Phosphorus Sorption by Purple Soils in Relation to Their Properties: Investigation, Characterization, and Explanation
Abstract
:1. Introduction
2. Materials and Methods
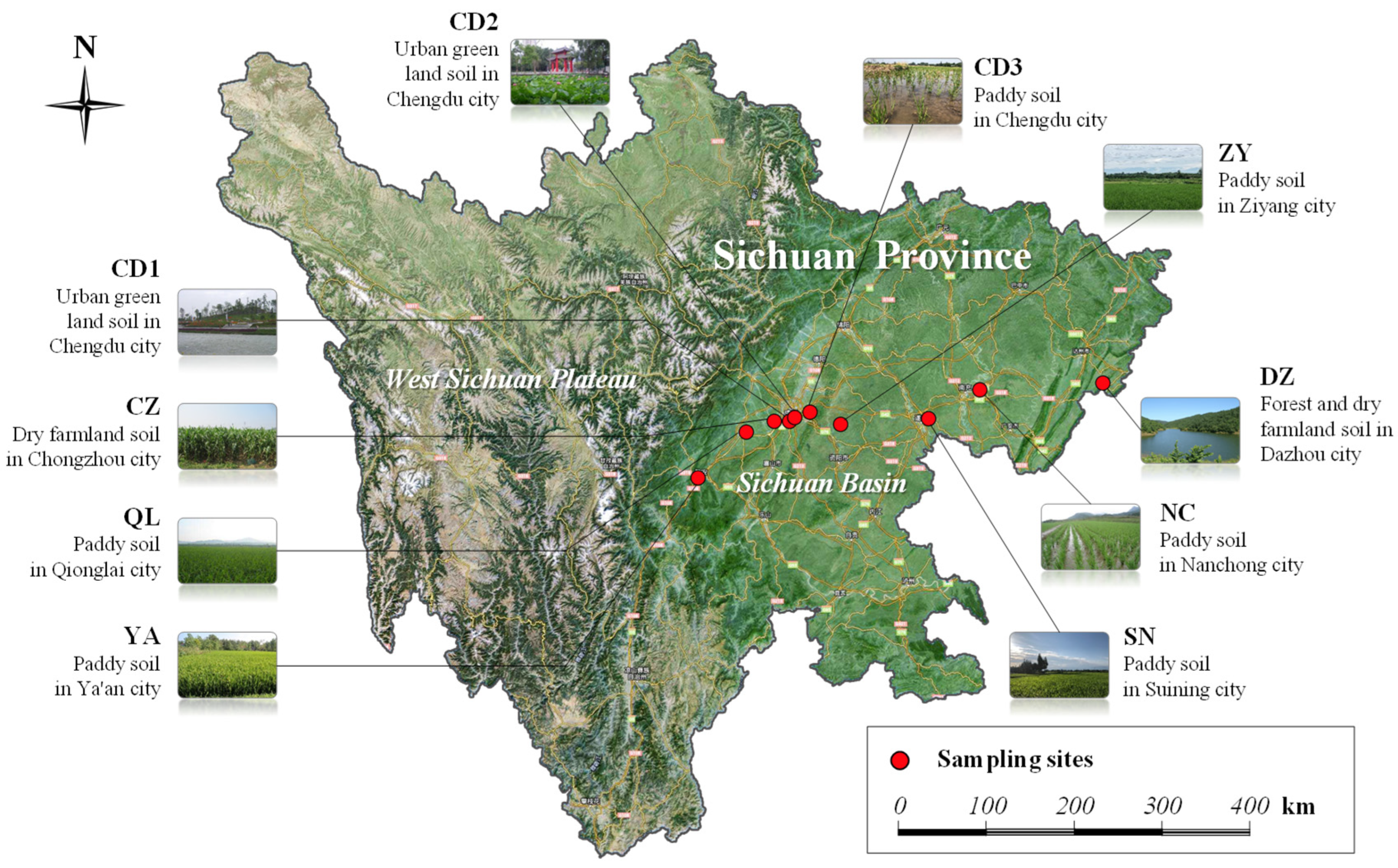
2.1. Soil Sampling and Treatment
2.2. Physical and Chemical Analysis of Sampled Soils
2.3. Sorption Experiments for Sampled Soils
2.4. Model Characterization
3. Results
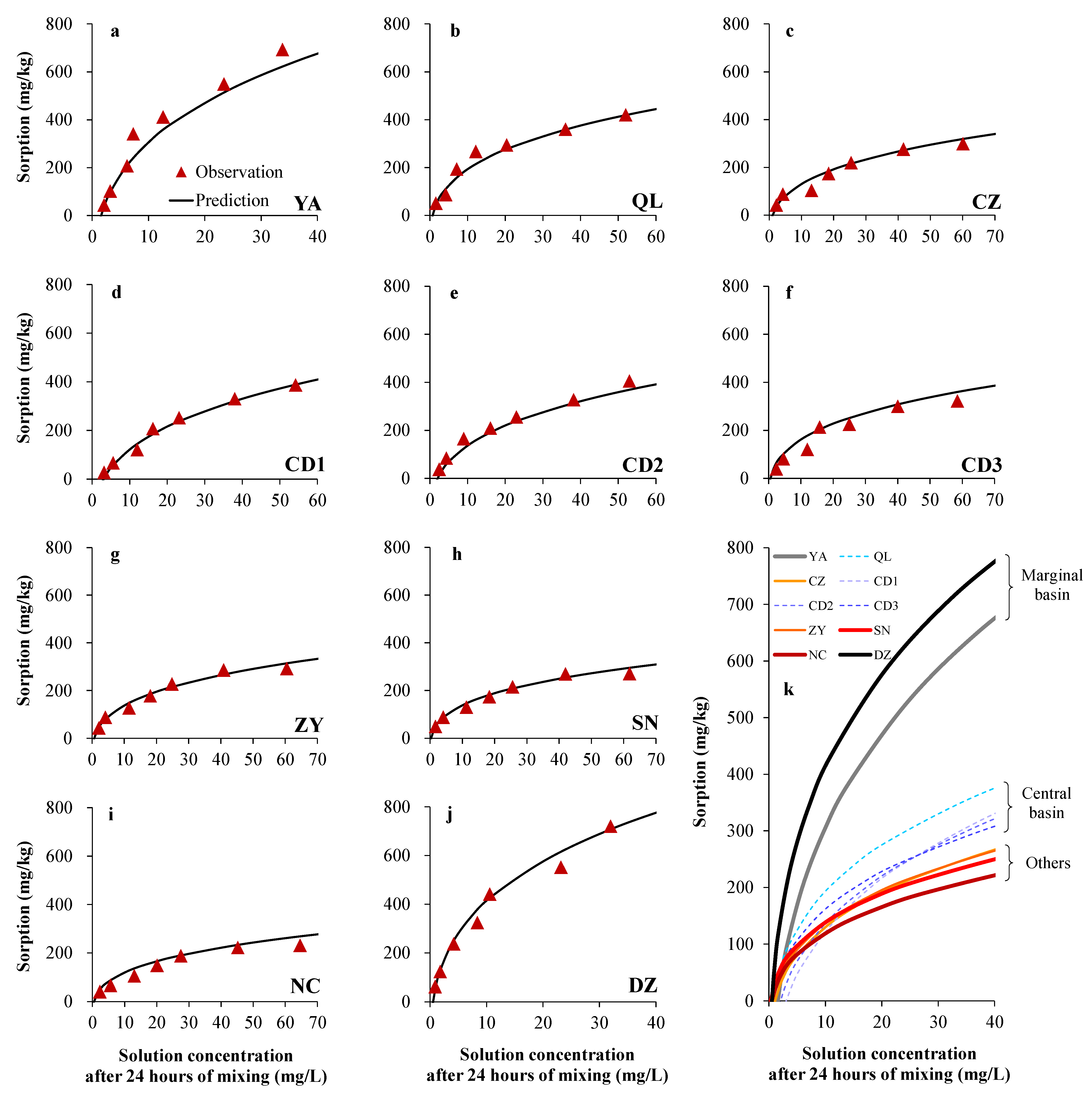
3.1. Phosphorus Sorption Properties
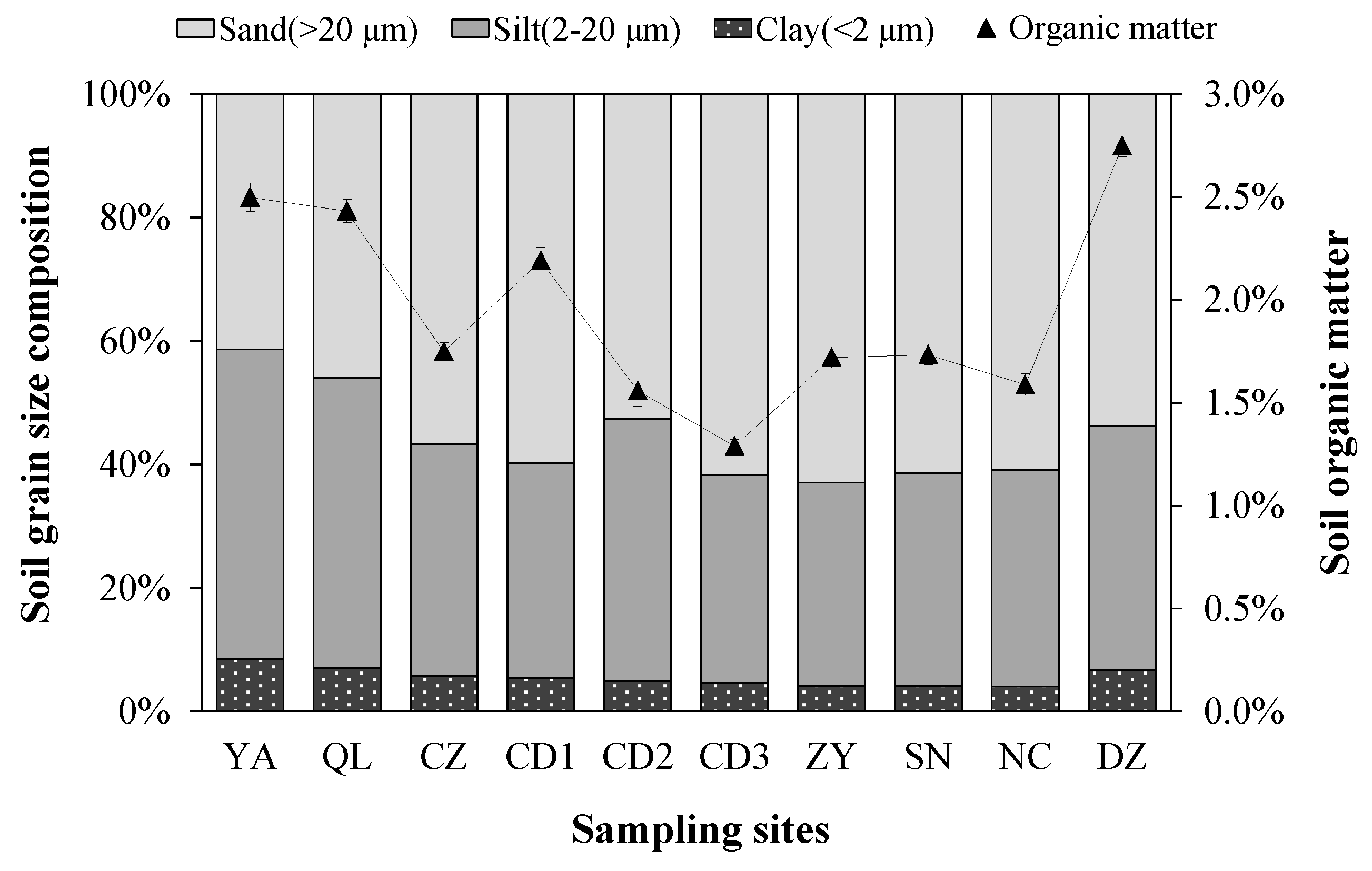
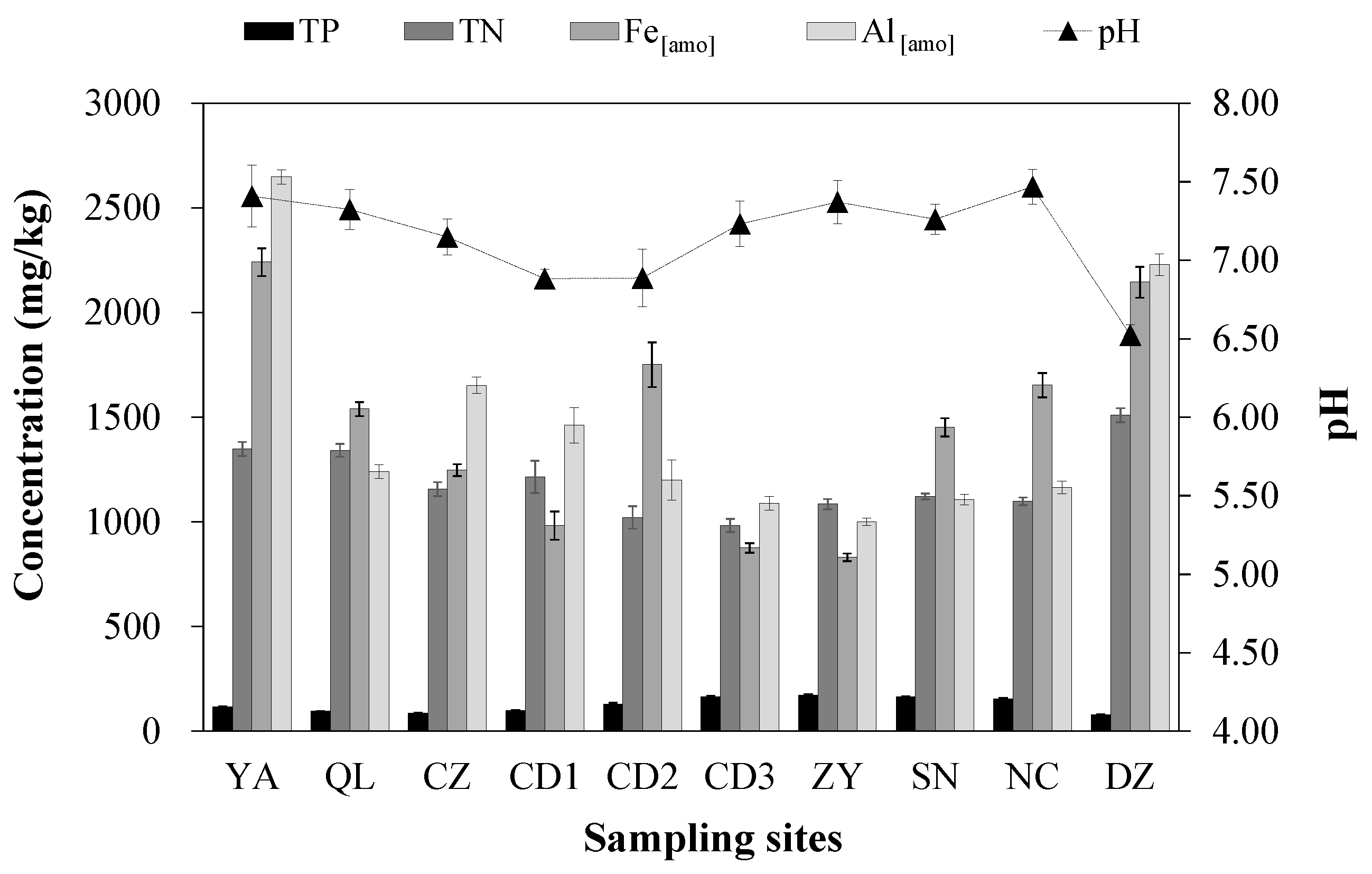
3.2. Soil Physical and Chemical Properties
4. Discussion
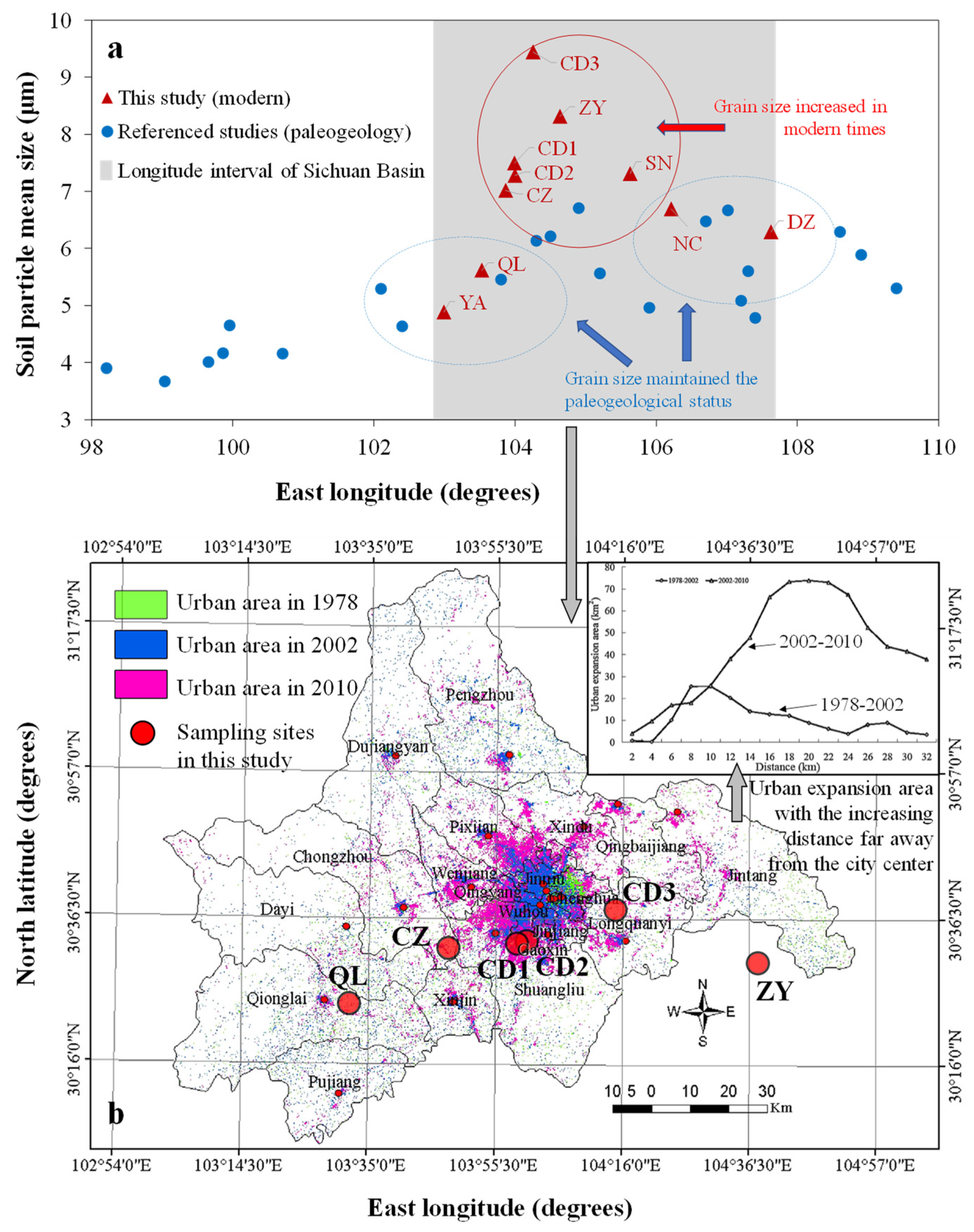
4.1. Historical Perspectives of Soil Particle Property Variation
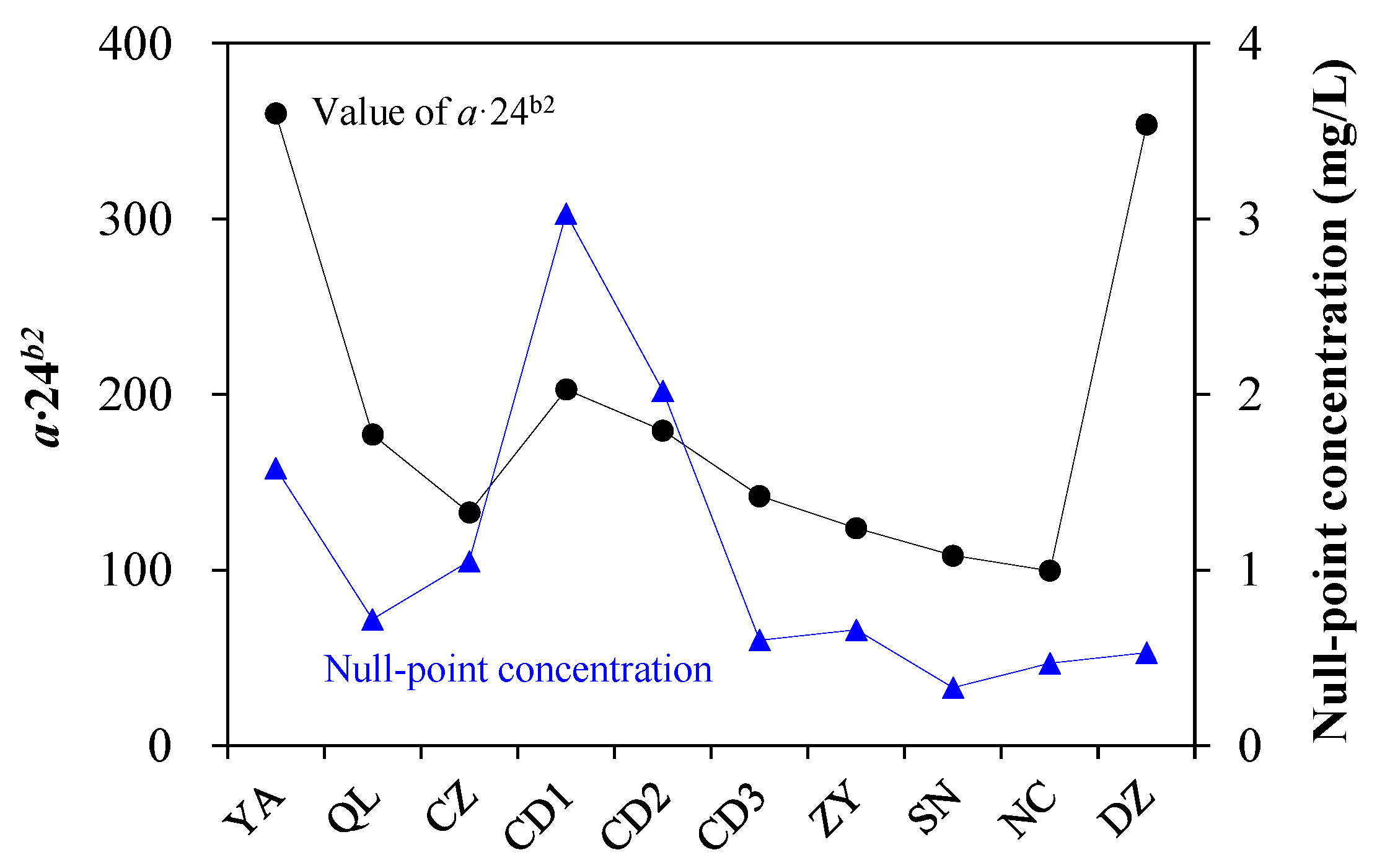
4.2. Interpretation for the Sorption Parameters
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Celik, I. Land-use effects on organic matter and physical properties of soil in a southern Mediterranean highland of Turkey. Soil Till. Res. 2005, 83, 270–277. [Google Scholar] [CrossRef]
- Lizaga, I.; Quijano, L.; Gaspar, L.; Concepcion Ramos, M.; Navas, A. Linking land use changes to variation in soil properties in a Mediterranean mountain agroecosystem. Catena 2019, 172, 516–527. [Google Scholar] [CrossRef]
- Tilman, D.; Lehman, C. Human-caused environmental change: Impacts on plant diversity and evolution. Proc. Natl. Acad. Sci. USA 2001, 98, 5433–5440. [Google Scholar] [CrossRef] [PubMed]
- Hinsinger, P. Bioavailability of soil inorganic P in the rhizosphere as affected by root-induced chemical changes: A review. Plant Soil 2001, 237, 173–195. [Google Scholar] [CrossRef]
- Barrow, N.J. The description of sorption curves. Eur. J. Soil Sci. 2008, 59, 900–910. [Google Scholar] [CrossRef]
