Simplified Model for Concentration Analysis of Catalytic Conversion of Carbon Gas into Hydrogen
Abstract
:1. Introduction
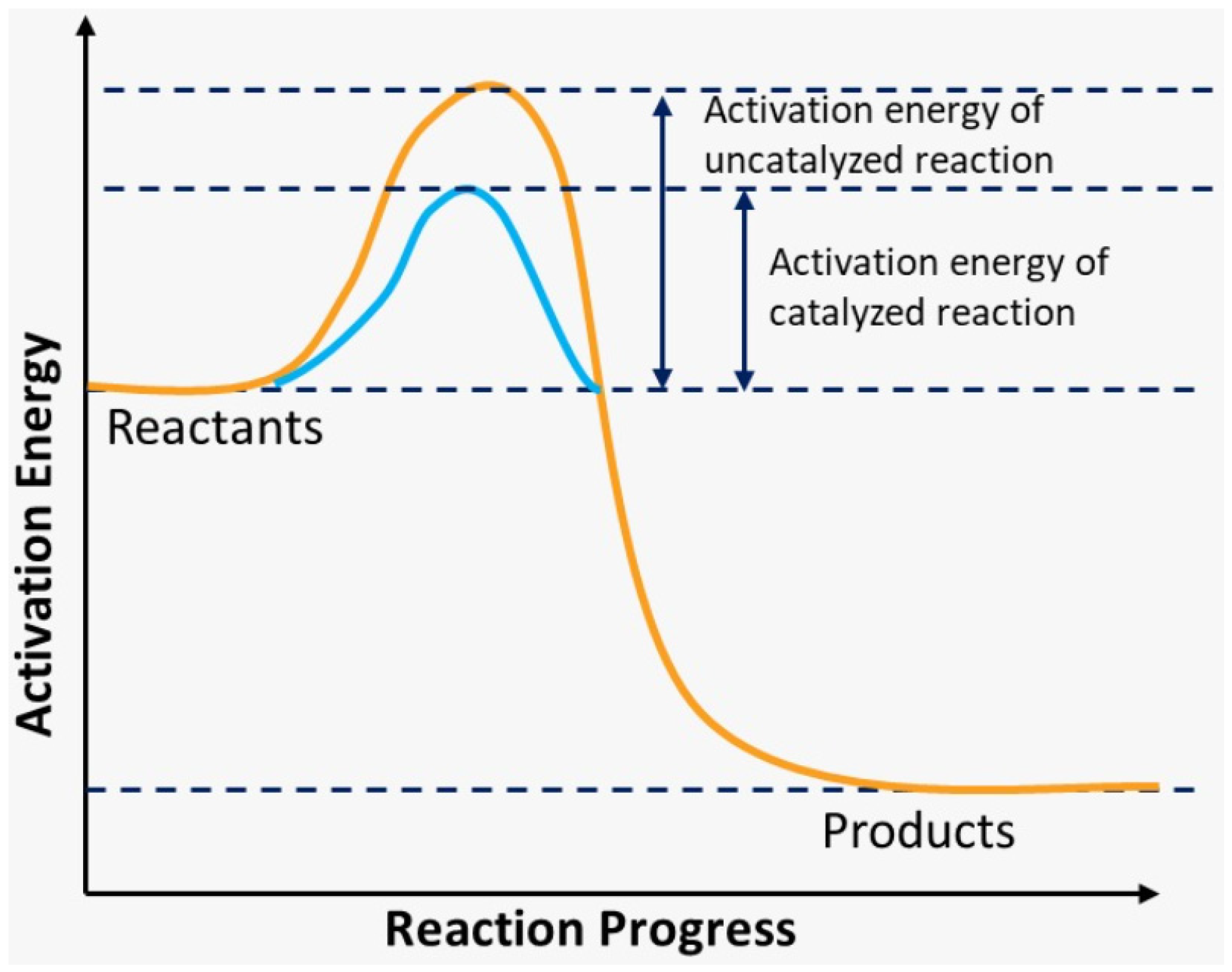
2. Chemistry Kinetic
- m and n make up the overall reaction order and are determined empirically;
- k is the speed constant.
