Abstract
Heat stress causes functional and metabolic alterations in different cells and tissues. There are several pathomorphological changes and biomarkers associated with head load in adaptive and productive organs of livestock. Heat stress-induced histopathological alterations in livestock were categorized as degenerative changes (fatty degeneration, steatosis, hydropic degeneration), necrosis (pyknosis, fibrosis), circulatory disturbances (hyperemia, edema, hemorrhage, congestion, thrombosis, ischemia), growth disturbances (hyperplasia, atrophy) and focal/diffuse inflammation (vascular changes, exudation). Upon immunohistochemical analysis, the biomarkers identified in growth-related organs were HSP70, HSP60, GABA, GABAAR, GABABR, HSP90, GnRH, LH, FSH, m6A, Nrf2, and C/EBPβ. The biomarkers in the reproductive organs were HSP70, Bax, Bcl-2, GABA, GABAAR, GABABR, Caspase-3, HSP90, HSPB9, HSPB10, HSF1, HSP40, T, E2, Cyt-C, CAT, BCL2L1, and VEGF. The identified biomarkers in the immune organs were CD3+ T cells, CD4+ T cells, CD8+ T cells, HSP70, and Bcl-2. All these biomarkers could serve as reliable variables in heat stress assessment in livestock. Further, HSP70, HSP90, HSP60, NPY, HSP27, Bcl-2, NF-κB, AQP2, Insulin, CD3+ T cells, CD4+ T cells, CD172a, EGF, AQP1, AQP3, AQP4, AQP5, CRYAB, GHR, 5-HT, CCK, and GLP-1 are heat stress-related biomarkers in adaptive organs that help in assessing the climate resilience of a livestock species and improving understanding about adaptive mechanisms. Among these biomarkers, HSP70 was established to be the ideal cellular biomarker for scaling heat response in livestock. Thus, examining heat-stressed organ histopathology and identifying cellular markers by immunohistochemistry may lay the foundation for screening climate-resilient livestock breeds in the challenging climatic scenario. Further, such an approach could help in developing concepts to combat the detrimental consequences of heat stress to ensure sustainability in livestock production.
1. Introduction
Livestock plays a significant role in determining the economy of poor and marginal farmers, especially in the developing parts of the world. Climate change is one of the most emphasized factors that negatively influence livestock production. Therefore, sustaining livestock production in the changing climate scenario is paramount. Climate change associated with increased frequency of heat waves and episodes of extreme temperature damages livestock production. It is widely established that the animal exhibits normal physiological functions within the thermo-neutral zone (TNZ). At the same time, the upper and lower critical temperature of the TNZ may alter these functions [1]. High metabolic heat production coupled with rising environmental temperatures, especially in the tropical region, may severely constrain livestock production. Thus climate change-associated heat stress can alter the economy of livestock farms. Therefore, it is very essential to identify more climate-resilient animals. Climate resilience refers to the ability of those animals that, apart from adapting to a specific environment, also produce optimally.
Heat stress may alter several biological functions, probably to change the behavior of animals to survive the stress. Of the various mechanisms exhibited by the animals to cope with heat stress, cellular and molecular changes are vital to impart the potential for the livestock to survive in extreme environmental conditions. The inability of the body to cope with increased temperatures may result in adverse pathological changes in vital organs at macroscopic and histologic levels. Thus, establishing these cellular changes could pave the way for understanding the mechanisms associated with livestock adaptation to heat stress. Establishing these changes in adaptive organs is very crucial to assessing climate resilience in livestock. The adaptive organs are those organs which govern heat dissipation mechanisms, such as adrenal gland, thyroid gland, heart, lung, skin, kidney and gastrointestinal tract (GIT). However, fewer studies have been carried out to examine heat stress-related pathologies in livestock. This warrants more efforts to understand the constraints linked to decreased productive and reproductive performance, and compromised immune functions in livestock.
This article aims to review and frame the current state of knowledge on heat stress-induced histological changes in productive and adaptive organs in livestock, and to pin down the knowledge gaps. This review also attempts to generate information on the sensitive immunohistochemistry changes associated biomarkers for heat stress. More nuanced analyses on the patterns of changes in stress markers related to heat stress are currently needed to enhance animal breeding.
2. Importance of Livestock from a Climate Change Perspective
The impact of climate change is global and is one of the defining and pressing anticipated challenges in the coming decades. The demand growth for livestock products has been escalating strikingly due to the rapid increase in global population, rising income, alterations in lifestyle and dietary shifts. Concurrently, this has paved the way to increasingly diverse livestock production to support the rising demand. On the verge of satisfying this demand, the livestock sector has significantly contributed to climate-related hazards.
It is reported that anthropogenic activities are the key drivers of the climate crisis due to global warming. The agriculture sector contributes substantially to the global greenhouse gas (GHGs) pool. The global release of methane (CH4) from agricultural sources accounts for two-thirds of the anthropogenic CH4 sources [2]. It is becoming clear that meat and dairy products produce the greatest environmental burden, accounting for approximately half of food-generated GHG emissions [3].
Direct GHG emissions from livestock refer to emissions produced by the animals, including enteric fermentation and manure and urine excretion [4]. The indirect emissions from the livestock sector refer to those sources such as the cultivation of feed crops used for animal feed, emissions from manure application, carbon dioxide (CO2) emissions during the production of fertilizer for feed production and CO2 emissions from the processing and transportation of refrigerated livestock products [5]. Other indirect emissions include net emissions from land linked to livestock, including deforestation (i.e., conversion of forest to pasture and cropland for livestock purposes), desertification (i.e., degradation of aboveground vegetation from livestock grazing), and release of carbon (C) from cultivated soils (i.e., loss of soil organic carbon (SOC) via tilling and natural processes) associated with livestock [6]. Therefore, global livestock production directly and indirectly impacts climate change, which has direct implications for the environment.
Although livestock contributes substantially to the global GHG pool, the increasing demand for livestock products makes it necessary to invest in the sector to meet the huge animal protein demand. There are reports that portray livestock as the most resilient climate sector among various sectors associated with agriculture. Further, livestock represent economic security for resource-poor farmers. Therefore, sustaining livestock production in the changing climate scenario could play a significant role in improving the income generated for the weaker sections of the human population.
3. Significance of Heat Stress to Livestock Production
Heat stress is recognized as a severe climate shock and an important stressor that acts as a direct threat to livestock production. Heat stress results from a combination of several weather variables, including high ambient temperature, humidity, solar radiation, and wind speed, with negative impacts on animal welfare and productivity [7]. As a result, the body temperature increases, which in succession initiates compensatory and adaptive mechanisms to restore homeothermy and homeostasis.
Heat stress increases respiration and mortality, reduces fertility, modifies animal behavior and suppresses the immune and endocrine systems, thereby increasing animal susceptibility to various diseases [8]. Additionally, heat stress is of great concern as it lowers the productivity of the livestock in terms of body weight, reproductive performance, milk yield, meat quality and egg production by causing a negative influence on the feed intake of the animals [9].
High ambient temperature causes functional and metabolic alterations in different cells and tissues. Heat stress may lead to the increased production of transition metal ions (TMI), which can make electron donations to oxygen-forming superoxide or H2O2, which is further reduced to a highly reactive oxygen species (ROS) causing oxidative stress [10]. Thus, the increased production and accumulation of ROS harms the immune system. Heat stress reduces the phagocytic and burst capacity of polymorphonuclear cells, which could lead to compromised immune functions in livestock. Therefore, various immune cells suffer the direct effects of heat load on functionality, and this is likely responsible for the observed impacts on disease incidence [11]. Heat stress activates the hypothalamo-pituitary adrenal (HPA) axis, resulting in increased glucocorticoid production in major livestock species, consequently decreasing the feed intake, body weight gain, relative immune organ weight and innate immunity [9,12].
Heat stress also results in hypofunction of the thyroid gland and affects the metabolism patterns of the animal to reduce metabolic heat production [13]. Heat stress affects cellular functions and causes impairments of the reproductive system in both sexes. In males, it reduces spermatogenic activity, while in females, it adversely impacts oogenesis, oocyte maturation, fertilization development and implantation rate [14]. Therefore, the detrimental effect of heat stress on various productive and adaptive organs negatively impacts their performance, resulting in reduced global livestock production.
In tropical and subtropical regions, it has been reported that the growth traits of farm animals—both males and females—are impaired as a result of the extreme changes in biological functions, such as disturbances in protein, water, energy and mineral metabolism. These drastic changes depress the growth traits of temperate animal breeds, at about 50% during the summer season when introduced to a tropical or sub-tropical environment due to heat-stressful conditions [15]. Consequently, heat stress, being a major environmental issue, has a detrimental impact on the growth, meat quality and productive efficiency of livestock, leading to huge economic losses [16].
The effects of heat stress on livestock growth performance are the products of the decrease in anabolic activity and the increase in tissue catabolism, mainly in fat depots and/or lean body mass, as well as decreases in thyroid hormone levels, decreased feed intake and increased tissue damage [15]. According to a study, the exposure of sheep to elevated temperatures results in a decrease in growth rate, body weight, average daily gain and total body solids [17]. Moreover, in the swine industry, the economic losses associated with heat stress are mainly explained by reduced and inconsistent growth, decreased feed efficiency, decreased carcass quality (increased lipid deposition and decreased protein accretion) and so on [18].
The heat stress-associated economic losses in the livestock sector include poor growth performance, carcass quality degradation and increased animal welfare issues. A study in heat-stressed goats assessed the meat quality variables of color, drip loss, cooking loss, water holding capacity, shear force, muscle fiber diameter, muscle pH, sarcoplasmic protein, collagen solubility and tenderness in Longissimus thoracis et lumborum and Semimembranosus muscles, reflecting that meat quality variables were compromised due to heat stress [19]. Extremes in summertime temperatures impose negative effects on animal growth performance, alter carcass composition and increase the risk of pale, soft, exudative (PSE) or PSE-like meat in pigs, poultry and beef cattle, and dark firm and dry (DFD) meat in ruminants [20]. The impacts of heat stress on meat quality among different species or breeds of animal are reported to be inconsistent, which might be attributed to alterations in the thermo-tolerance of the farm animals.
4. Importance of Studying Heat Stress-Induced Histopathological Changes of Various Organs
Thermal insult induces diverse alterations in the histology of different organs, and such histopathological studies are carried out with the aim of shedding light on the link between heat stress and observed lesions. Researchers prioritize studying the heat stress-incited changes in histology, as it offers the advantage of identifying the differences and categorizing lesions in both heat-stressed and healthy animals. In contrast to grossly affected tissues, pathological changes in histologic sections can be easily identified. In this sense, it offers an innovative approach to obtaining an efficient diagnosis. Histopathological diagnosis helps differentiate probable etiologies and establish differential changes from the field samples. This helps generate new information based on these histological changes, and is a requisite for generating advanced therapeutic policies for diagnosis based on these insights. Experimentation on the chronology of heat stress response and pathomorphological changes is necessary to gain an understanding of the mechanisms that lead to morbidity and mortality. Applications of novel biotechnological tools and the evaluation of pathomorphological changes associated with head load in livestock have to be investigated in detail, as this aids in creating new mitigation policies (genetic and managerial).
Due to the paucity of information specific to livestock, the descriptions in this review are organized according to the limited data available. They are also compared with studies in other species. This review evaluates the changes in the histology of various organs, as it is critical to enhancing our understanding of heat load-induced pathologies in major livestock production species.
5. Histopathological Changes in Productive Organs in Heat-Stressed Livestock
Heat stress is one of the limiting factors directly affecting the productive performance of livestock animals. It detrimentally influences the functioning of productive organs and thereby retards the animal’s growth, reproduction and immune competence. Histologic changes in lymphoid tissues could be the basis for understanding the changes in immunologic events responsible for the deleterious effects of heat stress [21].
The pathology is believed to be quite similar across different species of mammals, and hence when deprived of livestock-specific histopathological changes, examples from other experimental animal models are cited in this review.
5.1. Growth-Related Organs
5.1.1. Hypothalamus
The hypothalamus is a vital sub-cortical center and plays a crucial role in maintaining homeostasis, such as temperature regulation, regulation of a variety of stress responses, reproductive function integration, water and salt balance, etc. [22].
According to research in brown laying hens, morphological changes in neurons of heat-stressed hypothalamus were established, and the longer the elapsed time, the more serious the pathological damage. The oxidative stress and inflammation consequently damaged the structure of neurons and nerve fibers in the hypothalamus of the hens [23].
5.1.2. Pituitary
The histological picture of the pituitary glands of chickens under heat stress showed slight hyperplasia and hypertrophy of the basophil cells, suggesting an increase in adrenocorticotropic hormone (ACTH) output under these conditions [24]. The histological examination in an experiment using heat-stressed Japanese quails revealed that the pituicytes changed in conformation and size, with reduced lipid droplets [25].
On the rat pituitary gland, acute heat stress increased the thickness of hypothalamic axons in the posterior pituitary, and the dilation of small blood vessels with hyperemia was recorded in both anterior and posterior lobes [26]. This was in agreement with another study wherein pre-pubertal male rats were exposed to hyperthermic conditions, and the results revealed hyperemic adenohypophysis, along with hydropic degeneration and focal necrosis [27].
5.1.3. Thyroid Gland
The mass and volume of the thyroid depend on animal breed, body weight, nutrition, physiological status, season, and geographical and climatic region [28]. It has been reported that the exposure of goats to high environmental temperature decreased the thyroid activity, and consequently the level of thyroid hormones in serum declined [29]. Upon observing the ultrastructure of the thyroid gland of Zebu cattle, the extreme variability of the shape and size of follicular cells in the glands of all animals was noticed, even among the same follicle. Additionally, variable colloid droplets, lysosomes and microvilli in the thyroid suggest that the gland’s function is highly sensitive to several factors such as age, climate, feeding and environmental factors [30].
A study of thyroid gland histology in the summer and winter seasons in goats revealed that the parenchyma was formed from numerous inactive follicles, flattened to low cuboidal follicular cells with little cellular organelles in the summer season as opposed to the winter season. In contrast, the goats exposed to the winter season had active follicles, high columnar cells engorged with colloid droplets and cellular organelles [31]. Similarly, the histological structures of the thyroid glands of White Yorkshire piglets exposed to lower temperatures had more active follicles when compared to those exposed to a higher temperature. The low-temperature-exposed animals also showed increased epithelium with cuboidal or columnar cells. Additionally, the colloid stained uniformly in summer and lacked vacuoles, in contrast to non-uniformly stained abundant vacuoles in winter [32]. On the other hand, the thyroid gland during the summer season in a study using Buffalo tissue samples disclosed that colloid was more homogenous with a smoother periphery than in winter. In addition, follicle distension with colloid and few vacuoles were present in both seasons. Therefore, it was concluded in the study that the thyroid glands of buffaloes showed no significant differences in histological characteristics, as they are poor thermal regulators [33]. The resting (inactive) thyroid gland leads to low metabolism, exhibiting better resistance to heat stress [34]. This serves as an acclimatization and adaptive mechanism in buffaloes [33]. Furthermore, studies have recorded variations in follicular diameter during different seasons in buffaloes [35] and also in goats, wherein the diameters of thyroid follicles in the left and right thyroid lobes varied significantly according to the season [28].