- Mehadi, A.A.; Taylor, R.W.; Shuford, J.W. Prediction of Fertilizer Phosphate Requirement Using the Langmuir Adsorption Maximum. Plant Soil 1990, 122, 267–270. [Google Scholar] [CrossRef]
- Olsen, S.; Watanabe, F. A Method to Determine Phosphorus Adoption Maximum in Soils as Measured by the Langmuir Isotherm. Soil Sci. Soc. Am. J. 1957, 21, 144–149. [Google Scholar] [CrossRef]
- Yli-Halla, M.; Hartikainen, H.; Vaatainen, P. Depletion of soil phosphorus as assessed by several indices of phosphorus supplying power. Eur. J. Soil Sci. 2002, 53, 431–438. [Google Scholar] [CrossRef]
- Wang, Y.T.; Halloran, I.O.; Zhang, T.Q.; Hu, Q.C.; Tan, C.S. Langmuir Equation Modifications to Describe Phosphorus Sorption in Soils of Ontario, Canada. Soil Sci. 2014, 179, 536–546. [Google Scholar] [CrossRef]
- Abdu, N. Formulation of a first-order kinetic model and release of added phosphorus in a savanna Soil. Arch. Agron. Soil Sci. 2013, 59, 71–81. [Google Scholar] [CrossRef]
- Barrachina, A.C.; Carbonell, F.B.; Beneyto, J.M. Kinetics of arsenite sorption and desorption in Spanish soils. Commun. Soil Sci. Plan. 1996, 27, 3101–3117. [Google Scholar] [CrossRef]
- Chien, S.H.; Clayton, W.R. Application of Elovich Equation to the Kinetics of Phosphate Release and Sorption in Soils. Soil Sci. Soc. Am. J. 1980, 44, 265–268. [Google Scholar] [CrossRef]
- Halsey, G.D. The role of surface heterogeneity in adsorption. Adv. Catal. 1952, 4, 259–269. [Google Scholar] [CrossRef]
- Sposito, G. Derivation of the Freundlich equation for ion-exchange reactions in soils. Soil Sci. Soc. Am. J. 1980, 44, 652–654. [Google Scholar] [CrossRef]
- Barrow, N.J.; Cartes, P.; Mora, M.L. Modifications to the Freundlich equation to describe anion sorption over a large range and to describe competition between pairs of ions. Eur. J. Soil Sci. 2005, 56, 601–606. [Google Scholar] [CrossRef]
