Masonry Units with Portland Limestone Cement. Cem. Concr. Compos. 2015, 62, 168–177. [Google Scholar] [CrossRef]
- Rostami, V.; Shao, Y.; Boyd, A.J. Durability of Concrete Pipes Subjected to Combined Steam and Carbonation Curing. Constr. Build. Mater. 2011, 25, 3345–3355. [Google Scholar] [CrossRef]
- El-Hassan, H. Accelerated Carbonation Curing as a Means of Reducing Carbon Dioxide Emissions. In Cement Industry–Optimization, Characterization and Sustainable Application; El-Din Mostafa Saleh, H., Ed.; IntechOpen: London, UK, 2021; ISBN 978-1-83962-314-1. [Google Scholar]
- Hwalla, J.; Bawab, J.; El-Hassan, H.; Abu Obaida, F.; El-Maaddawy, T. Scientometric Analysis of Global Research on the Utilization of Geopolymer Composites in Construction Applications. Sustainability 2023, 15, 11340. [Google Scholar] [CrossRef]
- Hwalla, J.; El-Hassan, H.; Assaad, J.J.; El-Maaddawy, T. Performance of Cementitious and Slag-Fly Ash Blended Geopolymer Screed Composites: A Comparative Study. Case Stud. Constr. Mater. 2023, 18, e02037. [Google Scholar] [CrossRef]
- Assaad, J.J.; Saba, M. Suitability of Metakaolin-Based Geopolymers for Masonry Plastering. Mater. J. 2020, 117, 269–279. [Google Scholar] [CrossRef]
- Samantasinghar, S.; Singh, S.P. Fresh and Hardened Properties of Fly Ash–Slag Blended Geopolymer Paste and Mortar. Int. J. Concr. Struct. Mater. 2019, 13, 47. [Google Scholar] [CrossRef]
- Nassar, R.-U.-D.; Saeed, D.; Sufyan-Ud-Din, M.; Nassar, S. Production of Eco-Friendly Concrete Masonry Units Using Powder Waste Glass. Civ. Eng. Archit. 2022, 10, 415–424. [Google Scholar] [CrossRef]
- Concrete Block and Brick Manufacturing Market to Rise at CAGR of 5% Between 2020 and 2030: TMR Study. Available online: https://finance.yahoo.com/news/concrete-block-brick-manufacturing-market-193300499.html (accessed on 5 April 2023).
- ASTM-C-90-22; Standard Specification for Loadbearing Concrete Masonry Units. ASTM International: West Conshohocken, PA, USA, 2016; p. 5.
- ASTM-C-129-17; Standard Specification for Nonloadbearing Concrete Masonry Units. ASTM International: West Conshohocken, PA, USA, 2017; p. 3.
- Venugopal, K.; Radhakrishna; Sasalatti, V. Development of Alkali Activated Geopolymer Masonry Blocks. IOP Conf. Ser. Mater. Sci. Eng. 2016, 149, 12072. [Google Scholar] [CrossRef]
