Application of a Magnetic Field to Enhance the Environmental Sustainability and Efficiency of Microbial and Plant Biotechnological Processes
Abstract
:1. Introduction
2. Methods
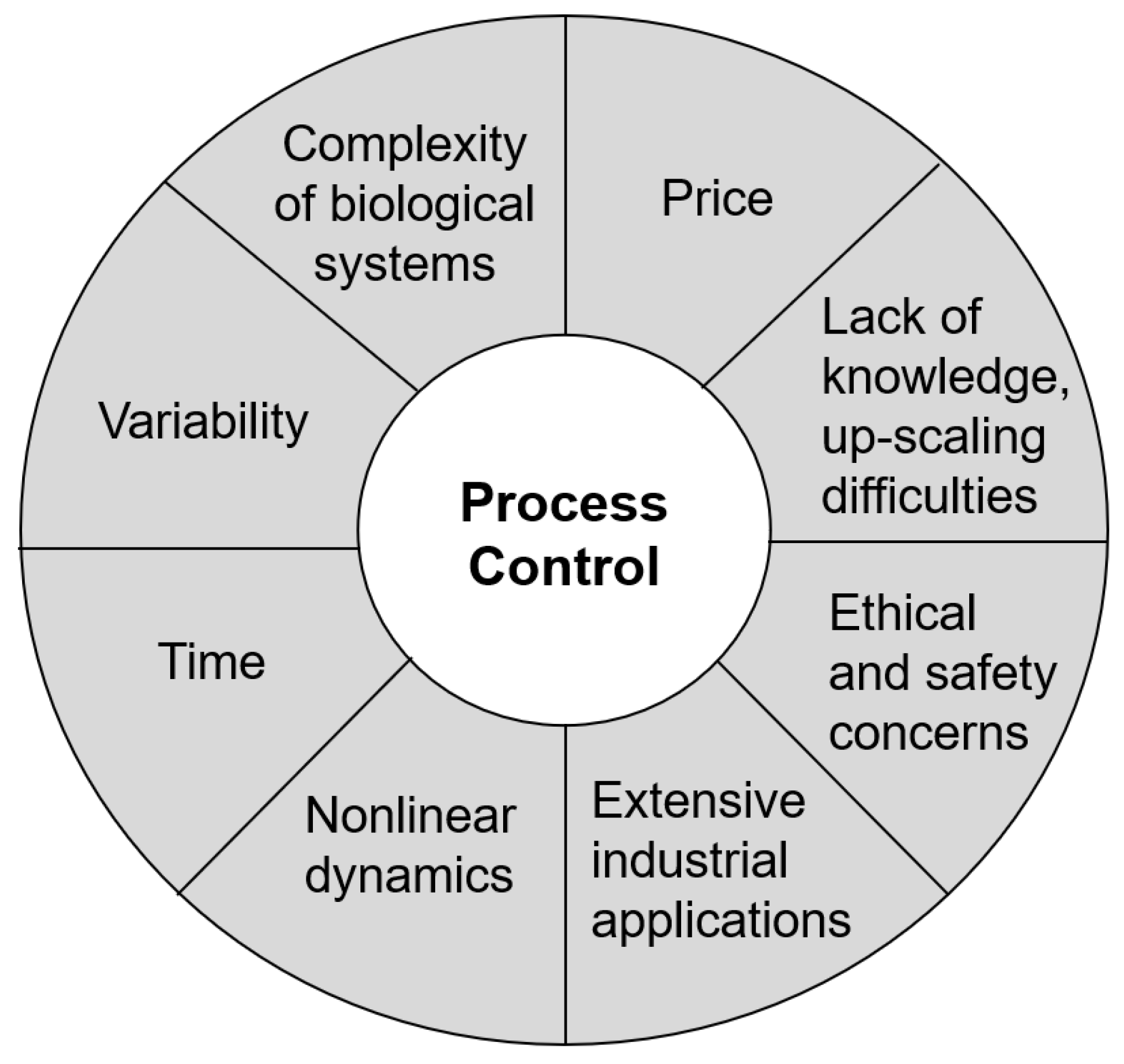
3. Control of Biotechnological Process
- Complexity of biological systems: Organisms commonly used in biotechnological applications are complex biological systems; therefore, it is difficult to predict how specific changes in the environment will affect the organism at multiple levels, and even subtle changes can lead to inconsistent outcomes or alterations in the quality or quantity of the resulting product [6].
- Variability: Biological systems used in biotechnology include bacteria, fungi, plants, as well as higher organisms. Therefore, a uniform method of controlling processes is impossible, and each biotechnological application, along with each organism used within it, requires specific conditions for the optimal growth and production of the target product [7,8].
- Nonlinear dynamics: Biological systems often exhibit nonlinear dynamics, meaning that small changes in one part of the system can have disproportionately large effects on the overall behavior of the system. This effect can make it difficult to predict how a biotechnological process will respond to changes in external or internal parameters [9,10].
- Lack of knowledge and up-scaling difficulties: Despite advances in biology, much remains unknown about how biological systems function and precisely respond to changes in the environmental conditions at the physiological or genetic levels. This can pose challenges in designing and controlling biotechnological processes with a high degree of precision [3]. It is important to ensure that the process can operate at larger volumes without losses in the efficiency or product quality [11].
- The price: Biotechnological processes used in certain industries, such as the pharmaceutical industry, may be less cost-effective compared to traditional industrial processes [12]. Furthermore, these processes often require specialized equipment to provide the optimal conditions for the used organisms, which can further increase their costs [13]. Seeking cost-effective strategies for the control of biotechnological processes is one important approach for making biotechnological processes more accessible and competitive [14].