3. Methodology
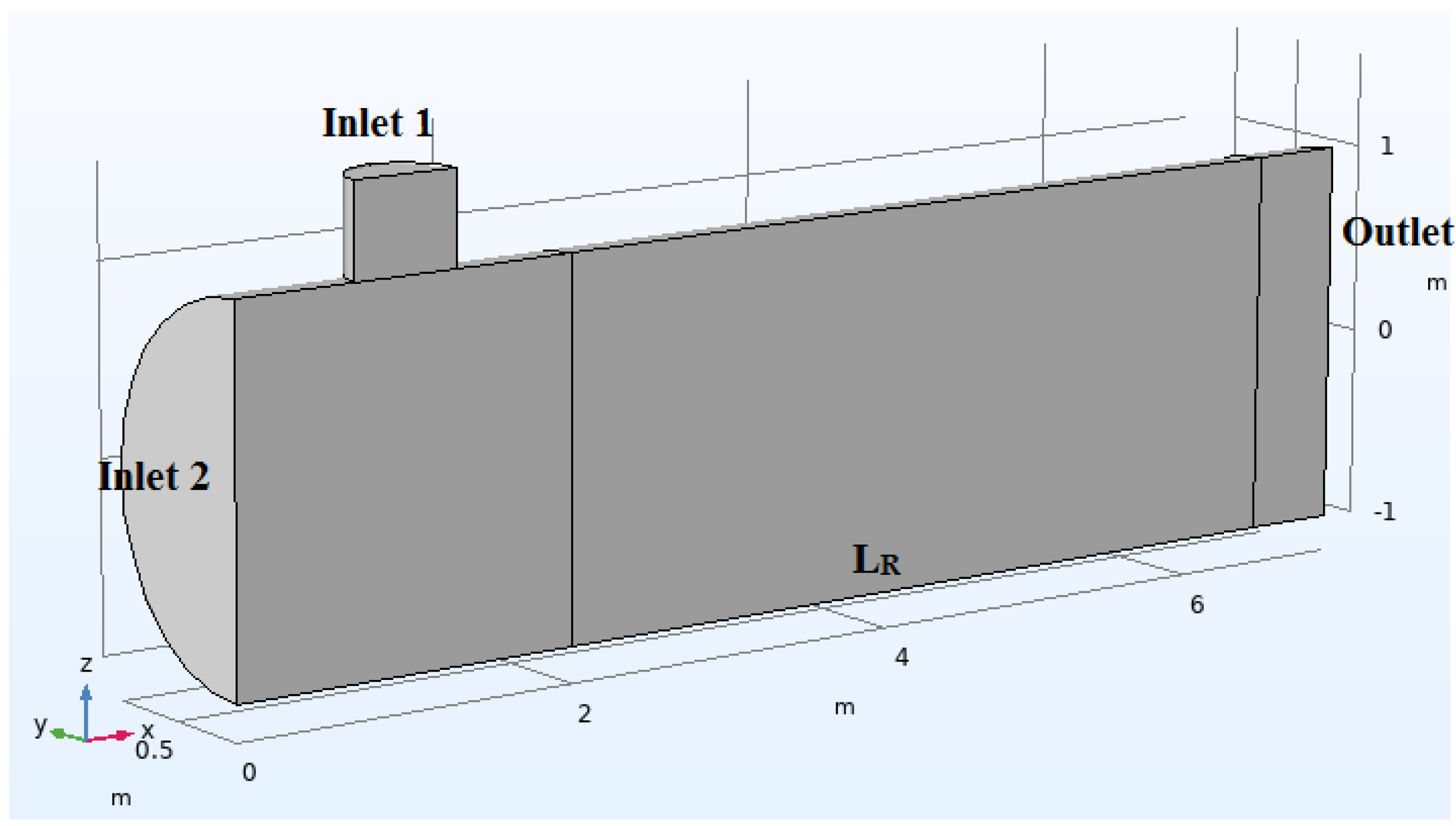
3.1. Parameters
- Due to the geometry of the reactor, symmetry in the plane will be considered;
- inlet concentration is equal to (Inlet 1) in all numerical simulations;
- Reactor diameter is equal to 1 m;
- The domain is entirely composed of air;
- The speeds at inputs 1 and 2 are the same;
- Isothermal analysis (T = 675 K);
- Porosity equal to 0.5 and permeability 1;
- The formula used to calculate the diffusivity between gases (), according to [9] is the following:where T is the temperature (K), P is the pressure (atm), is the molar mass of element A (, , or ), is the molar mass of element B (Air), and is defined by
- 9.
