Fermentation Characteristics and Nutritional Value of Avena sativa Genotypes Ensiled with or without Napier Grass (Pennisetum purpureum)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Site
2.2. Planting and Harvesting of Experimental Forages
2.3. Forage Preparation and Ensiling
2.4. Parameters of Treatment Silages Evaluation
2.5. Chemical Analysis
2.6. Statistical Analysis
3. Results
3.1. Chemical Composition of Forage and Treatment Silage
3.2. Silage pH, Temperature, and Dry Matter Loss
3.3. Physical Properties
3.4. Change in Dry Matter and Nutrient Composition
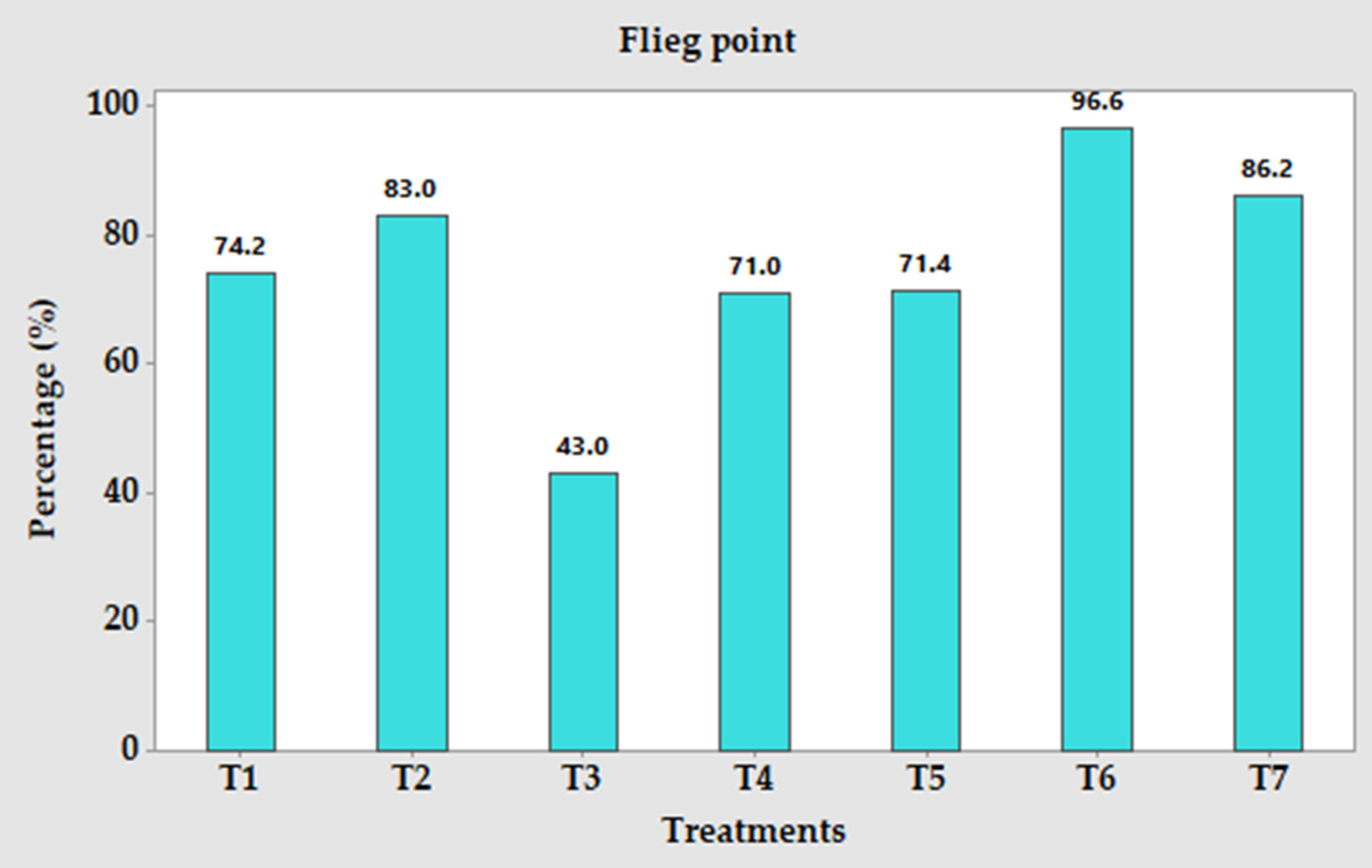
3.5. Flieg Score of Silage from A. sativa Genotypes with or without P. purpureum 16791 with 3% Added Molasses
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Rating Scores | Smell | Color | Texture | Moldiness | pH |
|---|---|---|---|---|---|
| 1. (Bad) | Rancid and musty smell/pungent | Dark/deep brown | Putrefactive and agglutinative | Highly moldy | >5.0 |
| 2. (Moderate) | Irritative/offensive; alcohol, acidic | Brown (Medium) | Slightly viscous/slimy | Medium | 4.4–5.0 |
| 3. (Good) | Light acidic (pleasant) | Brown, yellow | Medium (loose, soft, and firm) | Slightly moldy | 4.1–4.3 |
| 4. (Excellent) | Pleasant and sweet-acidic (very pleasant) | Light/greenish yellow/Olive green | Loose and soft, Firm | Without mold | ≤4.0 |
References
- IFAD Policy Reference Group on Climate Change; Rahman, A. Climate Change and the Future of Smallholder Agriculture. PLoS ONE 2008, 3, e25021. [Google Scholar]
- Anele, U.Y.; Südekum, K.H.; Hummel, J.; Arigbede, O.M.; Oni, A.O.; Olanite, J.A.; Böttger, C.; Ojo, V.O.; Jolaosho, A.O. Chemical Characterization, in Vitro Dry Matter and Ruminal Crude Protein Degradability and Microbial Protein Synthesis of Some Cowpea (Vigna unguiculata L. Walp) Haulm Varieties. Anim. Feed Sci. Technol. 2011, 163, 161–169. [Google Scholar] [CrossRef]
- Alemayehu, M.; Yohannes, F.; Dubale, P. Effect of Indigenous Stone Bunding (Kab) on Crop Yield at Mesobit-Gedeba, North Shoa, Ethiopia. Land Degrad. Dev. 2006, 17, 45–54. [Google Scholar] [CrossRef]
- McDonald, R.P.; Ho, M.H.R. Principles and Practice in Reporting Structural Equation Analyses. Psychol. Methods 2002, 7, 64–82. [Google Scholar] [CrossRef] [PubMed]
- Ososanya, T.O.; Olorunnisomo, O.A. Silage Characteristics and Preference of Sheep for Wet Brewer’s Grain Ensiled with Maize Cob. Livest. Res. Rural Dev. 2015, 27. Available online: https://www.lrrd.org/lrrd27/1/osos27012.htm (accessed on 17 November 2022).