- Time: Certain biotechnological processes, such as protein or metabolite production using microorganisms, bacterial bioleaching, and microbial waste treatment, can, in some cases, be slower compared to conventional processes [5]. This can pose a challenge for the industrial implementation of these biotechnological processes, which often require large quantities of a product in a short period [4].
- Ethical and safety concerns: Ethical and safety considerations still exist and must be taken into account when working with biological systems. The potential risks and unintended consequences of biotechnological processes can be subjects of public concern, which can present challenges in balancing the benefits of a particular process with its potential risks [15].
- Extensive industrial application of biotechnology: The use of living systems is not limited to a single industry but can be found in nearly every sector, from healthcare to environmental remediation and waste processing [16]. Since each of these industries uses different organisms and produces different target products, they require a varying process control complexity and face different challenges [17].
4. The Application of Magnetic Fields in Biotechnological Processes Using Eukaryotic Organisms
4.1. The Food Industry and Ethanol Production
4.2. Medical and Laboratory Applications
4.3. Pollutant Removal
5. Application of Magnetic Fields in Biotechnological Processes Involving Prokaryotic Organisms
5.1. Wastewater Treatment
5.2. Pollutant Removal
5.3. Food Industry Applications
5.4. Medical Applications
5.5. Metabolism Regulation and the Activation of Enzymes
5.6. Metal Recovery
6. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Zhang, B.; Tian, L. Reactive Oxygen Species: Potential Regulatory Molecules in Response to Hypomagnetic Field Exposure. Bioelectromagnetics 2020, 41, 573–580. [Google Scholar] [CrossRef] [PubMed]
- Saliev, T.; Begimbetova, D.; Masoud, A.-R.; Matkarimov, B. Biological effects of non-ionizing electromagnetic fields: Two sides of a coin. Prog. Biophys. Mol. Biol. 2018, 141, 25–36. [Google Scholar] [CrossRef] [PubMed]
- Dabros, M.; Schuler, M.M.; Marison, I.W. Simple control of specific growth rate in biotechnological fed-batch processes based on enhanced online measurements of biomass. Bioprocess Biosyst. Eng. 2010, 33, 1109–1118. [Google Scholar] [CrossRef]
- Sedlakova-Kadukova, J. Microorganisms in Metal Recovery—Tools or Teachers? Chapter 4, Microbial Syntrophy Mediated Eco-enterprising, Developments in Applied Microbiology and Biotechnology; Academic Press: New York, NY, USA, 2022; Volume 21, pp. 71–86. [Google Scholar]
- Panchal, T.M.; Patel, A.; Chauhan, D.; Thomas, M.; Patel, J.V. A methodological review on bio-lubricants from vegetable oil based resources. Renew. Sustain. Energy Rev. 2017, 70, 65–70. [Google Scholar] [CrossRef]
- Singh, S.; Rajam, M.V. Citrus biotechnology: Achievements, limitations and future directions. Physiol. Mol. Biol. Plants 2009, 15, 3–22. [Google Scholar] [CrossRef]
- Poulsen, L.K. Allergy assessment of foods or ingredients derived from biotechnology, gene-modified organisms, or novel foods. Mol. Nutr. Food Res. 2004, 48, 413–423. [Google Scholar] [CrossRef]
- Romano, D.; Donati, E.; Benelli, G.; Stefanini, C. A review on animal–robot interaction: From bio-hybrid organisms to mixed societies. Biol. Cybern. 2019, 113, 201–225. [Google Scholar] [CrossRef]
- Baby, B. Bio-bibliometric Analysis of Literature Output of Prof. M. Lakshmanan in the Subject of Nonlinear Dynamics. Asia Pac. J. Lib. Manag. Inf. Technol. 2012, 2. [Google Scholar]
- Telen, D.; Logist, F.; Quirynen, R.; Houska, B.; Diehl, M.; Van Impe, J. Optimal experiment design for nonlinear dynamic (bio)chemical systems using sequential semidefinite programming. AIChE J. 2014, 60, 1728–1739. [Google Scholar] [CrossRef]
- Karan, H.; Funk, C.; Grabert, M.; Oey, M.; Hankamer, B. Green Bioplastics as Part of a Circular Bioeconomy. Trends Plant Sci. 2019, 24, 237–249. [Google Scholar] [CrossRef]
- Gavrilescu, M.; Chisti, Y. Biotechnology—A sustainable alternative for chemical industry. Biotechnol. Adv. 2005, 23, 471–499. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Rahman, M.A.; Tashiro, Y.; Zendo, T.; Shibata, K.; Sonomoto, K. Isolation and characterization of lactic acid bacterium for effective fermentation of cellobiose into optically pure homo L-(+)-lactic acid. Appl. Microbiol. Biotechnol. 2011, 89, s00253-s010. [Google Scholar] [CrossRef] [PubMed]