- As we mentioned in the introduction, in this work the chemical reaction will be adopted as irreversible, and with that, we can use the simplified equation, which would be:
3.2. Governing Equations
4. Results and Discussion
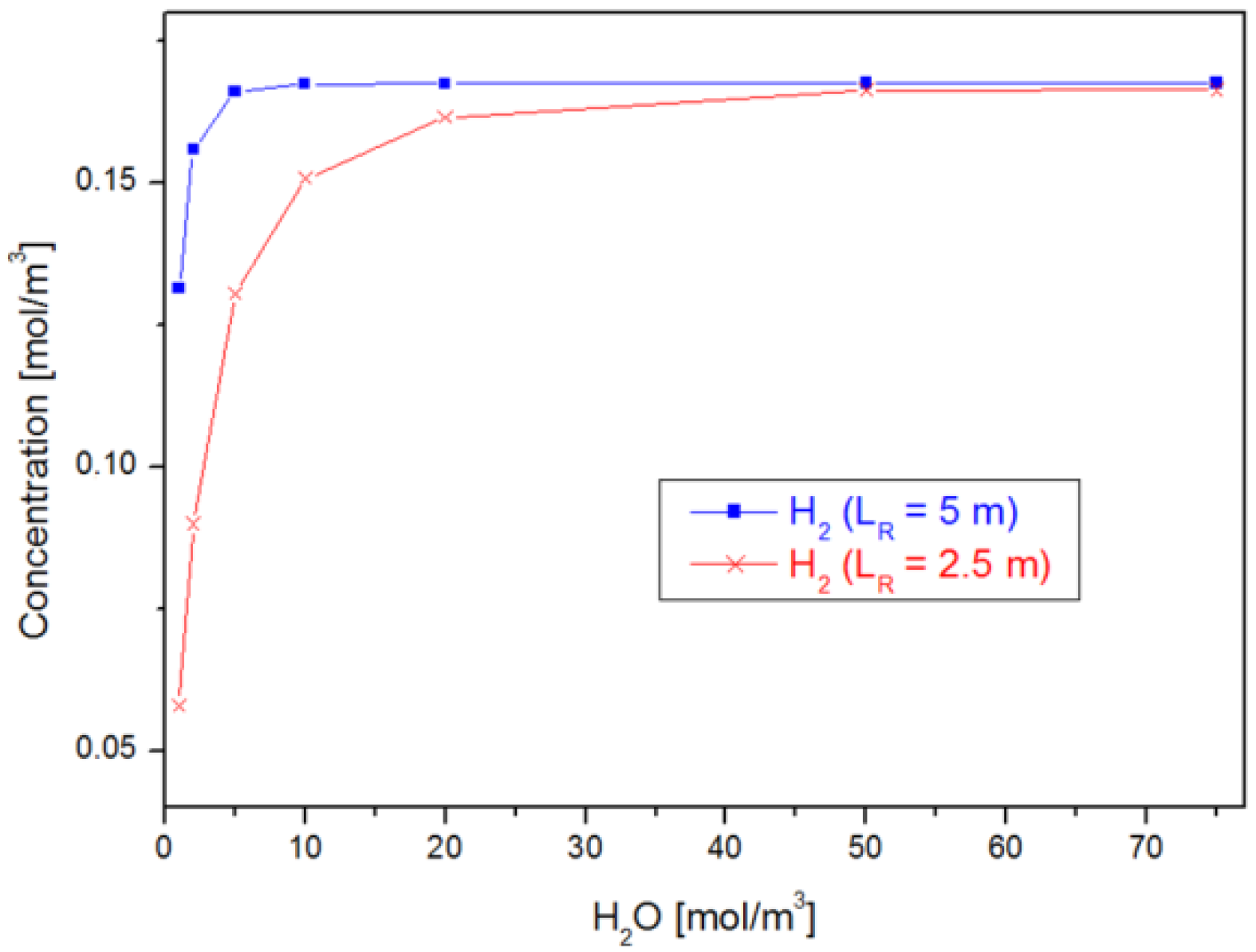
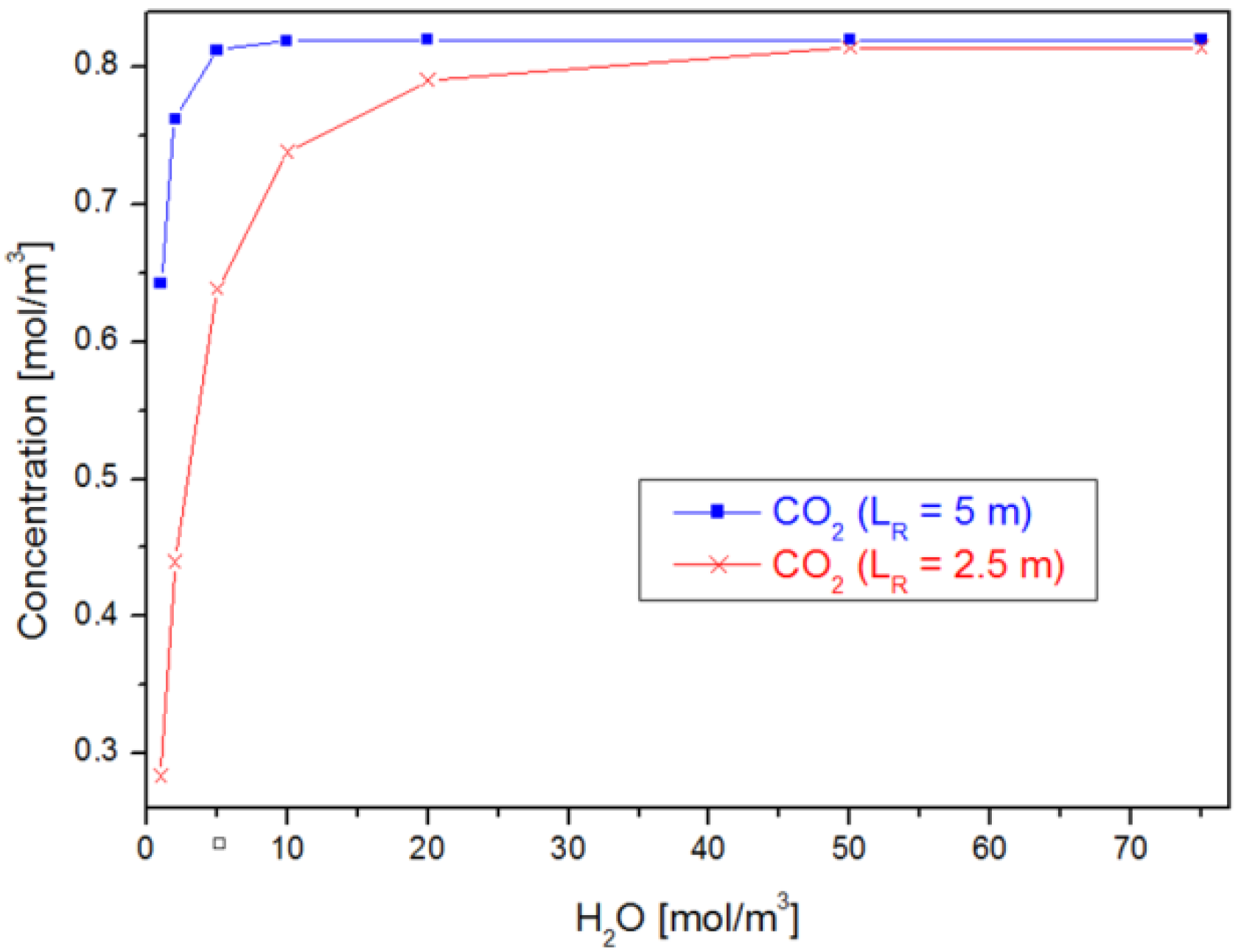
4.1. Case 1: Varying
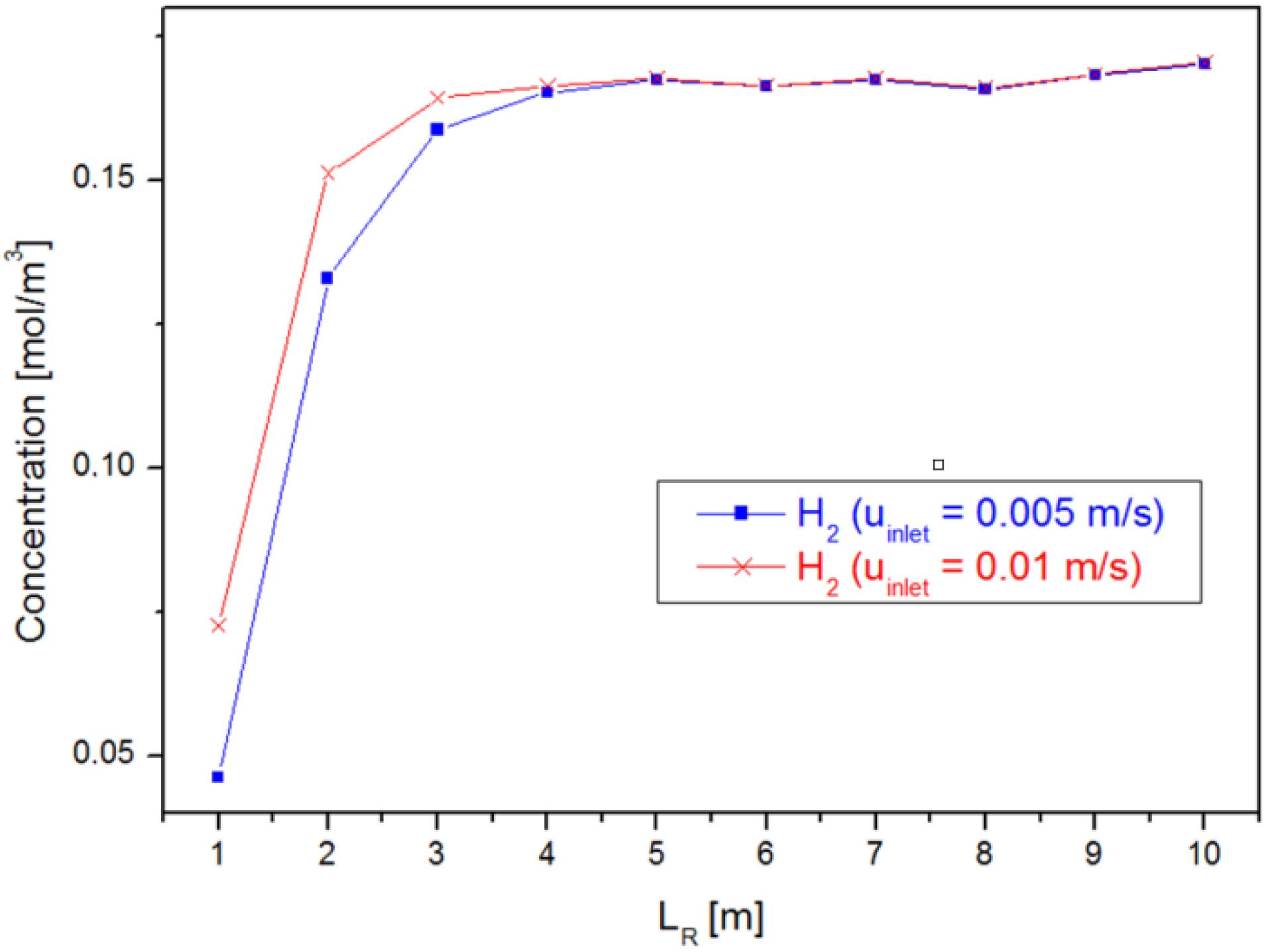
4.2. Case 2: Varying the Reactor Geometry ()
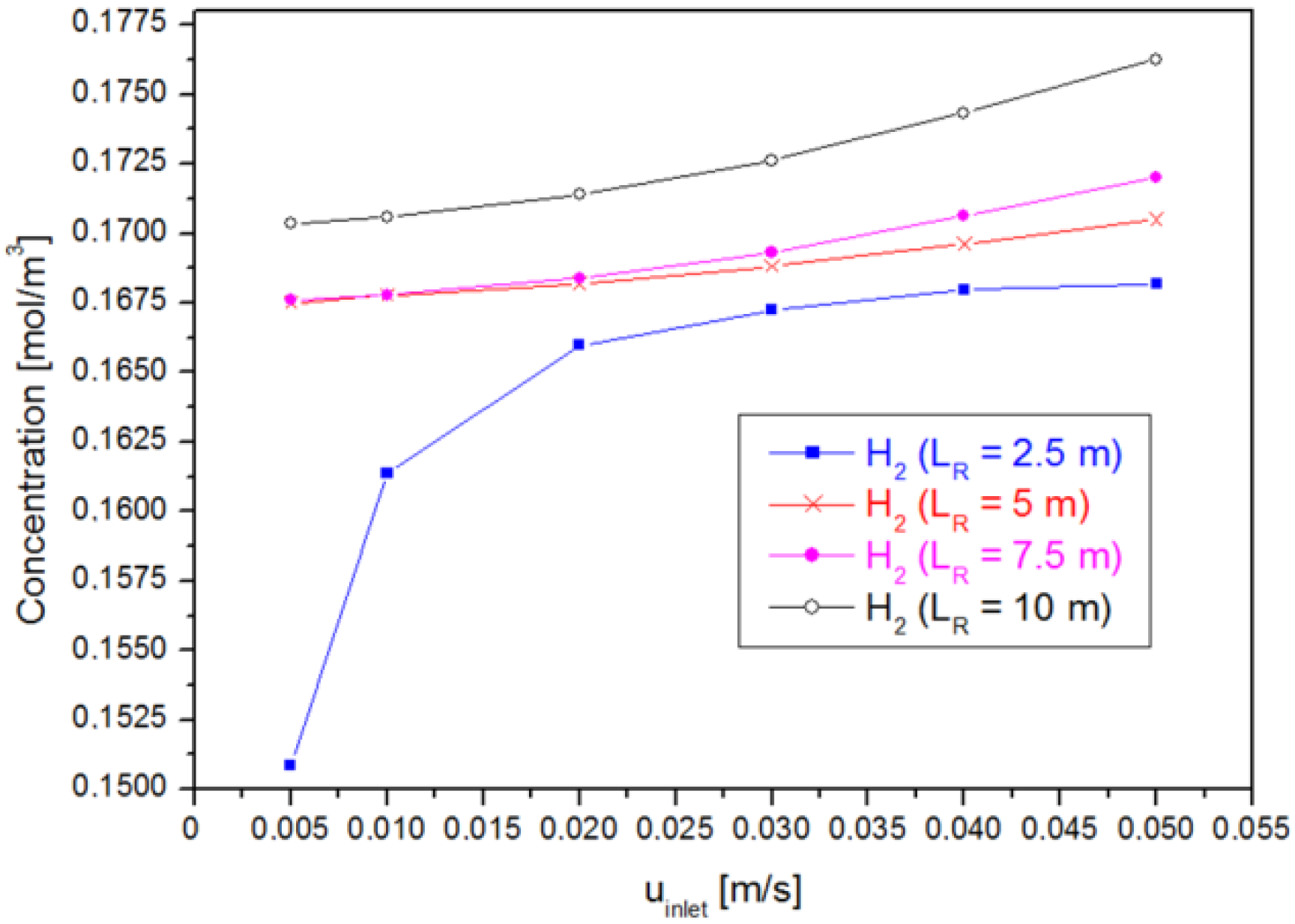
4.3. Case 3: Varying the Inlet Velocity ()