- Moran, B.J. Making Quality Silage. In Tropical Dairy Farming: Feeding Management for Small Holder Dairy Farmers in the Humid Tropics; Landlinks Press: Collingwood, Australia, 2005. [Google Scholar]
- Filya, I.; Ashbell, G.; Hen, Y.; Weinberg, Z.G. The Effect of Bacterial Inoculants on the Fermentation and Aerobic Stability of Whole Crop Wheat Silage. Anim. Feed Sci. Technol. 2000, 88, 39–46. [Google Scholar] [CrossRef]
- Ozata, E. The Determination of Silage Yield and Quality Traits of Candidate Maize Hybrids. Ekin J. Crop Breed. Genet. 2018, 4, 31–40. [Google Scholar]
- Juskiw, P.E.; Helm, J.H.; Salmon, D.F. Forage Yield and Quality for Monocrops and Mixtures of Small Grain Cereals. Crop Sci. 2000, 40, 138–147. [Google Scholar] [CrossRef]
- Ahmad, M.; Dar, Z.A.; Habib, M. A Review on Oat (Avena sativa L.) as a Dual-Purpose Crop. Sci. Res. Essays 2014, 9, 52–59. [Google Scholar] [CrossRef] [Green Version]
- Wallsten, J.; Bertilsson, J.; Nadeau, E.; Martinsson, K. Digestibility of Whole-Crop Barley and Oat Silages in Dairy Heifers. Animal 2010, 4, 432–438. [Google Scholar] [CrossRef] [Green Version]
- Shao, T.; Wang, T.; Shimojo, M.; Masuda, Y. Effect of Ensiling Density on Fermentation Quality of Guineagrass (Panicum maximum Jacq.) Silage during the Early Stage of Ensiling. Asian-Australas. J. Anim. Sci. 2005, 18, 1273–1278. [Google Scholar] [CrossRef]
- Yin, X.; Tian, J.; Zhang, J. Effects of Re-Ensiling on the Fermentation Quality and Microbial Community of Napier Grass (Pennisetum purpureum) Silage. J. Sci. Food Agric. 2021, 101, 5028–5037. [Google Scholar] [CrossRef] [PubMed]
- Kozloski, G.V.; Perottoni, J.; Sanchez, L.M.B. Influence of Regrowth Age on the Nutritive Value of Dwarf Elephant Grass Hay (Pennisetum purpureum Schum. Cv. Mott) Consumed by Lambs. Anim. Feed Sci. Technol. 2005, 119, 1–11. [Google Scholar] [CrossRef]
- Abebe, A.; Kuang, Q.F.; Evans, J.; Robinson, W.E.; Sugumaran, M. Oxidative Transformation of a Tunichrome Model Compound Provides New Insight into the Crosslinking and Defense Reaction of Tunichromes. Bioorg. Chem. 2017, 71, 219–229. [Google Scholar] [CrossRef] [PubMed]
- Jimma Metrology Station (JMS). Jimma Metrology Station, Weather Data Base Generation, and Management for Bonga Agricultural Research Center Agricultural Research System Information Accessing; JMS: Jimma, Ethiopia, 2022. [Google Scholar]