- Straathof, A.J.; Wahl, S.A.; Benjamin, K.R.; Takors, R.; Wierckx, N.; Noorman, H.J. Grand Research Challenges for Sustainable Industrial Biotechnology. Trends Biotechnol. 2019, 37, 1042–1050. [Google Scholar] [CrossRef] [PubMed]
- Dutfield, G. Intellectual property, biogenetic resources and traditional knowledge. Earthscan 2010, 2010, 847. [Google Scholar] [CrossRef]
- Chen, G.Q.; Jiang, X.R. Next-generation industrial biotechnology based on extremophilic bacteria. Curr. Opin. Biotechnol. 2018, 50, 94–100. [Google Scholar] [CrossRef]
- Chen, Y.; Nielsen, J. Biobased organic acids production by metabolically engineered microorganisms. Curr. Opin. Biotechnol. 2016, 37, 165–172. [Google Scholar] [CrossRef]
- Hayashi, K.; Nakamura, M.; Miki, H.; Ozaki, S.; Abe, M.; Matsumoto, T.; Sakamoto, W.; Yogo, T.; Ishimura, K. Magnetically responsive smart nanoparticles for cancer treatment with a combination of magnetic hyperthermia and remote-control drug release. Theranostics 2014, 4, 834. [Google Scholar] [CrossRef]
- Yuan, L.Q.; Wang, C.; Lu, D.F.; Zhao, X.D.; Tan, L.H.; Chen, X. Induction of apoptosis and ferroptosis by a tumor suppressing magnetic field through ROS-mediated DNA damage. Aging 2020, 12, 3662. [Google Scholar] [CrossRef]
- Saifzadeh, S.; Hobbennaghi, R.; Shokouhi Sabet Jalali, F. Effect of a static magnetic field on bone healing in the dog: Radiographic and histopathological studies. Iran. J. Vet. Res. 2007, 8, 8–15. [Google Scholar]
- Cheing, G.L.-Y.; Li, X.; Huang, L.; Kwan, R.L.-C.; Cheung, K.-K. Pulsed electromagnetic fields (PEMF) promote early wound healing and myofibroblast proliferation in diabetic rats. Bioelectromagnetics 2014, 35, 161–169. [Google Scholar] [CrossRef]
- Wosik, J.; Chen, W.; Qin, K.; Ghobrial, R.M.; Kubiak, J.Z.; Kloc, M. Magnetic Field Changes Macrophage Phenotype. Biophys. J. 2018, 114, 2001–2013. [Google Scholar] [CrossRef] [PubMed]
- Yu, B.; Choi, B.; Li, W.; Kim, D.-H. Magnetic field boosted ferroptosis-like cell death and responsive MRI using hybrid vesicles for cancer immunotherapy. Nat. Commun. 2020, 11, 805. [Google Scholar] [CrossRef]
- Luo, J.; He, W.; Xing, X.; Wu, J.; Gu, X.S. The phytoremediation efficiency of Eucalyptus globulus treated by static magnetic fields before sowing. Chemosphere 2019, 226, 891–897. [Google Scholar] [CrossRef] [PubMed]
- Luo, J.; He, W.; Yang, D.; Wu, J.; Gu, X.S. Magnetic field enhance decontamination efficiency of Noccaea caerulescens and reduce leaching of non-hyperaccumulated metals. J. Hazard. Mater. 2019, 368, 141–148. [Google Scholar] [CrossRef] [PubMed]
- Yang, P.; Gan, T.; Pi, W.; Cao, M.; Chen, D.; Luo, J. Effect of using Celosia argentea grown from seeds treated with a magnetic field to conduct Cd phytoremediation in drought stress conditions. Chemosphere 2021, 280, 130724. [Google Scholar] [CrossRef]
- Luo, J.; He, W.; Qi, S.; Wu, J.; Gu, X.S. A novel phytoremediation method assisted by magnetized water to decontaminate soil Cd based on harvesting senescent and dead leaves of Festuca arundinacea. J. Hazard. Mater. 2020, 383, 121115. [Google Scholar] [CrossRef]
- Tu, R.; Jin, W.; Xi, T.; Yang, Q.; Han, S.-F.; Abomohra, A.E.-F. Effect of static magnetic field on the oxygen production of Scenedesmus obliquus cultivated in municipal wastewater. Water Res. 2015, 86, 132–138. [Google Scholar] [CrossRef]
- Perez, V.H.; Reyes, A.F.; Justo, O.R.; Alvarez, D.C.; Alegre, R.M. Bioreactor Coupled with Electromagnetic Field Generator: Effects of Extremely Low Frequency Electromagnetic Fields on Ethanol Production by Saccharomyces cerevisiae. Biotechnol. Prog. 2007, 23, 1091–1094. [Google Scholar] [CrossRef]
- da Motta, M.A.; Muniz, J.B.F.; Schuler, A.; da Motta, M. Static Magnetic Fields Enhancement of Saccharomyces cerevisae Ethanolic Fermentation. Biotechnol. Prog. 2004, 20, 393–396. [Google Scholar] [CrossRef]
- Kladko, D.V.; Zakharzhevskii, M.A.; Vinogradov, V.V. Magnetic Field-Mediated Control of Whole-Cell Biocatalysis. J. Phys. Chem. Lett. 2020, 11, 8989–8996. [Google Scholar] [CrossRef]
- Canli, O.; Kurbanoglu, E.B. Application of low magnetic field on inulinase production by Geotrichum candidum under solid state fermentation using leek as substrate. Toxicol. Ind. Health 2012, 28, 894–900. [Google Scholar] [CrossRef]
