A Review on Melt-Spun Biodegradable Fibers
Abstract
:1. Introduction
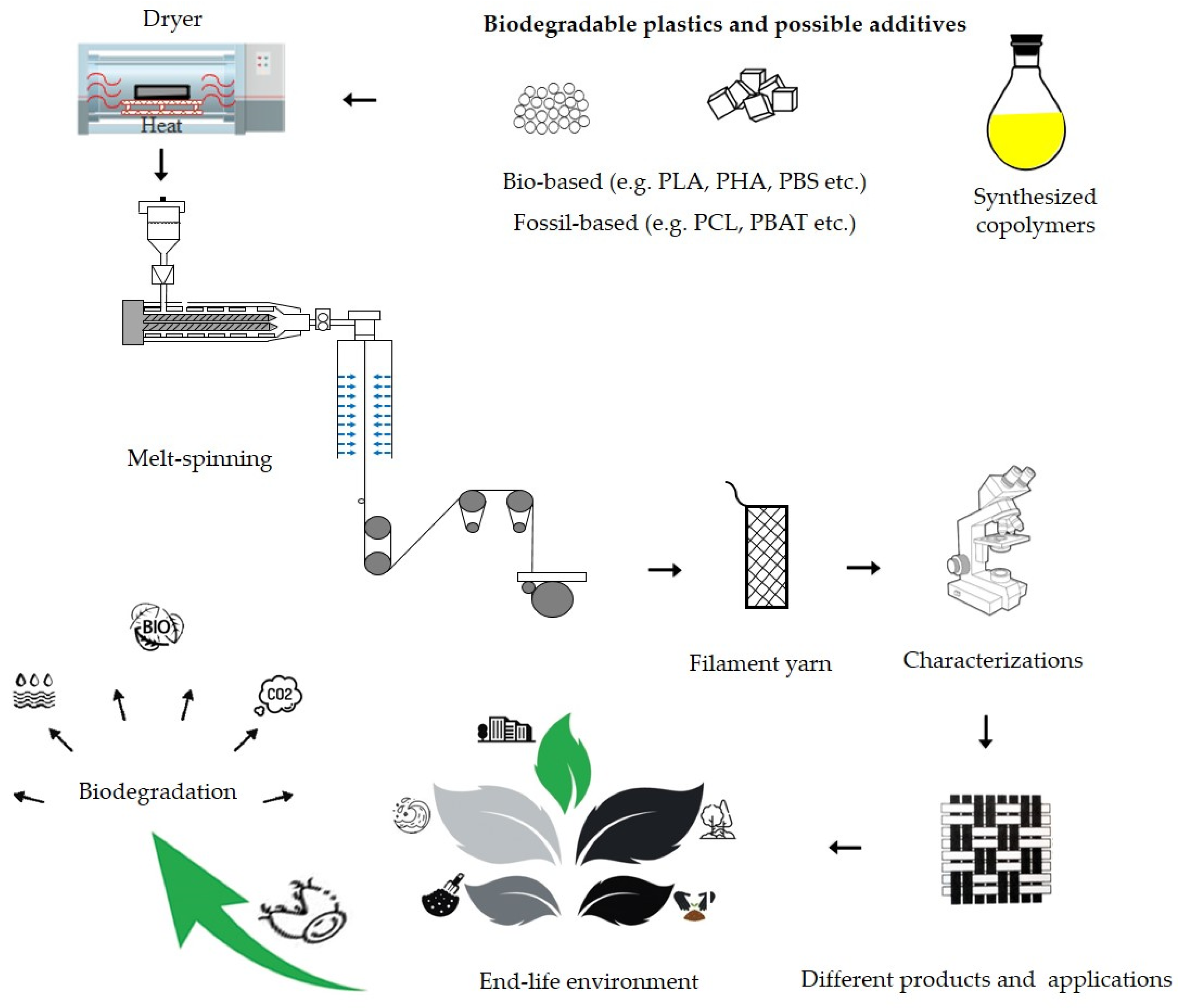
2. Bio-Based and Biodegradable Plastics
2.1. Biodegradable Thermoplastic Polymers
2.1.1. PLA
2.1.2. PHAs
2.1.3. TPS
2.1.4. PBS
2.1.5. PCL
2.1.6. PGA
2.1.7. PLGA
2.1.8. PBAT
2.1.9. PBSA
2.1.10. PBST
2.1.11. PBEAS
2.1.12. PBTSA
2.1.13. PIHO
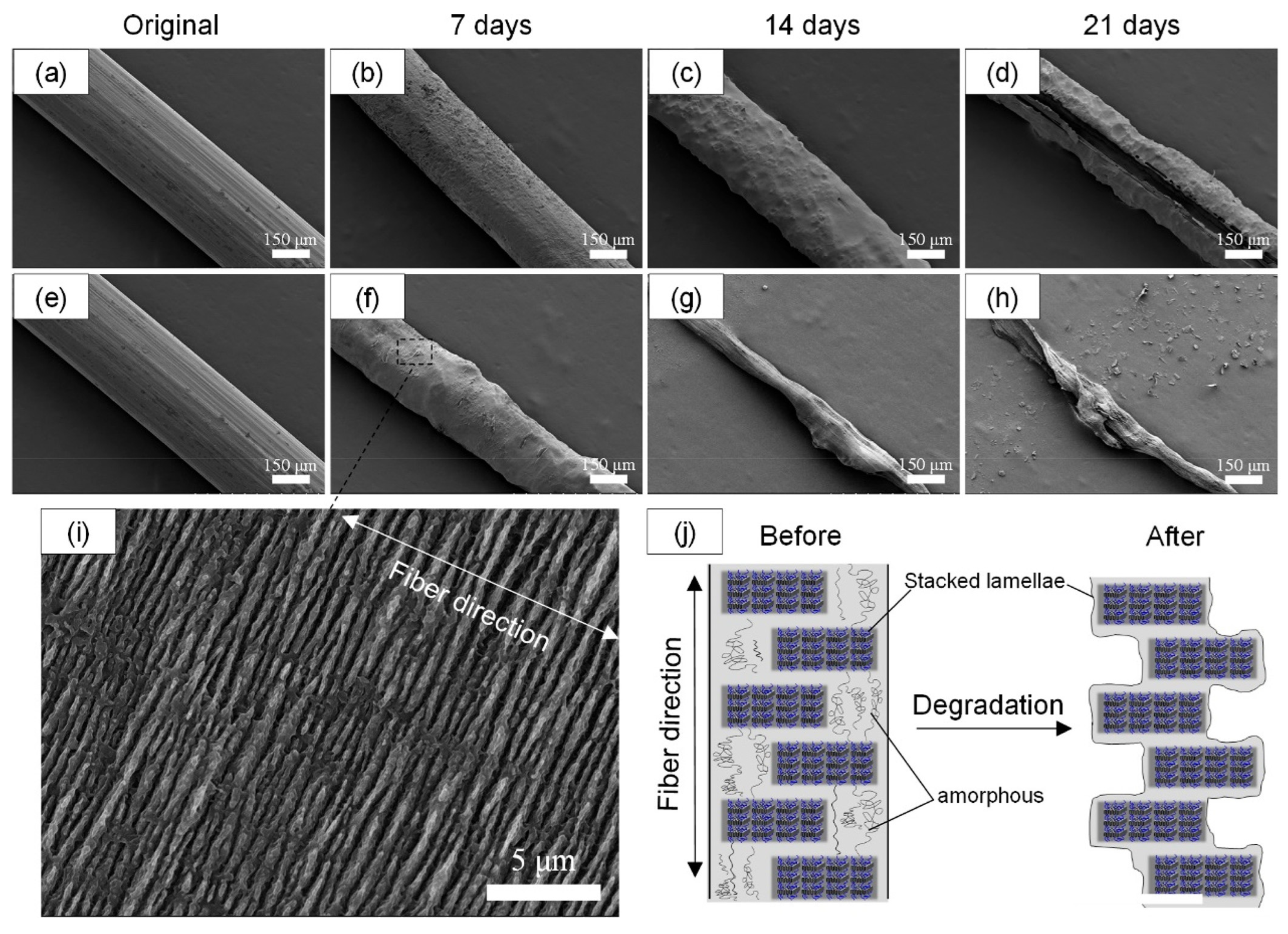
2.2. Biodegradation
2.3. Bio-Based and Biodegradable Plastics Market
| No | Polymer | Chemical Structure | Main Properties |
|---|---|---|---|
| 1 | PLA | 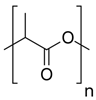 | Melting point = 160–180 °C Glass transition = 55–60 °C Density = 1.25 g/cm3 MW range = 20,000–200,000 g/mol or Daltons Biodegradation 1: Industrial compostable (<6 months) |
| 2 | PHAs | 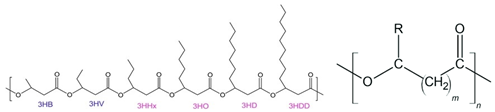 | Melting point = 140–171 °C Glass transition = 4 °C Density = 1.25 g/cm3 MW range = 10,000–1000,000 g/mol Biodegradation: Marine degradable (<12 months) |
| 3 | TPS | 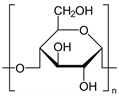 | Melting point = 150–170 °C Glass transition = 50–80 °C Density = 1.2–1.6 g/cm3 MW range = 10,000–500,000 g/mol Biodegradation: Marine degradable (<12 months) |
| 4 | PBS | 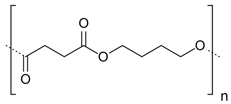 | Melting point = 110–120 °C Glass transition = −32 °C Density = 1.00 g/cm3 MW range = 10,000–200,000 g/mole Biodegradation: Soil degradable (<24 months) |
| 5 | PCL | 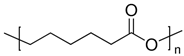 | Melting point = 60 °C Glass transition = −72 °C Density = 1.12 g/cm3 MW range = 3000 to 100,000 g/mol Biodegradation: Marine degradable (<6 months) |
| 6 | PGA | 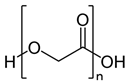 | Melting point = 225–230 °C Glass transition = 35–40 °C Density = 1.53 g/cm3 MW range = 10,000–300,000 g/mole Biodegradation: Marine and soil degradable (<3 months) |
| 7 | PLGA | mechanical strength (a significant increase of 78%) 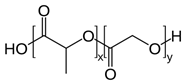 | Melting point = 262 °C Glass transition = 40–55 °C Density = 1.3 g/cm3 MW range = 10,000–110,000 g/mol Biodegradation: Marine and soil degradable (<6 months when G/L > 1) |
| 8 | PBAT |  | Melting point = 120 °C Glass transition = −28 °C Density = 1.26 g/cm3 MW range = 10,000 to 300,000 g/mol Biodegradation: Soil degradable (<24 months) |
| 9 | PBSA | 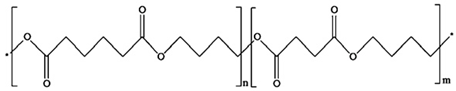 | Melting point = 112 °C Glass transition = −38 °C Density = 1.25 g/cm3 MW range = 10,000 to 100,000 g/mol Biodegradation: Soil degradable (<12 months) |
| 10 | PBST | 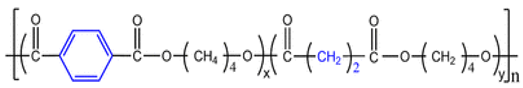 | Melting point = 115 °C Glass transition = 40 °C Density = 1.26 g/cm3 MW range = 10,000 to 200,000 g/mol Biodegradation: Soil degradable (<12 months) |
| 11 | PBEAS | 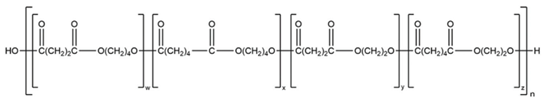 | Melting point = 50–60 °C Glass transition = −15 °C Density = 1.2 g/cm3 MW range = 10,000 to 100,000 g/mol Biodegradation: Soil degradable (<12 months) |
| 12 | PBTSA | 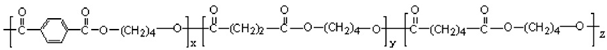 | Melting point = 150–200 °C Glass transition = 30–70 °C Density = 1.2–1.4 g/cm3 MW range = 20,000 to 100,000 g/mol Biodegradation: Soil degradable (<12 months) |
| 13 | PIHO | 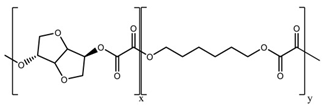 | Melting point = N/A Glass transition = 103 °C Density = 1.38 g/cm3 MW range = 10,000 to 100,000 g/mol Biodegradation: Marine and soil degradable (<6 months) |
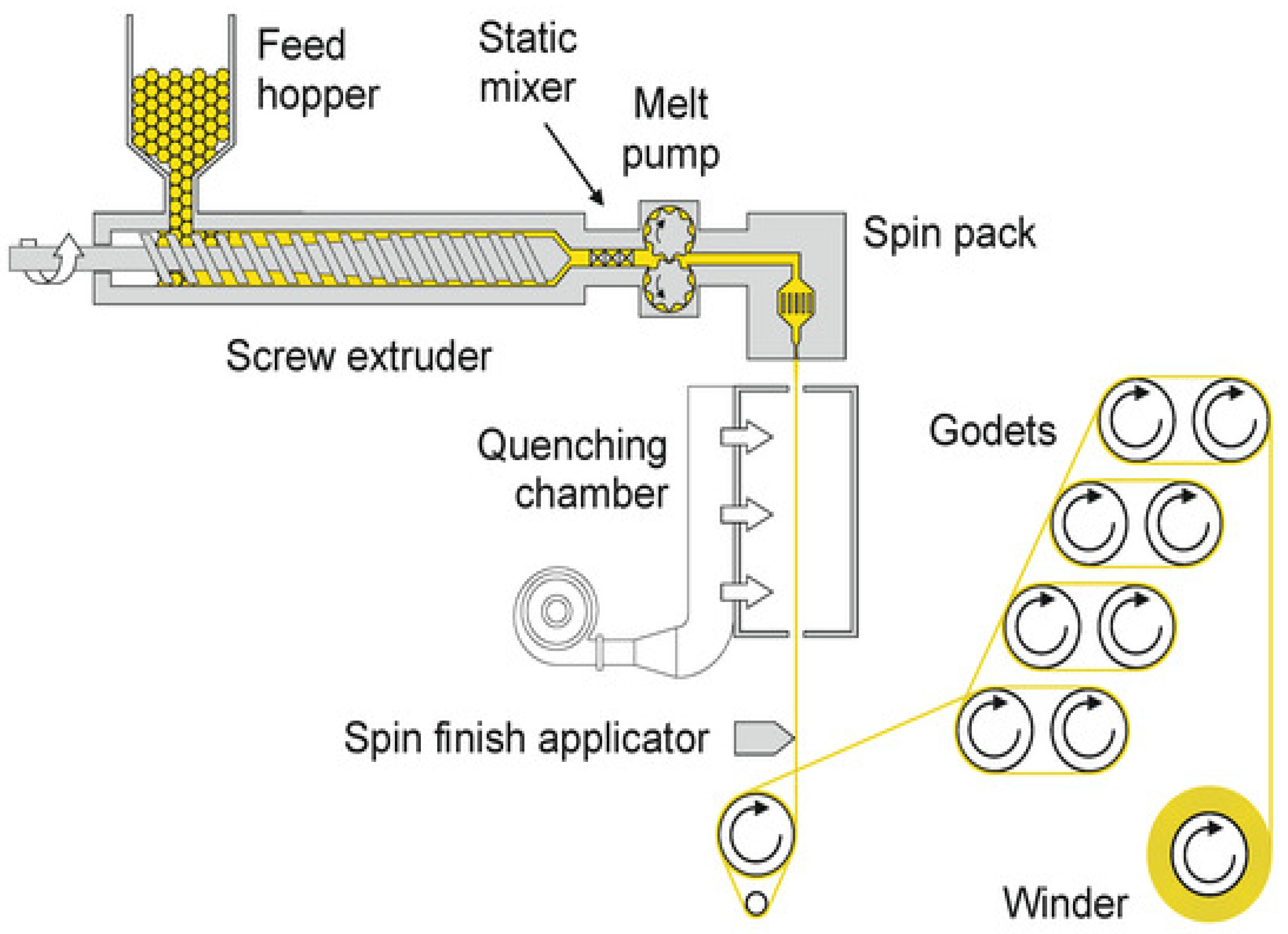
3. Melt-Spun Biodegradable Fibers
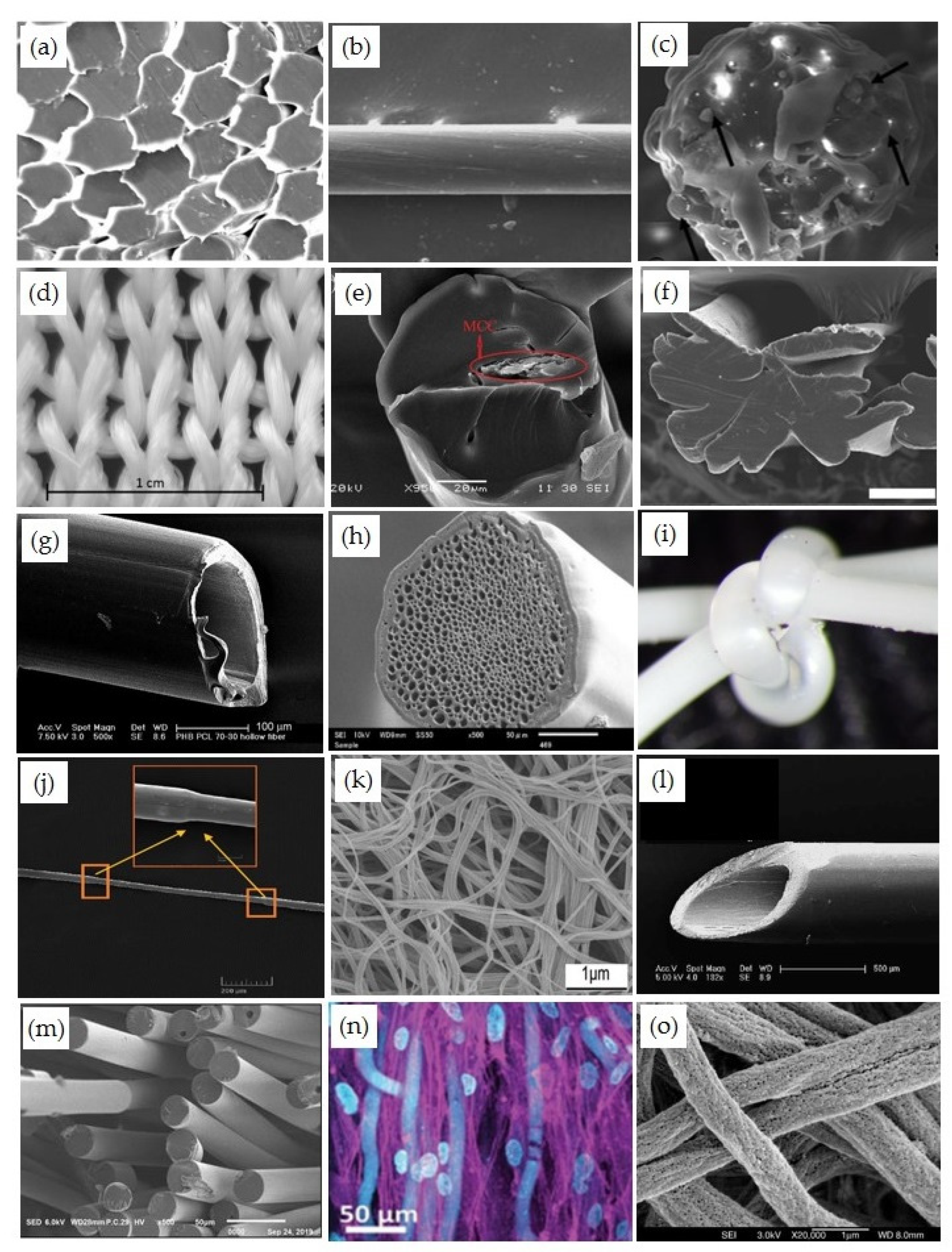
3.1. Monocomponent Filaments and Fibers
3.2. Blend and Composite Fibers
3.3. Bicomponent Filament Yarns and Staple Fibers
4. Discussion
4.1. Processing
- Thermal stability: The polymer should have sufficient thermal stability to withstand the extrusion temperature and shear strain during processing without significant degradation or cross-linking.