- Xiao, Y.; Tang, J.; Wang, M.; Zhai, L.; Zhang, X. Impacts of soil properties on phosphorus adsorption and fractions in purple soils. J. Mt. Sci. 2017, 14, 2420–2431. [Google Scholar] [CrossRef]
- Zheng, J.; He, X.; Walling, D.; Zhang, X.; Flanagan, D.; Qi, Y. Assessing soil erosion rates on manually-tilled hillslopes in the Sichuan Hilly Basin using 137Cs and 210Pbex measurements. Pedosphere 2007, 17, 273–283. [Google Scholar] [CrossRef]
- Luo, Z.; Zhu, B.; Tang, J.; Wang, T. Phosphorus retention capacity of agricultural headwater ditch sediments under alkaline condition in purple soils area, China. Ecol. Eng. 2009, 35, 57–64. [Google Scholar] [CrossRef]
- Zhang, J.H.; Lobb, D.A.; Li, Y.; Liu, G.C. Assessment of tillage translocation and tillage erosion by hoeing on the steep land in hilly areas of Sichuan, China. Soil Till. Res. 2004, 75, 99–107. [Google Scholar] [CrossRef]
- Zhu, B.; Wang, T.; You, X.; Gao, M. Nutrient release from weathering of purplish rocks in the Sichuan Basin, China. Pedosphere 2008, 18, 257–264. [Google Scholar] [CrossRef]
- Zhu, B.; Wang, Z.; Zhang, X. Phosphorus fractions and release potential of ditch sediments from different land uses in a small catchment of the upper Yangtze River. J. Soil Sediment. 2012, 12, 278–290. [Google Scholar] [CrossRef]
- Gao, Y.; Zhu, B.; Wang, T.; Wang, Y. Seasonal change of non-point source pollution-induced bioavailable phosphorus loss: A case study of Southwestern China. J. Hydrol. 2012, 420, 373–379. [Google Scholar] [CrossRef]
- Yang, X.; Shen, X.; Zhu, B. Characteristics of diffuse pollution of nitrogen and phosphorous from a small town in the hilly area of the central Sichuan Basin, China. J. Mt. Sci. 2016, 13, 292–301. [Google Scholar] [CrossRef]
- Zhu, B.; Wang, T.; Kuang, F.; Luo, Z.; Tang, J.; Xu, T. Measurements of Nitrate Leaching from a Hillslope Cropland in the Central Sichuan Basin, China. Soil Sci. Soc. Am. J. 2009, 73, 1419–1426. [Google Scholar] [CrossRef]