- Venugopal, K. Radhakrishna Development of Solid and Hollow Geopolymer Masonry Blocks. GSTF J. Eng. Technol. 2016, 4, 10. [Google Scholar] [CrossRef]
- Maruti, S.V.; Krishna, R.; Mounesh, M. Properties of Geopolymer Cement Mortar and Blocks with Calcium Carbonate. Mater. Today Proc. 2020, 24, 1518–1524. [Google Scholar] [CrossRef]
- Petrillo, A.; Cioffi, R.; Ferone, C.; Colangelo, F.; Borrelli, C. Eco-Sustainable Geopolymer Concrete Blocks Production Process. Agric. Agric. Sci. Procedia 2016, 8, 408–418. [Google Scholar] [CrossRef]
- Ban, C.C.; Ken, P.W.; Ramli, M. Effect of Sodium Silicate and Curing Regime on Properties of Load Bearing Geopolymer Mortar Block. J. Mater. Civ. Eng. 2017, 29, 4016237. [Google Scholar] [CrossRef]
- Lamaa, G.; Duarte, A.P.C.; Silva, R.V.; de Brito, J. Carbonation of Alkali-Activated Materials: A Review. Materials 2023, 16, 3086. [Google Scholar] [CrossRef]
- Law, D.W.; Adam, A.A.; Molyneaux, T.K.; Patnaikuni, I.; Wardhono, A. Long Term Durability Properties of Class F Fly Ash Geopolymer Concrete. Mater. Struct. 2015, 48, 721–731. [Google Scholar] [CrossRef]
- Hu, Y.; Tang, Z.; Li, W.; Li, Y.; Tam, V.W.Y. Physical-Mechanical Properties of Fly Ash/GGBFS Geopolymer Composites with Recycled Aggregates. Constr. Build. Mater. 2019, 226, 139–151. [Google Scholar] [CrossRef]
- Yang, H.; Liu, L.; Yang, W.; Liu, H.; Ahmad, W.; Ahmad, A.; Aslam, F.; Joyklad, P. A Comprehensive Overview of Geopolymer Composites: A Bibliometric Analysis and Literature Review. Case Stud. Constr. Mater. 2022, 16, e00830. [Google Scholar] [CrossRef]
- Li, Z.; Li, S. Carbonation Resistance of Fly Ash and Blast Furnace Slag Based Geopolymer Concrete. Constr. Build. Mater. 2018, 163, 668–680. [Google Scholar] [CrossRef]
- Mei, K.; Gu, T.; Zheng, Y.; Zhang, L.; Zhao, F.; Gong, P.; Huang, S.; Zhang, C.; Cheng, X. Effectiveness and Microstructure Change of Alkali-Activated Materials during Accelerated Carbonation Curing. Constr. Build. Mater. 2021, 274, 122063. [Google Scholar] [CrossRef]
- Nguyen, T.N.; Phung, Q.T.; Yu, Z.; Frederickx, L.; Jacques, D.; Sakellariou, D.; Dauzeres, A.; Elsen, J.; Pontikes, Y. Alteration in Molecular Structure of Alkali Activated Slag with Various Water to Binder Ratios under Accelerated Carbonation. Sci. Rep. 2022, 12, 5524. [Google Scholar] [CrossRef] [PubMed]