- Low polydispersity index: The polymer should have a relatively low polydispersity index to ensure consistent melt flow rheology. A polydispersity index below three is often desired for a stable melt-spinning process.
- Appropriate molecular weight: The polymer should have an appropriate molecular weight that provides enough melt strength to prevent filament breakage during processing. It should not be too viscous to impair processability.
- Uniformity and purity: The polymer should be uniform and free from impurities to prevent clogging of the processing equipment and fluctuations in the processing conditions.
- Linear structure: Linear polymers (versus branched) are preferred for melt-spinning because their molecular chains can easily unfold and align along the strain direction, facilitating orientation and crystallization and improving fiber properties.
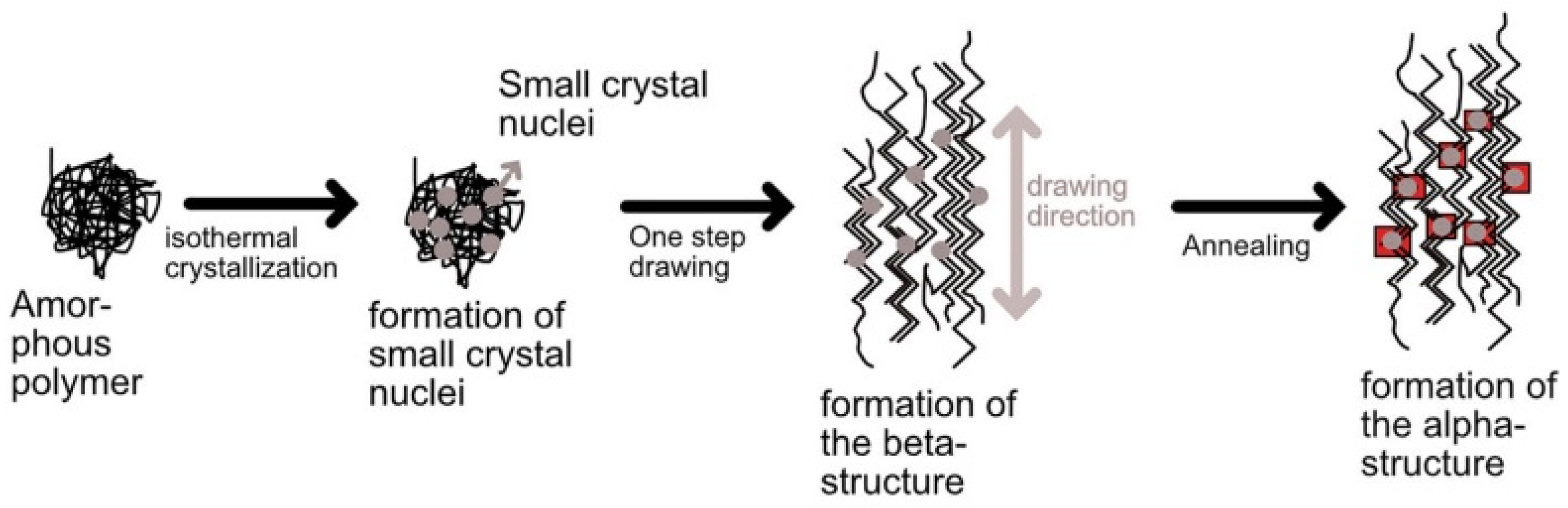
4.2. Crystallinity and Orientation
4.3. Physical-Mechanical Characteristics
4.4. Biodegradation
5. Applications
6. Conclusions
7. Outlook
- -
- Using bio-based materials in parallel with biodegradation is more preferable, while shifting to Carbon Capture Utilization (CCU) is considered.
- -
- Adjusting the final performance of biodegradable fibers based on the final application demands a balanced, smart strategy.
- -
- Finding the best biodegradable alternative for polyester fibers for textiles and fashion brands that is competitive in different aspects of physical properties, comfort, and price while also being more sustainable.
- -
- The influence of further textile-processing methods such as crimping or texturizing, twisting, waving, or knitting, and finishing and dying (especially chemicals) on the final textiles/clothes (from performance and biodegradation aspects) can be interesting and should be more intensively investigated.
- -
- Drawbacks such as low melting points and low glass transition temperatures, poor degradation resistance to high temperatures and poor hydrolytic resistance to strong alkaline conditions, high elongation requirements, and relatively poor storage stability should be taken into account when finding an appropriate solution in production and processing methods.
- -
- Field tests in actual application situations such as comfort, wearing, washing, and fastness of biodegradable textiles should be studied.
- -
- Decreasing the price of bio-based and biodegradable polymer fibrous products by increasing production volume can also pave the way for marketing and expansion of their applications.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rosenboom, J.-G.; Langer, R.; Traverso, G. Bioplastics for a circular economy. Nat. Rev. Mater. 2022, 7, 117–137. [Google Scholar] [CrossRef]
- Andrady, A.L.; Neal, M.A. Applications and societal benefits of plastics. Philos. Trans. R. Soc. B Biol. Sci. 2009, 364, 1977–1984. [Google Scholar] [CrossRef] [PubMed]
- Plastics Europe. Plastics—The Facts 2019; Plastics Europe: Brussels, Belgium, 2019. [Google Scholar]
- Available online: https://docs.european-bioplastics.org/publications/market_data/2022/Report_Bioplastics_Market_Data_2022_short_version.pdf (accessed on 15 August 2023).
- Geyer, R.; Jambeck, J.R.; Law, K.L. Production, use, and fate of all plastics ever made. Sci. Adv. 2017, 3, e1700782. [Google Scholar] [CrossRef] [PubMed]
- Gruter, G.-J.M. Using carbon above the ground as feedstock to produce our future polymers. Curr. Opin. Green Sustain. Chem. 2022, 40, 100743. [Google Scholar] [CrossRef]
- Narancic, T.; Cerrone, F.; Beagan, N.; O’Connor, K.E. Recent advances in bioplastics: Application and biodegradation. Polymers 2020, 12, 920. [Google Scholar] [CrossRef]
- Defruyt, S. Towards a new plastics economy. Field Actions Sci. Rep. J. Field Actions 2019, 78–81. [Google Scholar]
- Jambeck, J.R.; Geyer, R.; Wilcox, C.; Siegler, T.R.; Perryman, M.; Andrady, A.; Narayan, R.; Law, K.L. Plastic waste inputs from land into the ocean. Science 2015, 347, 768–771. [Google Scholar] [CrossRef]
- Narancic, T.; O’Connor, K.E. Microbial biotechnology addressing the plastic waste disaster. Microb. Biotechnol. 2017, 10, 1232. [Google Scholar] [CrossRef]
- Laborda, E.; Del-Busto, F.; Bartolomé, C.; Fernández, V. Analysing the Social Acceptance of Bio-Based Products Made from Recycled Absorbent Hygiene Products in Europe. Sustainability 2023, 15, 3008. [Google Scholar] [CrossRef]
- Bucci, K.; Tulio, M.; Rochman, C. What is known and unknown about the effects of plastic pollution: A meta-analysis and systematic review. Ecol. Appl. 2020, 30, e02044. [Google Scholar] [CrossRef]
- Hong, M.; Chen, E.Y.-X. Chemically recyclable polymers: A circular economy approach to sustainability. Green Chem. 2017, 19, 3692–3706. [Google Scholar] [CrossRef]
- Alberti, C.; Enthaler, S. Depolymerization of End-of-Life Poly (lactide) to Lactide via Zinc-Catalysis. ChemistrySelect 2020, 5, 14759–14763. [Google Scholar] [CrossRef]
- Wei, R.; Breite, D.; Song, C.; Gräsing, D.; Ploss, T.; Hille, P.; Schwerdtfeger, R.; Matysik, J.; Schulze, A.; Zimmermann, W. Biocatalytic degradation efficiency of postconsumer polyethylene terephthalate packaging determined by their polymer microstructures. Adv. Sci. 2019, 6, 1900491. [Google Scholar] [CrossRef] [PubMed]
- Roohi; Zaheer, M.R.; Kuddus, M. PHB (poly-β-hydroxybutyrate) and its enzymatic degradation. Polym. Adv. Technol. 2018, 29, 30–40. [Google Scholar] [CrossRef]
- European Commission. The Waste Incineration Directive. 2000. Available online: http://ec.europa.eu/environment/archives/air/stationary/wid/legislation.htm (accessed on 15 August 2023).
- European Commission. A European Strategy for Plastics in A Circular Economy; European Commission: Brussels, Belgium, 2018. [Google Scholar]
- Miri, S.; Saini, R.; Davoodi, S.M.; Pulicharla, R.; Brar, S.K.; Magdouli, S. Biodegradation of microplastics: Better late than never. Chemosphere 2022, 286, 131670. [Google Scholar] [CrossRef] [PubMed]
- United Nations. Strengthening Actions for Nature to Achieve the Sustainable Development Goals; United Nations: New York, NY, USA, 2022. [Google Scholar]
- Tian, L.; van Putten, R.J.; Gruter, G.J.M. Plastic pollution. The role of (bio) degradable plastics and other solutions. In Biodegradable Polymers in the Circular Plastics Economy; Wiley-VCH: Weinheim, Germany, 2022; pp. 59–81. [Google Scholar]
- Iheanacho, S.; Ogbu, M.; Bhuyan, M.S.; Ogunji, J. Microplastic pollution: An emerging contaminant in aquaculture. Aquac. Fish. 2023, 8, 603–616. [Google Scholar] [CrossRef]
- Amato-Lourenço, L.F.; Carvalho-Oliveira, R.; Júnior, G.R.; dos Santos Galvão, L.; Ando, R.A.; Mauad, T. Presence of airborne microplastics in human lung tissue. J. Hazard. Mater. 2021, 416, 126124. [Google Scholar] [CrossRef]
- Leslie, H.A.; Van Velzen, M.J.; Brandsma, S.H.; Vethaak, A.D.; Garcia-Vallejo, J.J.; Lamoree, M.H. Discovery and quantification of plastic particle pollution in human blood. Environ. Int. 2022, 163, 107199. [Google Scholar] [CrossRef]
- Center for International Environmental Law (CIEL). Plastic & Climate. The Hidden Costs of a Plastic Planet. 2020. Available online: https://www.ciel.org/plasticandclimate/ (accessed on 15 August 2023).
- Kershaw, P. Marine Plastic Debris and Microplastics—Global Lessons and Research to Inspire Action and Guide Policy Change; United Nations Environment Programme: New York, NY, USA, 2016. [Google Scholar]
- World Economic Forum (WEF). Top 10 Emerging Technologies 4–15 (WEF, 2019); World Economic Forum: Cologny, Switzerland, 2023. [Google Scholar]
- A Biodegradable Fibre as Polyester Replacement for Textiles, Maritime Forum. 2022. Available online: https://maritime-forum.ec.europa.eu/en/node/7480 (accessed on 15 August 2023).