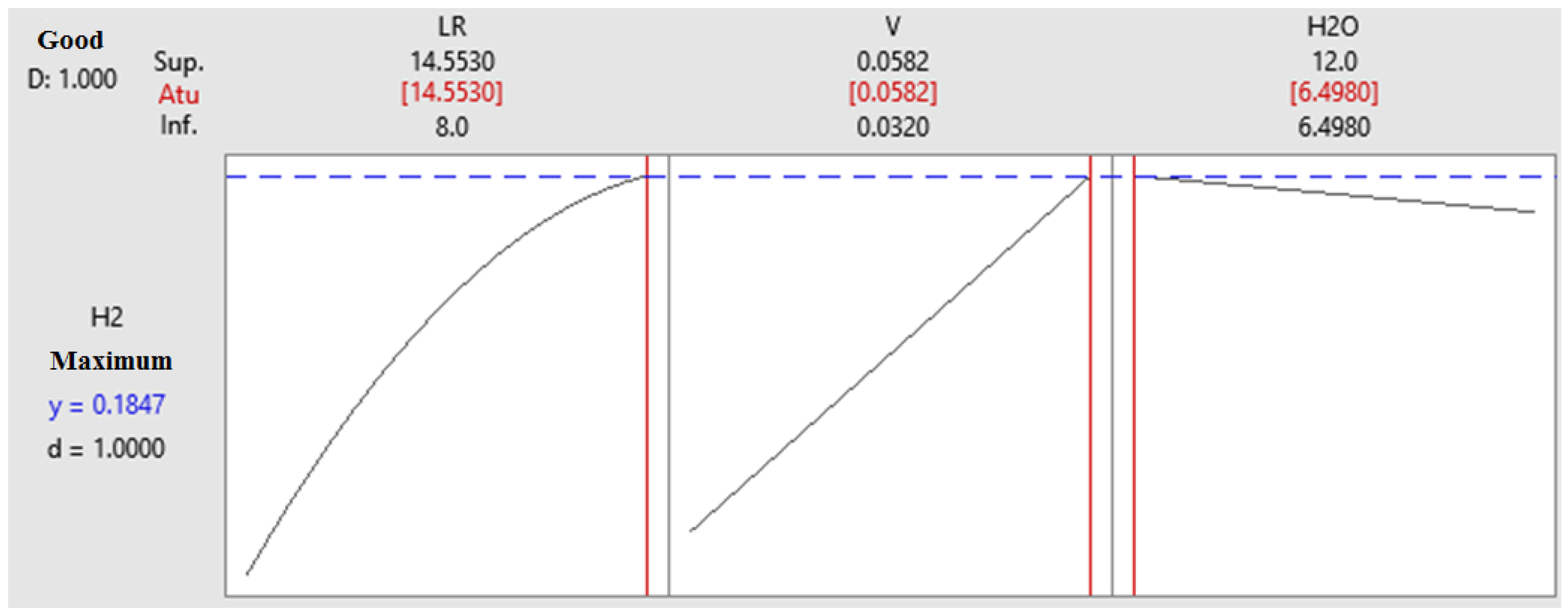
4.4. Optimization
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Teixeira, C.A.N.; Mendes, A.P.d.A.; Costa, R.C.d.; Rocio, M.A.R.; Prates, H.F. Natural Gas—A Key Fuel for a Low-Carbon Economy; Banco Nacional de Desenvolvimento Econômico e Social: Rio de Janeiro, Brazil, 2021; Volume 27, pp. 131–175. [Google Scholar]
- Santos, E.M.dos; Fagá, M.T.W.; Barufi, C.B.; Poulallion, P.L. Gás Natural: A construção de uma nova civilização. Estud. Avançados 2007, 21, 67–90. (In Portuguese) [Google Scholar] [CrossRef]