- dos Santos, R.J.C.; Lira, M.d.A.; Guim, A.; dos Santos, M.V.F.; Dubeux, J.C.B.; de Mello, A.C.d.L. Elephant Grass Clones for Silage Production. Sci. Agric. 2013, 70, 6–11. [Google Scholar] [CrossRef] [Green Version]
- Muck, R.E. Revista Brasileira de Zootecnia Silage Microbiology and Its Control through Additives. Rev. Bras. Zootec. 2010, 39, 183–191. [Google Scholar] [CrossRef] [Green Version]
- Guimarã, K.; da Silveira, T.C.; Ribeiro, K.G.; Roseira, J.P.S.; Alves, W.S.; Dos Anjos, A.J.; Coutinho, D.N.; Freitas, C.A.S.; Pereira, O.G. Cutting Time and Regrowth Age Affect the Quality of Elephant Grass Silage. J. Agric. Stud. 2021, 9, 64. [Google Scholar] [CrossRef]
- Yahaya, M.S.; Goto, M.; Yimiti, W.; Smerjai, B.; Kawamoto, Y. Evaluation of Fermentation Quality of a Tropical and Temperate Forage Crops Ensiled with Additives of Fermented Juice of Epiphytic Lactic Acid Bacteria (FJLB). Asian-Australas. J. Anim. Sci. 2004, 17, 942–946. [Google Scholar] [CrossRef]
- Rong, H.; Yu, C.Q.; Li, Z.; Shimojo, M.; Shao, T. Evaluation of fermentation dynamics and structural carbohydrate degradation of Napiergrass ensiled with additives of urea and molasses. Pak. Vet. J. 2013, 33, 374–377. [Google Scholar]
- da Silva, A.R.P.; Dias, F.J.; Rufino, J.P.F.; Tanaka, E.d.S.; Lopes, M.M. Effect of Using Inoculant on Elephant Grass Silage with Additives. Acta Sci.—Anim. Sci. 2020, 42, e50533. [Google Scholar] [CrossRef]
- Rêgo, M.M.T.; Neiva, J.N.M.; Rêgo, A.C.D.; Cândido, M.J.D.; Carneiro, M.S.D.S.; Lôbo, R.N.B. Chemical and Bromatological Characteristics of Elephant Grass Silages Containing a Mango By-Product 1/Características Bromatológicas e Fermentativas de Silagens de Capim-Elefante Contendo Subproduto Da Manga. Rev. Bras. Zootec. 2010, 39, 81–87. [Google Scholar] [CrossRef] [Green Version]
- Abdullah, M.; Javed, K.; Jabbar, M.A.; Shahid, M.Q.; Jan, P.S.; Ramzan, M.; Khan, M.A.; Ahmad, M. Comparison of Silo Types on Chemical Composition and Physical Quality of Silage Made from Maize, Sorghum and Oats Fodders. JAPS J. Anim. Plant Sci. 2017, 27, 771–775. [Google Scholar]
- Danso, A.; Nartey, M.A. Nutritive Value of Napier Grass Ensiled Using Molasses as an Additive. Int. J. Eng. Sci. 2018, 7, 45–50. [Google Scholar] [CrossRef]
- Kilic, A. Determined of Quality in Roughage; Hasat Poblication: Istanbul, Turkey, 2006. [Google Scholar]
- Junga, P.; Trávníček, P. Povrchová Teplota Odkrytého Čelního Profilu Siláže Jako Rychlý Indikátor Procesu Rozkladu Kukuřičné Siláže. J. Cent. Eur. Agric. 2015, 16, 76–91. [Google Scholar] [CrossRef]