It was reported in chickens that the thyroid gland apparently reduced in size when exposed to high environmental temperatures. Additionally, in the same study, histological examinations of thyroid tissue from birds revealed a decreased height of the follicular lining cells, and they appeared as flattened squamous cells [24].
5.1.4. Liver
The liver is one of the most crucial organs affected by high ambient temperature [36], and the exposure of conscious animal models to environmental heat stress increases portal venous radical content, which causes cellular hypoxic stress in the liver [37].
In the livers of heat-stressed Malabari goats, histopathological section revealed hepatocytes with fatty changes, and pronounced hepatic degenerative changes were also reported [38]. Similarly, in Osmanabadi goats, more degenerative changes were reported in heat-stressed livers [39]. These changes are attributed to the sensitivity of hepatocytes to heat stress, and the effects were found to be more pronounced in goats irrespective of breed [38].
Similarly, in a study using chickens exposed to heat stress, histological changes in hepatic tissue were characterized by fatty and parenchymatous degeneration, the congestion of hepatic vessels, the nuclear degeneration of various types and leucocytic infiltration, particularly around the blood vessels, as well as a few degrees of focal necrosis [24]. However, in the same study listed above, no liver cirrhosis was observed. It has been shown that under chronic heat stress, necrosis from oxygen deficit develops in centrilobular areas of the hypoxic liver in broilers. In addition, the liver cells of broilers showed fatty degeneration by vacuolation with the dilation of sinusoids and necrosis with leukocyte granulation tissue, especially in the centritubular region. This fatty degeneration could be due to the accumulation of neutral lipids in the cytoplasm, and thus it serves as the diagnostic clue for liver injury [40]. Similarly, studies have reported the results of liver pathology, wherein the fatty degeneration of liver cells along with dilation of broiler’s sinusoids and necrosis was observed with heterophils and lymphocytes [40,41]. Further, the histopathological analysis of the liver tissue of acute heat-stressed Kirin chickens showed the dilation of sinusoids and the central vein, increased inflammatory cell infiltration, vacuolar degeneration, congestion with mononuclear cells and few binuclear cells [42]. In the same study using another breed of chicken, mild dilation of the central vein and sinusoid with increased incidence of necrosis and hyperplasia was noted in the liver tissue. These established differences could be attributed to the differences in the thermo-tolerance between the breeds [42].
In a study in Japanese quail, it was reported that under heat stress, the livers of birds shrank, and the hepatocytes changed in conformation, size, and shape and decreased in the number of lipid droplets. Additionally, the nucleus conformation shrank, and the cytoplasm surrounding the nucleus showed a peripheral pallor and signs of necrosis with acidic composition [25]. In another study, morphological changes in quail liver were examined, and a typical hepatic macro-vesicular steatosis (fatty change) was found during exposure to heat stress. Similarly, other data presented indicated that chronic heat stress in broilers caused micro-vesicular steatosis in the liver, characterized by the presence of large fat droplets and vacuolar degeneration [43]. This abnormal status of the liver may be explained as heat stress accelerating the accumulation of fat in the liver [44]. The lipid accumulates in the hepatocytes as vacuoles and has a clear appearance with hematoxylin–eosin (H&E) staining [45]. Histopathology proved to be a more sensitive method to detect heat stress-induced liver tissue damage than the biochemical measurement of lipid peroxidation [46].
In a study using Bama miniature pigs that show high heat tolerance, no marked abnormal hepatic histology and hepatocyte ultrastructure were detected [36]. The histological picture of the liver of in utero heat-stressed bull calves contained significantly more cells than those of in utero cooled bull calves [47]. In a study using mice animal models, the livers of heat-stressed mice showed an increase in inflammatory macrophages and fibrosis [48].
5.1.5. Muscle
Muscle tissue plays a primary role in the metabolic adaptation to negative energy balance and heat stress [49]. Studies have revealed that sustained heat stress diminished muscle growth in lambs and pigs [50,51], and increased the production of reactive oxygen species in avian skeletal muscle [52].
Histopathological analyses of the leg muscle tissue of acute heat-stressed broilers in a study revealed increased fat tissue infiltration, degenerated muscle fiber and mild inflammatory cell infiltration, whereas in breast muscle tissue, the mild expansion of interstitial tissues with fibrosis and fat tissue infiltration were recorded [42]. The pectoralis muscle from experimental chicks had smaller myofibers and large fat deposition areas [53]. One of the common pathological changes in muscle is fat infiltration. Regarding increased fat and decreased protein content, altered meat quality was frequently reported in heat-stressed chicken meat [54]. Heat-stressed animals also exhibited changes in the composition of the muscles and adipose deposition, which were only partially mediated by inflammation [55]. Indeed, fat deposition in heat-stressed animals has been shown to shift away from surface areas to internal stores to reduce the insulating effect and improve heat exchange [56].
5.1.6. Thymus
The maturation of T-cells, which are responsible for mounting an immune response to foreign substances, occurs in the thymus. Abnormal development of the thymus might seriously affect an animal’s adaptive immune function [11]. The low weight of the thymus gland has been observed in laying hens under heat stress [57]. Similarly, the effect of in utero heat stress in calves revealed lower thymus weights and compromised post-natal immune functions [58]. Maternal heat stress could alter the typical development trajectory of immune cells and organs. However, the literature on immune outcomes is scarce in the Bovine model [11].
The thymus sections of heat-stressed broiler chickens were studied, and on examination, the reports displayed an unclear border between each lobe and a thinner thymic cortex. Moreover, under heat stress conditions, massive thymic cortex degradation with erythrocyte influx was observed in the thymus. Thus the atrophy of primary lymphoid tissues demonstrates the disruption of lymphocyte differentiation, proliferation, and the subsequent phase of immune abnormality in chickens bred under high ambient temperatures [59]. An experiment in broilers seeking to study the negative impacts of chronic heat stress on the thymus showed significant increases in the number of heterophils, apoptosis histiocytes and cell depletion [60]. Another study in Wenchang chicks revealed a reduced immune organ index accompanied by changes such as decreased thymic volume, reduced thymic lobule and lymphoid follicle. It reduced the medulla/cortex ratio, indicating a severe impact of heat stress on thymus development [61]. Continuous exposure of broiler chickens to heat stress-inducing temperatures caused lymphoid depletion of the thymus, a potential histologic marker for immunologic changes known to arise from heat stress [21]. The thymic tissue of the heat-stressed chickens showed severe necrosis with mononuclear inflammatory cell infiltration and loss of the normal architecture of thymic compartments [41].
5.2. Reproductive Organs
Heat stress reduces animal libido, fertility and embryonic survival [62]. As a non-specific environmental stress response, a negative effect on reproduction efficiency and growth performance in livestock and poultry has been observed [63]. High air temperature and humidity have a myriad of effects on cellular functions by the direct alteration and impairment of various tissues or organs of the reproductive system in both sexes of the animal [64].
5.2.1. Testes
In male animals, heat stress reduces sperm production and increases the number of deformed sperm by damaging testicular tissue [65]. Heat exposure triggers testicular degeneration, which is a process that causes deterioration of the testis structure, resulting in the loss of testicular function [66].
The histopathologic examination in heat-stressed lambs revealed a severe testicular degeneration, mainly in the germ line cells, without significant effects on somatic cells [67]. Similarly, another study also reported similar findings, which revealed testicular degeneration with significant germ line degeneration without any impact on somatic cells. Moreover, the vacuolation or disappearance of seminiferous tubules’ epithelium lining, the formation of intertubular multi-nucleate giant cells, spermatogenic arrest at the spermatocyte stage and decreasing thickness of the germinal epithelial layer, thickened basement membranes with interstitial fibrosis and increased peritubular connective tissues were observed [68]. Lower testicular weight and severe damage to testicular microstructures due to chronic heat stress have been reported in rams [67]. Similarly, reports suggested that an increase in apoptosis in spermatozoa subsequently reduced testes weight in developing lambs [68]. In heat-stressed mice, the histological results disclosed thin germinal epithelium with unclear boundaries, internal hemorrhage, decreased sperm count, tubular necrosis and degeneration in seminiferous tubule lumen, giving supporting evidence for the decreased testicular weight [69]. In a study, the histopathological changes in the testicular tubular compartment of heat-stressed goats comprised epithelial desquamation, clusters of Leydig cells with fatty degeneration, necrosis and apoptosis of germ cells, syncytial giant cells and macrophages, Sertoli cell vacuolization and thickening of the basal membrane [70]. This necrosis and apoptosis of germ cells leads to germinative epithelium degeneration, as reported by [71].
In heat-stressed bulls, the presence of twisted tubules of the correct round or round–oval form was observed. Additionally, a group of bulls had thickened linings, including numerous cells at different stages of differentiation. The sperm canals of some bulls were characterized by enlarged lumen, cavities in tubules with narrow spermatogenous epithelium, diluted and impaired cellular elements and a small number of sperms [72].
Buffaloes have higher performance in relation to other domestic species, demonstrating good adaptation skills [73]. In a study, the thermal factor did not result in significant changes in testicular histology in buffaloes [74]. Contrastingly, another histopathological study in buffaloes after prolonged testicular insulation revealed seminiferous tubules with the absence of vacuolized cells, numerous necrotic desquamated tubular lumen cells, and a lack of changes in interstitial cells and the basement membrane of seminiferous tubules. In the epididymis, epithelial cell vacuolization was desquamated and necrotic with pyknosis and cytoplasmic hyper eosinophilia, besides the presence of spermatozoa [73].
Testes histological evaluation in boars following scrotal insulation revealed a decrease in height of the seminiferous tubule epithelium, the presence of vacuoles in the seminiferous epithelium and a reduction in the number of primary spermatocytes and round spermatids, along with an increase in debris and remains of spermatids in the lumen of seminiferous tubules. Additionally, secondary spermatocytes were not found to be impacted [75].
The testicular structure of a heat-stressed male mice model had a disorganized germinal epithelium, with marked sloughing or obliteration of the lumen and the absence of many types of sperm cells [76]. A reduced number or loss of germ cells, such as spermatocytes and spermatids, has been reported to be due to the high sensitivity to heat in rams and adult male mice [71,77]. Heat stress disrupts the testicular tissue structure and induces oxidative stress and endoplasmic reticulum stress in chicken testes [63]. Further, histological sectioning in the same study revealed abnormalities in the distribution of spermatogenic cell layers and the absence of spermatids in the seminiferous tubules.
5.2.2. Ovary
Heat stress compromises fecundity through various mechanisms, including the induction of altered ovarian function [78]. Histological sections of goat ovaries during heat stress revealed abnormal antral follicles showing degenerating oocytes with few disorganized cumulus cells [79]. There are abundant reports about the detrimental effects of heat stress compromising oocyte quality and the alteration of follicular growth in cows [80,81,82] and in goats [79,83]. Another study of ovarian samples from heat-stressed buffaloes revealed a higher rate of degenerated oocytes and a low number of good-quality oocytes with a low maturation rate [84].
The histological evaluation of ovarian tissues from rabbits exposed to heat stress showed focal areas of the proliferation of fibroblast in the interstitial tissues, in addition to severe follicular degeneration characterized by oocyte lysis and enhanced follicular cell degeneration [85]. The morphology of stained follicles from ovaries of chronic heat-stressed mice revealed follicular atresia and granulosa cell apoptosis [86]. It has been found that the ovary and the oocyte operate in a unique flux between apoptosis and autophagy as a strategy to cope with thermal stress [87].
In pigs, heat stress had no impact on dominant follicle number or size [88]; however, in cows, it reduced the size of dominant follicles of the estrus cycle [89]. In laying hens exposed to short and long heat, reductions in ovarian weight and the number of large follicles were reported [90]. On the other hand, there was no significant change in ovarian weight and size in goats exposed to heat stress [79]. Although heat stress’s impact on ovarian tissues has been studied and well documented in lab animal models, information is very scarce in livestock.
5.3. Immunological Organs
The histologic changes in lymphoid tissues could be the basis for knowing the differences in immunologic events responsible for the deleterious effects of heat stress [21]. Additionally, gross changes such as significant reductions in organ weights (thymus, bursa and spleen) have also been associated with heat stress [91].
5.3.1. Lymph Node
As part of normal physiological functions, lymph nodes react to both endogenous and exogenous substances with various specific morphological and functional responses. The pathogen portal in animals is through the feco-oral route, thereby reaching the gastrointestinal tract, and therefore the mesenteric lymph node is considered to be essential for studying the immune system status in livestock [92].
Examining the mesenteric lymph nodes in Malabari goats upon exposure to a hot environment revealed changes such as a paucity of lymphocyte distribution in follicular areas and the decreased density of lymphocytes in germinal centers, reflecting compromised immune functions [92].
5.3.2. Spleen
The spleen is the largest secondary immune organ that elicits an immune response against blood-borne antigens and clears the blood of foreign entities [93]. Therefore, facilitating the understanding of histological changes in the spleen is necessary to correlate with the alteration in functionality.
The histological analysis of acute heat-stressed broiler spleens showed mild lymphoid depletion with mononuclear inflammatory cells, mild necrosis and fibrosis, mild vacuolar degeneration and hyperplasia with mononuclear cells [42]. Further, in another study, the negative impact of thermal stress caused the atrophy of the spleen in chickens [94]. In addition to the above changes, alterations in spleen weight, as well as spleen morphometry, were also noted in heat-stressed chickens [95]. Thus, spleen weight assessment in chickens might prove beneficial to identifying potential biomarkers of immune response during chronic heat stress exposure [96].
Histological changes in the heat-stressed spleens of adult Japanese quails were represented by necrosis, the degeneration and shrinkage fibrosis of lymphocytic nodules, cytoplasmic vacuolation, the infiltration and metamorphosis of lymphocytes, pyknotic nuclei, dilation of the central vein and sinusoids, edema and congested blood vessels [97].
5.3.3. Bone Marrow
The evaluation of the histological architecture in heat-challenged mice bone marrow revealed a wide dilation and thrombosis in vascular sinuses with a discontinuous endothelial cell lining. Further, ultrastructure examination revealed shrunken cells with degenerated cytoplasm, numerous apoptotic cells characterized by electron-dense pyknotic nuclei and the presence of apoptotic bodies of the nucleus [98]. These findings may be due to oxidative stress causing tissue injury through apoptosis and necrosis [99], and vascular endothelium injury during heat stress [100,101]. There is very limited literature on heat stress-associated changes in the bone marrow of farm animals, warranting more such research efforts in the future.
5.3.4. Gut-Associated Lymphoid Tissue (GALT)
The GALT is a major component of the immune system, diffusely scattered through the lamina propria and in organized lymphoid follicles such as the cecal tonsil, Peyer’s patch, the bursa of fabricius and Meckel’s diverticulum [102].