- Sichuan Provincial People’s Government. General Plan of Land Use of Sichuan Province for 2006–2020; Sichuan Provincial People’s Government: Chengdu, China, 2009. (In Chinese)
- USDA. Soil Taxonomy: A Basic System of Soil Classification for Making and Interpreting Soil Surveys. In USDA-NRCS Agriculture Handbook No. 436; U.S. Government Printing Office: Washington, DC, USA, 1988; Volume 114. [Google Scholar] [CrossRef]
- Eslamian, F.; Qi, Z.; Tate, M.J.; Romaniuk, N. Lime application to reduce phosphorus release in different textured intact and small repacked soil columns. J. Soil Sediments 2020, 20, 2053–2066. [Google Scholar] [CrossRef]
- Sommers, L.E.; Nelson, D.W. Determination of total phosphorus in soils: A rapid perchloric acid digestion procedure. Soil Sci. Soc. Am. J. 1972, 36, 902–904. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, X.; Huang, C. Spatial variability of soil total nitrogen and soil total phosphorus under different land uses in a small watershed on the Loess Plateau, China. Geoderma 2009, 150, 141–149. [Google Scholar] [CrossRef]
- He, Y.; Gu, F.; Xu, C.; Chen, J. Influence of iron/aluminum oxides and aggregates on plant available water with different amendments in red soils. J. Soil Water Conserv. 2019, 74, 145–159. [Google Scholar] [CrossRef]
- Mikhailova, E.A.; Noble, R.; Post, C.J. Comparison of soil organic carbon recovery by Walkley-Black and dry combustion methods in the Russian Chernozein. Commun. Soil Sci. Plan. 2003, 34, 1853–1860. [Google Scholar] [CrossRef]
- Barrow, N.J.; Debnath, A. Effect of phosphate status on the sorption and desorption properties of some soils of northern India. Plant Soil 2014, 378, 383–395. [Google Scholar] [CrossRef]