- Kung, L.; Shaver, R.D.; Grant, R.J.; Schmidt, R.J. Silage Review: Interpretation of Chemical, Microbial, and Organoleptic Components of Silages. J. Dairy Sci. 2018, 101, 4020–4033. [Google Scholar] [CrossRef] [PubMed]
- Paul, S.T.; Seglar, B. Fermentation Analysis and Silage Quality Testing; University of Minnesota: St. Paul, MN, USA, 2003. [Google Scholar]
- Cherney, J.H.; Cherney, D.J.R. Assessing Silage Quality. In Silage Science and Technology; Wiley: Hoboken, NJ, USA, 2015; pp. 141–198. [Google Scholar] [CrossRef]
- Association of Official Analytical Chemists (AOAC). Official Methods of Analysis of AOAC International; AOAC International: Arlington, VA, USA, 1995; Volume 16. [Google Scholar]
- Horst, E.H.; Neumann, M.; Mareze, J.; Leão, G.F.M.; Bumbieris Júnior, V.H.; Mendes, M.C. Nutritional Composition of Pre-Dried Silage of Different Winter Cereals. Acta Sci.—Anim. Sci. 2018, 40, e42500. [Google Scholar] [CrossRef] [Green Version]
- Van Soest, P.J.; Robertson, J.B.; Lewis, B.A. Methods for Dietary Fiber, Neutral Detergent Fiber, and Nonstarch Polysaccharides in Relation to Animal Nutrition. J. Dairy Sci. 1991, 74, 3583–3597. [Google Scholar] [CrossRef] [PubMed]
- Abate, A.L.; Mayer, M. Prediction of the Useful Energy in Tropical Feeds from Proximate Composition and In Vivo Derived Energetic Contents 1. Metabolisable Energy. Small Rumin. Res. 1997, 25, 51–59. [Google Scholar] [CrossRef]
- Pedroso, A.d.F.; de Andrade Rodrigues, A.; Barioni Júnior, W.; de Souza, G.B. Fermentation Parameters, Quality and Losses in Sugarcane Silages Treated with Chemical Additives and a Bacterial Inoculant. Rev. Bras. Zootec. 2011, 40, 2318–2322. [Google Scholar] [CrossRef] [Green Version]
- Zanine, A.d.M.; Santos, E.M.; Dórea, J.R.R.; Dantas, P.A.d.S.; da Silva, T.C.; Pereira, O.G. Evaluation of Elephant Grass Silage with the Addition of Cassava Scrapings. Rev. Bras. Zootec. 2010, 39, 2611–2616. [Google Scholar] [CrossRef] [Green Version]
- SAS Institute. Statistical Analysis System; SAS Institute: Cary, NC, USA, 2017. [Google Scholar]
- Knický, M. Possibilities to Improve Silage Conservation. Ph.D. Thesis, Swedish University of Agricultural Sciences, Uppsala, Sweden, 2005. [Google Scholar]
- McDonald, P.; Edwards, A.; Greenhalgh, J.F.D.; Morgan, C.A.; Sinclair, L.A.; Wilkinson, R.G. Animal Nutrition, 7th ed.; Prentice Hall: Hoboken, NJ, USA; Pearson Publishing Press Ltd.: London, UK, 2010; ISBN 0-582-21927-2. [Google Scholar]