In a study, the cecal tonsils of heat-challenged broiler chickens showed moderate lymphoid depletion with lymphocytolysis [103]. Another study in broilers revealed that exposure to heat stress increased the presence of cell debris, vacuolation, and the degeneration of epithelial cells in the cecal tonsil [104].
In the bursa, severe lymphocytic depletion of the cortex and medulla with heterophile infiltration of the bursa was observed in heat-challenged broiler chickens [103]. Additionally many studies proved that chickens subjected to heat stress exhibited lymphoid depletion in the bursa of fabricius tissue [91,105]. In addition to the aforementioned reports, another study demonstrated the occurrence of reduced bursa weight in broilers subjected to heat stress, and a decreased number of lymphocytes in the cortex and medulla areas of the bursa [106]. This histopathology results in broilers’ heat-stressed bursa manifesting intra-follicular and intra-epithelial cysts [107]. Similarly, another study in broiler chickens observed microcysts in mucosal folds, and the bursal follicles showed a small-sized cortex with slightly packed lymphocytes, medullary necrosis and hyalinization. Further, in the same study, interstitial edema with increased thickness of the inter-follicular connective tissue was also observed [41]. In heat-challenged broiler chickens, a decrease in immature B cells in bursal follicles has been reported. Further, the cell density in the medulla and cortex of bursal follicles decreased severely under heat stress [59].
6. Heat Stress-Associated Histopathological Changes in Adaptive Organs in Livestock
Livestock undergoes several morphological adaptations to cope with adverse environmental conditions. Morphological adaptation is considered crucial for the survival of animals exposed to adverse environmental conditions. In recent years, considerable progress has been made in describing the heat-induced histological changes in adaptive organs of farm animals. It is very vital to understand the significant histological changes in adaptive organs as it might help to differential the adaptive responses between normal and heat-stressed animals. This might help to differentiate between climate-sensitive and climate-resilient breeds. Therefore, understanding the histological changes in various organs linked with the adaptation process is crucial for assessing the climate resilience of livestock breeds.
6.1. Adrenal Gland
Environmental stress produces structural changes in the adrenal gland [108]; therefore, testing adrenal histology could provide valuable information for assessing heat stress [109].
In broiler chickens, faint cells with blurred cellular outlines in the adrenal gland were reported [110]. The histomorphology of adrenal glands from quails under heat-stressed conditions consisted of radial cords of cells in the fasciculate zone and the foamier cytoplasm of cells in the upper part than in the lower part [108]. In the same study, it was reported that the cells in the reticular zone were smaller and darker than cells in the fasciculate zone.
Only a few studies are available describing the histological changes in the adrenal glands in farm animals during heat stress exposure. A study wherein pre-pubertal male rats were exposed to hyperthermic conditions reported mild hydropic degeneration in adrenal glands [27]. Histological investigation of the adrenal gland of heat-stressed rats revealed the existence of dark and light regions in the adrenal cortex. The cells in the light regions were filled with large lipid droplets, but those in the dark cortical regions were deprived of them. Interstitial fibrosis in all parts of the cortical zone and medulla were observed, with no mitotic figures visible in the adrenal glands of heat-stressed rats [26]. Furthermore, another study in rats revealed exciting findings, such as the prolongation of temperature stress causing adrenal gland hypertrophy. Additionally, the volumes of individual zones in the adrenal gland differed with the increase in the volume of the adrenocortical zone, while the vascular tissue remained unchanged. Therefore, it could be concluded that the increase in the proliferation rate of adrenocortical cells was an adaptive response to meet the demands of stressful conditions [111].
6.2. Lung
In heat stress, the lung is one of the most affected and injured organs, as its unique structure and impaired functions promote damages in other organs and tissues, as anoxia [112].
The principal histopathologic lesions in the lung were related to vasculatures and the massive congestion of veins and arterioles, with hemorrhages in parabronchus and alveoli in broilers. It was also reported that the lungs might also be edematous and occasionally contain focal consolidations of bronchopneumonia [40]. Similarly, in heat-stressed sheep, broncho-interstitial pneumonia with bronchiolitis obliterans was reported [113]. In this context, many studies have reported acute interstitial pneumonia affecting cattle, and this is more common during hot and dry summers [114,115].
It has been found that an increased respiratory rate followed by the stimulation of the parabrachial nuclei has caused lung edema and hemorrhage in broilers due to accelerated evaporative heat loss by convection [116]. Similarly, hyperthermia-induced changes in lung histology were examined in piglets, which disclosed excess hemorrhage and alveolar edema [117].
6.3. Heart
As a response to heat stress, heart rate and blood flow to tissues for heat exchange increase, and decreased blood flow to organs takes place. This increase in heart rate maximizes the dissipation of heat and maintains perfusion in the face of vasodilation [118]. Further, it was reported that acute congestive heart failure secondary to heat stress causes death in sheep [119].
It has been reported that sub-endocardium and sub-pericardial hemorrhages are found in the heart with congestion in heat-stressed broilers. In addition, under light microscope examination, massive myofibrillar degeneration with hemorrhage, fatty degeneration, and the vacuolation of myofibers with diffuse myocarditis in some cases were observed [40]. Another study in broilers found that chronic heat stress caused pathological organ changes, including right atrial and ventricular hypertrophy, myofibrillar degeneration, general vacuolar degeneration of myofibers and diffuse myocarditis [120].
6.4. Skin
Skin is the first defense mechanism of the immune system that responds to and resists the hostile environment of temperature, humidity and radiation [121].
The histological result of a study in heat-stressed Iraqi Buffaloes during summer demonstrated that the sweat glands were poorly developed, and lie deeply in the dermis, the secretory acinus surrounded by a few myoepithelial cells and a basal layer of epidermis loaded with abundant melanocytes. On the other hand, sebaceous glands were large, lobulated and well-developed [122].
6.5. Kidney
The kidneys play a vital role in body homeostasis. In heat-stressed sheep, gross findings have revealed bilateral, moist, pale and moderately enlarged kidneys. Additionally, histological examination revealed asymmetrically severe, acute renal tubular necrosis, tubular epithelial regeneration, neutrophil influx and cellular basophilia [113].
Generalized edema and hemorrhages, especially in renal papillae, renal tubular and subrenal capsules with an accumulation of heterophils in many inflammatory areas, have been reported in heat-stressed broilers [40]. Besides this, fatty degeneration of the renal tubular epithelia and glomeruli damage were also reported in the same study. Another study in broilers revealed edema, hemorrhage in the sub-renal capsule with glomerular damage, and the vacuolar degeneration of kidneys [120]. Heat stroke might be associated with degenerative changes in the renal tubules in mammals. Several days after heat stroke, there is often evidence of renal failure caused by the degeneration and necrosis of renal tubules [123]. Similarly, renal histopathological alterations in broiler chicken were reported, revealing tubular necrosis, lumen dilation, the congestion of renal tubules, glomerular structural changes and lots of neutrophil granulocyte invasion in interstitial areas [124].
In a study using a mouse model, the histopathological findings of renal tissue specimens showed the degeneration of tubular epithelial cells and urinary casts [125], whereas the renal tissues from heat-stressed mice showed proximal tubular injury with brush border loss, low-grade inflammation and renal fibrosis [48].
6.6. Gastrointestinal Tract (GIT)
It has been reported that heat stress directly affects gut integrity and induces innate immune system activation, resulting in systemic inflammation in bovines [126] and laying hens [127]. The impact of heat stress in experimental goats showed significant changes in histological sections of the rumen, such as the reduction in length and thickness and the higher keratinization of rumen villi, influencing the fermentation pattern [128].
It is proven that heat stress severely affects the structure of the small intestine, including villi fracture, the shortening of the villus height, deeper crypts and mucosal epithelial cell exfoliation [129]. During heat stress, the blood flow to the intestine is reduced, leading to the ischemia and necrosis of intestinal epithelial cells [37]. Heat stress has been shown to disrupt tight junctions in the intestine, thereby increasing intestinal permeability in multiple mammalian species [130], including lactating goats [131,132], cows [126] and pigs [133]. Furthermore, it has been shown that heat stress increases the intestinal permeability of monogastric animals [134,135]. Nevertheless, it is unknown if the rumen epithelia’s permeability is increased during heat stress as it is structured differently than the intestinal epithelia [136]. However, a study was carried out, and specimens of rumen papillae were microscopically examined; it was found that the corneum sloughs off in heat-stressed cows [136]. Additionally, it has also been reported in the same study that the intercellular space of the granulosum and spinosum was enlarged. Another study using rumen histological sections of goats revealed that heat stress lowered the length and thickness, while keratinization was significantly higher [128].
A study reported that heat stress in cows triggers the migration of infiltrating cells, while jejunal morphology remains unaltered. However, the histological analysis revealed clusters of infiltrating cells of an unknown cell type in mucosa and submucosa [126]. Changes in the intestinal histology of broiler chickens showed a subtle increase in the cellularity of lamina propria in the acute heat stress group, characterized by subtle, acute, multifocal lympho-plasmocytic enteritis in the jejunum [137]. Another study in broiler chickens subjected to acute heat stress revealed a reduction in ileal crypt depth. Still, no change in villus height was found, and this study concluded that due to fast intestinal mucosal re-epithelialization, there is lack of structural changes in heat-stressed chickens [138]. Similarly, chronic heat stress in pigs caused marked morphological injuries in the small intestine, such as villi tip desquamation and exposure of the lamina propria [139]. Table 1 describes the histopathological changes in heat-stressed productive and adaptive organs of livestock and other animal models.

Table 1.
Heat stress-induced histopathological changes in productive and adaptive organs in livestock and other species.
The direct and indirect impacts of heat stress on ruminants also impose a detrimental influence on the rumen microbiome, which in itself is a separate niche. Significant alterations in rumen microbes due to heat stress were reported by researchers in cattle [140], buffalo [141], sheep [142] and goat [143]. A transition in the dominant phylum, from Firmicutes to Bacteroidetes, was observed in caprine rumen with increasing temperature humidity index (THI) [143]. Likewise, the relative abundance of the genus Prevotella gradually increased with rising THI, thereby being the dominant genera. Apart from significant alteration in the production influencing bacteria, heat stress was also proved to alter the relative abundance of pathogenic bacteria such as Erysipelotrichaceae_UCG-004 and Treponema_2, which increased with rising THI. Therefore, such intense association of rumen microbiota with the animals’ productive performance and health status highlight the potential risks associated with heat stress in ruminants.
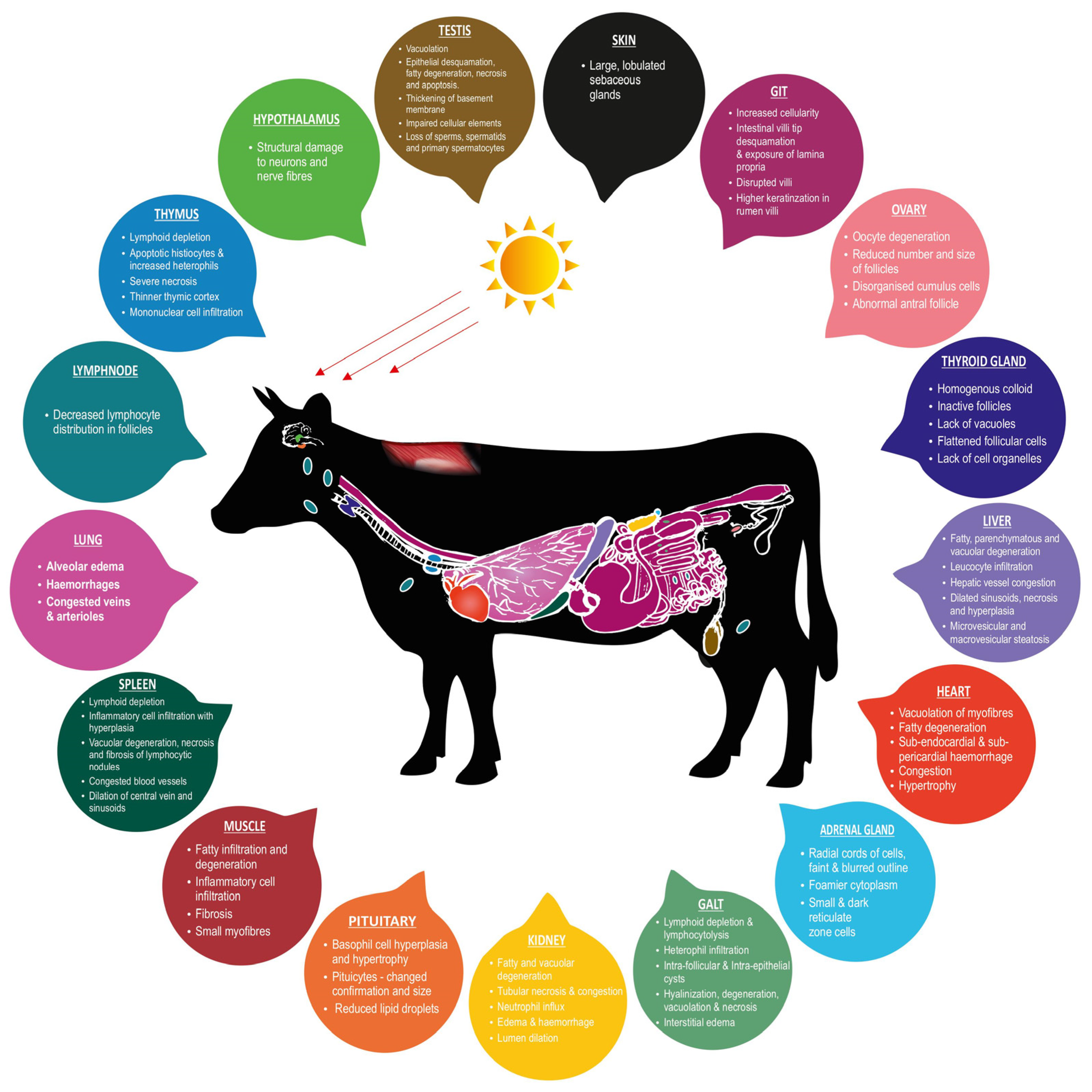
Figure 1 illustrates heat stress-induced histopathological changes in the productive and adaptive organs of livestock.
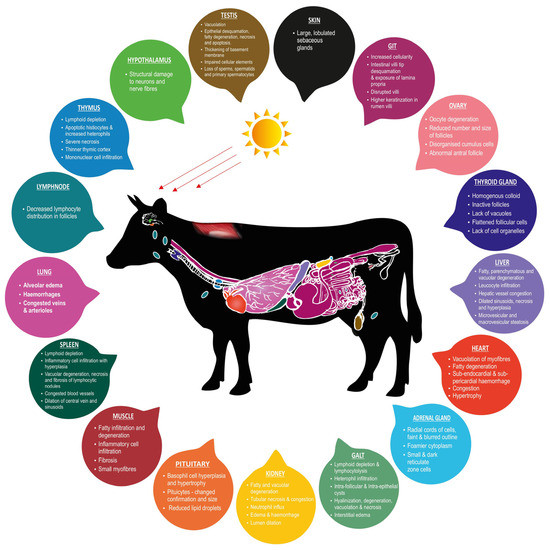
Figure 1.