- Barrow, N.J. Describing and explaining the reaction of soils with phosphate using existing observations. Eur. J. Soil Sci. 2020, 72, 234–242. [Google Scholar] [CrossRef]
- Barrow, N.J. A mechanistic model for describing the sorption and desorption of phosphate by Soil. Eur. J. Soil Sci. 2015, 66, 9–18. [Google Scholar] [CrossRef]
- Zhong, S.; Han, Z.; Duo, J.; Ci, E.; Ni, J.; Xie, D.; Wei, C. Relationships between the lithology of purple rocks and the pedogenesis of purple soils in the Sichuan Basin, China. Sci. Rep. 2019, 9, 13272. [Google Scholar] [CrossRef] [PubMed]
- Cui, Y.; Xiao, R.; Xie, Y.; Zhang, M. Phosphorus fraction and phosphate sorption-release characteristics of the wetland sediments in the Yellow River Delta. Phys. Chem. Earth 2018, 103, 19–27. [Google Scholar] [CrossRef]
- Meng, J.; Yao, Q.; Yu, Z. Particulate phosphorus speciation and phosphate adsorption characteristics associated with sediment grain size. Ecol. Eng. 2014, 70, 140–145. [Google Scholar] [CrossRef]
- Wang, S.; Jin, X.; Bu, Q.; Zhou, X.; Wu, F. Effects of particle size, organic matter and ionic strength on the phosphate sorption in different trophic lake sediments. J. Hazard. Mater. 2006, 128, 95–105. [Google Scholar] [CrossRef]
- Santamarina, J.C.; Klein, K.A.; Wang, Y.H.; Prencke, E. Specific surface: Determination and relevance. Can. Geotech. J. 2002, 39, 233–241. [Google Scholar] [CrossRef]
- Li, S.; Gong, Q.; Yang, S. Analysis of the Agricultural Economy and Agricultural Pollution Using the Decoupling Index in Chengdu, China. Int. J. Env. Res. Pub. Health 2019, 16, 4233. [Google Scholar] [CrossRef]
- Huang, J.; Xu, C.; Ridoutt, B.G.; Wang, X.; Ren, P. Nitrogen and phosphorus losses and eutrophication potential associated with fertilizer application to cropland in China. J. Clean. Prod. 2017, 159, 171–179. [Google Scholar] [CrossRef]