- Kung, L.; Shaver, R. Interpretation and Use of Silage Fermentation Analysis Reports. Focus Forage 2004, 3, 1–5. [Google Scholar]
- McDonald, P.; Henderson, A.R.; Heron, S.J.E. The Biochemistry of Silage, 2nd ed.; Chalcombe Publications: Marlow, UK, 1991. [Google Scholar]
- Kung, L. Silage Temperatures: How Hot Is Too Hot; University of Delaware: Newark, DE, USA, 2011; pp. 3–4. [Google Scholar]
- Bolsen, K.K.; Ashbell, G.; Weinberg, Z. Silage Fermentation and Silage Additives. Asian Australas. J. Anim. Sci. 1996, 9, 483–493. [Google Scholar] [CrossRef]
- Li, M.; Fan, X.; Cheng, Q.; Chen, Y.; Long, J.; Lei, Y.; Li, P.; Chen, C. Effect of Amomum Villosum Essential Oil as an Additive on the Chemical Composition, Fermentation Quality, and Bacterial Community of Paper Mulberry Silage. Front. Microbiol. 2022, 13, 951958. [Google Scholar] [CrossRef]
- Gebrehanna, M.M.; Gordon, R.J.; Madani, A.; VanderZaag, A.C.; Wood, J.D. Silage Effluent Management: A Review. J. Environ. Manag. 2014, 143, 113–122. [Google Scholar] [CrossRef] [PubMed]
- Zhao, K.; Tung, C.; Eizenga, G.C.; Wright, M.H.; Ali, M.L.; Price, A.H.; Norton, G.J.; Islam, M.R.; Reynolds, A.; Mezey, J.; et al. Genome-wide association mapping reveals a rich genetic architecture of complex traits in Oryza sativa. Nat. Commun. 2011, 2, 467. [Google Scholar] [CrossRef] [PubMed]
- Xie, Q.; Xu, Z. Sustainable agriculture: From sweet sorghum planting and ensiling to ruminant feeding. Mol. Plant 2019, 12, 603–606. [Google Scholar] [CrossRef] [PubMed]
- Van Soest, P. Nutrition Ecology of Ruminants; Cornell University Press: Ithaca, NY, USA, 1994. [Google Scholar]
- Lima, R.; Díaz, R.F.; Castro, A.; Hoedtke, S.; Fievez, V. Multifactorial Models to Assess Responses to Sorghum Proportion, Molasses and Bacterial Inoculant on in Vitro Quality of Sorghum-Soybean Silages. Anim. Feed Sci. Technol. 2011, 164, 161–173. [Google Scholar] [CrossRef]
- Stanley, D.F. Feeding Silage to Sheep. In Top Fodder Successful Silage Production Manual; Wagga Wagga Agricultural Institute: New South Wales, Australia, 2004. [Google Scholar]
- Ball, D.M.; Colins, M.; Lacefield, G.D.; Martin, N.P.; Mertens, D.A.; Olson, K.E.; Putnam, D.H.; Undersander, D.J.; Wolf, M.W. Understanding Forage Quality; American Farm Bureau Federation Publication 1-01: Park Ridge, IL, USA, 2001; p. 17. [Google Scholar]
- Tauqir, N.A.; Nisa, M.; Sarwar, M.; Bhatti, S.A. Impact of Varying Moisture Levels, Different Additives and Fermentation Periods on Nutritive Value of Leguminous and Non-Leguminous Fodder Silages in Lactating Nili-Ravi Buffaloes. Pak. J. Agric. Sci. 2008, 45, 386–402. [Google Scholar]