Heat stress-induced histopathological changes in productive and adaptive organs of livestock.
7. Immunohistochemistry (IHC) Derived Biomarkers in Adaptive and Productive Organs of Livestock
The cellular and molecular responses in livestock are fundamental, as they may lead to the identification of potential genetic biomarkers for heat stress in livestock [144]. Using molecular and physiological biomarkers for diagnosing diseases, monitoring physiological state and forecasting the risk of illness or low productive performance has become a valuable tool assisting agricultural animal production [145]. Immunohistochemistry (IHC) has been established as a solid and reliable methodology for routine diagnostic and research activities in veterinary medicine. The IHC is based on antibodies binding to a specific antigen in tissue sections [146]. The IHC technique is used in the search for cell or tissue antigens ranging from amino acids and proteins to infectious agents and specific cellular populations [147]. Various proteins, inflammation-related cytokines, blood cells and bacteria products in the bloodstream can also be used as biomarkers of heat stress [148].
It has been established that the susceptibility and tolerance to thermal load vary with individuals, breeds and species [149]. In livestock, diverse biomarkers have been identified in productive and adaptive organs as a response to heat stress due to altered cellular defense mechanisms. In growth-related organs of livestock, biomarkers, namely, the 70-kDa heat shock protein (HSP70), heat shock protein 60 (HSP60), Gamma-aminobutyric acid (GABA), GABAA receptors (GABAAR), GABAB receptors (GABABR), heat shock protein 90 (HSP90), Gonadotropin-Releasing Hormone (GnRH), Luteinizing Hormone (LH), Follicle Stimulating Hormone (FSH), N6-methyladenosine (m6A), Nuclear factor erythroid 2-related factor 2 (Nrf2), and CCAAT/enhancer binding-protein beta (C/EBPβ), were reported to change in response to heat stress. The expression pattern of HSP70 was reported mainly in the hypothalamus [150], liver [150,151] and muscle [152] of chickens. In response to heat stress, the expression of HSP60 in the liver was reported to be low in chickens [147] and high in cattle [153]. In the pituitary and hypothalamus of chickens, the expression of GABA, GABAAR, and GABABR biomarkers was reported [154]. Another study in chickens reported that the heat load affected the expression of reproductive hormones such as GnRH in the hypothalamus, LH and FSH in the pituitary [155]. On the evaluation of the effect of heat stress in chicken, HSP90 was found to be highly expressed in the liver [156]. Higher expression of m6A was reported in the hepatic tissue in sheep [157] and Nrf2 in pigs [36] exposed to heat stress. In the pectoralis muscle of heat-stressed chicken, the C/EBPβ biomarker was reported to be higher [53].
In testis, the expression of HSP70 was low in chicken [158] and cattle [159], whereas increased expression in the ovaries of pigs was reported [160] under heat-stressed conditions. Furthermore, Bcl-2 Associated X protein (BAX) and B-cell lymphoma-2 (Bcl-2) protein levels were reported to be highly expressed in sheep [161] and swine [162] testicular tissues. It has been established that testicular heating induced the apoptosis of germ cells by regulating the BAX and BCL-2 protein levels [162]. Further, in heat-stressed ovaries of chicken, varied expressions of GABA, GABAAR and GABABR biomarkers were reported [154]. Analyzing heat-challenged testicular tissue by IHC revealed higher expression patterns of Caspase-3 protein levels in goats [163] and pigs [164]. In chickens, the expression of testosterone (T) in the testis and estrogen (E2) in ovaries was reported to increase at the beginning of heat exposure, followed by a decrease [155]. Other reports in heat-stressed goats revealed higher expressions of HSPB9 and HSPB10 [165] and vascular endothelial growth factor (VEGF) [163] in testicular tissues. In heat-stressed testes, higher expressions of HSP90 has been reported in cattle [159] and cytochrome-C (Cyt-C) in pigs [164]. The expressions of heat shock factor 1 (HSF1), HSP40 [160] and BCL-2 like 1 (BCL2L1) [88] in heat-challenged pig ovaries were reported to be higher. In contrast, in cattle, catalase (CAT) enzyme levels were higher [166]. Moreover, microtubule-associated protein 1 light chain 3 (LC3B) expression levels in ovarian tissues have been reported with no difference in thermo-neutral and heat-stressed pigs [88]. In organs of the immune system, CD3+ T cells, CD4+ T cells, CD8+ T cells, HSP70 and Bcl-2 were reported to change in response to heat stress. In pigs’ heat-stressed spleens, CD3+ T cells, CD4+ T cells, HSP70 and Bcl-2 showed increased expression [167]. In the gut-associated lymphoid tissues (GALT) of chickens exposed to heat stress, CD3+ T, CD4+ and CD8+ T cells were reported [168].
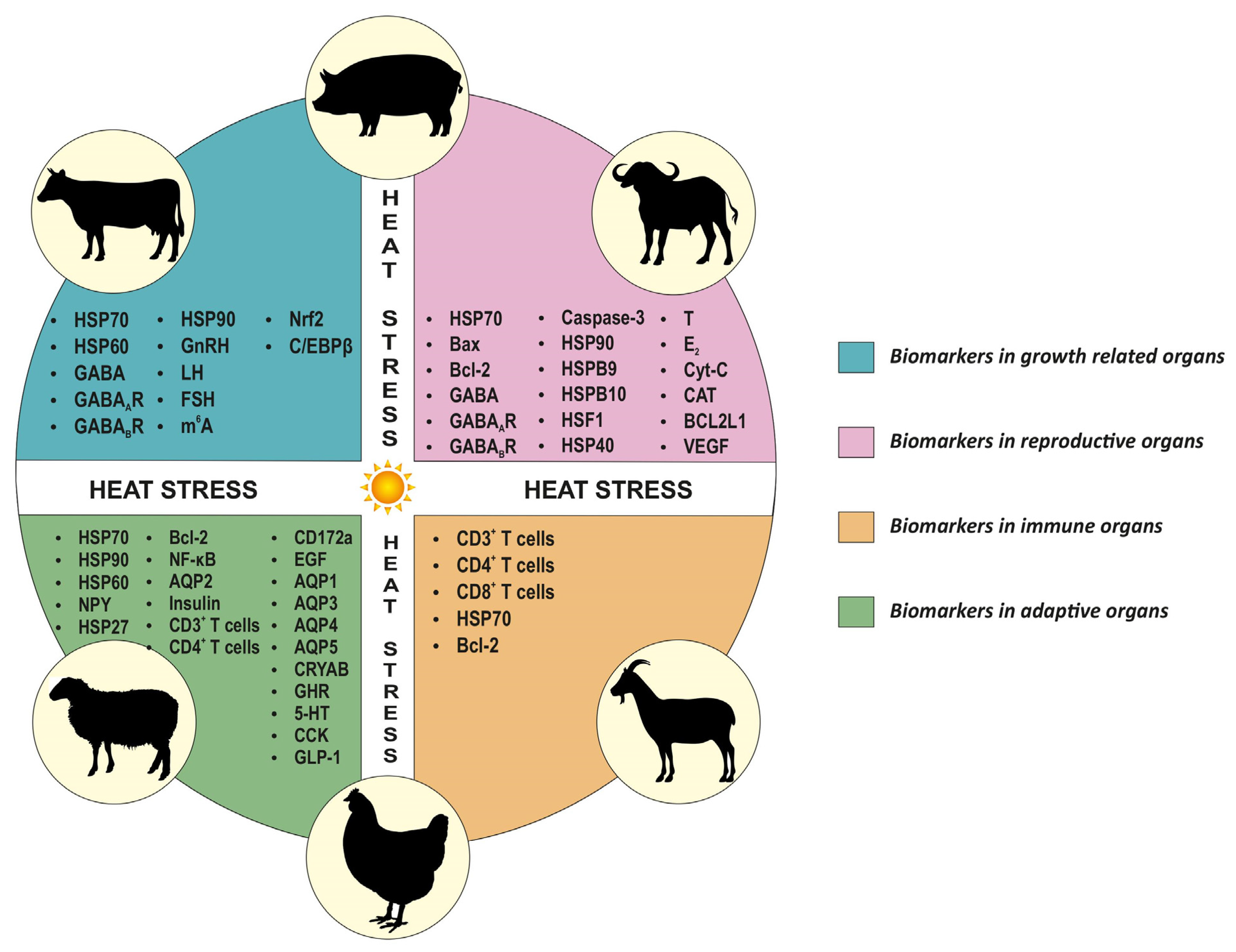
In the adaptive organs of heat-exposed livestock, cellular changes have been evaluated using IHC in many studies. The expression of HSP70 varied widely among different species and organs. A decreased expression of HSP70 levels was reported in heat-stressed lung tissues of cattle [159], as well as in the heart [169] and renal tissues [170] of chickens. In contrast, in other studies, the expression levels of HSP70 increased in the heart [151,171] and renal tissues [151,172] of chickens. Similarly, studies on heat-stressed heart tissues of cattle [159], the intestines of chickens [173,174] and pigs [167], and renal tissues of cattle [159] reported an increase in HSP70 protein levels. The levels of HSP90 in cattle lung and renal tissues increased, whereas a decrease in expression in cardiac tissues was reported [159]. Further, in chickens exposed to heat stress, HSP90 levels increased in the small intestine [173,174] and renal tissues [156]. The expression of HSP60 levels increased following heat stress in chicken [147] and cattle [153] heart tissues. The levels of alpha-B crystallin (CRYAB) and heat shock protein 27 (HSP27) decreased in the heart tissues of chicken [169,175]. The aquaporin 1, 4 and 5 (AQP1, AQP4 and AQP5) levels showed decreased expression in the respiratory tract of heat-stressed buffaloes, whereas AQP3 levels increased [176]. Another study in chickens reported increased AQP2 levels in renal tissues [172]. In heat-stressed chickens, Neuropeptide Y (NPY), ghrelin (GHR), serotonin (5-HT), cholecystokinin (CCK), glucagon-like peptide-1 (GLP-1), Nuclear factor kappa B (NF-κB) and Epidermal growth factor (EGF) protein levels in the gastrointestinal tract varied markedly [174,177]. In heat-stressed pigs, increased Bcl-2, CD3+ and CD4+ T cells in the small intestine (Huo et al., 2019), whereas decreased insulin in the pancreas [178], were reported. The CD172a biomarker in the jejunum of cattle showed increased expression in heat-stressed conditions [126]. Heat stress-related biomarkers in adaptive organs could help assess a livestock species’ resilience and improve the understanding of adaptive mechanisms. Figure 2 describes the heat stress-related biomarkers in productive and adaptive organs of livestock.
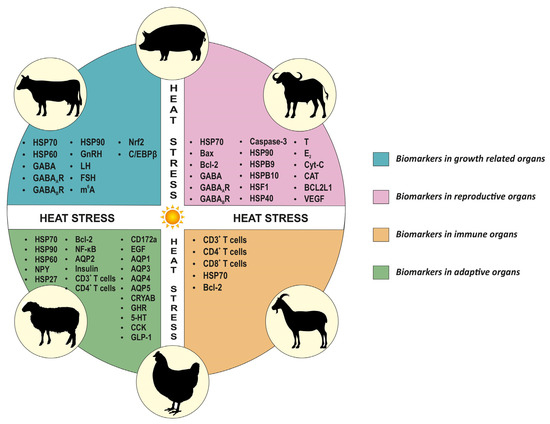
Figure 2.
Overview of heat stress-related biomarkers in productive and adaptive organs of livestock. This figure represents biomarkers for different organs common to all species.
Based on the findings from various studies on heat-stressed livestock, HSP70 was established to be the ideal cellular biomarker for scaling heat response in productive and adaptive organs. These HSPs are a family of proteins with diverse molecular weights, and they act to prevent thermal damage to proteins [179]. In general, it has been established that HSP70 expression is regarded as a reliable biomarker for changes in body temperature [180]. Veterinary pathologists face many challenges when performing IHC because of the diversity of species studied, and there are no guarantees that antibodies will cross-react among different species [146]. However, it is necessary to adopt advanced molecular biological techniques, as they are highly beneficial in understanding the cellular- and molecular-level changes in livestock thriving in extreme environments. Table 2 describes the biomarker expression pattern in livestock in response to heat stress.

Table 2.
Heat stress-associated biomarker expression pattern in livestock in response to heat stress.
8. Conclusions
Based on the available information in the literature, the present review highlights heat stress-induced histopathological alterations in productive and adaptive organs of livestock and the use of a panel of immunohistochemical biomarkers for diagnostic support and confirmation. To elucidate additional information, a brief account of heat stress-induced macroscopic lesions has also been catalogued. This review, citing results from different studies on heat-induced histopathological alterations in tissues, has revealed that the type and distribution of lesions varies depending on the pathologic processes encountered by the target organ. Thus, examining heat stressed-organs’ histopathology and IHC remains at the epicenter of thermal stress diagnosis and assessing climate resilience in farm animals. Further, this review has given a perspective about the broad knowledge deficits in understanding heat stress-induced pathologies in livestock. These findings suggest a lack of emphasis on species-specific animal models in climate change research. It is pivotal that more work be done to assess and emphasize the impact of heat stress on productive and adaptive organs in livestock animals from a pathological perspective. This way, the livestock sector can positively address the heat stress-related economic losses incurred, and such an approach may also help the farming community to make the sector more efficient and sustainable.
9. Future Perspectives
Our findings prove that there is still a need for more research effort, as the pathogenesis of tissue injury due to heat stress needs to be better understood primarily in farm animals. In future, it is essential to evaluate distinct changes in the target organs of livestock species as a result of thermal insults to postulate accurate and relevant information to prevent pitfalls in diagnosis. The broader adoption of histopathology independently or in conjunction with immunohistochemistry could facilitate efficient diagnoses and prognoses. There is an established variation in the pathology induced by thermal insult and the ability of a livestock species to tolerate the heat load. Hence, to further our understanding, more detailed studies in livestock animal models should be undertaken to inform specific microscopic lesions. Advanced technologies provide a dynamic and powerful approach to deriving a more detailed understanding of the molecular and cellular changes in heat-stressed tissues. Therefore, the exploration of unfamiliar cellular areas by adopting new molecular techniques bridges the gap in the literature. Furthermore, exploring the pathological changes at the cellular level improves the understanding of adaptive mechanisms.
Given climate breakdown, further research efforts and detailed investigations of pathologies using histopathological and immunohistochemical analysis are needed in diagnosing the irreversible detrimental effects of heat stress in livestock production. Additionally, the analysis of pathological changes serves as a reliable approach to identify potential biomarkers in assessing the impact of heat stress, and helps develop concepts to combat the detrimental consequences of heat stress in livestock. More research on molecular aspects may lay the foundation for commencing biomarkers-aided breeding programs, and assist in screening more resilient breeds in the challenging climatic scenario. These future research efforts can sustain livestock productivity and minimize the detrimental effects of heat stress on animal growth, production and reproduction.