- Prates, C.P.T.; Pierobon, E.C.; Costa, R.C.d.; Figueiredo, V.S.d. Evolução da Oferta e da Demanda de Gás Natural no Brasil; Banco Nacional de Desenvolvimento Econômico e Social: Rio de Janeiro, Brazil, 2006; pp. 35–68. (In Portuguese) [Google Scholar]
- Lacerda, A.; Leroux, T.; Morata, T. Efeitos Ototóxicos da Exposição ao Monóxido de Carbono: Uma Revisão. Pró-Fono Rev. Atualização Cient. 2005, 17, 403–412. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oliveira, N.M.B. Fundamentos de Cinética e Introdução ao Cálculo de Reatores; Editora e Distribuidora Educacional S.A.: Londrina, Brazil, 2017. (In Portuguese) [Google Scholar]
- Chang, R. Química Geral—Conceitos Essenciais; Bookman: Porto Alegre, Brazil, 2007; p. 778. (In Portuguese) [Google Scholar]
- Fernandes, R.F. Catalisador. Rev. Ciênc. Elem. 2015, 3, 36. [Google Scholar] [CrossRef]
- Atkins, P.; Jones, L. Princípios de Química: Questionando a Vida Moderna e o Meio Ambiente, 5th ed.; Bookman: Porto Alegre, Brazil, 2011; p. 1048. [Google Scholar]
- Cremasco, M.A. Difusão Mássica; Editora Blucher: São Paulo, Brazil, 2019; p. 285. [Google Scholar]
- Xu, J.; Froment, G.F. Methane Steam Reforming, Methanation and Water-Gas Shift: 1. Intrinsic Kinetics. AIChE J. 1989, 35, 88–96. [Google Scholar] [CrossRef]
- COMSOL. Available online: https://doc.comsol.com/5.6/doc/com.comsol.help.cfd/cfd_ug_fluidflow_porous.10.37.html (accessed on 23 November 2022).