- Jun, Y.; Han, S.H.; Kim, J.H. Performance of CO2-Cured Alkali-Activated Slag Pastes During Curing and Exposure. Int. J. Concr. Struct. Mater. 2023, 17, 3. [Google Scholar] [CrossRef]
- Hlaváček, P.; Gluth, G.J.G.; Reinemann, S.; Ebell, G.; Kühne, H.-C.; Mietz, J. Corrosion of Steel Reinforcement in Geopolymer Mortars–Carbonation Resistance, Chloride Migration, and Preliminary Corrosion Potential Data. In Proceedings of the European Corrosion Congress, Prague, Czech Republic, 3–7 September 2017. [Google Scholar]
- El-Mir, A.; Hwalla, J.; El-Hassan, H.; Assaad, J.J.; El-Dieb, A.; Shehab, E. Valorization of Waste Perlite Powder in Geopolymer Composites. Constr. Build. Mater. 2023, 368, 130491. [Google Scholar] [CrossRef]
- Zhang, D.; Zhu, H.; Wu, Q.; Yang, T.; Yin, Z.; Tian, L. Investigation of the Hydrophobicity and Microstructure of Fly Ash-Slag Geopolymer Modified by Polydimethylsiloxane. Constr. Build. Mater. 2023, 369, 130540. [Google Scholar] [CrossRef]
- Stefanidou, M. Crushed and River-Origin Sands Used as Aggregates in Repair Mortars. Geosciences 2016, 6, 23. [Google Scholar] [CrossRef]
- Alani, A.; MacMullen, J.; Telik, O.; Zhang, Z.Y. Investigation into the Thermal Performance of Recycled Glass Screed for Construction Purposes. Constr. Build. Mater. 2012, 29, 527–532. [Google Scholar] [CrossRef]
- Das, S.K.; Shrivastava, S. Influence of Molarity and Alkali Mixture Ratio on Ambient Temperature Cured Waste Cement Concrete Based Geopolymer Mortar. Constr. Build. Mater. 2021, 301, 124380. [Google Scholar] [CrossRef]
- Haruna, S.; Mohammed, B.S.; Shahir-Liew, M.; Alaloul, W.S.; Haruna, A. Effect of Water-Binder Ratio and Naoh Molarity on the Properties of High Calcium Fly Ash Geopolymer Mortars at Outdoor Curing. Int. J. Civ. Eng. Technol. 2018, 9, 1339–1352. [Google Scholar]
- Scrivener, K.L.; John, V.M.; Gartner, E.M. Eco-Efficient Cements: Potential Economically Viable Solutions for a Low-CO2 Cement-Based Materials Industry. Cem. Concr. Res. 2018, 114, 2–26. [Google Scholar] [CrossRef]
- Alzard, M.H.; El-Hassan, H.; El-Maaddawy, T. Environmental and Economic Life Cycle Assessment of Recycled Aggregates Concrete in the United Arab Emirates. Sustainability 2021, 13, 10348. [Google Scholar] [CrossRef]