- Li, T.; Zheng, W.; Zhang, S.; Jia, Y.; Li, Y.; Xu, X. Spatial variations in soil phosphorus along a gradient of central city-suburb-exurban satellite. Catena 2018, 170, 150–158. [Google Scholar] [CrossRef]
- Du, J.; Luo, Y.; Zhang, W.; Xu, C.; Wei, C. Major element geochemistry of purple soils/rocks in the red Sichuan Basin, China: Implications of their diagenesis and pedogenesis. Environ. Earth Sci. 2013, 69, 1831–1844. [Google Scholar] [CrossRef]
- Fang, X.M.; Li, J.J.; Van der Voo, R. Rock magnetic and grain size evidence for intensified Asian atmospheric circulation since 800,000 years BP Related to Tibetan uplift. Earth Planet. Sci. Lett. 1999, 165, 129–144. [Google Scholar] [CrossRef]
- Liu, D. Loess and the Environment; China Ocean Press: Beijing, China, 1985. [Google Scholar]
- Yang, S.; Fang, X.; Shi, Z.; Lehmkuhl, F.; Song, C.; Han, Y.; Han, W. Timing and provenance of loess in the Sichuan Basin, southwestern China. Palaeogeogr. Palaeoclimatol. Palaeoecol. 2010, 292, 144–154. [Google Scholar] [CrossRef]
- Peng, W.; Wang, G.; Zhou, J.; Zhao, J.; Yang, C. Studies on the temporal and spatial variations of urban expansion in Chengdu, western China, from 1978 to 2010. Sustain. Cities Soc. 2015, 17, 141–150. [Google Scholar] [CrossRef]
- Nearing, M.A.; Xie, Y.; Liu, B.; Ye, Y. Natural and anthropogenic rates of soil erosion. Int. Soil Water Conserv. Res. 2017, 5, 77–84. [Google Scholar] [CrossRef]
- Li, Q.; Li, A.; Yu, X.; Dai, T.; Peng, Y.; Yuan, D.; Zhao, B.; Tao, Q.; Wang, C.; Li, B.; et al. Soil acidification of the soil profile across Chengdu Plain of China from the 1980s to 2010s. Sci. Total Environ. 2020, 698, 134320. [Google Scholar] [CrossRef]
- Liu, X.; Li, T.; Zhang, S.; Jia, Y.; Li, Y.; Xu, X. The role of land use, construction and road on terrestrial carbon stocks in a newly urbanized area of western Chengdu, China. Landscape Urban. Plan. 2016, 147, 88–95. [Google Scholar] [CrossRef]