Author Contributions
Conceptualization, V.S.; formal analysis, E.B.R. and V.S.; writing—original draft preparation, E.B.R.; writing—review and editing, V.S., F.R.D. and M.V.S.; supervision, V.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Herbut, P.; Angrecka, S.; Godyń, D.; Hoffmann, G. The Physiological and Productivity Effects of Heat Stress in Cattle—A Review. Ann. Anim. Sci. 2019, 19, 579–593. [Google Scholar] [CrossRef]
- Moss, A.R.; Jouany, J.-P.; Newbold, J. Methane Production by Ruminants:Its Contribution to Global Warming. Ann. Zootech. 2000, 49, 231–253. [Google Scholar] [CrossRef]
- FAO. Moving Forward on Food Loss and Waste Reduction; The state of food and agriculture; Food and Agriculture Organization of the United Nations: Rome, Italy, 2019; ISBN 978-92-5-131789-1. [Google Scholar]
- Jungbluth, T.; Hartung, E.; Brose, G. Greenhouse Gas Emissions from Animal Houses and Manure Stores. Nutr. Cycl. Agroecosyst. 2001, 60, 133–145. [Google Scholar] [CrossRef]
- Mosier, A.; Kroeze, C.; Nevison, C.; Oenema, O.; Seitzinger, S.; van Cleemput, O. Closing the Global N2O Budget: Nitrous Oxide Emissions through the Agricultural Nitrogen Cycle: OECD/IPCC/IEA Phase II Development of IPCC Guidelines for National Greenhouse Gas Inventory Methodology. Nutr. Cycl. Agroecosyst. 1998, 52, 225–248. [Google Scholar] [CrossRef]
- IPCC. IPCC Guidelines for National Greenhouse Gas Inventories; IPCC: Geneva, Switzerland, 1997. [Google Scholar]
- Thornton, P.; Nelson, G.; Mayberry, D.; Herrero, M. Impacts of Heat Stress on Global Cattle Production during the 21st Century: A Modelling Study. Lancet Planet. Health 2022, 6, e192–e201. [Google Scholar] [CrossRef]
- Das, R.; Sailo, L.; Verma, N.; Bharti, P.; Saikia, J.; Imtiwati; Kumar, R. Impact of Heat Stress on Health and Performance of Dairy Animals: A Review. Vet. World 2016, 9, 260–268. [Google Scholar] [CrossRef]
- Sejian, V.; Bhatta, R.; Gaughan, J.B.; Dunshea, F.R.; Lacetera, N. Review: Adaptation of Animals to Heat Stress. Animal 2018, 12, s431–s444. [Google Scholar] [CrossRef]
- Agarwal, A.; Prabakaran, S.A. Mechanism, Measurement, and Prevention of Oxidative Stress in Male Reproductive Physiology. Indian J. Exp. Biol. 2005, 43, 963–974. [Google Scholar]
- Dahl, G.E.; Tao, S.; Laporta, J. Heat Stress Impacts Immune Status in Cows across the Life Cycle. Front. Vet. Sci. 2020, 7, 116. [Google Scholar] [CrossRef]
- Quinteiro-Filho, W.M.; Ribeiro, A.; Ferraz-de-Paula, V.; Pinheiro, M.L.; Sakai, M.; Sá, L.R.M.; Ferreira, A.J.P.; Palermo-Neto, J. Heat Stress Impairs Performance Parameters, Induces Intestinal Injury, and Decreases Macrophage Activity in Broiler Chickens. Poult. Sci. 2010, 89, 1905–1914. [Google Scholar] [CrossRef]
- Helal, A.; Hashem, A.L.S.; Abdel-Fattah, M.S.; El-Shaer, H.M. Effect of Heat Stress on Coat Characteristics and Physiological Responses of Balady and Damascus Goats in Sinai, Egypt. Am. Eurasian J. Agric. Environ. Sci. 2010, 7, 60–69. [Google Scholar]
- Takahashi, M. Heat Stress on Reproductive Function and Fertility in Mammals. Reprod. Med. Biol. 2012, 11, 37–47. [Google Scholar] [CrossRef] [PubMed]
- Habeeb, A.A.M. Deterioration Effects of Heat Stress on Farm Animals Performance in Tropical and Subtropical Regions. World J. Biol. Pharm. Health Sci. 2020, 4, 7–25. [Google Scholar] [CrossRef]
- Kumar, M.; Ratwan, P.; Dahiya, S.P.; Nehra, A.K. Climate Change and Heat Stress: Impact on Production, Reproduction and Growth Performance of Poultry and Its Mitigation Using Genetic Strategies. J. Therm. Biol. 2021, 97, 102867. [Google Scholar] [CrossRef]
- Kandemir, C.; Koşum, N.; Taşkin, T. Effects of Heat Stress on Physiological Traits in Sheep. Maced. J. Anim. Sci. 2013, 3, 25–29. [Google Scholar] [CrossRef]
- Mayorga, E.J.; Renaudeau, D.; Ramirez, B.C.; Ross, J.W.; Baumgard, L.H. Heat Stress Adaptations in Pigs. Anim. Front. 2019, 9, 54–61. [Google Scholar] [CrossRef]
- Spandan, P.V.; Ruban, W.; Sejian, V.; Devaraj, C.; Silpa, M.V.; Awachat, V.B.; Manjunathareddy, G.B.; Bhatta, R. Heat Stress Induced Changes in the Major Carcass Traits and Quantitative Expression Patterns of Selective Meat Quality Determining Genes in Kanni Aadu Goats. Food Chem. Adv. 2022, 1, 100053. [Google Scholar] [CrossRef]
- Zhang, M.; Dunshea, F.R.; Warner, R.D.; DiGiacomo, K.; Osei-Amponsah, R.; Chauhan, S.S. Impacts of Heat Stress on Meat Quality and Strategies for Amelioration: A Review. Int. J. Biometeorol. 2020, 64, 1613–1628. [Google Scholar] [CrossRef]
- Aguanta, B.N.; Fuller, A.L.; Milfort, M.C.; Williams, S.M.; Rekaya, R.; Aggrey, S.E. Histologic Effects of Concurrent Heat Stress and Coccidial Infection on the Lymphoid Tissues of Broiler Chickens. Avian Dis. 2018, 62, 345. [Google Scholar] [CrossRef]
- Yu, T.; Yong, Y.; Li, J.; Fang, B.; Hu, C.; Wu, L.; Liu, X.; Yu, Z.; Ma, X.; Patil, Y.; et al. Proteomic Study of Hypothalamus in Pigs Exposed to Heat Stress. BMC Vet. Res. 2020, 16, 286. [Google Scholar] [CrossRef]
- Zhao, Y.; Zhuang, Y.; Shi, Y.; Xu, Z.; Zhou, C.; Guo, L.; Liu, P.; Wu, C.; Hu, R.; Hu, G.; et al. Effects of N-Acetyl-l-Cysteine on Heat Stress-Induced Oxidative Stress and Inflammation in the Hypothalamus of Hens. J. Therm. Biol. 2021, 98, 102927. [Google Scholar] [CrossRef] [PubMed]
- Clark, C.E.; Das, G.P. Effect of High Environmental Temperature on Internal Organs of Chickens. Poult. Sci. 1974, 53, 859–863. [Google Scholar] [CrossRef] [PubMed]
- Sritharet, N.; Hara, H.; Yoshida, Y.; Hanzawa, K.; Watanabe, S. Effects of Heat Stress on Histological Features in Pituicytes and Hepatocytes, and Enzyme Activities of Liver and Blood Plasma in Japanese Quail (Coturnix japonica). J. Poult. Sci. 2002, 39, 167–178. [Google Scholar] [CrossRef]
- Koko, V.; Djordjeviæ, J.; Cvijiæ, G.; Davidoviæ, V. Effect of Acute Heat Stress on Rat Adrenal Glands: A Morphological and Stereological Study. J. Exp. Biol. 2004, 207, 4225–4230. [Google Scholar] [CrossRef]
- Mete, F.; Kilic, E.; Somay, A.; Yilmaz, B. Effects of Heat Stress on Endocrine Functions & Behaviour in the Pre-Pubertal Rat. Indian J. Med. Res. 2012, 135, 233–239. [Google Scholar]
- Hamad, E.S.A.-M. Seasonal Changes in the Morphology and Morphometry of the Thyroid Gland of the Nubian Goat. Master’s Thesis, University of Khartoum, Khartoum, Sudan, December 2008. [Google Scholar]
- Pragna, P.; Sejian, V.; Soren, N.M.; Bagath, M.; Krishnan, G.; Beena, V.; Devi, P.I.; Bhatta, R. Summer Season Induced Rhythmic Alterations in Metabolic Activities to Adapt to Heat Stress in Three Indigenous (Osmanabadi, Malabari and Salem Black) Goat Breeds. Biol. Rhythm. Res. 2018, 49, 551–565. [Google Scholar] [CrossRef]
- Igbokwe, C.O.; Ezeasor, D.N. Histologic and Ultrastructural Observations on the Thyroid Gland of the White Fulani (Zebu) Cattle in Northern Nigeria. Afr. J. Biotechnol. 2015, 14, 156–166. [Google Scholar] [CrossRef]
- Ali, S.A.; El-Sayed, S.A.; Goda, N.I.A.; Beheiry, R.R. Morphological Characteristics of the Goat Thyroid Glands among Summer and Winter Seasons. Adv. Anim. Vet. Sci. 2020, 8, 252–259. [Google Scholar] [CrossRef]
- Joshi, B.; Sanwal, P.; Agarwal, K. Effect of Cooling Large White Yorkshire Piglets during Summer Season on the Histological Structures of Their Adrenal Thyroid and Uterus. Indian J. Anim. Sci. 1977, 47, 134–144. [Google Scholar]
- Hussin, A.M.; Al-Taay, M.M. Histological Study of the Thyroid and Parathyroid Glands in Iraqi Buffalo “Bubalus Bubalis” with Referring to the Seasonal Changes. Basrah J. Vet. Res. 2009, 8, 13. [Google Scholar] [CrossRef]
- Verma, P.S.; Tyaqi, B.S.; Agarwal, V.K. Textbook of Animal Physiology and Ecology; S. Chand and Company LTD.: New Delhi, India, 1996. [Google Scholar]
- Bhatnagar, D.S.; Mukherjee, D.P.; Bhattacharya, P. Seasonal Changes in Histology of the Thyroid and Testis of Buffalo. Indian J. Vet. Sci. 1955, 42, 824–830. [Google Scholar]
- Chen, J.; Wang, F.; Zhou, X.; Cao, Y.; Li, Y.; Li, C. Bama Miniature Pigs’ Liver Possess Great Heat Tolerance through Upregulation of Nrf2-Mediated Antioxidative Enzymes. J. Therm. Biol. 2017, 67, 15–21. [Google Scholar] [CrossRef] [PubMed]
- Hall, D.M.; Baumgardner, K.R.; Oberley, T.D.; Gisolfi, C.V. Splanchnic Tissues Undergo Hypoxic Stress during Whole Body Hyperthermia. Am. J. Physiol. Gastrointest. Liver Physiol. 1999, 276, G1195–G1203. [Google Scholar] [CrossRef] [PubMed]
- Angel, S.P.; Bagath, M.; Sejian, V.; Krishnan, G.; Bhatta, R. Expression Patterns of Candidate Genes Reflecting the Growth Performance of Goats Subjected to Heat Stress. Mol. Biol. Rep. 2018, 45, 2847–2856. [Google Scholar] [CrossRef] [PubMed]
- Shaji, S.; Sejian, V.; Bagath, M.; Manjunathareddy, G.B.; Kurien, E.K.; Varma, G.; Bhatta, R. Summer Season Related Heat and Nutritional Stresses on the Adaptive Capability of Goats Based on Blood Biochemical Response and Hepatic HSP70 Gene Expression. Biol. Rhythm Res. 2017, 48, 65–83. [Google Scholar] [CrossRef]
- Aengwanich, W.; Simaraks, S. Pathology of Heart, Lung, Liver and Kidney in Broilers under Chronic Heat Stress. Songklanakarin J. Sci. Technol. 2004, 26, 8. [Google Scholar]
- Saad, A.H.; Ahmed, M.S.; Aboubakr, M.; Ghoneim, H.A.; Abdel-Daim, M.M.; Albadrani, G.M.; Arafat, N.; Fadl, S.E.; Abdo, W. Impact of Dietary or Drinking Water Ruminococcus Sp. Supplementation and/or Heat Stress on Growth, Histopathology, and Bursal Gene Expression of Broilers. Front. Vet. Sci. 2021, 8, 663577. [Google Scholar] [CrossRef] [PubMed]
- Adu-Asiamah, P.; Zhang, Y.; Amoah, K.; Leng, Q.Y.; Zheng, J.H.; Yang, H.; Zhang, W.L.; Zhang, L. Evaluation of Physiological and Molecular Responses to Acute Heat Stress in Two Chicken Breeds. Animal 2021, 15, 100106. [Google Scholar] [CrossRef]
- Ma, B.; Xing, T.; Li, J.; Zhang, L.; Jiang, Y.; Gao, F. Chronic Heat Stress Causes Liver Damage via Endoplasmic Reticulum Stress-Induced Apoptosis in Broilers. Poult. Sci. 2022, 101, 102063. [Google Scholar] [CrossRef]
- Lu, Z.; He, X.F.; Ma, B.B.; Zhang, L.; Li, J.L.; Jiang, Y.; Zhou, G.H.; Gao, F. Increased Fat Synthesis and Limited Apolipoprotein B Cause Lipid Accumulation in the Liver of Broiler Chickens Exposed to Chronic Heat Stress. Poult. Sci. 2019, 98, 3695–3704. [Google Scholar] [CrossRef]
- Pu, S.; Usuda, K.; Nagaoka, K.; Watanabe, G. Heat Challenge Influences Serum Metabolites Concentrations and Liver Lipid Metabolism in Japanese Quail (Coturnix Japonica). J. Vet. Med. Sci. 2019, 81, 77–83. [Google Scholar] [CrossRef] [PubMed]
- Jafargolipour, M.; Vahdatpour, T.; Mahmoodpour, H.; Vahdatpour, S. Effects of Antioxidants Consumption and Low Protein Diets on Liver and Intestine Histopathology and Performance of Japanese Quails (Coturnix Coturnix Japonica). Anim. Res. Int. 2017, 14, 2683–2690. [Google Scholar]
- Skibiel, A.L.; Peñagaricano, F.; Amorín, R.; Ahmed, B.M.; Dahl, G.E.; Laporta, J. In Utero Heat Stress Alters the Offspring Epigenome. Sci. Rep. 2018, 8, 14609. [Google Scholar] [CrossRef] [PubMed]