- COMSOL. Available online: https://doc.comsol.com/5.6/doc/com.comsol.help.chem/chem_ug_chemsptrans.08.050.html (accessed on 23 November 2022).





| Molecule | Molar Mass | |
|---|---|---|
| Air | 16.2 | 28.96 |
| 18.0 | 28.01 | |
| 13.1 | 18.01 | |
| 26.9 | 44.01 | |
| 6.12 | 2.016 |
| S | R2 | R2 (aj) | R2 (pred) | 10-Fold S | 10-Fold R2 |
|---|---|---|---|---|---|
| 0.0007019 | 98.16% | 97.81% | 97.40% | 0.0007624 | 97.33% |
| GL | SQ (Aj.) | QM (Aj.) | F-Value | p-Value | |
|---|---|---|---|---|---|
| Regression | 5 | 0.000684 | 0.000137 | 277.67 | 0.000 |
| 1 | 0.000018 | 0.000018 | 35.64 | 0.000 | |
| 1 | 0.000001 | 0.000001 | 1.53 | 0.227 | |
| 1 | 0.000002 | 0.000002 | 3.99 | 0.056 | |
| 1 | 0.000013 | 0.000013 | 25.40 | 0.000 | |
| 1 | 0.000006 | 0.000006 | 12.95 | 0.001 | |
| Error | 26 | 0.000013 | 0.000000 | ||
| Total | 31 | 0.000697 |
| n | Prediction | LIMP | LISP | ||||
|---|---|---|---|---|---|---|---|
| 1 | 15 | 0.065 | 6 | 0.190 | 0.188 | 0.1854 | 0.1902 |
| 2 | 15.5 | 0.07 | 5.5 | 0.193 | 0.190 | 0.1875 | 0.1932 |
| 3 | 16 | 0.08 | 5 | 0.201 | 0.195 | 0.1912 | 0.1992 |
| 4 | 18 | 0.09 | 4 | 0.207 | 0.201 | 0.1947 | 0.2080 |
| 5 | 19 | 0.15 | 3.5 | 0.266 | 0.236 | 0.2158 | 0.2559 |
| 6 | 20 | 0.2 | 3 | 0.336 | 0.268 | 0.2337 | 0.3032 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Furlan, J.; Siqueira, A.F.; Romão, E.C. Simplified Model for Concentration Analysis of Catalytic Conversion of Carbon Gas into Hydrogen. Sustainability 2023, 15, 1126. https://doi.org/10.3390/su15021126
Furlan J, Siqueira AF, Romão EC. Simplified Model for Concentration Analysis of Catalytic Conversion of Carbon Gas into Hydrogen. Sustainability. 2023; 15(2):1126. https://doi.org/10.3390/su15021126
Chicago/Turabian StyleFurlan, Jacqueline, Adriano Francisco Siqueira, and Estaner Claro Romão. 2023. "Simplified Model for Concentration Analysis of Catalytic Conversion of Carbon Gas into Hydrogen" Sustainability 15, no. 2: 1126. https://doi.org/10.3390/su15021126