- Boeira, C.Z.; Silvello, M.A.d.C.; Remedi, R.D.; Feltrin, A.C.P.; Santos, L.O.; Garda-Buffon, J. Mitigation of nivalenol using alcoholic fermentation and magnetic field application. Food Chem. 2021, 340, 127935. [Google Scholar] [CrossRef] [PubMed]
- Anton-Leberre, V.; Haanappel, E.; Marsaud, N.; Trouilh, L.; Benbadis, L.; Boucherie, H.; Massou, S.; François, J.M. Exposure to high static or pulsed magnetic fields does not affect cellular processes in the yeast Saccharomyces cerevisiae. Bioelectromagnetics 2010, 31, 28–38. [Google Scholar] [CrossRef] [PubMed]
- Binninger, D.M.; Ungvichian, V. Effects of 60 Hz AC magnetic fields on gene expression following exposure over multiple cell generations using Saccharomyces cerevisiae. Bioelectrochem. Bioenerg. 1997, 43, 83–89. [Google Scholar] [CrossRef]
- Sotirios-Spyridon, V.; Kapolos, J. Factors affecting yeast ethanol tolerance and fermentation efficiency. World J. Microbiol. Biotechnol. 2020, 36, 818. [Google Scholar] [CrossRef]
- Walker, G.M.; Stewart, G.G. Saccharomyces cerevisiae in the Production of Fermented Beverages. Beverages 2016, 2, 30. [Google Scholar] [CrossRef]
- Abo, B.O.; Gao, M.; Wang, Y.; Wu, C.; Ma, H.; Wang, Q. Lignocellulosic biomass for bioethanol: An overview on pretreatment, hydrolysis and fermentation processes. Rev. Environ. Health 2019, 34, 57–68. [Google Scholar] [CrossRef]
- Juutilainen, J.; Höytö, A.; Kumlin, T.; Naarala, J. Review of possible modulation-dependent biological effects of radiofrequency fields. Bioelectromagnetics 2011, 32, 511–534. [Google Scholar] [CrossRef]
- Nathanail, A.V.; Gibson, B.; Han, L.; Peltonen, K.; Ollilainen, V.; Jestoi, M.; Laitila, A. The lager yeast Saccharomyces pastorianus removes and transforms Fusarium trichothecene mycotoxins during fermentation of brewer’s wort. Food Chem. 2016, 203, 448–455. [Google Scholar] [CrossRef]
- Wang, Y.; Zhao, W.; Hao, J.; Xu, W.; Luo, Y.; Wu, W.; Yang, Z.; Liang, Z.; Huang, K. Changes in biosynthesis and metabolism of glutathione upon ochratoxin A stress in Arabidopsis thaliana. Plant Physiol. Biochem. 2014, 79, 10–18. [Google Scholar] [CrossRef]
- Santos, L.O.; Alegre, R.M.; Garcia-Diego, C.; Cuellar, J. Effects of magnetic fields on biomass and glutathione production by the yeast Saccharomyces cerevisiae. Process. Biochem. 2010, 45, 1362–1367. [Google Scholar] [CrossRef]
- Feng, C.; Yu, B.; Song, C.; Wang, J.; Zhang, L.; Ji, X.; Wang, Y.; Fang, Y.; Liao, Z.; Wei, M.; et al. Static Magnetic Fields Reduce Oxidative Stress to Improve Wound Healing and Alleviate Diabetic Complications. Cells 2022, 11, 443. [Google Scholar] [CrossRef] [PubMed]
- Mathieu, P.S.; Loboa, E.G. Cytoskeletal and focal adhesion influences on mesenchymal stem cell shape, mechanical properties, and differentiation down osteogenic, adipogenic, and chondrogenic pathways. Tissue Eng. Part B Rev. 2012, 18, 436–444. [Google Scholar] [CrossRef] [PubMed]
- Cieślińska-Świder, J. Przegląd Metod Fizykoterapeutycznych Stosowanych w Reumatoidalnym Zapaleniu Stawów; The Jerzy Kukuczka Academy of Physical Education: Katowice, Poland, 2014; pp. 64–69. [Google Scholar]
- Marycz, K.; Kornicka, K.; Röcken, M. Static Magnetic Field (SMF) as a Regulator of Stem Cell Fate – New Perspectives in Regenerative Medicine Arising from an Underestimated Tool. Stem Cell Rev. Rep. 2018, 14, 785–792. [Google Scholar] [CrossRef] [PubMed]
- Ross, C.L. The use of electric, magnetic, and electromagnetic field for directed cell migration and adhesion in regenerative medicine. Biotechnol. Prog. 2017, 33, 5–16. [Google Scholar] [CrossRef]
- Kaszuba-Zwoinska, J.; Chorobik, P.; Juszczak, K.; Zaraska, W.; Thor, P.J. Pulsed electromagnetic field affects intrinsic and endoplasmatic reticulum apoptosis induction pathways in MonoMac6 cell line culture. J. Physiol. Pharmacol. 2012, 63, 537–545. [Google Scholar]
- Pesce, M.; Patruno, A.; Speranza, L.; Reale, M. Extremely low frequency electromagnetic field and wound healing: Implication of cytokines as biological mediators. Eur. Cytokine Netw. 2013, 24, 332. [Google Scholar] [CrossRef]
- Ganeva, V.; Galutzov, B.; Teissie, J. Evidence that Pulsed Electric Field Treatment Enhances the Cell Wall Porosity of Yeast Cells. Appl. Biochem. Biotechnol. 2014, 172, 1540–1552. [Google Scholar] [CrossRef]
- Fang, D.-D.; Zhang, L.-Z.; Wang, Q. Effects of Superparamagnetic Nanomaterials on Soil Microorganisms and Enzymes in Cadmium-Contaminated Paddy Fields. Huan Jing ke Xue Huanjing Kexue 2021, 42, 1523–1534. [Google Scholar] [CrossRef]