- Roncal-Jimenez, C.A.; Sato, Y.; Milagres, T.; Andres Hernando, A.; García, G.; Bjornstad, P.; Dawson, J.B.; Sorensen, C.; Newman, L.; Krisher, L.; et al. Experimental Heat Stress Nephropathy and Liver Injury Are Improved by Allopurinol. Am. J. Physiol. Ren. Physiol. 2018, 315, F726–F733. [Google Scholar] [CrossRef] [PubMed]
- Koch, F.; Lamp, O.; Kuhla, B. Heat Stress Involves Activation of PAMPK and FOXO3 Regulating Glycolysis and Proteolysis in the Skeletal Muscle of Dairy Cows. In Engergy and Protein Metabolism and Nutrition; Skomiał, J., Lapierre, H., Eds.; Wageningen Academic Publishers: Wageningen, The Netherlands, 2016; pp. 99–100. [Google Scholar]
- Barnes, T.L.; Cadaret, C.N.; Beede, K.A.; Schmidt, T.B.; Petersen, J.L.; Yates, D.T. Hypertrophic Muscle Growth and Metabolic Efficiency Were Impaired by Chronic Heat Stress, Improved by Zilpaterol Supplementation, and Not Affected by Ractopamine Supplementation in Feedlot Lambs. J. Anim. Sci. 2019, 97, 4101–4113. [Google Scholar] [CrossRef] [PubMed]
- Close, W.H.; Mount, L.E. Energy Retention in the Pig at Several Environmental Temperatures and Levels of Feeding. Proc. Nutr. Soc. 1971, 30, 33A–34A. [Google Scholar]
- Kikusato, M.; Toyomizu, M. Crucial Role of Membrane Potential in Heat Stress-Induced Overproduction of Reactive Oxygen Species in Avian Skeletal Muscle Mitochondria. PLoS ONE 2013, 8, e64412. [Google Scholar] [CrossRef]
- Patael, T.; Piestun, Y.; Soffer, A.; Mordechay, S.; Yahav, S.; Velleman, S.G.; Halevy, O. Early Posthatch Thermal Stress Causes Long-Term Adverse Effects on Pectoralis Muscle Development in Broilers. Poult. Sci. 2019, 98, 3268–3277. [Google Scholar] [CrossRef]
- Zhang, Z.Y.; Jia, G.Q.; Zuo, J.J.; Zhang, Y.; Lei, J.; Ren, L.; Feng, D.Y. Effects of Constant and Cyclic Heat Stress on Muscle Metabolism and Meat Quality of Broiler Breast Fillet and Thigh Meat. Poult. Sci. 2012, 91, 2931–2937. [Google Scholar] [CrossRef]
- Most, M. Targeting Inflammation in Heat-Stressed Wethers Improves Growth and Efficiency and Alters Body Composition: A Brief Exploration and Application of Extension Principles. Master’s Thesis, University of Nebraska, Lincoln, NE, USA, May 2022. [Google Scholar]
- Mader, T.L.; Davis, M.S. Effect of Management Strategies on Reducing Heat Stress of Feedlot Cattle: Feed and Water Intake. J. Anim. Sci. 2004, 82, 3077–3087. [Google Scholar] [CrossRef]
- Ghazi, S.; Habibian, M.; Moeini, M.M.; Abdolmohammadi, A.R. Effects of Different Levels of Organic and Inorganic Chromium on Growth Performance and Immunocompetence of Broilers under Heat Stress. Biol. Trace Elem. Res. 2012, 146, 309–317. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, B.M.S.; Younas, U.; Asar, T.O.; Monteiro, A.P.A.; Hayen, M.J.; Tao, S.; Dahl, G.E. Maternal Heat Stress Reduces Body and Organ Growth in Calves: Relationship to Immune Status. JDS Commun. 2021, 2, 295–299. [Google Scholar] [CrossRef] [PubMed]
- Hirakawa, R.; Nurjanah, S.; Furukawa, K.; Murai, A.; Kikusato, M.; Nochi, T.; Toyomizu, M. Heat Stress Causes Immune Abnormalities via Massive Damage to Effect Proliferation and Differentiation of Lymphocytes in Broiler Chickens. Front. Vet. Sci. 2020, 7, 46. [Google Scholar] [CrossRef] [PubMed]
- Andriani, R.; Arimbi, A.; Rahardjo, D.; Plumeriastuti, H.; Legowo, D.; Hestianah, E.P. Histopathological Appearance of Thymus on Broiler under Chronic Heat Stress. JBMV 2020, 8, 13. [Google Scholar] [CrossRef]
- Tang, J.; Chen, Z. The Protective Effect of γ-Aminobutyric Acid on the Development of Immune Function in Chickens under Heat Stress. J. Anim. Physiol. Anim. Nutr. 2016, 100, 768–777. [Google Scholar] [CrossRef]
- Kumar, S.B.V.; Kumar, A.; Kataria, M. Effect of Heat Stress in Tropical Livestock and Different Strategies for Its Amelioration. J. Stress Physiol. Biochem. 2011, 7, 45–54. [Google Scholar]
- Xiong, Y.; Yin, Q.; Li, J.; He, S. Oxidative Stress and Endoplasmic Reticulum Stress Are Involved in the Protective Effect of Alpha Lipoic Acid Against Heat Damage in Chicken Testes. Animals 2020, 10, 384. [Google Scholar] [CrossRef]
- Singh, K.P.; Singh, P. Management of Heat Stress in Livestock. Pashudhan Praharee 2021. Available online: https://www.pashudhanpraharee.com/management-of-heat-stress-in-livestock/ (accessed on 21 November 2022).
- Kastelic, J.P.; Wilde, R.E.; Bielli, A.; Genovese, P.; Rizzoto, G.; Thundathil, J. Hyperthermia Is More Important than Hypoxia as a Cause of Disrupted Spermatogenesis and Abnormal Sperm. Theriogenology 2019, 131, 177–181. [Google Scholar] [CrossRef]
- Turner, R.M.O. Pathogenesis, Diagnosis, and Management of Testicular Degeneration in Stallions. Clin. Tech. Equine Pract. 2007, 6, 278–284. [Google Scholar] [CrossRef]
- Gomes, W.R.; Butler, W.R.; Johnson, A.D. Effect of Elevated Ambient Temperature on Testis and Blood Levels and In Vitro Biosynthesis of Testosterone in the Ram. J. Anim. Sci. 1971, 33, 804–807. [Google Scholar] [CrossRef] [PubMed]
- Rasooli, A.; Taha Jalali, M.; Nouri, M.; Mohammadian, B.; Barati, F. Effects of Chronic Heat Stress on Testicular Structures, Serum Testosterone and Cortisol Concentrations in Developing Lambs. Anim. Reprod. Sci. 2010, 117, 55–59. [Google Scholar] [CrossRef] [PubMed]
- Kang, D.; Kim, D. Antioxidant Effect of Lonicera Caerulea on Heat Stress-Treated Male Mice. J. Anim. Reprod. Biotechnol. 2021, 36, 220–229. [Google Scholar] [CrossRef]
- Xavier, G.C.; Soares, P.C.; da Silva, V.A., Jr.; de Torres, S.M.; Maymone, A.C.M.; de Morais, R.N.; Moura, C.S.; Guerra, M.M.P. Effect of Dietary Selenium and Vitamin E Supplementation on Testicular Morphology and Serum Testosterone Concentration in Goats Following Scrotal Insulation. Acta Sci. Vet. 2016, 44, 8. [Google Scholar] [CrossRef]
- Rockett, J.C.; Mapp, F.L.; Garges, J.B.; Luft, J.C.; Mori, C.; Dix, D.J. Effects of Hyperthermia on Spermatogenesis, Apoptosis, Gene Expression, and Fertility in Adult Male Mice. Biol. Reprod. 2001, 65, 229–239. [Google Scholar] [CrossRef]
- Bordunova, O.; Shulzhenko, N.; Mylostyvyi, R.; Chernenko, O.; Prishedko, V.; Chernenko, O. Comparison of Morphometric and Histological Properties of Testicles and Sperm Production in Breeding Bulls with Different Reaction to Stress. Vet. Stanica 2022, 54, 193–209. [Google Scholar] [CrossRef]
- Garcia, O.S.; Vale, W.G.; Garcia, A.R.; Ribeiro, H.F.L.; Assunção, W.L.P.; Ferro, R.S.; Rolim Filho, S.T.; Sales, M.S.; Barbosa, E.M.; Ayala, H.D.M.; et al. Histopathology of Buffalo Testis Submitted to Experimental Testicular Insulation. Rev. Vet. 2010, 21, 905–907. [Google Scholar]
- Chacur, M.G.M.; Oba, E.; Ferreira, J.C.P.; Velloso, N.M. Physiological Changes Occurred in Buffalo Bulls (Bubalus Bubalis) Subject to Thermal Stress. Rev. Vet. 2010, 21, 936–937. [Google Scholar]
- Parrish, J.J.; Willenburg, K.L.; Gibbs, K.M.; Yagoda, K.B.; Krautkramer, M.M.; Loether, T.M.; Melo, F.C.S.A. Scrotal Insulation and Sperm Production in the Boar. Mol. Reprod. Dev. 2017, 84, 969–978. [Google Scholar] [CrossRef]
- Dang-Cong, T.; Nguyen-Thanh, T. Testicular Histopathology and Spermatogenesis in Mice with Scrotal Heat Stress. In Male Reproductive Anatomy; Wu, W., Ed.; IntechOpen: London, UK, 2022; ISBN 978-1-83968-524-8. [Google Scholar]
- Reviers, M.T.H.-D.; Locatelli, A.; Perreau, C.; Pisselet, C.; Setchell, B.P. Effects of a Single Brief Period of Moderate Heating of the Testes on Seminiferous Tubules in Hypophysectomized Rams Treated with Pituitary Extract. Reproduction 1993, 97, 381–387. [Google Scholar] [CrossRef]
- Hansen, P.J. Effects of Heat Stress on Mammalian Reproduction. Phil. Trans. R. Soc. B 2009, 364, 3341–3350. [Google Scholar] [CrossRef] [PubMed]
- Bari, M.; Kabir, M.; Sarker, M.; Khan, A.; Momiruzzaman, M. Morphometric Analysis of Ovarian Follicles of Black Bengal Goats during Winter and Summer Season. Bangladesh J. Anim. Sci. 2012, 40, 51–55. [Google Scholar] [CrossRef]
- Roth, Z.; Meidan, R.; Braw-Tal, R.; Wolfenson, D. Impaired Reproduction in Heat-Stressed Cattle: Basic and Applied Aspects. Anim. Reprod. Sci. 2000, 60–61, 535–547. [Google Scholar] [CrossRef]
- Roth, Z.; Wolfenson, D. Comparing the Effects of Heat Stress and Mastitis on Ovarian Function in Lactating Cows: Basic and Applied Aspects. Domest. Anim. Endocrinol. 2016, 56, S218–S227. [Google Scholar] [CrossRef]
- López-Gatius, F.; Hunter, R. Clinical Relevance of Pre-Ovulatory Follicular Temperature in Heat-Stressed Lactating Dairy Cows. Reprod. Dom. Anim. 2017, 52, 366–370. [Google Scholar] [CrossRef]
- Ozawa, M.; Tabayashi, D.; Latief, T.A.; Shimizu, T.; Oshima, I.; Kanai, Y. Alterations in Follicular Dynamics and Steroidogenic Abilities Induced by Heat Stress during Follicular Recruitment in Goats. Reproduction 2005, 129, 621–630. [Google Scholar] [CrossRef]
- El-Naby, A.A.-H.H.; Mahmoud, K.G.H.M.; Ahmed, Y.F.; Abouel-Roos, M.E.A.; Abdel-Ghaffar, A.E. Effect of Season of the Year and Ovarian Structures on Oocytes Recovery Rate, Quality and Meiotic Competence in Egyptian Buffaloes. Glob. Vet. 2013, 10, 408–412. [Google Scholar]
- Mutwedu, V.B.; Nyongesa, A.W.; Kitaa, J.M.; Ayagirwe, R.B.B.; Baharanyi, C.; Mbaria, J.M. Effects of Moringa Oleifera Aqueous Seed Extracts on Reproductive Traits of Heat-Stressed New Zealand White Female Rabbits. Front. Vet. Sci. 2022, 9, 883976. [Google Scholar] [CrossRef]
- Li, J.; Gao, H.; Tian, Z.; Wu, Y.; Wang, Y.; Fang, Y.; Lin, L.; Han, Y.; Wu, S.; Haq, I.; et al. Effects of Chronic Heat Stress on Granulosa Cell Apoptosis and Follicular Atresia in Mouse Ovary. J. Anim. Sci. Biotechnol. 2016, 7, 57. [Google Scholar] [CrossRef]
- Mayorga, E.J.; Ross, J.W.; Keating, A.F.; Rhoads, R.P.; Baumgard, L.H. Biology of Heat Stress; the Nexus between Intestinal Hyperpermeability and Swine Reproduction. Theriogenology 2020, 154, 73–83. [Google Scholar] [CrossRef]
- Hale, B.J.; Hager, C.L.; Seibert, J.T.; Selsby, J.T.; Baumgard, L.H.; Keating, A.F.; Ross, J.W. Heat Stress Induces Autophagy in Pig Ovaries during Follicular Development. Biol. Reprod. 2017, 97, 426–437. [Google Scholar] [CrossRef] [PubMed]
- Wilson, S.J.; Kirby, C.J.; Koenigsfeld, A.T.; Keisler, D.H.; Lucy, M.C. Effects of Controlled Heat Stress on Ovarian Function of Dairy Cattle. 2. Heifers. J. Dairy Sci. 1998, 81, 2132–2138. [Google Scholar] [CrossRef] [PubMed]
- Rozenboim, I.; Tako, E.; Gal-Garber, O.; Proudman, J.A.; Uni, Z. The Effect of Heat Stress on Ovarian Function of Laying Hens. Poult. Sci. 2007, 86, 1760–1765. [Google Scholar] [CrossRef] [PubMed]
- Bartlett, J.R.; Smith, M.O. Effects of Different Levels of Zinc on the Performance and Immunocompetence of Broilers under Heat Stress. Poult. Sci. 2003, 82, 1580–1588. [Google Scholar] [CrossRef]
- Rashamol, V.P.; Sejian, V.; Bagath, M.; Krishnan, G.; Beena, V.; Bhatta, R. Effect of Heat Stress on the Quantitative Expression Patterns of Different Cytokine Genes in Malabari Goats. Int. J. Biometeorol. 2019, 63, 1005–1013. [Google Scholar] [CrossRef]
- Kapoor, M.; Ranjan, N.; Chauhan, B.N.; Singh, S.B. Structural Alterations in Spleen and Kidney in Response to Graded Heat Stress. Int. J. Pharmacol. Res. 2015, 5, 138–142. [Google Scholar]