- Hannover, I. Biopolymers—Facts and Statistics; Institute for Bioplastics and Biocomposites—Hochschule Hannover: Hannover, Germany, 2021. [Google Scholar]
- Arikan, E.B.; Ozsoy, H.D. A review: Investigation of bioplastics. J. Civ. Eng. Arch. 2015, 9, 188–192. [Google Scholar]
- Motloung, M.P.; Mofokeng, T.G.; Mokhena, T.C.; Ray, S.S. Recent advances on melt-spun fibers from biodegradable polymers and their composites. Int. Polym. Process. 2022, 37, 523–540. [Google Scholar] [CrossRef]
- Spierling, S.; Knüpffer, E.; Behnsen, H.; Mudersbach, M.; Krieg, H.; Springer, S.; Albrecht, S.; Herrmann, C.; Endres, H.-J. Bio-based plastics-A review of environmental, social and economic impact assessments. J. Clean. Prod. 2018, 185, 476–491. [Google Scholar] [CrossRef]
- Nofal, R.M. Biodegradable Textiles, Recycling, and Sustainability Achievement. In Handbook of Biodegradable Materials; Springer: Berlin/Heidelberg, Germany, 2023; pp. 1449–1485. [Google Scholar]
- Chen, X.; Memon, H.A.; Wang, Y.; Marriam, I.; Tebyetekerwa, M. Circular Economy and sustainability of the clothing and textile Industry. Mater. Circ. Econ. 2021, 3, 12. [Google Scholar] [CrossRef]
- Sharma, V.; Sehgal, R.; Gupta, R. Polyhydroxyalkanoate (PHA): Properties and modifications. Polymer 2021, 212, 123161. [Google Scholar] [CrossRef]
- Acharjee, S.A.; Bharali, P.; Gogoi, B.; Sorhie, V.; Walling, B. Alemtoshi PHA-based bioplastic: A potential alternative to address microplastic pollution. Water Air Soil Pollut. 2023, 234, 21. [Google Scholar] [CrossRef]
- Cai, Y.; Yang, T.; Mitrano, D.M.; Heuberger, M.; Hufenus, R.; Nowack, B. Systematic study of microplastic fiber release from 12 different polyester textiles during washing. Environ. Sci. Technol. 2020, 54, 4847–4855. [Google Scholar] [CrossRef]
- Cai, Y.; Lin, J.; Gimeno, S.; Begnaud, F.; Nowack, B. Country-Specific Environmental Risks of Fragrance Encapsulates Used in Laundry Care Products. Environ. Toxicol. Chem. 2022, 41, 905–916. [Google Scholar] [CrossRef]
- Cai, Y.; Mitrano, D.M.; Hufenus, R.; Nowack, B. Formation of fiber fragments during abrasion of polyester textiles. Environ. Sci. Technol. 2021, 55, 8001–8009. [Google Scholar] [CrossRef]
- Patti, A.; Acierno, D. Towards the sustainability of the plastic industry through biopolymers: Properties and potential applications to the textiles World. Polymers 2022, 14, 692. [Google Scholar] [CrossRef]
- De Haan, W.P.; Quintana, R.; Vilas, C.; Cózar, A.; Canals, M.; Uviedo, O.; Sanchez-Vidal, A. The dark side of artificial greening: Plastic turfs as widespread pollutants of aquatic environments. Environ. Pollut. 2023, 334, 122094. [Google Scholar] [CrossRef]
- Armstrong, M. Where the Ocean’s Microplastics Come From, 2022. Available online: https://www.statista.com/chart/17957/where-the-oceans-microplastics-come-from/ (accessed on 15 August 2023).
- Stanvay, D. Plastic entering oceans could nearly triple by 2040 if left unchecked—Research. Reuters, 8 March 2023. [Google Scholar]
- Michael, C. Plastic in the depths: How pollution took over our oceans. The Guardian, 25 July 2022. [Google Scholar]
- Manshoven, S.; Smeets, A.; Malarciuc, C.; Tenhunen-Lunkka, A.; Mortensen, L.F. Microplastic Pollution from Textile Consumption in Europe; VTT: Espoo, Finland, 2022. [Google Scholar]
- Microplastics from Textiles: Towards a Circular Economy for Textiles in Europe; European Environment Agency: Copenhagen, Denmark, 2022.
- Cesa, F.S.; Turra, A.; Baruque-Ramos, J. Synthetic fibers as microplastics in the marine environment: A review from textile perspective with a focus on domestic washings. Sci. Total Environ. 2017, 598, 1116–1129. [Google Scholar] [CrossRef]
- Habib, R.Z.; Thiemann, T. Microplastic in the marine environment of the Red Sea—A short review. Egypt. J. Aquat. Res. 2022, 48, 383–388. [Google Scholar] [CrossRef]
- MacArthur, E. Beyond Plastic Waste; American Association for the Advancement of Science: Washington, DC, USA, 2017; Volume 358, p. 843. [Google Scholar]
- Babaahmadi, V.; Amid, H.; Naeimirad, M.; Ramakrishna, S. Biodegradable and multifunctional surgical face masks: A brief review on demands during COVID-19 pandemic, recent developments, and future perspectives. Sci. Total Environ. 2021, 798, 149233. [Google Scholar] [CrossRef] [PubMed]
- Wiedemann, S.G.; Nguyen, Q.V.; Clarke, S.J. Using LCA and Circularity Indicators to Measure the Sustainability of Textiles—Examples of Renewable and Non-Renewable Fibres. Sustainability 2022, 14, 16683. [Google Scholar] [CrossRef]
- Dusselier, M.; Lange, J.-P. Biodegradable Polymers in the Circular Plastics Economy. John Wiley & Sons: Hoboken, NJ, USA, 2022. [Google Scholar]
- Science Advice for Policy by European Academies. Biodegradability of Plastics in the Open Environment; Evidence Review Report No. 8; SAPEA: Brussels, Belgium, 2020; pp. 52–57. [Google Scholar]
- Hufenus, R.; Yan, Y.; Dauner, M.; Kikutani, T. Melt-spun fibers for textile applications. Materials 2020, 13, 4298. [Google Scholar] [CrossRef] [PubMed]
- Bao, H.; Hong, Y.; Yan, T.; Xie, X.; Zeng, X. A systematic review of biodegradable materials in the textile and apparel industry. J. Text. Inst. 2023, 1–20. [Google Scholar] [CrossRef]
- Flury, M.; Narayan, R. Biodegradable plastic as an integral part of the solution to plastic waste pollution of the environment. Curr. Opin. Green Sustain. Chem. 2021, 30, 100490. [Google Scholar] [CrossRef]
- Narayanan, M.; Kandasamy, S.; Kumarasamy, S.; Gnanavel, K.; Ranganathan, M.; Kandasamy, G. Screening of polyhydroxybutyrate producing indigenous bacteria from polluted lake soil. Heliyon 2020, 6, e05381. [Google Scholar] [CrossRef]
- Narancic, T.; Verstichel, S.; Reddy Chaganti, S.; Morales-Gamez, L.; Kenny, S.T.; De Wilde, B.; Babu Padamati, R.; O’Connor, K.E. Biodegradable plastic blends create new possibilities for end-of-life management of plastics but they are not a panacea for plastic pollution. Environ. Sci. Technol. 2018, 52, 10441–10452. [Google Scholar] [CrossRef]
- Nakajima, H.; Dijkstra, P.; Loos, K. The recent developments in biobased polymers toward general and engineering applications: Polymers that are upgraded from biodegradable polymers, analogous to petroleum-derived polymers, and newly developed. Polymers 2017, 9, 523. [Google Scholar] [CrossRef]
- Mecking, S. Nature or petrochemistry?—Biologically degradable materials. Angew. Chem. Int. Ed. 2004, 43, 1078–1085. [Google Scholar] [CrossRef]
- Rai, P.; Mehrotra, S.; Priya, S.; Gnansounou, E.; Sharma, S.K. Recent advances in the sustainable design and applications of biodegradable polymers. Bioresour. Technol. 2021, 325, 124739. [Google Scholar] [CrossRef] [PubMed]
- Leja, K.; Lewandowicz, G. Polymer biodegradation and biodegradable polymers—A review. Pol. J. Environ. Stud. 2010, 19, 255–266. [Google Scholar]
- Polman, E.M.; Gruter, G.-J.M.; Parsons, J.R.; Tietema, A. Comparison of the aerobic biodegradation of biopolymers and the corresponding bioplastics: A review. Sci. Total Environ. 2021, 753, 141953. [Google Scholar] [CrossRef] [PubMed]
- Nanda, S.; Patra, B.R.; Patel, R.; Bakos, J.; Dalai, A.K. Innovations in applications and prospects of bioplastics and biopolymers: A review. Environ. Chem. Lett. 2022, 20, 379–395. [Google Scholar] [CrossRef]
- Emadian, S.M.; Onay, T.T.; Demirel, B. Biodegradation of bioplastics in natural environments. Waste Manag. 2017, 59, 526–536. [Google Scholar] [CrossRef]
- Chen, S.; Wu, Z.; Chu, C.; Ni, Y.; Neisiany, R.E.; You, Z. Biodegradable elastomers and gels for elastic electronics. Adv. Sci. 2022, 9, 2105146. [Google Scholar] [CrossRef]
- Vinod, A.; Sanjay, M.; Suchart, S.; Jyotishkumar, P. Renewable and sustainable biobased materials: An assessment on biofibers, biofilms, biopolymers and biocomposites. J. Clean. Prod. 2020, 258, 120978. [Google Scholar] [CrossRef]
- Ganesh, S.; Lakshmanan Saraswathy, J.; Raghunathan, V.; Sivalingam, C. Extraction and characterization chemical treated and untreated lycium ferocissimum fiber for epoxy composites. J. Nat. Fibers 2022, 19, 6509–6520. [Google Scholar] [CrossRef]
- Chang, C.; Ginn, B.; Livingston, N.K.; Yao, Z.; Slavin, B.; King, M.W.; Chung, S.; Mao, H.-Q. Medical fibers and biotextiles. In Biomaterials Science; Academic Press: Cambridge, MA, USA, 2020; pp. 575–600. [Google Scholar]
- Kopf, S.; Åkesson, D.; Skrifvars, M. Textile Fiber Production of Biopolymers–A Review of Spinning Techniques for Polyhydroxyalkanoates in Biomedical Applications. Polym. Rev. 2023, 63, 200–245. [Google Scholar] [CrossRef]
- Pivsa-Art, S.; Srisawat, N.; Narongchai, O.; Pavasupree, S.; Pivsa-Art, W. Preparation of knitting socks from poly (lactic acid) and poly [(R)-3-hydroxybutyrate-co-(R)-3-hydroxyvalerate](PHBV) blends for textile industrials. Energy Procedia 2011, 9, 589–597. [Google Scholar] [CrossRef]
- Alaswad, S.O.; Mahmoud, A.S.; Arunachalam, P. Recent Advances in Biodegradable Polymers and Their Biological Applications: A Brief Review. Polymers 2022, 14, 4924. [Google Scholar] [CrossRef] [PubMed]
- Nair, N.; Sekhar, V.; Nampoothiri, K.; Pandey, A. Biodegradation of biopolymers. In Current Developments in Biotechnology and Bioengineering; Elsevier: Amsterdam, The Netherlands, 2017; pp. 739–755. [Google Scholar]
- Narayan, R.; Balakrishnan, S.; Kubik, D.A.; Gencer, M.A. Biodegradable Polymer Masterbatch, and a Composition Derived Therefrom Having Improved Physical Properties. U.S. Patent US8008373B2, 30 August 2011. [Google Scholar]
- Yang, Y.; Zhang, M.; Ju, Z.; Tam, P.Y.; Hua, T.; Younas, M.W.; Kamrul, H.; Hu, H. Poly (lactic acid) fibers, yarns and fabrics: Manufacturing, properties and applications. Text. Res. J. 2021, 91, 1641–1669. [Google Scholar] [CrossRef]
- Van den Oever, M.; Molenveld, K.; van der Zee, M.; Bos, H. Bio-Based and Biodegradable Plastics: Facts and Figures: Focus on Food Packaging in the Netherlands; Wageningen Food & Biobased Research: Wageningen, The Netherlands, 2017. [Google Scholar]
- Van der Zee, M. 1. Methods for evaluating the biodegradability of environmentally degradable polymers. In Handbook of Biodegradable Polymers; De Gruyter: Berlin, Germany, 2020; pp. 1–22. [Google Scholar]
- Van der Zee, M.; Stoutjesdijk, J.; Van der Heijden, P.; De Wit, D. Structure-biodegradation relationships of polymeric materials. 1. Effect of degree of oxidation on biodegradability of carbohydrate polymers. J. Environ. Polym. Degrad. 1995, 3, 235–242. [Google Scholar]
- Mochizuki, M.; Hirami, M. Biodegradable fibers made from truly-biodegradable thermoplastics. In Polymers and Other Advanced Materials: Emerging Technologies and Business Opportunities; Springer: Berlin/Heidelberg, Germany, 1995; pp. 589–596. [Google Scholar]
- Tavanaie, M.A. Engineered biodegradable melt-spun fibers. In Engineered Polymeric Fibrous Materials; Elsevier: Amsterdam, The Netherlands, 2021; pp. 191–232. [Google Scholar]
- Available online: https://docs.european-bioplastics.org/publications/EUBP_Facts_and_figures.pdf (accessed on 20 August 2023).
- Shi, X.; Ito, H.; Kikutani, T. Characterization on mixed-crystal structure and properties of poly (butylene adipate-co-terephthalate) biodegradable fibers. Polymer 2005, 46, 11442–11450. [Google Scholar] [CrossRef]
- Espinosa, M.J.C.; Blanco, A.C.; Schmidgall, T.; Atanasoff-Kardjalieff, A.K.; Kappelmeyer, U.; Tischler, D.; Pieper, D.H.; Heipieper, H.J.; Eberlein, C. Toward biorecycling: Isolation of a soil bacterium that grows on a polyurethane oligomer and monomer. Front. Microbiol. 2020, 11, 404. [Google Scholar] [CrossRef]
- Terzopoulou, Z.; Tsanaktsis, V.; Bikiaris, D.N.; Exarhopoulos, S.; Papageorgiou, D.G.; Papageorgiou, G.Z. Biobased poly (ethylene furanoate-co-ethylene succinate) copolyesters: Solid state structure, melting point depression and biodegradability. RSC Adv. 2016, 6, 84003–84015. [Google Scholar] [CrossRef]
- Olabisi, O.; Adewale, K. Handbook of Thermoplastics; CRC Press: Boca Raton, FL, USA, 2016; Volume 41. [Google Scholar]
- Gruter, G.J.M.; Lange, J.P. Tutorial on Polymers—Manufacture, Properties, and Applications. In Biodegradable Polymers in the Circular Plastics Economy; Wiley-VCH: Weinheim, Germany, 2022; pp. 83–111. [Google Scholar]
- Schick, S.; Groten, R.; Seide, G.H. Performance Spectrum of Home-Compostable Biopolymer Fibers Compared to a Petrochemical Alternative. Polymers 2023, 15, 1372. [Google Scholar] [CrossRef]
- Zhang, H.; Bai, H.; Liu, Z.; Zhang, Q.; Fu, Q. Toward high-performance poly (L-lactide) fibers via tailoring crystallization with the aid of fibrillar nucleating agent. ACS Sustain. Chem. Eng. 2016, 4, 3939–3947. [Google Scholar] [CrossRef]
- Mezghani, K.; Spruiell, J. High speed melt spinning of poly (L-lactic acid) filaments. J. Polym. Sci. Part B Polym. Phys. 1998, 36, 1005–1012. [Google Scholar] [CrossRef]
- Roungpaisan, N.; Takasaki, M.; Takarada, W.; Kikutani, T. Mechanism of fiber structure development in melt spinning of PLA. In Poly (Lactic Acid) Synthesis, Structures, Properties, Processing, Applications, and End of Life; John Wiley & Sons: Hoboken, NJ, USA, 2022; pp. 425–438. [Google Scholar]
- Teijin News Release, 7 December 20223. Available online: https://www.teijin.com/news/2022/12/07/20221207_01.pdf (accessed on 15 August 2023).