- Soghomonyan, D.; Trchounian, K.; Trchounian, A. Millimeter waves or extremely high frequency electromagnetic fields in the environment: What are their effects on bacteria? Appl. Microbiol. Biotechnol. 2016, 100, 4761–4771. [Google Scholar] [CrossRef]
- Zaidi, N.S.; Muda, K.; Sohaili, J.; Loan, L.W.; Sillanpää, M. Enhancement of nitrification efficiency during sludge bulking by magnetic field under long sludge retention time. 3 Biotech 2020, 10, 989. [Google Scholar] [CrossRef]
- Wang, Z.; Chen, C.; Liu, H.; Hrynshpan, D.; Savitskaya, T.; Chen, J.; Chen, J. Effects of carbon nanotube on denitrification performance of Alcaligenes sp. TB: Promotion of electron generation, transportation and consumption. Ecotoxicol. Environ. Saf. 2019, 183, 109507. [Google Scholar] [CrossRef] [PubMed]
- Hou, L.; Liu, Y.; Fan, S.; Li, J. Magnetic field enhanced denitrification efficiency of immobilized bacterial particles. Water Sci. Technol. 2020, 81, 622–629. [Google Scholar] [CrossRef] [PubMed]
- Lu, H.; Wang, X.; Hu, S.; Han, T.; He, S.; Zhang, G.; He, M.; Lin, X. Bioeffect of static magnetic field on photosynthetic bacteria: Evaluation of bioresources production and wastewater treatment efficiency. Water Environ. Res. 2020, 92, 1131–1141. [Google Scholar] [CrossRef]
- Mansouri, A.; Abbes, C.; Ben Mouhoub, R.; Ben Hassine, S.; Landoulsi, A. Enhancement of mixture pollutant biodegradation efficiency using a bacterial consortium under static magnetic field. PLoS ONE 2019, 14, e0208431. [Google Scholar] [CrossRef]
- Qu, M.; Chen, J.; Huang, Q.; Chen, J.; Xu, Y.; Luo, J.; Wang, K.; Gao, W.; Zheng, Y. Bioremediation of hexavalent chromium contaminated soil by a bioleaching system with weak magnetic fields. Int. Biodeterior. Biodegrad. 2018, 128, 41–47. [Google Scholar] [CrossRef]
- Ren, Z.; Leng, X.; Liu, Q. Effect of a static magnetic field on the microscopic characteristics of highly efficient oil-removing bacteria. Water Sci. Technol. 2018, 77, 296–303. [Google Scholar] [CrossRef]
- Lin, L.; Wang, X.; Cui, H. Synergistic efficacy of pulsed magnetic fields and Litseacubeba essential oil treatment against Escherichia coli O157:H7 in vegetable juices. Food Control. 2019, 106, 106686. [Google Scholar] [CrossRef]
- Bandara, H.; Nguyen, D.; Mogarala, S.; Osiñski, M.; Smyth, H. Magnetic fields suppress Pseudomonas aeruginosa biofilms and enhance ciprofloxacin activity. Biofouling 2015, 31, 443–457. [Google Scholar] [CrossRef]
- Fan, W.; Huang, Z.; Fan, B. Effects of prolonged exposure to moderate static magnetic field and its synergistic effects with alkaline pH on Enterococcus faecalis. Microb. Pathog. 2018, 115, 117–122. [Google Scholar] [CrossRef]
- Brkovic, S.; Postic, S.; Ilic, D. Influence of the magnetic field on microorganisms in the oral cavity. J. Appl. Oral Sci. 2015, 23, 179–186. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Zhang, K.; Wang, M.; Zhang, Z.; Feng, Y. Enhancement of magnetic field on fermentative hydrogen production by Clostridium pasteurianum. Bioresour. Technol. 2021, 341, 125764. [Google Scholar] [CrossRef]
- Lu, L.L.; Li, D.W.; Yu, L. Study on Intensified Bioleaching Technology by Static Magnetic Field of Chalcopyrite Tailings. Appl. Mech. Mater. 2011, 84–85, 476–479. [Google Scholar] [CrossRef]
- Lu, L.L.; Li, D.W.; Wei, X.D.; Liao, C.J.; Jiang, J.L.; Su, L. The Effects of Static Magnetic Field in Leaching Cadmium and Arsenic by Acidthiobacillus ferrooxidans. Appl. Mech. Mater. 2014, 675-677, 90–93. [Google Scholar] [CrossRef]
- Gu, W.; Miller, S.; Chiu, C.Y. Clinical Metagenomic Next-Generation Sequencing for Pathogen Detection. Annu. Rev. Pathol. Mech. Dis. 2019, 14, 319–338. [Google Scholar] [CrossRef] [PubMed]
- Le, D.W.; Huang, T.; Lu, L. Research on the intensified bioleaching technology based on the optimized model of the orthogonal method in the static electromagnetic field. Disaster Adv. 2013, 6, 299–307. [Google Scholar]
- Wei, X.; Liu, D.; Huang, W.; Lei, Z. Simultaneously enhanced Cu bioleaching from E-wastes and recovered Cu ions by direct current electric field in a bioelectrical reactor. Bioresour. Technol. 2020, 298, 122566. [Google Scholar] [CrossRef]
- Korolczuk, J.; Mc Keag, J.R.; Fernandez, J.C.; Baron, F.; Grosset, N.; Jeantet, R. Effect of pulsed electric field processing parameters on Salmonella enteritidis inactivation. J. Food Eng. 2006, 75, 11–20. [Google Scholar] [CrossRef]