- Ohtsu, H.; Yamazaki, M.; Abe, H.; Murakami, H.; Toyomizu, M. Heat Stress Modulates Cytokine Gene Expression in the Spleen of Broiler Chickens. J. Poult. Sci. 2015, 52, 282–287. [Google Scholar] [CrossRef]
- Calefi, A.S.; de Siqueira, A.; Namazu, L.B.; Costola-de-Souza, C.; Honda, B.B.T.; Ferreira, A.J.P.; Quinteiro-Filho, W.M.; da Silva Fonseca, J.G.; Palermo-Neto, J. Effects of Heat Stress on the Formation of Splenic Germinal Centres and Immunoglobulins in Broilers Infected by Clostridium Perfringens Type A. Vet. Immunol. Immunopathol. 2016, 171, 38–46. [Google Scholar] [CrossRef]
- Strong, R.A. The Effects of Heat Stress on Immunity in Laying Hens and Dairy Cattle. Ph.D. Thesis, Purdue University, West Lafayette, IN, USA, December 2014. [Google Scholar]
- Al-Ali, Z.A.; Faysal, M.; Yasin, J. Histological and Molecular Study of Spleen in Japanese Quail under Thermal Condition. Basrah J. Vet. Res. 2017, 16, 1–10. [Google Scholar]
- Ramadan, N.K.; Badr, G.; Abdel-Tawab, H.S.; Ahmed, S.F.; Mahmoud, M.H. Camel Whey Protein Enhances Lymphocyte Survival by Modulating the Expression of Survivin, Bim/Bax, and Cytochrome C and Restores Heat Stress-Mediated Pathological Alteration in Lymphoid Organs. Iran. J. Basic Med. Sci. 2018, 21, 896–904. [Google Scholar] [CrossRef]
- Amira, A.M.A. Oxidative Stress and Disease: An Updated Review. Res. J. Immunol. 2010, 3, 129–145. [Google Scholar] [CrossRef]
- Roberts, G.T.; Ghebeh, H.; Chishti, M.A.; Al-Mohanna, F.; El-Sayed, R.; Al-Mohanna, F.; Bouchama, A. Microvascular Injury, Thrombosis, Inflammation, and Apoptosis in the Pathogenesis of Heatstroke: A Study in Baboon Model. Arterioscler. Thromb. Vasc. Biol. 2008, 28, 1130–1136. [Google Scholar] [CrossRef]
- Brinton, M.R.; Tagge, C.A.; Stewart, R.J.; Cheung, A.K.; Shiu, Y.-T.E.; Christensen, D.A. Thermal Sensitivity of Endothelial Cells on Synthetic Vascular Graft Material. Int. J. Hyperth. 2012, 28, 163–174. [Google Scholar] [CrossRef] [PubMed]
- Nochi, T.; Jansen, C.A.; Toyomizu, M.; van Eden, W. The Well-Developed Mucosal Immune Systems of Birds and Mammals Allow for Similar Approaches of Mucosal Vaccination in Both Types of Animals. Front. Nutr. 2018, 5, 60. [Google Scholar] [CrossRef] [PubMed]
- Awaad, M.; Elmenawey, M.; Bashandy, M.; Mohamed, F.; Salem, H.; Morsy, E.; Gossens, T. Heat Stress Impedance by Acidifiers in Broiler Chickens. Acta Sci. Med. Sci. 2018, 2, 10. [Google Scholar]
- Ebtasam, J.A.; Balqees, H.A. Histopathological Study of Cecal Tonsils in Broilers Exposed to Heat Stress after Treatment with Lactobacillus Acidophilus: Balqees, H.A.; Ebtasam, J.A. and Nawals, J. Iraqi J. Vet. Med. 2015, 39, 119–124. [Google Scholar] [CrossRef]
- Lola, M.S.; Paraso, M.G.; Divina, B.P.; Gobonseng, D.T.; Bombio, A.M.; Collantes, T.M. Heat Stress Induces Histopathological Changes in Lymphoid Organs of Broiler and Philippine Native Chickens. Philipp. J. Vet. Anim. Sci. 2017, 42, 85–90. [Google Scholar]
- Aengwanich, W. Pathological Changes and the Effects of Ascorbic Acid on Lesion Scores of Bursa of Fabricius in Broilers under Chronic Heat Stress. Res. J. Vet. Sci. 2010, 3, 74–78. [Google Scholar]
- Rajan, R.A.; Edwin, S.C.; Rajendran, K.; Murali, N.; Pramod, R.K. Effect of Heat Stress on Internal Organs of Four Chicken Varieties. Int. J. Sci. Res. 2014, 3, 467–468. [Google Scholar] [CrossRef]
- Özdemir, D.; Ozudogru, Z.; İmik, H.; Can, M.; Sunar, M. Effects of Dietary Antioxidant Supplementation on the Adrenal Glands in Quails (Coturnix Coturnix Japonica) Reared under Heat Stress. Rev. Med. Vet. 2011, 162, 8–12. [Google Scholar]
- Rosas-Trigueros, A.P.; Candanosa-Aranda, I.E.; Ducoing-Watty, A.E.; Gutiérrez-Molotla, J.; Galindo, F.; Sisto-Burt, A.M. Histological Differences in the Adrenal Glands and Cortisol Levels of Suckling Dairy Goat Kids in Enriched and Non-Enriched Environments. Res. Vet. Sci. 2017, 115, 221–225. [Google Scholar] [CrossRef] [PubMed]
- Majekodunmi, B.C.; Ogunwole, O.A.; Sokunbi, O.A. Plasma Corticosterone and Adrenal Gland Histomorphometry of Heat Stressed Broiler Chickens given Supplemental Electrolytes or Vitamin C. Arch. Zootec. 2016, 65, 535–540. [Google Scholar]
- Popovska-Perčinić, F.; Manojlović-Stojanoski, M.; Pendovski, L.; Dinevska Kjovkarovska, S.; Miova, B.; Grubin, J.; Milošević, V.; Ajdžanović, V. A Moderate Increase in Ambient Temperature Influences the Structure and Hormonal Secretion of Adrenal Glands in Rats. Cell J. 2020, 22, 415. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.-H.; Hou, C.-C.; Lin, M.-T.; Chang, C.-P. Attenuating Heat-Induced Acute Lung Inflammation and Injury by Dextromethorphan in Rats. Am. J. Respir. Cell Mol. Biol. 2012, 46, 407–413. [Google Scholar] [CrossRef]
- Sula, M.J.M.; Winslow, C.M.; Boileau, M.J.; Barker, L.D.; Panciera, R.J. Heat-Related Injury in Lambs. J. Vet. Diagn. Investig. 2012, 24, 772–776. [Google Scholar] [CrossRef]
- Ayroud, M.; Popp, J.D.; VanderKop, M.A.; Yost, G.S.; Haines, D.M.; Majak, W.; Karren, D.; Yanke, L.J.; McAllister, T.A. Characterization of Acute Interstitial Pneumonia in Cattle in Southern Alberta Feedyards. Can. Vet. J. 2000, 41, 547–554. [Google Scholar]
- Woolums, A.R.; Loneragan, G.H.; Hawkins, L.L.; Williams, S.M. A Survey of the Relationship between Management Practices and Risk of Acute Interstitial Pneumonia at US Feedlots. Bov. Pract. 2005, 39, 125–133. [Google Scholar] [CrossRef]
- Gregory, N.G. Animal Welfare and Meat Science; CAB International: Wallingford, UK, 1998; ISBN 978-0-85199-296-9. [Google Scholar]
- Elder, D.; Bolton, D.; Dempster, A.; Taylor, B.; Broadbent, R. Pathophysiology of Overheating in a Piglet Model: Findings Compared with Sudden Infant Death Syndrome. J. Paediatr. Child Health 1996, 32, 113–119. [Google Scholar] [CrossRef]
- Hales, J.R.S. Effects of Exposure to Hot Environments on the Regional Distribution of Blood Flow and on Cardiorespiratory Function in Sheep. Pflugers Arch. 1973, 344, 133–148. [Google Scholar] [CrossRef]
- Garofolo, S.Q.; Watson, C.; Bianco, R.W.; Robinson, N.A. Cases of Sudden Death in a Sheep Model Implanted with Aortic Bioprostheses. J. Vet. Sci. Med. 2014, 2, 4. [Google Scholar]
- Xie, S. The Physiology and Pathology of Heat Stress in Australian Desert Birds. Ph.D. Thesis, The University of Adelaide, Adelaide, Australia, December 2018. [Google Scholar]
- Shandilya, U.K.; Sharma, A.; Sodhi, M.; Mukesh, M. Heat Stress Modulates Differential Response in Skin Fibroblast Cells of Native Cattle (Bos Indicus) and Riverine Buffaloes (Bubalus Bubalis). Biosci. Rep. 2020, 40, BSR20191544. [Google Scholar] [CrossRef] [PubMed]
- Hussain, A.M. The Histological Relationship between the Environmental Stress and Milk Production in Iraqi Buffaloes; Bubalus Bubalis. J. Entomol. Zool. Stud. 2016, 4, 19–22. [Google Scholar]
- Cheville, N.F. Introduction to Veterinary Pathology, 2nd ed.; Iowa State University Press: Ames, IA, USA, 1999. [Google Scholar]
- Huang, S.C.; Fu, Y.F.; Lan, Y.F.; Rehman, M.U.; Tong, Z.X. Histopathological and Biochemical Evaluations of the Kidney Inbroiler Chickens under Acute Heat Stress Conditions. Indian J. Anim. Res. 2017, 52, 637–639. [Google Scholar] [CrossRef]
- Miyamoto, K.; Suzuki, K.; Ohtaki, H.; Nakamura, M.; Yamaga, H.; Yagi, M.; Honda, K.; Hayashi, M.; Dohi, K. A Novel Mouse Model of Heatstroke Accounting for Ambient Temperature and Relative Humidity. J. Intensive Care 2021, 9, 35. [Google Scholar] [CrossRef]
- Koch, F.; Thom, U.; Albrecht, E.; Weikard, R.; Nolte, W.; Kuhla, B.; Kuehn, C. Heat Stress Directly Impairs Gut Integrity and Recruits Distinct Immune Cell Populations into the Bovine Intestine. Proc. Natl. Acad. Sci. USA 2019, 116, 10333–10338. [Google Scholar] [CrossRef]
- Zhu, L.; Liao, R.; Wu, N.; Zhu, G.; Yang, C. Heat Stress Mediates Changes in Fecal Microbiome and Functional Pathways of Laying Hens. Appl. Microbiol. Biotechnol. 2019, 103, 461–472. [Google Scholar] [CrossRef]
- Chaidanya, K.; Soren, N.M.; Sejian, V.; Bagath, M.; Manjunathareddy, G.B.; Kurien, E.K.; Varma, G.; Bhatta, R. Impact of Heat Stress, Nutritional Stress and Combined (Heat and Nutritional) Stresses on Rumen Associated Fermentation Characteristics, Histopathology and HSP70 Gene Expression in Goats. JABB 2017, 5, 36–48. [Google Scholar] [CrossRef]
- Yu, J.; Yin, P.; Liu, F.; Cheng, G.; Guo, K.; Lu, A.; Zhu, X.; Luan, W.; Xu, J. Effect of Heat Stress on the Porcine Small Intestine: A Morphological and Gene Expression Study. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 2010, 156, 119–128. [Google Scholar] [CrossRef]
- Baumgard, L.H.; Rhoads, R.P. Effects of Heat Stress on Postabsorptive Metabolism and Energetics. Annu. Rev. Anim. Biosci. 2013, 1, 311–337. [Google Scholar] [CrossRef]
- Wang, L.; Xue, B.; Wang, K.; Li, S.; Li, Z. Effect of Heat Stress on Endotoxin Flux across Mesenteric-Drained and Portal-Drained Viscera of Dairy Goat: Effect of Heat Stress on Endotoxin Absorption. J. Anim. Physiol. Anim. Nutr. 2011, 95, 468–477. [Google Scholar] [CrossRef]
- Contreras-Jodar, A.; Nayan, N.H.; Hamzaoui, S.; Caja, G.; Salama, A.A.K. Heat Stress Modifies the Lactational Performances and the Urinary Metabolomic Profile Related to Gastrointestinal Microbiota of Dairy Goats. PLoS ONE 2019, 14, e0202457. [Google Scholar] [CrossRef] [PubMed]
- Pearce, S.C.; Mani, V.; Weber, T.E.; Rhoads, R.P.; Patience, J.F.; Baumgard, L.H.; Gabler, N.K. Heat Stress and Reduced Plane of Nutrition Decreases Intestinal Integrity and Function in Pigs. J. Anim. Sci. 2013, 91, 5183–5193. [Google Scholar] [CrossRef] [PubMed]
- Sakurada, S.; Hales, J.R.S. A Role for Gastrointestinal Endotoxins in Enhancement of Heat Tolerance by Physical Fitness. J. Appl. Physiol. 1998, 84, 207–214. [Google Scholar] [CrossRef] [PubMed]
- Leon, L.R.; Helwig, B.G. Heat Stroke: Role of the Systemic Inflammatory Response. J. Appl. Physiol. 2010, 109, 1980–1988. [Google Scholar] [CrossRef]
- Guo, Z.; Gao, S.; Ding, J.; He, J.; Ma, L.; Bu, D. Effects of Heat Stress on the Ruminal Epithelial Barrier of Dairy Cows Revealed by Micromorphological Observation and Transcriptomic Analysis. Front. Genet. 2022, 12, 768209. [Google Scholar] [CrossRef]
- Quinteiro-Filho, W.M.; Rodrigues, M.V.; Ribeiro, A.; Ferraz-de-Paula, V.; Pinheiro, M.L.; Sá, L.R.M.; Ferreira, A.J.P.; Palermo-Neto, J. Acute Heat Stress Impairs Performance Parameters and Induces Mild Intestinal Enteritis in Broiler Chickens: Role of Acute Hypothalamic-Pituitary-Adrenal Axis Activation. J. Anim. Sci. 2012, 90, 1986–1994. [Google Scholar] [CrossRef]
- Burkholder, K.M.; Thompson, K.L.; Einstein, M.E.; Applegate, T.J.; Patterson, J.A. Influence of Stressors on Normal Intestinal Microbiota, Intestinal Morphology, and Susceptibility to Salmonella Enteritidis Colonization in Broilers. Poult. Sci. 2008, 87, 1734–1741. [Google Scholar] [CrossRef]
- Xiong, Y.; Cao, S.; Xiao, H.; Wu, Q.; Yi, H.; Jiang, Z.; Wang, L. Alterations in Intestinal Microbiota Composition Coincide with Impaired Intestinal Morphology and Dysfunctional Ileal Immune Response in Growing-Finishing Pigs under Constant Chronic Heat Stress. J. Animal. Sci. Biotechnol. 2022, 13, 1. [Google Scholar] [CrossRef]