- Fattahi, F.S.; Khoddami, A.; Izadian, H. Review on production, properties, and applications of poly (lactic acid) fibers. J. Text. Sci. Technol. 2015, 5, 11–17. [Google Scholar]
- Yang, J.; Liu, X.; Zhao, J.; Pu, X.; Shen, Z.; Xu, W.; Liu, Y. The Structural Evolution of β-to-α Phase Transition in the Annealing Process of Poly (3-hydroxybutyrate-co-3-hydroxyvalerate). Polymers 2023, 15, 1921. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Guo, B.H. Poly (butylene succinate) and its copolymers: Research, development and industrialization. Biotechnol. J. 2010, 5, 1149–1163. [Google Scholar] [CrossRef]
- Chen, Y.; Tan, L.; Chen, L.; Yang, Y.; Wang, X. Study on biodegradable aromatic/aliphatic copolyesters. Braz. J. Chem. Eng. 2008, 25, 321–335. [Google Scholar] [CrossRef]
- Guimarães, T.C.; Araújo, E.S.; Hernández-Macedo, M.L.; López, J.A. Polyhydroxyalkanoates: Biosynthesis from alternative carbon sources and analytic methods: A short review. J. Polym. Environ. 2022, 30, 2669–2684. [Google Scholar] [CrossRef]
- Tavanaie, M.A.; Gevari, A. Biodegradability study of polypropylene fibers blended with disposable recycled poly (lactic acid) plastic flakes. Turk. J. Chem. 2019, 43, 424–434. [Google Scholar] [CrossRef]
- Robledo-Ortíz, J.R.; González-López, M.E.; Martín del Campo, A.S.; Pérez-Fonseca, A.A. Lignocellulosic materials as reinforcement of polyhydroxybutyrate and its copolymer with hydroxyvalerate: A review. J. Polym. Environ. 2021, 29, 1350–1364. [Google Scholar] [CrossRef]
- Dilkes-Hoffman, L.S.; Lant, P.A.; Laycock, B.; Pratt, S. The rate of biodegradation of PHA bioplastics in the marine environment: A meta-study. Mar. Pollut. Bull. 2019, 142, 15–24. [Google Scholar] [CrossRef]
- Van der Walle, G.A.M.; De Koning, G.J.M.; Weusthuis, R.A.; Eggink, G. Properties, modifications and applications of biopolyesters. Biopolyesters 2001, 71, 263–291. [Google Scholar]
- Koller, M.; Mukherjee, A. A new wave of industrialization of PHA biopolyesters. Bioengineering 2022, 9, 74. [Google Scholar] [CrossRef]
- Bond, E.B.; Autran, J.-P.M.; Mackey, L.N.; Noda, I.; O’donnell, H.J. Fibers comprising starch and biodegradable polymers. U.S. Patent US6946506B2, 20 September 2005. [Google Scholar]
- Kaseem, M.; Hamad, K.; Deri, F. Thermoplastic starch blends: A review of recent works. Polym. Sci. Ser. A 2012, 54, 165–176. [Google Scholar] [CrossRef]
- Martinez Villadiego, K.; Arias Tapia, M.J.; Useche, J.; Escobar Macías, D. Thermoplastic starch (TPS)/polylactic acid (PLA) blending methodologies: A review. J. Polym. Environ. 2022, 30, 75–91. [Google Scholar] [CrossRef]
- Zhang, Y.; Rempel, C.; Liu, Q. Thermoplastic starch processing and characteristics—A review. Crit. Rev. Food Sci. Nutr. 2014, 54, 1353–1370. [Google Scholar] [CrossRef] [PubMed]
- Heisig, C.; Diedenhoven, J.; Jensen, C.; Gehrke, H.; Turek, T. Selective hydrogenation of biomass-derived succinic acid: Reaction network and kinetics. Chem. Eng. Technol. 2020, 43, 484–492. [Google Scholar] [CrossRef]
- Varghese, S.A.; Pulikkalparambil, H.; Rangappa, S.M.; Siengchin, S.; Parameswaranpillai, J. Novel biodegradable polymer films based on poly (3-hydroxybutyrate-co-3-hydroxyvalerate) and Ceiba pentandra natural fibers for packaging applications. Food Packag. Shelf Life 2020, 25, 100538. [Google Scholar] [CrossRef]
- Wang, Y.; Davey, C.J.; Van der Maas, K.; Van Putten, R.-J.; Tietema, A.; Parsons, J.R.; Gruter, G.-J.M. Biodegradability of novel high Tg poly (isosorbide-co-1, 6-hexanediol) oxalate polyester in soil and marine environments. Sci. Total Environ. 2022, 815, 152781. [Google Scholar] [CrossRef]
- Li, F.; Luo, S.; Yu, J. Mechanical, thermal properties and isothermal crystallization kinetics of biodegradable poly (butylene succinate-co-terephthalate)(PBST) fibers. J. Polym. Res. 2010, 17, 279–287. [Google Scholar] [CrossRef]
- Rafiqah, S.A.; Khalina, A.; Harmaen, A.S.; Tawakkal, I.A.; Zaman, K.; Asim, M.; Nurrazi, M.; Lee, C.H. A review on properties and application of bio-based poly (butylene succinate). Polymers 2021, 13, 1436. [Google Scholar] [CrossRef]
- Azimi, B.; Nourpanah, P.; Rabiee, M.; Arbab, S. Poly (∊-caprolactone) fiber: An overview. J. Eng. Fibers Fabr. 2014, 9, 155892501400900309. [Google Scholar] [CrossRef]
- Meng, Q.; Hu, J. Study on poly (ε-caprolactone)-based shape memory copolymer fiber prepared by bulk polymerization and melt spinning. Polym. Adv. Technol. 2008, 19, 131–136. [Google Scholar] [CrossRef]
- Hayashi, T.; Nakayama, K.; Mochizuki, M.; Masuda, T. Studies on biodegradable poly (hexano-6-lactone) fibers. Part 3. Enzymatic degradation in vitro (IUPAC Technical Report). Pure Appl. Chem. 2002, 74, 869–880. [Google Scholar] [CrossRef]
- Mochizuki, M.; Hayashi, T.; Nakayama, K.; Masuda, T. Studies on biodegradable poly (hexano-6-lactone) fibers. Part 2: Environmental degradation. Pure Appl. Chem. 1999, 71, 2177–2188. [Google Scholar] [CrossRef]
- Charuchinda, A.; Molloy, R.; Siripitayananon, J.; Molloy, N.; Sriyai, M. Factors influencing the small-scale melt spinning of poly (ε-caprolactone) monofilament fibres. Polym. Int. 2003, 52, 1175–1181. [Google Scholar] [CrossRef]
- Douglas, P.; Andrews, G.; Jones, D.; Walker, G. Analysis of in vitro drug dissolution from PCL melt extrusion. Chem. Eng. J. 2010, 164, 359–370. [Google Scholar] [CrossRef]
- Veit, D. Fibers: History, Production, Properties, Market; Springer Nature: Berlin/Heidelberg, Germany, 2023. [Google Scholar]
- Saigusa, K.; Saijo, H.; Yamazaki, M.; Takarada, W.; Kikutani, T. Influence of carboxylic acid content and polymerization catalyst on hydrolytic degradation behavior of Poly (glycolic acid) fibers. Polym. Degrad. Stab. 2020, 172, 109054. [Google Scholar] [CrossRef]
- Murcia Valderrama, M.A.; van Putten, R.-J.; Gruter, G.-J.M. PLGA barrier materials from CO2. The influence of lactide co-monomer on glycolic acid polyesters. ACS Appl. Polym. Mater. 2020, 2, 2706–2718. [Google Scholar] [CrossRef]
- Wang, Y.; Murcia Valderrama, M.A.; van Putten, R.-J.; Davey, C.J.; Tietema, A.; Parsons, J.R.; Wang, B.; Gruter, G.-J.M. Biodegradation and non-enzymatic hydrolysis of poly (lactic-co-glycolic acid)(PLGA12/88 and PLGA6/94). Polymers 2021, 14, 15. [Google Scholar] [CrossRef]
- Ginde, R.M.; Gupta, R.K. In vitro chemical degradation of poly (glycolic acid) pellets and fibers. J. Appl. Polym. Sci. 1987, 33, 2411–2429. [Google Scholar] [CrossRef]
- Samantaray, P.K.; Little, A.; Haddleton, D.M.; McNally, T.; Tan, B.; Sun, Z.; Huang, W.; Ji, Y.; Wan, C. Poly (glycolic acid)(PGA): A versatile building block expanding high performance and sustainable bioplastic applications. Green Chem. 2020, 22, 4055–4081. [Google Scholar] [CrossRef]
- Budak, K.; Sogut, O.; Aydemir Sezer, U. A review on synthesis and biomedical applications of polyglycolic acid. J. Polym. Res. 2020, 27, 208. [Google Scholar] [CrossRef]
- Bansode, S.H.; Khare, P.V.; Mahanwar, P.A. Synthesis of PLGA and Its Fabrication for the Tissue Engineering by Electro and Melt Spinning.
- Xu, W. In A study on the synthesis, modification and current market status of PBAT. E3S Web Conf. 2023, 385, 04007. [Google Scholar] [CrossRef]
- Shi, X.; Ito, H.; Kikutani, T. Structure development and properties of high-speed melt spun poly (butylene terephthalate)/poly (butylene adipate-co-terephthalate) bicomponent fibers. Polymer 2006, 47, 611–616. [Google Scholar] [CrossRef]
- Zhang, W.; Wang, Q.; Wang, G.; Liu, S. Synthesis and characterization of bio-based poly (ethylene 2, 5-furandicarboxylate)-b-poly (butylene adipate-co-terephthalate) copolymers. J. Appl. Polym. Sci. 2022, 139, e52803. [Google Scholar] [CrossRef]
- Zhou, W.; Wang, X.; Yang, B.; Xu, Y.; Zhang, W.; Zhang, Y.; Ji, J. Synthesis, physical properties and enzymatic degradation of bio-based poly (butylene adipate-co-butylene furandicarboxylate) copolyesters. Polym. Degrad. Stab. 2013, 98, 2177–2183. [Google Scholar] [CrossRef]
- Baidurah, S. Methods of analyses for biodegradable polymers: A review. Polymers 2022, 14, 4928. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Park, S.; Bang, J.; Jin, H.J.; Kwak, H.W. Biodegradation in Composting Conditions of PBEAS Monofilaments for the Sustainable End-Use of Fishing Nets. Glob. Chall. 2023, 7, 2300020. [Google Scholar] [CrossRef]
- Prambauer, M.; Wendeler, C.; Weitzenböck, J.; Burgstaller, C. Biodegradable geotextiles—An overview of existing and potential materials. Geotext. Geomembr. 2019, 47, 48–59. [Google Scholar] [CrossRef]
- Austin, H.P.; Allen, M.D.; Donohoe, B.S.; Rorrer, N.A.; Kearns, F.L.; Silveira, R.L.; Pollard, B.C.; Dominick, G.; Duman, R.; El Omari, K. Characterization and engineering of a plastic-degrading aromatic polyesterase. Proc. Natl. Acad. Sci. USA 2018, 115, E4350–E4357. [Google Scholar] [CrossRef]
- Mohee, R.; Unmar, G. Determining biodegradability of plastic materials under controlled and natural composting environments. Waste Manag. 2007, 27, 1486–1493. [Google Scholar] [CrossRef]
- Viera, J.S.; Marques, M.R.; Nazareth, M.C.; Jimenez, P.C.; Sanz-Lázaro, C.; Castro, Í.B. Are biodegradable plastics an environmental rip off? J. Hazard. Mater. 2021, 416, 125957. [Google Scholar] [CrossRef]
- EN 13432; Packaging—Requirements for Packaging Recoverable through Composting and Biodegradation—Test Scheme and Evaluation Criteria for the Final Acceptance of Packaging. Institute for Standardization of Serbia: Belgrade, Serbia, 2000.
- ASTM D6400; Standard Specification for Labeling of Plastics Designed to be Aerobically Composted in Municipal or Industrial Facilities. ATSM International: West Conshohocken, PA, USA, 2021.
- Available online: https://www.tuv-at.be/green-marks/certifications/ok-biodegradable/ (accessed on 20 August 2023).
- Haider, T.P.; Völker, C.; Kramm, J.; Landfester, K.; Wurm, F.R. Plastics of the future? The impact of biodegradable polymers on the environment and on society. Angew. Chem. Int. Ed. 2019, 58, 50–62. [Google Scholar] [CrossRef]
- Available online: https://nova-institute.eu/press/ (accessed on 20 August 2023).
- SkyQuest Technology. Biodegradable Plastics Market Size, Share & Trends Analysis, by Type (PLA, Starch Blends, PHA, Biodegradable Polyesters), End Use Industry (Packaging, Consumer Goods, Textile, Agriculture & Horticulture), Region and Forecast Period 2022–2030; SkyQuest Technology: Westford, MA, USA, 2022. [Google Scholar]
- Khatami, K.; Perez-Zabaleta, M.; Owusu-Agyeman, I.; Cetecioglu, Z. Waste to bioplastics: How close are we to sustainable polyhydroxyalkanoates production? Waste Manag. 2021, 119, 374–388. [Google Scholar] [CrossRef] [PubMed]
- Naeimirad, M.; Zadhoush, A.; Kotek, R.; Esmaeely Neisiany, R.; Nouri Khorasani, S.; Ramakrishna, S. Recent advances in core/shell bicomponent fibers and nanofibers: A review. J. Appl. Polym. Sci. 2018, 135, 46265. [Google Scholar] [CrossRef]
- Krins, B. Biodegradable yarns—Overview of the state-of-the-art. In Proceedings of the Dornbirn Global Fiber Congress, Dornbirn, Austria, 15–17 September 2021. [Google Scholar]
- Park, C.H.; Hong, E.Y.; Kang, Y.K. Effects of spinning speed and heat treatment on the mechanical properties and biodegradability of poly (lactic acid) fibers. J. Appl. Polym. Sci. 2007, 103, 3099–3104. [Google Scholar] [CrossRef]
- Warnock, M.; Davis, K.; Wolf, D.; Gbur, E. Soil Burial Effects on Biodegradation and Properties of Three Cellulosic Fabrics. AATCC Rev. 2011, 11, 53–57. [Google Scholar]
- Situ Biosciences. AATCC 30—Antifungal Test—Textiles; Situ Biosciences: Wheeling, IL, USA, 2020. [Google Scholar]
- Ali, A.; El-Dessouky, H. An insight on the process–property relationships of melt spun polylactic acid fibers. Text. Res. J. 2019, 89, 4959–4966. [Google Scholar] [CrossRef]
- Gajjar, C.R.; Stallrich, J.W.; Pasquinelli, M.A.; King, M.W. Process–Property Relationships for Melt-Spun Poly (lactic acid) Yarn. ACS Omega 2021, 6, 15920–15928. [Google Scholar] [CrossRef]
- Ebrahimi, H. Poly (Lactic Acid) Structure-Process-Property Relationships. Ph.D. Thesis, North Carolina State University, Raleigh, NC, USA, 2021. [Google Scholar]
- Schmack, G.; Tändler, B.; Vogel, R.; Beyreuther, R.; Jacobsen, S.; Fritz, H.G. Biodegradable fibers of poly (L-lactide) produced by high-speed melt spinning and spin drawing. J. Appl. Polym. Sci. 1999, 73, 2785–2797. [Google Scholar] [CrossRef]
- Schmack, G.; Tändler, B.; Optiz, G.; Vogel, R.; Komber, H.; Häußler, L.; Voigt, D.; Weinmann, S.; Heinemann, M.; Fritz, H.G. High-speed melt spinning of various grades of polylactides. J. Appl. Polym. Sci. 2004, 91, 800–806. [Google Scholar] [CrossRef]
- Takasaki, M.; Ito, H.; Kikutani, T. Structure development of polylactides with various D-lactide contents in the high-speed melt spinning process. J. Macromol. Sci. Part B 2003, 42, 57–73. [Google Scholar] [CrossRef]
- Yuan, X.; Mak, A.F.; Kwok, K.W.; Yung, B.K.; Yao, K. Characterization of poly (L-lactic acid) fibers produced by melt spinning. J. Appl. Polym. Sci. 2001, 81, 251–260. [Google Scholar] [CrossRef]
- Nishimura, Y.; Takasu, A.; Inai, Y.; Hirabayashi, T. Melt spinning of poly (L-lactic acid) and its biodegradability. J. Appl. Polym. Sci. 2005, 97, 2118–2124. [Google Scholar] [CrossRef]
- Paakinaho, K.; Ellä, V.; Syrjälä, S.; Kellomäki, M. Melt spinning of poly (l/d) lactide 96/4: Effects of molecular weight and melt processing on hydrolytic degradation. Polym. Degrad. Stab. 2009, 94, 438–442. [Google Scholar] [CrossRef]
- Fambri, L.; Bragagna, S.; Migliaresi, C. Biodegradable Fibers of Poly-L, DL-Lactide 70/30 Produced by Melt Spinning; Macromolecular symposia, 2006; Wiley Online Library: Hoboken, NJ, USA, 2006; pp. 20–25. [Google Scholar]
- Liu, Q.; Zhang, H.; Deng, B.; Zhao, X. Poly (3-hydroxybutyrate) and Poly (3-hydroxybutyrate-co-3-hydroxyvalerate): Structure, Property, and Fiber. Int. J. Polym. Sci. 2014, 2014, 374368. [Google Scholar] [CrossRef]
- Qin, Q.; Takarada, W.; Kikutani, T. Fiber structure formation in melt spinning of bio-based aliphatic co-polyesters. AIP Conf. Proc. 2015, 1664, 080004. [Google Scholar]
- Qin, Q.; Takarada, W.; Kikutani, T. Fiber structure development of PHBH through stress-induced crystallization in high-speed melt spinning process. J. Fiber Sci. Technol. 2017, 73, 49–60. [Google Scholar] [CrossRef]
- Iwata, T.; Tanaka, T. Manufacturing of PHA as Fibers. In Plastics from Bacteria: Natural Functions and Applications; Springer: Berlin/Heidelberg, Germany, 2010; pp. 257–282. [Google Scholar]
- Imre, B.; Pukánszky, B. Compatibilization in bio-based and biodegradable polymer blends. Eur. Polym. J. 2013, 49, 1215–1233. [Google Scholar] [CrossRef]
- Saito, Y.; Doi, Y. Microbial synthesis and properties of poly (3-hydroxybutyrate-co-4-hydroxybutyrate) in Comamonas acidovorans. Int. J. Biol. Macromol. 1994, 16, 99–104. [Google Scholar] [CrossRef]
- Saito, Y.; Nakamura, S.; Hiramitsu, M.; Doi, Y. Microbial synthesis and properties of poly (3-hydroxybutyrate-co-4-hydroxybutyrate). Polym. Int. 1996, 39, 169–174. [Google Scholar] [CrossRef]
- Omura, T.; Komiyama, K.; Maehara, A.; Kabe, T.; Iwata, T. Elastic marine biodegradable fibers produced from poly [(R)-3-hydroxybutylate-co-4-hydroxybutylate] and evaluation of their biodegradability. ACS Appl. Polym. Mater. 2021, 3, 6479–6487. [Google Scholar] [CrossRef]