- Mok, J.H.; Choi, W.; Park, S.H.; Lee, S.H.; Jun, S. Emerging pulsed electric field (PEF) and static magnetic field (SMF) combination technology for food freezing. Int. J. Refrig. 2015, 50, 137–145. [Google Scholar] [CrossRef]
- Jia, W.; Fan, Z.; Du, A.; Shi, L. Molecular mechanism of Mare Nectaris and magnetic field on the formation of ethyl carbamate during 19 years aging of Feng-flavor Baijiu. Food Chem. 2022, 382, 132357. [Google Scholar] [CrossRef]
- Hardiansyah, A.; Yang, M.-C.; Liao, H.-L.; Cheng, Y.-W.; Destyorini, F.; Irmawati, Y.; Liu, C.-M.; Yung, M.-C.; Hsu, C.-C.; Liu, T.-Y. Magnetic Graphene-Based Sheets for Bacteria Capture and Destruction Using a High-Frequency Magnetic Field. Nanomaterials 2020, 10, 674. [Google Scholar] [CrossRef] [PubMed]
- Woroszyło, M.; Ciecholewska-Juśko, D.; Junka, A.; Wardach, M.; Chodaczek, G.; Dudek, B.; Fijałkowski, K. The Effect of Rotating Magnetic Field on Susceptibility Profile of Methicillin-Resistant Staphylococcus aureus Strains Exposed to Activity of Different Groups of Antibiotics. Int. J. Mol. Sci. 2021, 22, 11551. [Google Scholar] [CrossRef] [PubMed]
- Junka, A.F.; Rakoczy, R.; Szymczyk, P.; Bartoszewicz, M.; Sedghizadeh, P.P.; Fijałkowski, K. Application of Rotating Magnetic Fields Increase the Activity of Antimicrobials Against Wound Biofilm Pathogens. Sci. Rep. 2018, 8, 167. [Google Scholar] [CrossRef]
- Quan, K.; Zhang, Z.; Chen, H.; Ren, X.; Ren, Y.; Peterson, B.W.; van der Mei, H.C.; Busscher, H.J. Artificial Channels in an Infectious Biofilm Created by Magnetic Nanoparticles Enhanced Bacterial Killing by Antibiotics. Small 2019, 15, 1902313. [Google Scholar] [CrossRef] [PubMed]
- Silva, J.; Dias, R.; Cardoso, V.; de Resende, M.M. Biolixiviation of metals of computers printed circuit boards by Acidithiobacillus ferrooxidans and metals bioremoval by mixed culture subjected to a magnetic field. Res. Square 2022, 2022, 7v1. [Google Scholar] [CrossRef] [PubMed]
- Almomani, F.; Bhosale, R.; Khraisheh, M.; Kumar, A.; Almomani, T. Heavy metal ions removal from industrial wastewater using magnetic nanoparticles (MNP). Appl. Surf. Sci. 2020, 506, 144924. [Google Scholar] [CrossRef]
- Dunkel, T.; De León Gallegos, E.L.; Bock, C.; Lange, A.; Hoffmann, D.; Boenigk, J.; Denecke, M. Illumina sequencing for the identification of filamentous bulking and foaming bacteria in industrial activated sludge plants. Int. J. Environ. Sci. Technol. 2017, 15, 1139–1158. [Google Scholar] [CrossRef]
- Sayqal, A.; Ahmed, O.B. Advances in Heavy Metal Bioremediation: An Overview. Appl. Bionics Biomech. 2021, 2021, 149. [Google Scholar] [CrossRef]
- Lucena, G.N.; dos Santos, C.C.; Pinto, G.C.; Piazza, R.D.; Guedes, W.N.; Júnior, M.J.; de Paula, A.V.; Marques, R.F.C. Synthesis and characterization of magnetic cross-linked enzyme aggregate and its evaluation of the alternating magnetic field (AMF) effects in the catalytic activity. J. Magn. Magn. Mater. 2020, 516, 167326. [Google Scholar] [CrossRef]
- Letuta, U.G.; Berdinskiy, V.L. Magnetosensitivity of bacteria E. coli: Magnetic isotope and magnetic field effects. Bioelectromagnetics 2017, 38, 581–591. [Google Scholar] [CrossRef]
- Mouhoub, R.B.; El May, A.; Boujezza, I.; Sethom, M.M.; Feki, M.; Landoulsi, A. Viability and membrane lipid composition under a 57 mT static magnetic field in Salmonella Hadar. Bioelectrochemistry 2018, 122, 134–141. [Google Scholar] [CrossRef] [PubMed]
- Konopacki, M.; Rakoczy, R. The analysis of rotating magnetic field as a trigger of Gram-positive and Gram-negative bacteria growth. Biochem. Eng. J. 2019, 141, 259–267. [Google Scholar] [CrossRef]
- Liu, Z.; Gao, X.; Zhao, J.; Xiang, Y. The Sterilization Effect of Solenoid Magnetic Field Direction on Heterotrophic Bacteria in Circulating Cooling Water. Procedia Eng. 2017, 174, 1296–1302. [Google Scholar] [CrossRef]
- Xu, F.; Wang, B.; Hong, C.; Telebielaigen, S.; Nsor-Atindana, J.; Duan, Y.; Zhong, F. Optimization of spiral continuous flow-through pulse light sterilization for Escherichia coli in red grape juice by response surface methodology. Food Control 2019, 105, 8–12. [Google Scholar] [CrossRef]
- Zhang, J.; Zeng, D.; Xu, C.; Gao, M. Effect of low-frequency magnetic field on formation of pigments of Monascus purpureus. Eur. Food Res. Technol. 2015, 240, 577–582. [Google Scholar] [CrossRef]
- Alvarez, D.C.; Pérez, V.H.; Justo, O.R.; Alegre, R.M. Effect of the extremely low frequency magnetic field on nisin production by Lactococcus lactis subsp. lactis using cheese whey permeate. Process. Biochem. 2006, 41, 1967–1973. [Google Scholar] [CrossRef]