- Luo, Y.; Li, Q.; Wang, C.; Li, B.; Stomph, T.; Yang, J.; Tao, Q.; Yuan, S.; Tang, X.; Ge, J.; et al. Negative effects of urbanization on agricultural soil easily oxidizable organic carbon down the profile of the Chengdu Plain, China. Land. Degrad. Dev. 2020, 31, 404–416. [Google Scholar] [CrossRef]
- Han, W.; Li, Y.; Yin, H. The Influence of Mechanical Composition and Mineral Composition of Calcareous Soil on Slope Farmland on Phosphorus Fixation. Appl. Sci. 2021, 11, 3731. [Google Scholar] [CrossRef]
- Troitino, F.; Gil-Sotres, F.; Leiros, M.C.; Trasar-Cepeda, C.; Seoane, S. Effect of land use on some soil properties related to the risk of loss of soil phosphorus. Land Degrad. Dev. 2008, 19, 21–35. [Google Scholar] [CrossRef]
- Bai, J.; Ye, X.; Jia, J.; Zhang, G.; Zhao, Q.; Cui, B.; Liu, X. Phosphorus sorption-desorption and effects of temperature, pH and salinity on phosphorus sorption in marsh soils from coastal wetlands with different flooding conditions. Chemosphere 2017, 188, 677–688. [Google Scholar] [CrossRef]
- Sun, T.; Deng, L.; Fei, K.; Zhang, L.; Fan, X. Characteristics of phosphorus adsorption and desorption in erosive weathered granite area and effects of soil properties. Environ. Sci. Pollut. Res. 2020, 27, 28780–28793. [Google Scholar] [CrossRef] [PubMed]
- Rojas, R.; Morillo, J.; Usero, J.; Delgado-Moreno, L.; Gan, J. Enhancing soil sorption capacity of an agricultural soil by addition of three different organic wastes. Sci. Total Environ. 2013, 458, 614–623. [Google Scholar] [CrossRef] [PubMed]
- Vareda, J.P.; Valente, A.J.M.; Duraes, L. Assessment of heavy metal pollution from anthropogenic activities and remediation strategies: A review. J. Environ. Manag. 2019, 246, 101–118. [Google Scholar] [CrossRef]