- Miyao, Y.; Takarada, W.; Kikutani, T. In Improvement of mechanical properties of biodegradable PHBH fibers through high-speed melt spinning process equipped with a liquid isothermal bath. AIP Conf. Proc. 2020, 2289, 020038. [Google Scholar]
- Tanaka, T.; Fujita, M.; Takeuchi, A.; Suzuki, Y.; Uesugi, K.; Ito, K.; Fujisawa, T.; Doi, Y.; Iwata, T. Formation of highly ordered structure in poly [(R)-3-hydroxybutyrate-co-(R)-3-hydroxyvalerate] high-strength fibers. Macromolecules 2006, 39, 2940–2946. [Google Scholar] [CrossRef]
- Rebia, R.A.; Shizukuishi, K.; Tanaka, T. Characteristic changes in PHBH isothermal crystallization monofilaments by the effect of heat treatment and dip-coating in various solvents. Eur. Polym. J. 2020, 134, 109808. [Google Scholar] [CrossRef]
- Selli, F.; Hufenus, R.; Gooneie, A.; Erdoğan, U.H.; Perret, E. Structure–property relationship in melt-spun poly (hydroxybutyrate-co-3-hexanoate) monofilaments. Polymers 2022, 14, 200. [Google Scholar] [CrossRef]
- Leonés, A.; Mujica-Garcia, A.; Arrieta, M.P.; Salaris, V.; Lopez, D.; Kenny, J.M.; Peponi, L. Organic and inorganic PCL-based electrospun fibers. Polymers 2020, 12, 1325. [Google Scholar] [CrossRef]
- Gurarslan, A.; Caydamli, Y.; Shen, J.; Tse, S.; Yetukuri, M.; Tonelli, A.E. Coalesced poly (ε-caprolactone) fibers are stronger. Biomacromolecules 2015, 16, 890–893. [Google Scholar] [CrossRef]
- Kikutani, T. Fiber Formation through Melt Spinning of Bio-polymers. In Proceedings of the International Conference on Technology and Social Science, Kiryu, Japan, 2–4 December 2020. [Google Scholar]
- Yang, Q.; Shen, X.; Tan, Z. Investigations of the preparation technology for polyglycolic acid fiber with perfect mechanical performance. J. Appl. Polym. Sci. 2007, 105, 3444–3447. [Google Scholar] [CrossRef]
- Ziabicki, A. Fundamentals of Fibre Formation: The Science of Fibre Spinning and Drawing; John Wiley & Sons: Hoboken, NJ, USA, 1976. [Google Scholar]
- Fourné, F. Synthetic Fibers: Machines and Equipment, Manufacture, Properties: Handbook for Plant Engineering, Machine Design, and Operation; Hanser Publication: Cincinnati, OH, USA, 1999. [Google Scholar]
- Saigusa, K.; Takarada, W.; Kikutani, T. Improvement of the mechanical properties of poly (glycolic acid) fibers through control of molecular entanglements in the melt spinning process. J. Macromol. Sci. Part B 2020, 59, 399–414. [Google Scholar] [CrossRef]
- Tracy, M.; Ward, K.; Firouzabadian, L.; Wang, Y.; Dong, N.; Qian, R.; Zhang, Y. Factors affecting the degradation rate of poly (lactide-co-glycolide) microspheres in vivo and in vitro. Biomaterials 1999, 20, 1057–1062. [Google Scholar] [CrossRef]
- Malafeev, K.; Moskalyuk, O.; Yudin, V.; Sedush, N.; Chvalun, S.; Elokhovskii, V.Y.; Popova, E.; Ivan’kova, E. Synthesis and properties of fibers prepared from lactic acid–glycolic acid copolymer. Polym. Sci. Ser. A 2017, 59, 53–57. [Google Scholar] [CrossRef]
- Fu, S.; Lu, Y.; Zhang, P. Development and characteristics of novel polyglycolic acid (PGA) monofilaments for acupoint catgut-embedding therapy applications. Text. Res. J. 2019, 89, 845–854. [Google Scholar] [CrossRef]
- Fu, S.; Yang, D.; Zhang, P. Development and characterizations of polylactic acid (PLA) and polyglycolide acid (PGA) monofilaments for acupoint catgut embedding therapy applications. J. Text. Inst. 2019, 110, 1580–1587. [Google Scholar] [CrossRef]
- Khare, P.V.; Bansode, S.H.; Mahanwar, P. Synthesis of Poly (Lactic-co-glycolic) acid and its micro fabrication by Centrifugal force melt spinning Technique. Int. J. Adv. Eng. Manag. IJAEM 2022, 4, 587–600. [Google Scholar]
- Guo, Z.; Chen, S.H.; Zhang, P.H. The Effect of Melt-Spinning Technology on the Degradation of Poly (Glycolic Acid) Fiber In Vitro. Adv. Mater. Res. 2011, 152, 1240–1243. [Google Scholar] [CrossRef]
- Miao, Y.; Cui, H.; Dong, Z.; Ouyang, Y.; Li, Y.; Huang, Q.; Wang, Z. Structural Evolution of Polyglycolide and Poly (glycolide-co-lactide) Fibers during In Vitro Degradation with Different Heat-Setting Temperatures. ACS Omega 2021, 6, 29254–29266. [Google Scholar] [CrossRef] [PubMed]
- Younes, B. A statistical investigation of the influence of the multi-stage hot-drawing process on the mechanical properties of biodegradable linear aliphatic-aromatic co-polyester fibers. Adv. Mater. Sci. Appl. 2014, 3, 186–202. [Google Scholar] [CrossRef]
- Younes, B.; Fotheringham, A. Factorial optimization of the effects of extrusion temperature profile and polymer grade on as-spun aliphatic–aromatic copolyester fibers. II. Crystallographic order. J. Appl. Polym. Sci. 2011, 119, 1896–1904. [Google Scholar] [CrossRef]
- Younes, B.; Fotheringham, A. Factorial optimization of the effects of melt-spinning conditions on biodegradable as-spun aliphatic–aromatic copolyester fibers. III. Diameter, tensile properties, and thermal shrinkage. J. Appl. Polym. Sci. 2011, 122, 1434–1449. [Google Scholar] [CrossRef]
- Younes, B.; Fotheringham, A.; El-Dessouky, H.M. Birefringent approach for assessing the influence of the extrusion temperature profile on the overall orientation of as-spun aliphatic-aromatic co-polyester fibers. Polym. Eng. Sci. 2009, 49, 2492–2500. [Google Scholar] [CrossRef]
- Younes, B.; Fotheringham, A.; El-Dessouky, H.M. Factorial optimization of the effects of extrusion temperature profile and polymer grade on as-spun aliphatic–aromatic copolyester fibers. I. Birefringence and overall orientation. J. Appl. Polym. Sci. 2010, 118, 1270–1277. [Google Scholar] [CrossRef]
- Younes, B.; Fotheringham, A.; El-Dessouky, H.M.; Haddad, G. Factorial optimization of the effects of melt-spinning conditions on as-spun aliphatic-aromatic copolyester fibers I. Spin draw ratio, overall orientation and drawability. Int. J. Polym. Mater. 2011, 60, 316–339. [Google Scholar] [CrossRef]
- Younes, B.; Fotheringham, A.; Mather, R. Factorial Optimisation of the Effects of Melt Spinning Conditions on Biodegradable As-spun Aliphatic-Aromatic Co-Polyester Fibres: II. Die Head Pressure, Crystallographic Order and Thermo-graphic Measurement. Int. Polym. Process. 2011, 26, 150–163. [Google Scholar] [CrossRef]
- Jung Kang, H.; Soon Park, S. Characterization and biodegradability of poly (butylene adipate-co-succinate)/poly (butylene terephthalate) copolyester. J. Appl. Polym. Sci. 1999, 72, 593–608. [Google Scholar] [CrossRef]
- Fuoco, T.; Mathisen, T.; Finne-Wistrand, A. Poly (l-lactide) and poly (l-lactide-co-trimethylene carbonate) melt-spun fibers: Structure–processing–properties relationship. Biomacromolecules 2019, 20, 1346–1361. [Google Scholar] [CrossRef] [PubMed]
- Baimark, Y.; Molloy, R.; Molloy, N.; Siripitayananon, J.; Punyodom, W.; Sriyai, M. Synthesis, characterization and melt spinning of a block copolymer of L-lactide and ε-caprolactone for potential use as an absorbable monofilament surgical suture. J. Mater. Sci. Mater. Med. 2005, 16, 699–707. [Google Scholar] [CrossRef] [PubMed]
- Murayama, A.; Yoneda, H.; Maehara, A.; Shiomi, N.; Hirata, H. A highly elastic absorbable monofilament suture fabricated from poly (3-hydroxybutyrate-co-4-hydroxybutyrate). Sci. Rep. 2023, 13, 3275. [Google Scholar] [CrossRef]
- He, Y.; Qian, Z.; Zhang, H.; Liu, X. Alkaline degradation behavior of polyesteramide fibers: Surface erosion. Colloid Polym. Sci. 2004, 282, 972–978. [Google Scholar] [CrossRef]
- Naeimirad, M.; Zadhoush, A.; Esmaeely Neisiany, R.; Salimian, S.; Kotek, R. Melt-spun PLA liquid-filled fibers: Physical, morphological, and thermal properties. J. Text. Inst. 2019, 110, 89–99. [Google Scholar] [CrossRef]
- Selli, F.; Erdugan, Ü.H. Production and characterization of melt-spun (Ԑ-caprolactone) fibers having different cross sections. In Proceedings of the TexTeh9—Advanced Textiles for a Better World Conference, Bucharest, Romania, 24–25 October 2019; p. 42. [Google Scholar]
- El-Salmawy, A.; Kitagawa, T.; Ko, I.K.; Murakami, A.; Kimura, Y.; Yamaoka, T.; Iwata, H. Preparation and properties of ProNectin F-coated biodegradable hollow fibers. J. Artif. Organs 2005, 8, 245–251. [Google Scholar] [CrossRef]
- Bauer, B.; Emonts, C.; Bonten, L.; Annan, R.; Merkord, F.; Vad, T.; Idrissi, A.; Gries, T.; Blaeser, A. Melt-Spun, Cross-Section Modified Polycaprolactone Fibers for Use in Tendon and Ligament Tissue Engineering. Fibers 2022, 10, 23. [Google Scholar] [CrossRef]
- Park, S.; Lee, B.-K.; Na, M.; Kim, D. Melt-spun shaped fibers with enhanced surface effects: Fiber fabrication, characterization and application to woven scaffolds. Acta Biomater. 2013, 9, 7719–7726. [Google Scholar] [CrossRef]
- Tavanaie, M.A. Melt recycling of poly (lactic acid) plastic wastes to produce biodegradable fibers. Polym.-Plast. Technol. Eng. 2014, 53, 742–751. [Google Scholar] [CrossRef]
- Niaounakis, M. Biopolymers: Processing and Products; William Andrew: Norwich, NY, USA, 2014. [Google Scholar]
- Huang, Y.; Brünig, H.; Müller, M.T.; Wießner, S. Melt spinning of PLA/PCL blends modified with electron induced reactive processing. J. Appl. Polym. Sci. 2022, 139, 51902. [Google Scholar] [CrossRef]
- Furuhashi, Y.; Kimura, Y.; Yoshie, N.; Yamane, H. Higher-order structures and mechanical properties of stereocomplex-type poly (lactic acid) melt spun fibers. Polymer 2006, 47, 5965–5972. [Google Scholar] [CrossRef]
- Padee, S.; Thumsorn, S.; On, J.W.; Surin, P.; Apawet, C.; Chaichalermwong, T.; Kaabbuathong, N.; Narongchai, O.; Srisawat, N. Preparation of poly (lactic acid) and poly (trimethylene terephthalate) blend fibers for textile application. Energy Procedia 2013, 34, 534–541. [Google Scholar] [CrossRef]
- Jompang, L.; Thumsorn, S.; On, J.W.; Surin, P.; Apawet, C.; Chaichalermwong, T.; Kaabbuathong, N.; Narongchai, O.; Srisawat, N. Poly (lactic acid) and poly (butylene succinate) blend fibers prepared by melt spinning technique. Energy Procedia 2013, 34, 493–499. [Google Scholar] [CrossRef]
- Panichsombat, K.; Panbangpong, W.; Poompiew, N.; Potiyaraj, P. Biodegradable fibers from poly (lactic acid)/poly (butylene succinate) blends. IOP Conf. Ser. Mater. Sci. Eng. 2019, 600, 012004. [Google Scholar] [CrossRef]
- Park, S.-W.; Kim, S.-H.; Choi, H.-S.; Cho, H.-H. Preparation and physical properties of biodegradable polybutylene succinate/polybutylene adipate-co-terephthalate blend monofilament by melt spinning. J. Korean Soc. Fish. Ocean Technol. 2010, 46, 257–264. [Google Scholar] [CrossRef]
- Hassan, E.A.; Elarabi, S.E.; Wei, Y.; Yu, M. Biodegradable poly (lactic acid)/poly (butylene succinate) fibers with high elongation for health care products. Text. Res. J. 2018, 88, 1735–1744. [Google Scholar] [CrossRef]
- Pivsa-Art, W.; Pivsa-Art, S.; Fujii, K.; Nomura, K.; Ishimoto, K.; Aso, Y.; Yamane, H.; Ohara, H. Compression molding and melt-spinning of the blends of poly (lactic acid) and poly (butylene succinate-co-adipate). J. Appl. Polym. Sci. 2015, 132, 41856. [Google Scholar] [CrossRef]
- Li, J.; Wang, Y.; Wang, X.; Wu, D. Crystalline characteristics, mechanical properties, thermal degradation kinetics and hydration behavior of biodegradable fibers melt-spun from polyoxymethylene/poly (l-lactic acid) blends. Polymers 2019, 11, 1753. [Google Scholar] [CrossRef]
- Hinüber, C.; Häussler, L.; Vogel, R.; Brünig, H.; Heinrich, G.; Werner, C. Hollow fibers made from a poly (3-hydroxybutyrate)/poly-ε-caprolactone blend. Express Polym. Lett. 2011, 5, 643–652. [Google Scholar] [CrossRef]
- Barral, V.; Dropsit, S.; Cayla, A.; Campagne, C.; Devaux, É. Study of the influence of pcl on the in vitro degradation of extruded pla monofilaments and melt-spun filaments. Polymers 2021, 13, 171. [Google Scholar] [CrossRef] [PubMed]
- Vieira, A.; Medeiros, R.; Guedes, R.M.; Marques, A.; Tita, V. Visco-elastic-plastic properties of suture fibers made of PLA-PCL. Mater. Sci. Forum 2013, 730, 56–61. [Google Scholar] [CrossRef]
- Vieira, A.; Vieira, J.; Guedes, R.; Marques, A. Degradation and viscoelastic properties of PLA-PCL, PGA-PCL, PDO and PGA fibres. Mater. Sci. Forum 2010, 636, 825–832. [Google Scholar] [CrossRef]
- Visco, A.; Scolaro, C.; Giamporcaro, A.; De Caro, S.; Tranquillo, E.; Catauro, M. Threads made with blended biopolymers: Mechanical, physical and biological features. Polymers 2019, 11, 901. [Google Scholar] [CrossRef]
- Chen, Z.; Zhao, Z.; Hong, J.; Pan, Z. Novel bioresource-based poly (3-Hydroxybutyrate-co-4-Hydroxybutyrate)/poly (LacticAcid) blend fibers with high strength and toughness via melt-spinning. J. Appl. Polym. Sci. 2020, 137, 48956. [Google Scholar] [CrossRef]
- Vogel, R.; Tändler, B.; Voigt, D.; Jehnichen, D.; Häußler, L.; Peitzsch, L.; Brünig, H. Melt spinning of bacterial aliphatic polyester using reactive extrusion for improvement of crystallization. Macromol. Biosci. 2007, 7, 820–828. [Google Scholar] [CrossRef]
- Li, L.; Huang, W.; Wang, B.; Wei, W.; Gu, Q.; Chen, P. Properties and structure of polylactide/poly (3-hydroxybutyrate-co-3-hydroxyvalerate)(PLA/PHBV) blend fibers. Polymer 2015, 68, 183–194. [Google Scholar] [CrossRef]
- Tavanaie, M.; Mahmudi, A. Green engineered polypropylene biodegradable fibers through blending with recycled poly (lactic) acid plastic wastes. Polym. Plast. Technol. Eng. 2014, 53, 1506–1517. [Google Scholar] [CrossRef]
- Okihara, T.; Tsuji, M.; Kawaguchi, A.; Katayama, K.-I.; Tsuji, H.; Hyon, S.-H.; Ikada, Y. Crystal structure of stereocomplex of poly (L-lactide) and poly (D-lactide). J. Macromol. Sci. Part B Phys. 1991, 30, 119–140. [Google Scholar] [CrossRef]
- Takasaki, M.; Ito, H.; Kikutani, T. Development of stereocomplex crystal of polylactide in high-speed melt spinning and subsequent drawing and annealing processes. J. Macromol. Sci. Part B 2003, 42, 403–420. [Google Scholar] [CrossRef]
- Mazrouei-Sebdani, Z.; Naeimirad, M.; Peterek, S.; Begum, H.; Galmarini, S.; Pursche, F.; Baskin, E.; Zhao, S.; Gries, T.; Malfait, W.J. Multiple assembly strategies for silica aerogel-fiber combinations—A review. Mater. Des. 2022, 223, 111228. [Google Scholar] [CrossRef]
- Reyhani, R.; Zadhoush, A.; Tabrizi, N.S.; Nazockdast, H.; Naeimirad, M. Synthesis and characterization of powdered CNT-doped carbon aerogels. J. Non-Cryst. Solids 2021, 571, 121058. [Google Scholar] [CrossRef]
- Güzdemir, Ö.; Bermudez, V.; Kanhere, S.; Ogale, A.A. Melt-spun poly (lactic acid) fibers modified with soy fillers: Toward environment-friendly disposable nonwovens. Polym. Eng. Sci. 2020, 60, 1158–1168. [Google Scholar] [CrossRef]
- Xue, W.; Chen, P.; Wang, F.; Wang, L. Melt spinning of nano-hydroxyapatite and polycaprolactone composite fibers for bone scaffold application. J. Mater. Sci. 2019, 54, 8602–8612. [Google Scholar] [CrossRef]
- Chen, X.-X.; Yu, J.-C.; Chen, K.; Ji, P.; Chen, X.-L.; Pan, Z.-J. Facile and large-scale fabrication of biodegradable thermochromic fibers based on poly (lactic acid). Chin. J. Polym. Sci. 2022, 40, 1242–1251. [Google Scholar] [CrossRef]
- Hufenus, R.; Reifler, F.A.; Fernández-Ronco, M.P.; Heuberger, M. Molecular orientation in melt-spun poly (3-hydroxybutyrate) fibers: Effect of additives, drawing and stress-annealing. Eur. Polym. J. 2015, 71, 12–26. [Google Scholar] [CrossRef]
- Hufenus, R.; Reifler, F.A.; Haenggi, U.J. Biodegradable Fibers from Renewable Resources-Melt-spinning of Poly (3-hydroxybutyrate).