- Davidovits, J. False Values on CO2 Emission for Geopolymer Cement/Concrete Published in Scientific Papers; Geopolymer Institute Library: Saint-Quentin, France, 2015. [Google Scholar]
- Alhassan, M.; Alkhawaldeh, A.; Betoush, N.; Alkhawaldeh, M.; Huseien, G.F.; Amaireh, L.; Elrefae, A. Life Cycle Assessment of the Sustainability of Alkali-Activated Binders. Biomimetics 2023, 8, 58. [Google Scholar] [CrossRef] [PubMed]
- Sun, B.; Ye, G.; de Schutter, G. A Review: Reaction Mechanism and Strength of Slag and Fly Ash-Based Alkali-Activated Materials. Constr. Build. Mater. 2022, 326, 126843. [Google Scholar] [CrossRef]
- Jun, Y.; Han, S.H.; Shin, T.Y.; Kim, J.H. Effects of CO2 Curing on Alkali-Activated Slag Paste Cured in Different Curing Conditions. Materials 2019, 12, 3513. [Google Scholar] [CrossRef]
- Bernal, S.A.; de Gutierrez, R.M.; Provis, J.L.; Rose, V. Effect of Silicate Modulus and Metakaolin Incorporation on the Carbonation of Alkali Silicate-Activated Slags. Cem. Concr. Res. 2010, 40, 898–907. [Google Scholar] [CrossRef]
- Nedeljković, M.; Zuo, Y.; Arbi, K.; Ye, G. Carbonation Resistance of Alkali-Activated Slag Under Natural and Accelerated Conditions. J. Sustain. Metall. 2018, 4, 33–49. [Google Scholar] [CrossRef]
- Nath, P.; Sarker, P.K. Effect of GGBFS on Setting, Workability and Early Strength Properties of Fly Ash Geopolymer Concrete Cured in Ambient Condition. Constr. Build. Mater. 2014, 66, 163–171. [Google Scholar] [CrossRef]
- Ahmad, S.; Assaggaf, R.A.; Maslehuddin, M.; Al-Amoudi, O.S.B.; Adekunle, S.K.; Ali, S.I. Effects of Carbonation Pressure and Duration on Strength Evolution of Concrete Subjected to Accelerated Carbonation Curing. Constr. Build. Mater. 2017, 136, 565–573. [Google Scholar] [CrossRef]
- Albitar, M.; Mohamed Ali, M.S.; Visintin, P.; Drechsler, M. Durability Evaluation of Geopolymer and Conventional Concretes. Constr. Build. Mater. 2017, 136, 374–385. [Google Scholar] [CrossRef]
- Sakr, M.R.; Bassuoni, M.T. Performance of Concrete under Accelerated Physical Salt Attack and Carbonation. Cem. Concr. Res. 2021, 141, 106324. [Google Scholar] [CrossRef]
- Jiao, Z.; Li, X.; Yu, Q.; Yao, Q.; Guan, X.; Hu, P. Chloride Resistance of Class C/Class F Fly Ash-Based Geopolymer Mortars with Different Strength Grades. Case Stud. Constr. Mater. 2023, 18, e01811. [Google Scholar] [CrossRef]