- Wang, Y.; Li, L.; Zou, X.; Shu, R.; Ding, L.; Yao, K.; Lv, W.; Liu, G. Impact of Humin on Soil Adsorption and Remediation of Cd(II), Pb(II), and Cu(II). Soil Sediment. Contam. 2016, 25, 700–715. [Google Scholar] [CrossRef]
- Rao, P.; He, M. Adsorption of anionic and nonionic surfactant mixtures from synthetic detergents on soils. Chemosphere 2006, 63, 1214–1221. [Google Scholar] [CrossRef]

| Residual Sum of Squares | Degree of Freedom | Mean Square | VR | |
|---|---|---|---|---|
| 3-parameter | 398.6300 | 130 | 3.3219 | |
| 4-parameter | 325.1200 | 120 | 2.7093 | 2.7100 |
| Improvement | 73.5100 | 10 | 7.3510 |
| Parameters | a·24b2 | b2 | q |
|---|---|---|---|
| Clay | 0.820 ** | 0.043 | 0.656 * |
| Silt | 0.644 * | 0.087 | 0.526 |
| Sand | −0.693 * | −0.080 | −0.564 |
| Mean grain size | −0.595 | −0.353 | −0.390 |
| pH | −0.463 | 0.390 | −0.458 |
| Al[amo] | 0.910 ** | −0.030 | 0.726 * |
| Fe[amo] | 0.708 * | 0.136 | 0.439 |
| TN | 0.792 ** | 0.272 | 0.466 |
| TP | −0.579 | 0.098 | −0.524 |
| SOM | 0.813 ** | 0.221 | 0.569 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tan, B.; Barrow, N.J.; Li, L.; Zhou, P.; Zhuang, W. Phosphorus Sorption by Purple Soils in Relation to Their Properties: Investigation, Characterization, and Explanation. Sustainability 2023, 15, 14609. https://doi.org/10.3390/su151914609
Tan B, Barrow NJ, Li L, Zhou P, Zhuang W. Phosphorus Sorption by Purple Soils in Relation to Their Properties: Investigation, Characterization, and Explanation. Sustainability. 2023; 15(19):14609. https://doi.org/10.3390/su151914609
Chicago/Turabian StyleTan, Bo, N. J. Barrow, Longguo Li, Ping Zhou, and Wenhua Zhuang. 2023. "Phosphorus Sorption by Purple Soils in Relation to Their Properties: Investigation, Characterization, and Explanation" Sustainability 15, no. 19: 14609. https://doi.org/10.3390/su151914609