- Xiang, H.; Chen, Z.; Zheng, N.; Zhang, X.; Zhu, L.; Zhou, Z.; Zhu, M. Melt-spun microbial poly (3-hydroxybutyrate-co-3-hydroxyvalerate) fibers with enhanced toughness: Synergistic effect of heterogeneous nucleation, long-chain branching and drawing process. Int. J. Biol. Macromol. 2019, 122, 1136–1143. [Google Scholar] [CrossRef]
- Broekman, S. Crystallization of PHAs for melt-spinning (Internship report). In 2023.
- Siebert, S.; Berghaus, J.; Seide, G. Nucleating agents to enhance poly (l-Lactide) fiber crystallization during industrial-scale melt spinning. Polymers 2022, 14, 1395. [Google Scholar] [CrossRef]
- Gu, J.; Wei, X.; Hou, X.; Wei, Z. Biodegradable Poly (butylene succinate-co-terephthalate) Fibers Incorporated with Nanoparticles under High Drawing Temperatures for Enhanced Mechanical Properties. Macromol. Mater. Eng. 2019, 304, 1900168. [Google Scholar] [CrossRef]
- Aouat, T.; Kaci, M.; Devaux, E.; Campagne, C.; Cayla, A.; Dumazert, L.; Lopez-Cuesta, J.M. Morphological, mechanical, and thermal characterization of poly (lactic acid)/cellulose multifilament fibers prepared by melt spinning. Adv. Polym. Technol. 2018, 37, 1193–1205. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, X.; Yang, Y.; Lan, A.; He, X.; Yu, M. In situ graft copolymerization of L-lactide onto cellulose and the direct melt spinning. RSC Adv. 2014, 4, 34584–34590. [Google Scholar] [CrossRef]
- An Tran, N.H.; Brünig, H.; Hinüber, C.; Heinrich, G. Melt spinning of biodegradable nanofibrillary structures from poly (lactic acid) and poly (vinyl alcohol) blends. Macromol. Mater. Eng. 2014, 299, 219–227. [Google Scholar] [CrossRef]
- Zhang, X.; Jin, G.; Ma, W.; Meng, L.; Yin, H.; Zhu, Z.; Dong, Z.; Wang, R. Fabrication and properties of poly (l-lactide) nanofibers via blend sea-island melt spinning. J. Appl. Polym. Sci. 2015, 132, 41228. [Google Scholar] [CrossRef]
- Huang, W.; Huang, X.; Wang, P.; Chen, P. Poly (glycolic acid) Nanofibers via Sea-Island Melt-Spinning. Macromol. Mater. Eng. 2018, 303, 1800425. [Google Scholar] [CrossRef]
- You, Y.; Youk, J.H.; Lee, S.W.; Min, B.-M.; Lee, S.J.; Park, W.H. Preparation of porous ultrafine PGA fibers via selective dissolution of electrospun PGA/PLA blend fibers. Mater. Lett. 2006, 60, 757–760. [Google Scholar] [CrossRef]
- Magazzini, L.; Grilli, S.; Fenni, S.E.; Donetti, A.; Cavallo, D.; Monticelli, O. The Blending of Poly (glycolic acid) with Polycaprolactone and Poly (l-lactide): Promising Combinations. Polymers 2021, 13, 2780. [Google Scholar] [CrossRef]
- Liu, D.; Xie, Q.; Liu, Z.; Chen, J.; Zou, X.; Jing, B. Improvement of melt viscosity and compatibility of polyglycolic acid (PGA)/polylactic acid (PLA) blend via reactive blending with bifunctional and multifunctional hybrid chain extender. J. Appl. Polym. Sci. 2023, 140, e54501. [Google Scholar] [CrossRef]
- Deshpande, S.; Bhati, P.; Ghosh, A.; Bhatnagar, N. Effect of PGA/PCL on the structure–property relation of PLA during tube extrusion. In Proceedings of the 10th World Biomaterials Congress, Montreal, QC, Canada, 17–22 May 2016; pp. 17–22. [Google Scholar]
- Sharma, D.; Satapathy, B.K. Performance evaluation of electrospun nanofibrous mats of polylactic acid (PLA)/poly (ε-caprolactone)(PCL) blends. Mater. Today Proc. 2019, 19, 188–195. [Google Scholar] [CrossRef]
- Akhir, N.A.M.; Othman, M.; Buys, Y.F.; Shaffiar, N.; Jimat, D.N.; Shaharuddin, S.I.S. Characterisation and production of poly (lactic acid)/poly (ethylene glycol) microfiber via melt drawn spinning process. IIUM Eng. J. 2021, 22, 201–212. [Google Scholar] [CrossRef]
- Vayshbeyn, L.I.; Mastalygina, E.E.; Olkhov, A.A.; Podzorova, M.V. Poly (lactic acid)-Based Blends: A Comprehensive Review. Appl. Sci. 2023, 13, 5148. [Google Scholar] [CrossRef]
- Krishnan, S.; Pandey, P.; Mohanty, S.; Nayak, S.K. Toughening of polylactic acid: An overview of research progress. Polym. Plast. Technol. Eng. 2016, 55, 1623–1652. [Google Scholar] [CrossRef]
- Gupta, B.; Geeta; Ray, A.R. Preparation of poly (ε-caprolactone)/poly (ε-caprolactone-co-lactide)(PCL/PLCL) blend filament by melt spinning. J. Appl. Polym. Sci. 2012, 123, 1944–1950. [Google Scholar] [CrossRef]
- Li, X.; Liu, J.; Lu, Y.; Hou, T.; Zhou, J.; Zhang, X.; Zhou, L.; Sun, M.; Xue, J.; Yang, B. Melting centrifugally spun ultrafine poly butylene adipate-co-terephthalate (PBAT) fiber and hydrophilic modification. RSC Adv. 2021, 11, 27019–27026. [Google Scholar] [CrossRef]
- Hufenus, R.; Yan, Y.; Dauner, M.; Yao, D.; Kikutani, T. Bicomponent fibers. In Handbook of Fibrous Materials; Wiley: Hoboken, NJ, USA, 2020; pp. 281–313. [Google Scholar]
- Elahi, M.F.; Lu, W.; Guoping, G.; Khan, F. Core-shell fibers for biomedical applications—A review. J. Bioeng. Biomed. Sci 2013, 3, 1–14. [Google Scholar] [CrossRef]
- Hufenus, R. Novel synthetic fibers by multocomponent melt-spinning. In Proceedings of the XII International İzmir Textile and Apparel Symposium, Izmir, Turkey, 28–30 October 2010; pp. 299–307. [Google Scholar]
- Hufenus, R. Fiber Development by Multicomponent Melt-Spinning; Research Gate: Berlin, Germany, 2011. [Google Scholar]
- Zinn, M.; Dilettoso, S.; Lischer, S.; Maniura, K.; Milz, S.; Weisse, B.; Hufenus, R. Melt-Spun Fibers From Polyhydroxyalkanoate And Polylactate For Fiber Implant Applications. Eur. Cells Mater. 2010, 20, 12. [Google Scholar]
- Hufenus, R.; Reifler, F.A.; Maniura-Weber, K.; Spierings, A.; Zinn, M. Biodegradable bicomponent fibers from renewable sources: Melt-spinning of poly (lactic acid) and poly [(3-hydroxybutyrate)-co-(3-hydroxyvalerate)]. Macromol. Mater. Eng. 2012, 297, 75–84. [Google Scholar] [CrossRef]
- Prahsarn, C.; Klinsukhon, W.; Padee, S.; Suwannamek, N.; Roungpaisan, N.; Srisawat, N. Hollow segmented-pie PLA/PBS and PLA/PP bicomponent fibers: An investigation on fiber properties and splittability. J. Mater. Sci. 2016, 51, 10910–10916. [Google Scholar] [CrossRef]
- Yang, B.; Wang, R.; Dong, Z.; Wu, J.; Kuang, M.; Jin, G.; Ma, H.; Wang, Y.; Zhang, Q.; Zhang, X. Three-dimensional crimped biodegradable poly (lactic acid) fibers prepared via melt spinning and controlled structural reorganization. RSC Adv. 2020, 10, 42890–42896. [Google Scholar] [CrossRef]
- Song, B.; Cao, Y.; Wang, L.; Shen, Y.; Qian, X. Properties and Structure of Thermoplastic Polyvinyl Alcohol/Polyamide Sea-Island Fibers. Polymers 2023, 15, 2071. [Google Scholar] [CrossRef]
- Pal, J.; Kankariya, N.; Sanwaria, S.; Nandan, B.; Srivastava, R.K. Control on molecular weight reduction of poly (ε-caprolactone) during melt spinning—A way to produce high strength biodegradable fibers. Mater. Sci. Eng. C 2013, 33, 4213–4220. [Google Scholar] [CrossRef] [PubMed]
- Hinüber, C.; Häussler, L.; Vogel, R.; Brünig, H.; Werner, C. Hollow poly (3-hydroxybutyrate) fibers produced by melt spinning. Macromol. Mater. Eng. 2010, 295, 585–594. [Google Scholar] [CrossRef]
- Nazhat, S.; Kellomäki, M.; Törmälä, P.; Tanner, K.; Bonfield, W. Dynamic mechanical characterization of biodegradable composites of hydroxyapatite and polylactides. J. Biomed. Mater. Res. 2001, 58, 335–343. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.S.; Kim, J.C.; Kim, Y.H. Effects of take-up speed on the structure and properties of melt-spun poly (L-lactic acid) fibers. Polym. Adv. Technol. 2008, 19, 748–755. [Google Scholar] [CrossRef]
- Gordeyev, S.; Nekrasov, Y.P. Processing and mechanical properties of oriented poly (β-hydroxybutyrate) fibers. J. Mater. Sci. Lett. 1999, 18, 1691–1692. [Google Scholar] [CrossRef]
- Yamane, H.; Terao, K.; Hiki, S.; Kimura, Y. Mechanical properties and higher order structure of bacterial homo poly (3-hydroxybutyrate) melt spun fibers. Polymer 2001, 42, 3241–3248. [Google Scholar] [CrossRef]
- Schmack, G.; Jehnichen, D.; Vogel, R.; Tändler, B. Biodegradable fibers of poly (3-hydroxybutyrate) produced by high-speed melt spinning and spin drawing. J. Polym. Sci. Part B Polym. Phys. 2000, 38, 2841–2850. [Google Scholar] [CrossRef]
- Iwata, T.; Aoyagi, Y.; Fujita, M.; Yamane, H.; Doi, Y.; Suzuki, Y.; Takeuchi, A.; Uesugi, K. Processing of a strong biodegradable poly [(R)-3-hydroxybutyrate] fiber and a new fiber structure revealed by micro-beam X-ray diffraction with synchrotron radiation. Macromol. Rapid Commun. 2004, 25, 1100–1104. [Google Scholar] [CrossRef]
- Antipov, E.; Dubinsky, V.; Rebrov, A.; Nekrasov, Y.P.; Gordeev, S.; Ungar, G. Strain-induced mesophase and hard-elastic behaviour of biodegradable polyhydroxyalkanoates fibers. Polymer 2006, 47, 5678–5690. [Google Scholar] [CrossRef]
- Krins, B. Effect of PHA-material structure on yarn and filament spinning. In Proceedings of the 2nd PHA-Platform World Congress, Cologne, Germany, 22–23 September 2021. [Google Scholar]
- Perret, E.; Reifler, F.A.; Gooneie, A.; Chen, K.; Selli, F.; Hufenus, R. Structural response of melt-spun poly (3-hydroxybutyrate) fibers to stress and temperature. Polymer 2020, 197, 122503. [Google Scholar] [CrossRef]
- Perret, E.; Reifler, F.A.; Gooneie, A.; Hufenus, R. Tensile study of melt-spun poly (3-hydroxybutyrate) P3HB fibers: Reversible transformation of a highly oriented phase. Polymer 2019, 180, 121668. [Google Scholar] [CrossRef]
- Perret, E.; Reifler, F.A.; Gooneie, A.; Chen, K.; Selli, F.; Hufenus, R. X-ray data about the structural response of melt-spun poly (3-hydroxybutyrate) fibers to stress and temperature. Data Brief 2020, 31, 105675. [Google Scholar] [CrossRef] [PubMed]
- Perret, E.; Reifler, F.A.; Gooneie, A.; Hufenus, R. X-ray data from a cyclic tensile study of melt-spun poly (3-hydroxybutyrate) P3HB fibers: A reversible mesophase. Data Brief 2019, 25, 104376. [Google Scholar] [CrossRef]
- Perret, E.; Sharma, K.; Tritsch, S.; Hufenus, R. WAXD, polarized ATR-FTIR and DSC data of stress-annealed poly (3-hydroxybutyrate) fibers. Data Brief 2021, 39, 107523. [Google Scholar] [CrossRef]
- Krishnanand, K.; Deopura, B.L.; Gupta, B. Determination of intrinsic birefringence values of polycaprolactone filaments. Polym. Int. 2013, 62, 49–53. [Google Scholar] [CrossRef]
- Selli, F.; Erdoğan, U.; Hufenus, R.; Perret, E. Mesophase in melt-spun poly (ϵ-caprolactone) filaments: Structure–mechanical property relationship. Polymer 2020, 206, 122870. [Google Scholar] [CrossRef]
- La Mantia, F.; Ceraulo, M.; Mistretta, M.; Morreale, M. Effect of hot drawing on the mechanical properties of biodegradable fibers. J. Polym. Environ. 2016, 24, 56–63. [Google Scholar] [CrossRef]
- La Mantia, F.P.; Ceraulo, M.; Mistretta, M.C.; Morreale, M. Effect of cold drawing on mechanical properties of biodegradable fibers. J. Appl. Biomater. Funct. Mater. 2017, 15, 70–76. [Google Scholar] [CrossRef]
- Mujica-Garcia, A.; Hooshmand, S.; Skrifvars, M.; Kenny, J.M.; Oksman, K.; Peponi, L. Poly (lactic acid) melt-spun fibers reinforced with functionalized cellulose nanocrystals. RSC Adv. 2016, 6, 9221–9231. [Google Scholar] [CrossRef]
- Persson, M.; Lorite, G.S.; Cho, S.-W.; Tuukkanen, J.; Skrifvars, M. Melt spinning of poly (lactic acid) and hydroxyapatite composite fibers: Influence of the filler content on the fiber properties. ACS Appl. Mater. Interfaces 2013, 5, 6864–6872. [Google Scholar] [CrossRef] [PubMed]
- John, M.J.; Anandjiwala, R.; Oksman, K.; Mathew, A.P. Melt-spun polylactic acid fibers: Effect of cellulose nanowhiskers on processing and properties. J. Appl. Polym. Sci. 2013, 127, 274–281. [Google Scholar] [CrossRef]
- Rizvi, R.; Tong, L.; Naguib, H. Processing and properties of melt spun polylactide–multiwall carbon nanotube fiber composites. J. Polym. Sci. Part B Polym. Phys. 2014, 52, 477–484. [Google Scholar] [CrossRef]
- Gilmore, J.; Yin, F.; Burg, K.J. Evaluation of permeability and fluid wicking in woven fiber bone scaffolds. J. Biomed. Mater. Res. Part B Appl. Biomater. 2019, 107, 306–313. [Google Scholar] [CrossRef]
- Kabe, T.; Tsuge, T.; Kasuya, K.-I.; Takemura, A.; Hikima, T.; Takata, M.; Iwata, T. Physical and structural effects of adding ultrahigh-molecular-weight poly [(R)-3-hydroxybutyrate] to wild-type poly [(R)-3-hydroxybutyrate]. Macromolecules 2012, 45, 1858–1865. [Google Scholar] [CrossRef]