- Wang, Z.; Liu, X.; Ni, S.Q.; Zhang, J.; Zhang, X.; Ahmad, H.A.; Gao, B. Weak magnetic field: A powerful strategy to enhance partial nitrification. Water Res. 2017, 120, 190–198. [Google Scholar] [CrossRef]
- Armenia, I.; Grazú Bonavia, M.V.; De Matteis, L.; Ivanchenko, P.; Martra, G.; Gornati, R.; de la Fuente, J.M.; Bernardini, G. Enzyme activation by alternating magnetic field: Importance of the bioconjugation methodology. J. Colloid Interface Sci. 2019, 537, 615–628. [Google Scholar] [CrossRef]
- Luptakova, A.; Macingova, E.; Slesarova, A.; Ubaldini, S.; Abbruzzese, C. Solubilization and immobilization of toxic metals by bacteria (Slovak Republic and Italy). Environ. Sci. 2007, 2007, 38326245. [Google Scholar]
- Sedlakova-Kadukova, J.; Marcincakova, R.; Mrazikova, A.; Willner, J.; Fornalczyk, A. Closing the Loop: Key Role of Iron in Metal-Bearing Waste Recycling. Arch. Met. Mater. 2017, 62, 1459–1466. [Google Scholar] [CrossRef]
- Dev, S.; Sachan, A.; Dehghani, F.; Ghosh, T.; Briggs, B.R.; Aggarwal, S. Mechanisms of biological recovery of rare-earth elements from industrial and electronic wastes: A review. Chem. Eng. J. 2020, 397, 124596. [Google Scholar] [CrossRef]
- Zhang, S.; Yan, L.; Xing, W.J.; Chen, P.; Zhang, Y.; Wang, W.D. Acidithiobacillus ferrooxidans and its potential application. Extremophiles 2018, 22, 563–579. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Yan, L.; Li, H.; Liu, H. Optimal conditions for growth and magnetosome formation of Acidithiobacillus ferrooxidans. Afr. J. Microbiol. Res. 2012, 6, 6142–6151. [Google Scholar] [CrossRef]
- Panda, S. Magnetic separation of ferrous fractions linked to improved bioleaching of metals from waste-to-energy incinerator bottom ash (IBA): A green approach. Environ. Sci. Pollut. Res. 2020, 27, 9475–9489. [Google Scholar] [CrossRef]
- Cui, X.L.; Zuo, H.E.; Wen, J.K. The Effect of pH on Bioleaching of Deerni Pyrite Roasting Residues as Magnetic Materials. Key Eng. Mater. 2017, 730, 226–230. [Google Scholar] [CrossRef]
- Yan, L.; Da, H.Y.; Zhang, S.; López, V.M.; Wang, W.D. Bacterial magnetosome and its potential application. Microbiol. Res. 2017, 203, 19–28. [Google Scholar] [CrossRef]
- He, S.; Yang, J.; Fan, X.; Lu, D.; Zhang, S.; Yan, L. Magnetosome yield characteristics modeling of Acidithiobacillus ferrooxidans in airlift bioreactor using response surface methodology. J. Biomater. Appl. 2022, 2022, 364. [Google Scholar] [CrossRef]
| Impact on | Effect | Magnetic Field Properties | |||||
|---|---|---|---|---|---|---|---|
| Organism | Magnetic/Electromagnetic Field | B (mT) | Frequency (Hz) | Duration | References | ||
| cancer treatment | promotion | mice | alternating | not stated | 230 kHz | 2–8 days | [18] |
| promotion | cancer cell cultures | alternating | 5.1 | 50 Hz | 2 h in 3 days | [19] | |
| bone healing | promotion | dogs | static | 100 | - | 8 weeks | [20] |
| wound healing | promotion | mice | static | 5 | 25 Hz | 1h/10 days | [21] |
| M1 macrophages transformation to macrophages M2 | promotion | mouse macrophages | static | 1240 | - | not stated | [22] |
| immune response | promotion | human tumor cells | alternating (rotatory) | not stated | 2 Hz | 10 min | [23] |
| metal accumulation | promotion | Noccaea caerulescens | alternating | 30, 60, 120, 150 | not stated | 20 min/7 days | [24] |
| promotion | Noccaea caerulescens | alternating | 400 | not stated | 20 min/7 days | [24] | |
| phytoremediation | promotion | Eucaliptus globulus | static | 30, 60, 120, 150 | - | 20 min/7 days | [25] |
| promotion | Celosia argentea | static | 30–150 | - | 20 min/7 days | [26] | |
| promotion | Eucaliptus globulus | static | 400 | - | 20 min/7 days | [25] | |
| no effect | Celosia argentea | static | 30–150 | - | 20 min/7 days | [26] | |
| promotion | water for watering of Festuca arundinacea | static | 100 | - | 10 s/5 min | [27] | |
| wastewater treatment | promotion | Scenedesmus obliquus | static | 50–500 | - | 3 days | [28] |
| ethanol production | promotion | Saccharomyces cerevisiae | alternating | 8–10 | <50 Hz | 24 h | [29] |
| promotion | Saccharomyces cerevisiae | static | 200 | - | 24 h | [30] | |
| no effect | Saccharomyces cerevisiae | alternating (rotatory) | 1 | 100 Hz | not stated | [31] | |
| fermentation | promotion | Geotrichum candidum | static | 7 | - | 24–120h | [32] |
| mycotoxins content | no effect | Saccharomyces cerevisiae | static | 35 | - | 48h | [33] |