- Özcan, A.; Karakoç, M.B. Evaluation of Sulfate and Salt Resistance of Ferrochrome Slag and Blast Furnace Slag-Based Geopolymer Concretes. Struct. Concr. 2019, 20, 1607–1621. [Google Scholar] [CrossRef]
- Anderson, L.G. Carbonate Dissolution. In Encyclopedia of Marine Geosciences; Harff, J., Meschede, M., Petersen, S., Thiede, J., Eds.; Springer: Dordrecht, The Netherlands, 2013; pp. 1–4. ISBN 978-94-007-6644-0. [Google Scholar]
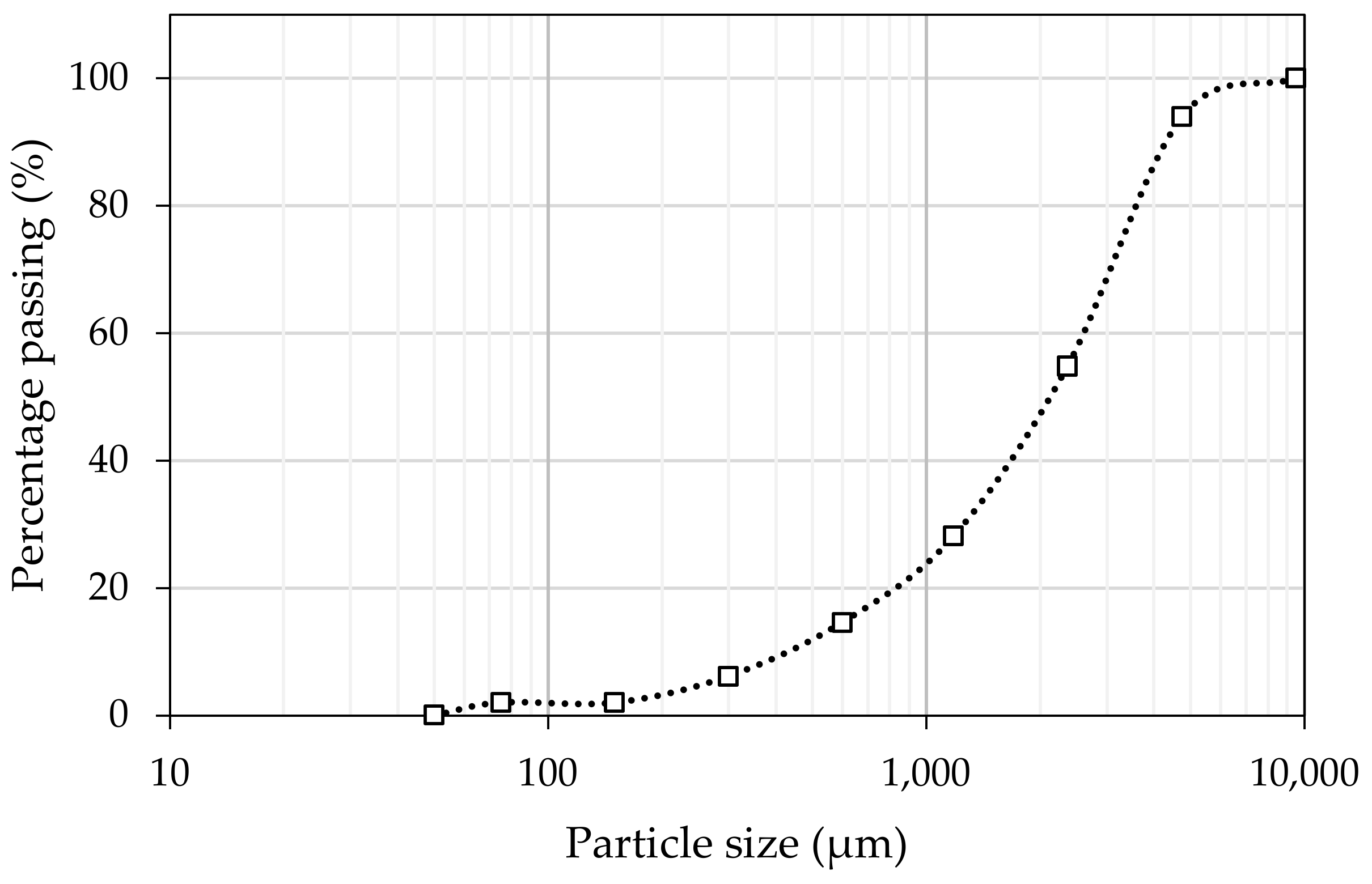





| Oxide | Slag | Crushed Limestone |
|---|---|---|
| CaO, % | 59.7 | 89.3 |
| SiO2, % | 27.0 | 8.5 |
| Al2O3, % | 7.5 | - |
| SO3, % | 2.5 | - |
| Fe2O3, % | 1.2 | 1.5 |
| Loss on ignition, % | 1.1 | - |
| Others, % | 1.0 | 0.7 |
| Scheme No. | Mix Designation | Initial Curing (h) | Carbonation Curing (h) | Pressure (bars) |
|---|---|---|---|---|
| 1 | 0a-0c-0b | - | - | - |
| 2 | 0a-4c-1b | 0 | 4 | 1 |
| 3 | 0a-24c-1b | 0 | 24 | 1 |
| 4 | 2a-4c-1b | 2 | 4 | 1 |
| 5 | 4a-4c-1b | 4 | 4 | 1 |
| 6 | 4a-20c-1b | 4 | 20 | 1 |
| 7 | 20a-4c-1b | 20 | 4 | 1 |
| 8 | 0a-4c-5b | 0 | 4 | 5 |
| 9 | 4a-4c-5b | 4 | 4 | 5 |
| 10 | 4a-20c-5b | 4 | 20 | 5 |
| 11 | 20a-4c-5b | 20 | 4 | 5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hwalla, J.; Al-Mazrouei, M.; Al-Karbi, K.; Al-Hebsi, A.; Al-Ameri, M.; Al-Hadrami, F.; El-Hassan, H. Performance of Alkali-Activated Slag Concrete Masonry Blocks Subjected to Accelerated Carbonation Curing. Sustainability 2023, 15, 14291. https://doi.org/10.3390/su151914291
Hwalla J, Al-Mazrouei M, Al-Karbi K, Al-Hebsi A, Al-Ameri M, Al-Hadrami F, El-Hassan H. Performance of Alkali-Activated Slag Concrete Masonry Blocks Subjected to Accelerated Carbonation Curing. Sustainability. 2023; 15(19):14291. https://doi.org/10.3390/su151914291
Chicago/Turabian StyleHwalla, Joud, Mahra Al-Mazrouei, Khalood Al-Karbi, Afraa Al-Hebsi, Mariam Al-Ameri, Fatima Al-Hadrami, and Hilal El-Hassan. 2023. "Performance of Alkali-Activated Slag Concrete Masonry Blocks Subjected to Accelerated Carbonation Curing" Sustainability 15, no. 19: 14291. https://doi.org/10.3390/su151914291
APA StyleHwalla, J., Al-Mazrouei, M., Al-Karbi, K., Al-Hebsi, A., Al-Ameri, M., Al-Hadrami, F., & El-Hassan, H. (2023). Performance of Alkali-Activated Slag Concrete Masonry Blocks Subjected to Accelerated Carbonation Curing. Sustainability, 15(19), 14291. https://doi.org/10.3390/su151914291