- Zhou, M.; Fan, M.; Zhao, Y.; Jin, T.; Fu, Q. Effect of stretching on the mechanical properties in melt-spun poly (butylene succinate)/microfibrillated cellulose (MFC) nanocomposites. Carbohydr. Polym. 2016, 140, 383–392. [Google Scholar] [CrossRef]
- Naeimirad, M.; Krins, B. Structure-Properties of Melt-Spun Bioplastic Fibers in Correlation with Their Biodegradation Behaviour and Mechanical Performance (PolyBioDeg-101110810); European Commission: Brussels, Belgium, 2023. [Google Scholar]
- Iwata, T.; Tanaka, T.; Doi, Y. High-Strength Fiber of Biodegradable Aliphatic Polyester and Process for Producing the Same. U.S. Patent WO2006038373, 13 April 2006. [Google Scholar]
- Iwata, T.; Hongo, C.; Tamura, M. Biodegradable Polyester Fiber Having Excellent Thermal Stability and Strength, and Method for Producing Same. U.S. Patent US2014/0088288, 27 March 2014. [Google Scholar]
- Zhang, J.; Wang, L.; Sun, J.; Jiang, S.; Li, H.; Zhang, S.; Yang, W.; Gu, X.; Qiao, H. A novel hollow microsphere acting on crystallization, mechanical, and thermal performance of poly (3-hydroxybutyrate-co-4-hydroxybutyrate). Polym. Cryst. 2021, 4, e10204. [Google Scholar] [CrossRef]
- Qian, J.; Zhu, L.; Zhang, J.; Whitehouse, R.S. Comparison of different nucleating agents on crystallization of poly (3-hydroxybutyrate-co-3-hydroxyvalerates). J. Polym. Sci. Part B Polym. Phys. 2007, 45, 1564–1577. [Google Scholar] [CrossRef]
- Barham, P.; Keller, A. The relationship between microstructure and mode of fracture in polyhydroxybutyrate. J. Polym. Sci. Part B Polym. Phys. 1986, 24, 69–77. [Google Scholar] [CrossRef]
- Shah, D.T.; Tran, M.; Berger, P.A.; Aggarwal, P.; Asrar, J.; Madden, L.A.; Anderson, A.J. Synthesis and properties of hydroxy-terminated poly (hydroxyalkanoate) s. Macromolecules 2000, 33, 2875–2880. [Google Scholar] [CrossRef]
- Melo-Lopez, L.; Cabello-Alvarado, C.J.; Andrade-Guel, M.L.; Medellín-Banda, D.I.; Fonseca-Florido, H.A.; Avila-Orta, C.A. Synthetic Fibers from Renewable Sources. In Handbook of Nanomaterials and Nanocomposites for Energy and Environmental Applications; Springer: Berlin/Heidelberg, Germany, 2020; pp. 1–25. [Google Scholar]
- Perret, E.; Hufenus, R. Insights into strain-induced solid mesophases in melt-spun polymer fibers. Polymer 2021, 229, 124010. [Google Scholar] [CrossRef]
- Tanaka, T.; Yabe, T.; Teramachi, S.; Iwata, T. Mechanical properties and enzymatic degradation of poly [(R)-3-hydroxybutyrate] fibers stretched after isothermal crystallization near Tg. Polym. Degrad. Stab. 2007, 92, 1016–1024. [Google Scholar] [CrossRef]
- Aminlashgari, N.; Pal, J.; Sanwaria, S.; Nandan, B.; Srivastava, R.K.; Hakkarainen, M. Degradation product profiles of melt spun in situ cross-linked poly (ε-caprolactone) fibers. Mater. Chem. Phys. 2015, 156, 82–88. [Google Scholar] [CrossRef]
- Stevens, E. Green Plastics, Plastics and the Environment; Princeton University Press: Princeton, NJ, USA, 2001. [Google Scholar]
- Gonsalves, K.; Chen, X.; Cameron, J. Degradation of nonalternating poly (ester amides). Macromolecules 1992, 25, 3309–3312. [Google Scholar] [CrossRef]
- Aaliya, B.; Sunooj, K.V.; Lackner, M. Biopolymer composites: A review. Int. J. Biobased Plast. 2021, 3, 40–84. [Google Scholar] [CrossRef]
- Available online: https://www.senbis.com/sustainable-products/ (accessed on 20 August 2023).
- Miranda, C.S.; Ribeiro, A.R.; Homem, N.C.; Felgueiras, H.P. Spun biotextiles in tissue engineering and biomolecules delivery systems. Antibiotics 2020, 9, 174. [Google Scholar] [CrossRef]
- Fan, X.; Keynton, R.S. Fabrication and characterization of biopolymer fibers for 3D oriented microvascular structures. J. Micromech. Microeng. 2019, 29, 083003. [Google Scholar] [CrossRef]
- Koller, M. Biodegradable and biocompatible polyhydroxy-alkanoates (PHA): Auspicious microbial macromolecules for pharmaceutical and therapeutic applications. Molecules 2018, 23, 362. [Google Scholar] [CrossRef]
- Andriano, K.P.; Pohjonen, T.; Törmälä, P. Processing and characterization of absorbable polylactide polymers for use in surgical implants. J. Appl. Biomater. 1994, 5, 133–140. [Google Scholar] [CrossRef]
- Naeimirad, M. Marine degradable fishing net protection. Technical Textiles, 4 May; 2023.
- Li, J.; Lemstra, P.J.; Ma, P. Can high-performance fibers be (come) bio-based and also biocompostable? Adv. Ind. Eng. Polym. Res. 2022, 5, 117–132. [Google Scholar] [CrossRef]
- Manfra, L.; Marengo, V.; Libralato, G.; Costantini, M.; De Falco, F.; Cocca, M. Biodegradable polymers: A real opportunity to solve marine plastic pollution? J. Hazard. Mater. 2021, 416, 125763. [Google Scholar] [CrossRef] [PubMed]
- Naeimirad, M.; Krins, B. Melt-spun high-tenacity marine-degradable filament yarns. In Proceedings of the European Bioplastics Conferece (EBC-23), Berlin, Germany, 12–13 December 2023. [Google Scholar]
- Eck, M.; Schwab, S.T.; Nelson, T.F.; Wurst, K.; Iberl, S.; Schleheck, D.; Link, C.; Battagliarin, G.; Mecking, S. Biodegradable High-Density Polyethylene-like Material. Angew. Chem. 2023, 135, e202213438. [Google Scholar] [CrossRef]
- Liu, Y.; Mecking, S. A synthetic polyester from plant oil feedstock by functionalizing polymerization. Angew. Chem. Int. Ed. 2019, 58, 3346–3350. [Google Scholar] [CrossRef] [PubMed]
- Ortmann, P.; Mecking, S. Long-spaced aliphatic polyesters. Macromolecules 2013, 46, 7213–7218. [Google Scholar] [CrossRef]
- Cristina, J.; de Ilarduya, A.M.; Abdelilah, A.; Yi, J.; Katja, L.; Muñoz-Guerra, S. Copolyesters Made from 1, 4-Butanediol, Sebacic Acid, and d-Glucose by Melt and Enzymatic Polycondensation. Biomacromolecules 2015, 16, 868–879. [Google Scholar]
- Fodor, C.; Golkaram, M.; Woortman, A.J.; van Dijken, J.; Loos, K. Enzymatic approach for the synthesis of biobased aromatic–aliphatic oligo-/polyesters. Polym. Chem. 2017, 8, 6795–6805. [Google Scholar] [CrossRef]
- Jiang, Y.; Loos, K. Enzymatic synthesis of biobased polyesters and polyamides. Polymers 2016, 8, 243. [Google Scholar] [CrossRef]
- Biodegradation of biopolymeric fibers. Man Made Fibers Int. 2023, 73, 13.
- Ghosh, K.; Jones, B.H. Roadmap to biodegradable plastics—Current state and research needs. ACS Sustain. Chem. Eng. 2021, 9, 6170–6187. [Google Scholar] [CrossRef]



| Author(s) | Polymer(s) | Processing | Performance(s) | Application | Ref. |
|---|---|---|---|---|---|
| Monocomponent fibers | |||||
| Mezghani et al. (1997) | PLLA | High-speed melt-spinning (up to 5000 m/min) | Best results at 3000 m/min, 43% crystallinity, 385 MPa, 6 GPa | Development for textiles | [89] |
| Schmack et al. (1998) | PLA | Yarn melt-spinning (12 F 2, 300 µm, DR 3 6, 6000 m/min) | UTS 4 430 MPa, UTM 5 6 GPa, 20% crystallinity | Textiles | [150] |
| Yuan et al. (2000) | PLLA | Monofilament spinning (1 mm, 1 m/min), drawing | MW reduction, Ø 110 µm, 63% crystallinity, UTS 600 MPa | Textiles | [153] |
| Nazhat et al. (2001) | PLLA | Monofilament spinning through 1 mm die, DR of 4.8 | Fibers with 260–300 µm diameter | Composite for bone tissue | [259] |
| Schmack et al. (2003) | PLA (1–8% D, 2–7 PDI) | Yarn melt-spinning (12 F, 300 µm, DR 6, 5000 m/min) | Better fibers at lower D content and PDI, higher speed, and DR | Textiles | [151] |
| Takasaki et al. (2003) | PLA (1.5, 8.1, 16.4% D) | Monofilament spinning (500 µm, 5000–10,000 m/min) | Better at low D%, high speed and DR (45% crystallinity, 570 MPa) | Textiles | [152] |
| Nishimura et al. (2004) | PLLA | 12 Filaments spinning and 2-stage drawing in water | 18 times drawing, 70% crystallinity, UTS 810 MPa | Technical | [154] |
| EL-Salmawy et al. (2004) | PLLA, P(LA-co-CL) | ProNectin F-coated hollow monofilament spinning | Higher adhesion PLLA fibers, better orientation hollow fibers | Nerve tissue regeneration | [197] |
| Baimark et al. (2005) | P(LL-co-CL) | 2-step synthesis, piston-spinning 1 mm, water bath | Drawn filament, 160 µm, 530 MPa, 168 °C MP, | Absorbable surgical suture | [192] |
| Fambri et al. (2006) | PLDLA | Melt-spinning at below 100 m/min speed | Filaments 120 µm in diameter and UTS 200 MPa | Tissue engineering | [156] |
| Park et al. (2007) | PLA | Spinning at high speeds and batch heat treatment | Speed and heat, 69% crystallinity, 6 g/den, lower biodegradation | N/A | [144] |
| Kim et al. (2008) | PLLA | One-step melt-spinning process | Well-developed α-crystallites at high take-up speeds (3500 m/min) | Textile | [260] |
| Paakinaho et al. (2009) | PLDLA | Multifilament (8 and 12 F) spinning and hot drawing | High MW, faster thermal degradation, hydrolysis effect of lactide | Tissue repair | [155] |
| Tavanaie et al. (2014) | r-PLA | Monofilament spinning (1 mm, 70 m/min), cold draw | Strength: 491 MPa, orientation: 0.96, durability | Textiles and clothing | [200] |
| Naeimirad et al. (2018) | PLA | Hollow multifilament yarn | Liquid-filled, higher hollowness by increasing throughput | Agriculture and drug delivery | [195] |
| Ali et al. (2019) | PLA | POY and FDY spun filament yarns | Lower diameter and higher crystallinity @ 600 m/min | Textile and technical | [147] |
| Fuoco et al. (2019) | PLA, PLA-co-TCM | Melt-spinning (1800 m/min) and after drawing | 17 µm filaments (125 den), 60% crystallinity, 302–610 MPa | Clothing textiles | [191] |
| Fu et al. (2019) | PLA | Spinning (300 m/min, DR 6) and chitosan dip-coating | Ø 253 µm, Strength 78.6 cN, EAB 57% | Acupoint catgut embedding | [179] |
| Chirag et al. (2021) | PLA | 69 filaments melt-spinning with different parameters | 62–1000 dtex, 25–340 mN/tex, 14–62% crystallinity | Textiles | [148] |
| Gordeyev et al. (2000) | PHB | High-drawn melt-spun yarns | DR of 2, and hot drawing and annealing resulted in 330 MPa | Research only | [261] |
| Yamane et al. (2001) | Non-pure PHB | Melt-spinning | Impurities for nucleating, fast crystallization, 300 micron fibers | Load bearing in medicine | [262] |
| Schmack et al. (2000) | PHB | Spinning with DR of 6.9 and speed up to 3500 m/min | Low crystal size with high rate, Strength of 27 cN/tex | Development | [263] |
| Iwata et al. (2004) | UHMW-PHB | Spinning, ice quenching, drawing, and annealing | Fiber diameter 40 µm, strength 1.3 GPa, modulus of 18.1 GPa | High-performance | [264] |
| Antipov et al. (2006) | PHB and copolymers | Optimized melt-spinning | Min thermal degradation, UTS 330 MPa, UTM 7.7 GPa | R&D | [265] |
| Tanaka et al. (2006) | PHBV | Spinning, ice quenching, drawing, and annealing | Suitable copolymer and spinning: UTS 1 GPa, UTM 8 GPa | High performance | [166] |
| Qing et al. (2015) | PHBH | Spinning at different temperatures, 500 µm spinneret | Better crystallization (α-crystal orientation) at high speeds | Technical | [158] |
| Krins et al. (2021) | PHA | Melt-spinning of different biodegradable filaments | Marine degradable | Maritime | [143,266] |
| Perret et al. (2019, 2020) | P3HB | Melt-spinning, stress-annealing | Low stress: viscoelastic (α crystal to mesophase transformation) high stress: high strength (UTS 184 MPa) | Shock-absorbing ductile textiles | [267,268,269,270,271] |
| Rebia et al. (2020) | PHBH | Monofilament-spinning, isothermal crystallization, and drawing | Dip-coated biodegradable fibers | Drug delivery and the dying approach | [167] |
| Miyao et al. (2020) | PHBH | Liquid isothermal bath, mono (1 mm) melt-spinning | Better crystallization, 1000 m/min speed, UTS 170 MPa | Technical and textile | [165] |
| Omura et al. (2021) | PHBH | Gram scale spinner, 1 hole 1 mm, 1.8 m/min, DR 5 | Elastic, marine biodegradable, UTS 200 MPa, 200% elongation | Agricultural, fishery, or medical | [164] |
| Selli et al. (2022) | PHBH | Monofilament spinning (500 µm, L/D 4, 140–160 °C) | Ø 130 µm, UTS 291 MPa, orientation 0.98, 35% crystallinity | Textile and medical | [168] |
| Murayama et al. (2023) | PHBH | Monofilament spinning, knot making | 250 µm knots, UTS 167 MPa, biodegradable (in vitro and in vivo) | Absorbable and safe suture | [193] |
| Mochizuki et al. (1997) | PCL | Melt-spinning | Effect of draw ratio on the mechanical properties of PCL filament | Biomedical | [113,114] |