- Kim, D.-H.; Kim, M.-H.; Kim, S.-B.; Son, J.-K.; Lee, J.-H.; Joo, S.-S.; Gu, B.-H.; Park, T.; Park, B.-Y.; Kim, E.-T. Differential Dynamics of the Ruminal Microbiome of Jersey Cows in a Heat Stress Environment. Animals 2020, 10, 1127. [Google Scholar] [CrossRef]
- Yadav, B.; Yadav, P.; Kumar, M.; Vasvani, S.; Anand, M.; Kumar, A.; Swain, D.K.; Yadav, S.; Madan, A.K. Effect of Heat Stress on Rumen Microbial Diversity and Fermentation Pattern in Buffalo. Adv. Gut Microbiome Res. 2022, 2022, 1–14. [Google Scholar] [CrossRef]
- Duffy, E.M.; Tietze, S.M.; Knoell, A.L.; Aluthge, N.D.; Fernando, S.C.; Schmidt, T.S.; Yates, D.T.; Petersen, J.L. Rumen Bacterial Composition in Lambs Is Affected by β-Adrenergic Agonist Supplementation and Heat Stress at the Phylum Level. Transl. Anim. Sci. 2018, 2, S145–S148. [Google Scholar] [CrossRef] [PubMed]
- Zhong, S.; Ding, Y.; Wang, Y.; Zhou, G.; Guo, H.; Chen, Y.; Yang, Y. Temperature and Humidity Index (THI)-Induced Rumen Bacterial Community Changes in Goats. Appl. Microbiol. Biotechnol. 2019, 103, 3193–3203. [Google Scholar] [CrossRef] [PubMed]
- Qu, H. Mechanism of Adipose Tissue Specific Response to Heat Stress in Pigs. Ph.D. Thesis, Purdue University, West Lafayette, IN, USA, May 2018. [Google Scholar]
- Califf, R.M. Biomarker Definitions and Their Applications. Exp. Biol. Med. 2018, 243, 213–221. [Google Scholar] [CrossRef] [PubMed]
- Ramos-Vara, J.A. Technical Aspects of Immunohistochemistry. Vet. Pathol. 2005, 42, 405–426. [Google Scholar] [CrossRef] [PubMed]
- Yan, J.; Bao, E.; Yu, J. Heat Shock Protein 60 Expression in Heart, Liver and Kidney of Broilers Exposed to High Temperature. Res. Vet. Sci. 2009, 86, 533–538. [Google Scholar] [CrossRef]
- De Matos, L.L.; Trufelli, D.C.; De Matos, M.G.L.; Da Silva Pinhal, M.A. Immunohistochemistry as an Important Tool in Biomarkers Detection and Clinical Practice. Biomark. Insights 2010, 5, 9–20. [Google Scholar] [CrossRef]
- Sejian, V.; Bagath, M.; Krishnan, G.; Rashamol, V.P.; Pragna, P.; Devaraj, C.; Bhatta, R. Genes for Resilience to Heat Stress in Small Ruminants: A Review. Small Rumin. Res. 2019, 173, 42–53. [Google Scholar] [CrossRef]
- Jiang, S.; Mohammed, A.A.; Jacobs, J.A.; Cramer, T.A.; Cheng, H.W. Effect of Synbiotics on Thyroid Hormones, Intestinal Histomorphology, and Heat Shock Protein 70 Expression in Broiler Chickens Reared under Cyclic Heat Stress. Poult. Sci. 2020, 99, 142–150. [Google Scholar] [CrossRef]
- Yu, J.; Bao, E. Effect of Acute Heat Stress on Heat Shock Protein 70 and Its Corresponding MRNA Expression in the Heart, Liver, and Kidney of Broilers. Asian Australas. J. Anim. Sci. 2008, 21, 1116–1126. [Google Scholar] [CrossRef]
- Ali, A.M.; Dalab, A.S.; Althnaian, T.A.; Alkhodair, K.M.; Al-Ramadan, S.Y. Molecular Investigations of the Effect of Thermal Manipulation during Embryogenesis on Muscle Heat Shock Protein 70 and Thermotolerance in Broiler Chickens. Rev. Bras. Zootec. 2022, 51, e20210011. [Google Scholar] [CrossRef]
- Zou, S.; Liu, P.; Yu, S.; Cui, Y.; He, J.; Afedo, S.Y.; Zhang, H.; Niayale, R.; Zhao, K. Heat Shock Protein 60 Expression and Localisation in Different Tissues and Testis Development of Male Cattle (Cattle-Yak and Yak). Folia Morphol. 2021, 80, 857–869. [Google Scholar] [CrossRef] [PubMed]
- Liang, W.; Lu, B.; Liang, C.; Yu, Z.; Xie, X.; Chen, Z. Effects of Heat Stress on the Development of GABAergic Neurons in the HPG Axis of Wenchang Chicks. Braz. J. Poult. Sci. 2019, 21, eRBCA-2018-0954. [Google Scholar] [CrossRef]
- Lu, B.; Liang, W.; Liang, C.; Yu, Z.; Xie, X.; Chen, Z. Effect of Heat Stress on Expression of Main Reproductive Hormone in Hypothalamic-Pituitary-Gonadal Axis of Wenchang Chicks. Braz. J. Poult. Sci. 2021, 23, eRBCA-2019-1056. [Google Scholar] [CrossRef]
- Lei, L.; Yu, J.; Bao, E. Expression of Heat Shock Protein 90 (Hsp90) and Transcription of Its Corresponding MRNA in Broilers Exposed to High Temperature. Br. Poult. Sci. 2009, 50, 504–511. [Google Scholar] [CrossRef]
- Lu, Z.; Ma, Y.; Li, Q.; Liu, E.; Jin, M.; Zhang, L.; Wei, C. The Role of N6-Methyladenosine RNA Methylation in the Heat Stress Response of Sheep (Ovis Aries). Cell Stress Chaperones 2019, 24, 333–342. [Google Scholar] [CrossRef]
- Terim Kapakin, K.; İmik, H.; Gumus, R.; Kapakin, S.; Sağlam, Y. Effect of Vit E on Secretion of HSP-70 in Testes of Broilers Exposed to Heat Stress. Kafkas Univ. Vet. Fak. Derg. 2013, 19, 305–310. [Google Scholar] [CrossRef]
- Liu, P.; Yu, S.; Cui, Y.; He, J.; Zhang, Q.; Liu, J.; Song, L.; Xu, Y.; Wang, T.; Zou, S.; et al. Regulation by HSP70/90 in the Different Tissues and Testis Development of Male Cattle (Cattle-Yak and Yak). bioRxiv 2018, 393371. [Google Scholar] [CrossRef]
- Pennarossa, G.; Maffei, S.; Rahman, M.M.; Berruti, G.; Brevini, T.A.L.; Gandolfi, F. Characterization of the Constitutive Pig Ovary Heat Shock Chaperone Machinery and Its Response to Acute Thermal Stress or to Seasonal Variations. Biol. Reprod. 2012, 87, 119. [Google Scholar] [CrossRef]
- Hamilton, T.R.D.S.; Siqueira, A.F.P.; de Castro, L.S.; Mendes, C.M.; Delgado, J.D.C.; de Assis, P.M.; Mesquita, L.P.; Maiorka, P.C.; Nichi, M.; Goissis, M.D.; et al. Effect of Heat Stress on Sperm DNA: Protamine Assessment in Ram Spermatozoa and Testicle. Oxid. Med. Cell. Longev. 2018, 2018, 5413056. [Google Scholar] [CrossRef]
- Xi, H.; Fan, X.; Zhang, Z.; Liang, Y.; Li, Q.; He, J. Bax and Bcl-2 Are Involved in the Apoptosis Induced by Local Testicular Heating in the Boar Testis. Reprod. Dom. Anim. 2017, 52, 359–365. [Google Scholar] [CrossRef]
- Yousef, M.S.; Megahed, G.A.; Abozed, G.F.; Hayder, M.; Abd-Elhafeez, H.H.; Rawy, M.S. Exogenous Gonadotropin-Releasing Hormone Counteracts the Adverse Effect of Scrotal Insulation on Testicular Functions in Bucks. Sci. Rep. 2022, 12, 7869. [Google Scholar] [CrossRef] [PubMed]
- Fan, X.; Xi, H.; Zhang, Z.; Liang, Y.; Li, Q.; He, J. Germ Cell Apoptosis and Expression of Bcl-2 and Bax in Porcine Testis under Normal and Heat Stress Conditions. Acta Histochem. 2017, 119, 198–204. [Google Scholar] [CrossRef] [PubMed]
- Xun, W.; Shi, L.; Cao, T.; Zhao, C.; Yu, P.; Wang, D.; Hou, G.; Zhou, H. Dual Functions in Response to Heat Stress and Spermatogenesis: Characterization of Expression Profile of Small Heat Shock Proteins 9 and 10 in Goat Testis. BioMed Res. Int. 2015, 2015, 686239. [Google Scholar] [CrossRef] [PubMed]
- Khan, A.; Khan, M.Z.; Dou, J.; Umer, S.; Xu, H.; Sammad, A.; Zhu, H.-B.; Wang, Y. RNAi-Mediated Silencing of Catalase Gene Promotes Apoptosis and Impairs Proliferation of Bovine Granulosa Cells under Heat Stress. Animals 2020, 10, 1060. [Google Scholar] [CrossRef] [PubMed]
- Huo, C.; Xiao, C.; She, R.; Liu, T.; Tian, J.; Dong, H.; Tian, H.; Hu, Y. Chronic Heat Stress Negatively Affects the Immune Functions of Both Spleens and Intestinal Mucosal System in Pigs through the Inhibition of Apoptosis. Microb. Pathog. 2019, 136, 103672. [Google Scholar] [CrossRef]
- Kum, S.; Eren, U.; Korkmaz, D.; Sandikci, M.; Aydemir, I. Effects of Vitamin E on T Cells in Gut-Associated Lymphoid Tissue (GALT) of Broiler Chickens under Heat Stress. Vet. Ir Zootech. 2015, 71, 39–47. [Google Scholar]
- Tang, S.; Yin, B.; Xu, J.; Bao, E. Rosemary Reduces Heat Stress by Inducing CRYAB and HSP70 Expression in Broiler Chickens. Oxid. Med. Cell. Longev. 2018, 2018, 7014126. [Google Scholar] [CrossRef]
- Kapakin, T.; Kübra, K.; Recep, R.; Halit, H.; Samet, S.; Selim, Y. Effects of ascorbic and α-lipoic acid on secretion of HSP- 70 and apoptosis in liver and kidneys of broilers exposed to heat stress. Ank. Üniv. Vet. Fakültesi Derg. 2012, 59, 279–287. [Google Scholar] [CrossRef]
- Alves, V.L.F.; Braga, W.M.T.; Zanatta, D.B.; de Carvalho, F.; de Lourdes Chauffaille, M.; Strauss, B.E.; Colleoni, G.W.B. Functional Studies of Hsp70 Gene Expression In Multiple Myeloma Cell Lines Suggest That This Gene Inhibition Can Be a Potential Therapeutic Target, Independent Of 17p/P53 Status. Blood 2013, 122, 3771. [Google Scholar] [CrossRef]
- Sugito, S.; Etriwati, E.; Akmal, M.; Rahmi, E.; Delima, M.; Muchlisin, Z.A.; Hasan, D.I. Immunohistochemical Expression of AQP2 and HSP70 in Broiler Kidney Tissue Treated with Salix Tetrasperma Roxb. Extract under Heat Exposure. Sci. World J. 2021, 2021, 8711286. [Google Scholar] [CrossRef]
- Yu, Z.; Tian, J.; Wen, J.; Chen, Z. Effects of Heat Stress on Expression of Heat Shock Proteins in the Small Intestine of Wenchang Chicks. Braz. J. Poult. Sci. 2021, 23, eRBCA-2020-1430. [Google Scholar] [CrossRef]
- Liu, L.; Fu, C.; Yan, M.; Xie, H.; Li, S.; Yu, Q.; He, S.; He, J. Resveratrol Modulates Intestinal Morphology and HSP70/90, NF-ΚB and EGF Expression in the Jejunal Mucosa of Black-Boned Chickens on Exposure to Circular Heat Stress. Food Funct. 2016, 7, 1329–1338. [Google Scholar] [CrossRef] [PubMed]
- Wu, D.; Xu, J.; Song, E.; Tang, S.; Zhang, X.; Kemper, N.; Hartung, J.; Bao, E. Acetyl Salicylic Acid Protected against Heat Stress Damage in Chicken Myocardial Cells and May Associate with Induced Hsp27 Expression. Cell Stress Chaperones 2015, 20, 687–696. [Google Scholar] [CrossRef]
- Debbarma, S.; Ludri, A.; Saini, S.; Devi, P.; Kumar, G. Expression of AQP1, AQP3, AQP4 and AQP5 in Upper Respiratory Tract of Buffaloes during Different Seasons. Biol. Rhythm Res. 2022, 53, 1660–1669. [Google Scholar] [CrossRef]
- Mazzoni, M.; Zampiga, M.; Clavenzani, P.; Lattanzio, G.; Tagliavia, C.; Sirri, F. Effect of Chronic Heat Stress on Gastrointestinal Histology and Expression of Feed Intake-Regulatory Hormones in Broiler Chickens. Animal 2022, 16, 100600. [Google Scholar] [CrossRef] [PubMed]
- Victoria Sanz Fernandez, M.; Johnson, J.S.; Abuajamieh, M.; Stoakes, S.K.; Seibert, J.T.; Cox, L.; Kahl, S.; Elsasser, T.H.; Ross, J.W.; Clay Isom, S.; et al. Effects of Heat Stress on Carbohydrate and Lipid Metabolism in Growing Pigs. Physiol. Rep. 2015, 3, e12315. [Google Scholar] [CrossRef]
- Saadeldin, I.M.; Swelum, A.A.-A.; Elsafadi, M.; Mahmood, A.; Osama, A.; Shikshaky, H.; Alfayez, M.; Alowaimer, A.N.; Magdeldin, S. Thermotolerance and Plasticity of Camel Somatic Cells Exposed to Acute and Chronic Heat Stress. J. Adv. Res. 2020, 22, 105–118. [Google Scholar] [CrossRef]
- Gaughan, J.B.; Bonner, S.L.; Loxton, I.; Mader, T.L. Effects of Chronic Heat Stress on Plasma Concentration of Secreted Heat Shock Protein 70 in Growing Feedlot Cattle. J. Anim. Sci. 2013, 91, 120–129. [Google Scholar] [CrossRef]
- Shen, H.; Fan, X.; Zhang, Z.; Xi, H.; Ji, R.; Liu, Y.; Yue, M.; Li, Q.; He, J. Effects of Elevated Ambient Temperature and Local Testicular Heating on the Expressions of Heat Shock Protein 70 and Androgen Receptor in Boar Testes. Acta Histochem. 2019, 121, 297–302. [Google Scholar] [CrossRef]
- Zhang, X.; Zhu, H.; Qian, Z.; Tang, S.; Wu, D.; Kemper, N.; Hartung, J.; Bao, E. The Association of Hsp90 Expression Induced by Aspirin with Anti-Stress Damage in Chicken Myocardial Cells. J. Vet. Sci. 2016, 17, 35. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).