| Impact on | Effect | Magnetic Field Properties | |||||
|---|---|---|---|---|---|---|---|
| Organism | Magnetic/Electromagnetic Field | B (mT) | Frequency (Hz) | Duration | References | ||
| nitrogen removal, bacterial survival | promotion | active sludge | static | 88 | - | 12 h | [53] |
| reduction | active sludge | static | 88 | - | 6 days | [53] | |
| denitrification | promotion | active sludge | static | 39.5–65.3 | - | 8 and 12 h | [54] |
| promotion | active sludge | static | 30 | - | 8 and 12 h | [55] | |
| concentration of COD * | promotion | active sludge | static | 350 | - | 48 h | [56] |
| nitrogen removal | no effect | active sludge | static | 350 | - | 48 h | [56] |
| contaminants removal ** | promotion | Pseudomonas spp., Cupriavidus spp., Rhodococcus spp. | static | 200 | - | 5 h | [57] |
| lignocellulose degradation | promotion | environmental microorganism | alternating | 230~260 | electric field (0.3~0.8 V) | 3–21 days | [58] |
| oil removal | promotion | Acinetobacter sp. B11 | static | 25 | - | 7 days | [59] |
| vinegar aging | promotion | vinegar-producing bacteria | alternating | 0–5 | not stated | 3 h | [60] |
| biofilm removal | promotion | Pseudomonas aeruginosa | static and alternating | 444 | 474 kHz | 3–24 h | [61] |
| biofilm removal | promotion | Enterococcus faecalis | static | 170 | - | 24–72 h | [62] |
| reduction | bacteria of teeth biofilm | static | 60 | - | 24 and 48 h | [63] | |
| hydrogen production | promotion | Clostridium pasteurianum | static | 3.2 | - | 60 h | [64] |
| bioleaching (Cu, Fe) | promotion | Acidithiobacillus ferrooxidans | static | 3.14 | - | 24 days | [65] |
| bioleaching (As, Cd) As a Cd | promotion | Acidithiobacillus ferrooxidans | static | 8–10 | - | 30 min | [66] |
| bioleaching (Cr) | promotion | Geotrichum sp. a Bacillus sp. | static | 7 | - | 24 h | [67] |
| bioleaching (Cu) | promotion | Acidithiobacillus ferrooxidans, Thiobacillus thiooxidans | static | 9.6 | - | 27 days | [68] |
| bioleachin (Cu) | promotion | mix culture of iron-oxidating bacteria (most prevalent genus Acidithiobacillus) | static | (electric field) 40 mA, 0.2–2.7 V | - | 2–6 days | [69] |
| bacterial inactivation | promotion | Salmonella enteritidis | pulse | (electric field) 5–50 kV cm−1, 2 kW | 50 ns to 3 μs | [70] | |
| quality of frozen food | promotion | various food products | combination of static and pulsed | 480 | 20 KHz | 1443 ± 2 s | [71] |
| aging of Baijiu | promotion | Feng-flavored Baijiu (fermentation product) | static | 210 | - | 24 h | [72] |
| bacterial destruction | promotion | Staphylococcus aureus | alternating | not stated | 15 kHz | 20 min | [73] |
| antibiotics resistance | reduction | Staphylococcus aureus | alternating (rotatory) | 8.1 | 5–50 Hz | 120 h | [74] |
| biofilm formation | reduction | Pseudomonas aeruginosa | alternating (rotatory) | 23–34 | 10–50 Hz | 5 min | [75] |
| reduction | Staphylococcus aureus | static + magnetics nanoparticles | ≂1000 | - | 1 min | [76] | |
| metal bioremediation (Al, Cu, Pb) | promotion | mixed culture (industrial sludge) | alternating | not stated | 5 Hz | 6–12 days | [77] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sincak, M.; Luptakova, A.; Matusikova, I.; Jandacka, P.; Sedlakova-Kadukova, J. Application of a Magnetic Field to Enhance the Environmental Sustainability and Efficiency of Microbial and Plant Biotechnological Processes. Sustainability 2023, 15, 14459. https://doi.org/10.3390/su151914459
Sincak M, Luptakova A, Matusikova I, Jandacka P, Sedlakova-Kadukova J. Application of a Magnetic Field to Enhance the Environmental Sustainability and Efficiency of Microbial and Plant Biotechnological Processes. Sustainability. 2023; 15(19):14459. https://doi.org/10.3390/su151914459
Chicago/Turabian StyleSincak, Miroslava, Alena Luptakova, Ildiko Matusikova, Petr Jandacka, and Jana Sedlakova-Kadukova. 2023. "Application of a Magnetic Field to Enhance the Environmental Sustainability and Efficiency of Microbial and Plant Biotechnological Processes" Sustainability 15, no. 19: 14459. https://doi.org/10.3390/su151914459
APA StyleSincak, M., Luptakova, A., Matusikova, I., Jandacka, P., & Sedlakova-Kadukova, J. (2023). Application of a Magnetic Field to Enhance the Environmental Sustainability and Efficiency of Microbial and Plant Biotechnological Processes. Sustainability, 15(19), 14459. https://doi.org/10.3390/su151914459