| Charuchinda et al. (2003) | PCL | Small-scale monofilament melt-spinning | Effect of processing parameters on fiber properties like diameter | Biomedical and others | [115] |
| Park et al. (2013) | PCL | Profiled fibers via piston spinner | Faster degradation in NaOH solution due to high SSA | Tissue engineering | [199] |
| Krishnanand at al. (2013) | PCL | Intrinsic birefringence | UTM 3.473 GPa for crystalline and 0.071 GPa for amorphous | Amorphous orientation | [272] |
| Pal et al. (2013) | PCL | Reactive monofilament extrusion (BCY crosslinking) | Fiber diameter 66 µm, UTS 2500 MPa, lower degradation | Tissue engineering | [257] |
| Gurarslan et al. (2014) | PCL-coalesced | Treatment in urea, monofilament spinning, drawing | Higher modulus and crystallinity due to intrinsic alignment | Tissue engineering | [170] |
| Selli et al. (2020) | PCL | Monofilament spinning, modified drawing | 600 m/min, mesophase, 55% crystallinity, 59 µm, 315 mN/tex | Technical and mechical | [273] |
| Selli et al. (2019) | PCL | Melt-spinning with different cross sections | Hollow fibers and solid fibers with smooth surface | Technical and medical | [196] |
| Bauer et al. (2022) | PCL | Cross-section modified mono and multifilament | DR 9.25, 65% crystallinity, 690 mN/tex, Hermans orientation 0.9 | Tendon and Ligament | [198] |
| Yang et al. (2007) | PGA | Melt-spinning (255 °C, 30 m/min) | Less internal stress, DR 5, UTS 654 mN/tex | Medical sutures | [215] |
| Guo et al. (2011) | PGA | Melt-spinning (240 °C, 20 m/min), DR (2–6 @40–50 °C) | Different degradation rates via IV, DR, and Drawing temperature | Biomedical | [181] |
| Fu et al. (2018) | PGA | Monofilament spinning and chitosan dip-coating | Higher mechanical, swelling, antibacterial, lower biodegradation | Acupoint catgut embedding | [178] |
| Fu et al. (2019) | PGA | Spinning (300 m/min, DR 6) and chitosan dip-coating | Ø 245 µm, Strength 57.3 cN, EAB 18% | Acupoint catgut embedding | [179] |
| Saigusa et al. (2020) | PGA | Optimized melt-spinning (24F, 250 µm, 245 °C) | Guide roller at 300 m/min, take-up at 1500 m/min, UTS 780 mN/tex | Biomedical | [118,175] |
| Miao et al. (2021) | PGA, PLGA (8% LA) | Extrusion (250 °C, DR 4.5 @ 60 °C, set @100–140 °C) | Faster degradation of PGA in PBS starting from amorphous | Biomedical | [182] |
| Shi et al. (2005) | PBAT (44% BT) | High speed melt-spinning (5000 m/min) | PBT-like crystal structure and good mechanical properties | Tough products | [82] |
| Yunes et al. (2011) | PBAT | Factoriaal DOE, spinning (30/55 F, 0.4 mm, 36 m/min) | Simulation (temperature, MFI, speed, orientation, crys%, etc.) | Biodegradable textiles | [184,185,186,187,188,189] |
| Mantia et al. (2017) | PBAT (Bioflex, Mater-Bi) | Extrusion via capillary, cold and hot after drawing | Orientation, high modulus, strength, and thermomechanical resistance | High performance mission | [274,275] |
| He et al. (2004) | PEA | Copolymerization, monofilament spinning, drawing | 125 µm, UTS 125 MPa, Alkaline reduction | N/A | [194] |
| Shi et al. (2006) | PBTSA | 4 filaments yarn, 2000 m/min | Elastic yarn | Testing new copolymer | [126] |
| Li et al. (2010) | PBST | 36F melt-spinning (1 mm), isothermal crystallization | UTS 360 MPa, 35% crystallinity, Tm 180 °C | Sustainable textiles | [109] |
| Bansode et al. (N/A) | PLGA | Melt-spinning and electro-spinning | Fine fibers (21–27 µm), biocompatible, and degradable | Tissue engineering, drug delivery | [124,180] |
| Malafeev et al. (2017) | PLGA (90/10) | Microextruder spinning (Ø 1 mm, 20 m/min) | UTS 91 MPa | Bio-resorbable suture | [177] |
| Schick et al. (2023) | PBS TPS, and PBAT | Melt-spinning via FET-100, 48F, max 1000 m/min | DPF 4, 100 mN/tex < PP (500 mN/tex), 70% crystallinity | Lower mechanical performance | [87] |
| Kim et al. (2023) | PBSA | Provided by National Institute of Fisheries Science | 80% biodegradation and physiochemical reduction via composting | Fish nets | [130] |
| Blends and composite fibers | |||||
| Takasaki et al. (2003) | r-PLA (PLLA + PDLA) | Monofilament spinning (0.5 mm, 7500 m/min) | Ø 40 µm, 40% crystallinity, including stereocomplex, UTS 500 MPa | Technical | [221] |
| Furuhashi et al. (2006) | PLLA/PDLA | Melt-spinning, drawing, and annealing | Draw @90 °C, stereocomplex crystals, UTS 520 MPa, UTM 8.5 GPa | Textiles | [203] |
| P-Art et al. (2011) | PLA/PHBV (90/10) | Blend biofiber multifilament spinning | Effect of draw speed on tenacity and linear density | Socks knitting | [71] |
| M-Garcia et al. (2016) | PLA/(CNC)-g-PLLA | Composite fiber spinning along with grafting | Smooth surface, alignment of the CNC and PLA molecular chains | Specific high properties | [276] |
| Jompang et al. (2013) | PLA/PBS | Multifilament blend yarns | Miscibility at 10% of PBS | Modified textile yarns | [205] |
| Padee et al. (2013) | PLA/PTT | Blend fiber spinning via different ratios | Successful spinning at 10% PTT, Crystallinity improvement | Textile fibers | [204] |
| Persson et al. (2013) | PLA/HAP | Composite fibers | Rough surface | Biomedical | [277] |
| John et al. (2013) | PLA/CNW | Compounding (10 wt%) and melt-spinning (1–3 wt%) | Rough fibers, 90–95 µm, concentration of CNW, crystallinity | Biomedicine | [278] |
| Zhang et al. (2014) | PLLA-g-Cellulose | Reactive co-extrusion, direct spinning 500 m/min | Smooth surface, ductile cross-section, better properties 41 mN/tex | Textile | [234] |
| Tavanaie et al. (2014) | r-PLA/PP (0–50%) | Monofilament spinning (1 mm, 70 m/min), cold draw | Tenacity 430 mN/tex, 42% crystallinity, dyability | Textiles and clothing | [97,219] |
| Zhang et al. (2014) | PLLA/TMC-306 NA | Compounding (0.1–0.5 wt%) and spinning (18 F, 0.4 mm, 150 m/min), hot drawing (DR 2.5) | Optimum at 0.3 wt%, 57% crystallinity, UTS 600 MPa, | High performance textile fibers | [88] |
| Rizvi et al. (2014) | PLA/MWCNT | Composite fibers | Rough and irregular surface | Smart textiles | [279] |
| Hoai et al. (2014) | PLA/PVA | Blending, spinning (<100 m/min), water dissolving | Nanofibrils 60 nm in diameter, filament Ø 164 µm (243 DPF) | Scaffolds for tissue engineering | [235] |
| Zhang et al. (2014) | PLA/LDPE | Blend spinning (24 F, 0.3 mm, 400 m/min) and DR 2 | Immiscible, Extraction, 92 nm PLA fiber, 60% crystallinity | Filtration | [236] |
| Li et al. (2015) | PLA/PHBV | Blend melt-spinning and hot drawing | Higher heat-resistance, softness, and tenacity | Textile | [218] |
| Pisva-Art et al. (2016) | PLA/PBSA | Blending, fiber spinning, drawing 4 times, annealing | Modify brittleness of PLA, UTS 300 MPa | Textile | [209] |
| Hassan et al. (2017) | PLA/PBS | Blend fiber spinning | Effect of PBS ratio (ductility) | Healthcare products | [208] |
| Huang et al. (2018) | PLA (92%)/PGA (8%) | Blend spinning (24F, 260 µm, DR 1.4, 2500 m/min) | 93 nm diameter nanofibers | Tissue Engineering | [237] |
| Aouat et al. (2018) | PLA/MA/CNW, MCC | Composite fiber spinning | Poor dispersion for MCC (Vs. CNW), fillers alter the diameter | Biomedical, technical | [233] |
| Panichsombat et al. (2019) | PLA/PBS | Blend fibers | Miscibility via PBS below 10% | Textile fibers | [206] |
| Gilmore et al. (2019) | PLA/PLA-co-PCL | Blend modified process | Grooved fibers with wicking performance | Wicking | [280] |
| Visco et al. (2019) | PLA/LTI/PCL | Reactive extrusion (160 °C, air quench, after drawing) | Thread filament 300 µm diameter, UTS 45 MPa, EAB 450% | Absorbable antibacterial sutures | [215] |
| Li et al. (2019) | PLLA/POM | Blending, melt-spinning (72 F, 300 µm), Drawing | Modification, higher crystallinity via drawing, 791 MPa, hydration | Technical | [210] |
| Chen et al. (2020) | PLA/PHBH | 65/35 the best ratio for crystallization | UTS 904 MPa, UTM 28.42 GPa, EAB 81% | Balanced performance | [216] |
| Güzdemir et al. (2020) | PLA/Soy | Composite biofiber, 3 spinning holes-500 μm | Larger and non-uniform diameter, 56 MPa | Disposable nonwoven fabrics | [224] |
| Akhir et al. (2021) | PLA/PEG | Fine melt-spun fibers | Diameter reduction by PEG concentration (18 µm) | Ductility, and surface roughness | [243] |
| Barral et al. (2021) | PLA/PCL (10 to 40%) | Mono (1 mm), and multifilament (80 F, 50–70 µm) | Adding PCL reduced hydrolytic degradation of PLA in DMEM | Bioresorbable implants | [212] |
| Chen et al. (2022) | PLLA/microcapsules | Compounding, powder mixing, melt-spinning | Mesophase content, 470 mN/tex, Thermochromic effect | Smart textiles | [226] |
| Siebert et al. (2022) | PLA/BHET | 48 F, 250 µm, spinning through FET-100, 3000 m/min | Slow nucleating effect, 50% crystallinity, 270 mN/tex | Textile and technical | [231] |
| Huang et al. (2022) | PLA/PCL | EIReP, piston spinning (0.3 mm, 2000 m/min) | Better melt strength and elastic behavior, lower crystallinity | Testing the compound | [202] |
| Vogel et al. (2007) | P3HB/DCP | Melt-spinning and drawing by max ratio of 7 | Nucleating effect, about 200 MPa UTS | Textile | [217] |
| Hinüber et al. (2011) | PHB/PCL | Blend hollow fibers | Phase separation in spinning due to Tm difference | Biomedical | [211] |
| Hinuber et al. (2011) | P3HB/NA | Nucleating-assisted hollow fiber piston-spinning | Regular surface and an inner diameter of between 50 and 500 µm | Nerve repair with artificial tubes | [211,258] |
| Kabe et al. (2012) | PHB/UHMW-PHB | Blend film casting, nucleation via 5–10% UHMW | Faster crystallization, β-form crystals, slower degradation | Biodegradable alternatives | [281] |
| Hufenus et al. (2015) | P3HB/NA | Nucleating, intermediate draw-off spinning | Induced oriented crystallization, stability, and 215 MPa strength | Textile yarns | [227] |
| Xiang et al. (2019) | PHBV/DCP/WS2 | Monofilament spinning (0.5 mm, 550 m/min) | UTS 189 MPa, heterogeneous nucleation, branching, 73% crys | Textiles | [229] |
| Gupta et al. (2012) | PCL/PLCL (10–50%) | Monofilament extrusion and drawing | UTS 500 MPa, 60% crystallinity. Reduction by PLCL content. | Biomedical | [246] |
| Xue et al. (2019) | PCL/HAP | Composite fibers | Enhanced mechanical performance | Bone scaffold | [225] |
| Park et al. (2010) | PBS/PBAT | Blend monofilament melt-spinning (80 m/min) | Tensile strength reduction in more than 5% PBAT content | Technical | [207] |
| Zhou et al. (2016) | PBS/MFC | Composite yarns | Effect of drawing and take-up speed on dispersion of filler | Extensive | [282] |
| Gu et al. (2019) | PBST/NP | In situ polymerization and spinning | 150 dtex yarn, UTS 240 MPa, 42.3% crystallinity, orientation 0.54 | High-performance | [232] |
| Li et al. (2021) | PBAT/HBP | Centrifugal melt-spinning | Hydrophilic microfibers | Medical | [247] |
| Multicomponent fibers | |||||
| Shi et al. (2006) | PBAT/PBT | Bico (core/sheath) | Structure development of PBT (via PBAT) | Thermo-bonds | [126] |
| M. Zinn et al. (2010) | PHBV/PLA | Bico (PHBV only in core possible) | No toxicity | Medical tendon repair | [252] |
| Hufenus et. al. (2012) | PHBV/PLA | Bico melt-spinning (PHBV core and PLA sheath) | UTS 410 MPa for PLA, 340 MPa for bico | Medical | [228,253] |
| Prahsarn et al. (2016) | PLA/PBS | Hollow segmented-pie bicomponent fibers | Good compatibility, 132 mN/tex, EAB 101%, 25 µm diameter | Biomedical, technical | [254] |
| Yang et al. (2020) | PLA/LM-PLA | Side-by-Side bicomponent melt-spinning (24 F) | 3D structure after self-crimping, 400 µm, 300 mN/tex, | Alternative of PET/PTT | [255] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Naeimirad, M.; Krins, B.; Gruter, G.-J.M. A Review on Melt-Spun Biodegradable Fibers. Sustainability 2023, 15, 14474. https://doi.org/10.3390/su151914474
Naeimirad M, Krins B, Gruter G-JM. A Review on Melt-Spun Biodegradable Fibers. Sustainability. 2023; 15(19):14474. https://doi.org/10.3390/su151914474
Chicago/Turabian StyleNaeimirad, Mohammadreza, Bas Krins, and Gert-Jan M. Gruter. 2023. "A Review on Melt-Spun Biodegradable Fibers" Sustainability 15, no. 19: 14474. https://doi.org/10.3390/su151914474
APA StyleNaeimirad, M., Krins, B., & Gruter, G.-J. M. (2023). A Review on Melt-Spun Biodegradable Fibers. Sustainability, 15(19), 14474. https://doi.org/10.3390/su151914474