- Fisher, D.S.; Burns, J.C. Quality Analysis of Summer-Annual Forages. II. Effects of Forage Carbohydrate Constituents on Silage Fermentation. Agron. J. 1987, 79, 242–248. [Google Scholar] [CrossRef]
| Treatments |
|---|
| Sole Avena sativa SRCPX80AB2806 (T1) |
| Sole Avena sativa ILRI_5527A (T2) |
| Sole Avena sativa ILRI_5526A (T3) |
| Sole Pennisetum purpureum 16791 (T4) |
| 50% Avena sativa SRCPX80AB2806 + 50% Pennisetum purpureum 16791 (T5) |
| 50% Avena sativa ILRI_5527A + 50% Pennisetum purpureum 16791 (T6) |
| 50% Avena sativa ILRI_5526A + 50% Pennisetum Purpureum 16791 (T7) |
| Treatments (n = 3) | DM | Ash | EE | OM | CP | NDF | ADF | ADL | ME |
|---|---|---|---|---|---|---|---|---|---|
| Avena sativa SRCPX80AB2806 | 91.33 | 6.14 | 1.97 | 93.86 | 11.46 | 42.70 | 19.28 | 3.43 | 8.44 |
| Avena sativa ILRI_5527A | 91.66 | 4.70 | 2.57 | 95.30 | 12.35 | 39.33 | 14.57 | 2.70 | 8.41 |
| Avena sativa ILRI_5526A | 91.25 | 7.22 | 1.28 | 92.78 | 9.97 | 51.11 | 21.88 | 4.02 | 8.30 |
| Pennisetum purpureum 16791 | 90.96 | 6.35 | 1.94 | 93.65 | 14.37 | 57.31 | 28.71 | 4.80 | 8.30 |
| Molasses | 72.43 | 13.38 | ND | 86.62 | 3.27 | 0.43 | 0.12 | 0.09 | 14 |
| T1 | 23.91 | 5.64 | 1.81 | 95.10 | 11.45 | 40.70 | 18.28 | 3.23 | 9.75 |
| T2 | 24.76 | 6.72 | 2.47 | 93.28 | 12.33 | 37.11 | 13.88 | 2.32 | 9.92 |
| T3 | 22.96 | 4.75 | 2.5 | 95.25 | 10.07 | 50.16 | 20.84 | 4.12 | 9.70 |
| T4 | 23.19 | 6.16 | 2.62 | 93.84 | 14.25 | 55.15 | 26.44 | 5.17 | 9.88 |
| T5 | 25.37 | 5.85 | 2.13 | 94.15 | 11.37 | 47.31 | 24.71 | 4.8 | 9.88 |
| T6 | 26.70 | 4.2 | 3.07 | 95.80 | 13.29 | 42.33 | 22.57 | 3.70 | 10.11 |
| T7 | 22.96 | 4.29 | 3.03 | 95.71 | 10.21 | 52.21 | 25.75 | 4.06 | 9.68 |
| Parameter | T1 | T2 | T3 | T4 | T5 | T6 | T7 | SE | p |
|---|---|---|---|---|---|---|---|---|---|
| pHi | 5.72 d | 5.72 d | 5.80 a | 5.74 b | 5.71 e | 5.70 f | 5.73 c | 0.01 | <0.0001 |
| pH 45th day | 4.71 d | 4.52 e | 5.01 a | 4.81 c | 4.22 f | 3.52 g | 4.91 b | 0.01 | <0.0001 |
| Temperature (°C) | 27.53 d | 26.70 e | 30.22 a | 28.60 c | 25.80 f | 25.00 g | 29.73 b | 0.20 | <0.0001 |
| TDML% | 2.47 d | 2.33 e | 2.55 b | 2.53 c | 2.23 f | 2.17 g | 2.67 a | 0.01 | <0.0001 |
| GL (%DM) | 4.18 d | 4.04 e | 4.55 a | 4.31 c | 3.94 f | 3.74 g | 4.35 b | 0.01 | <0.0001 |
| EL (kg/t FM) | 7.09 d | 6.38 e | 10.04 a | 8.52 c | 5.57 f | 4.28 g | 9.28 b | 0.20 | <0.0001 |
| DMRR (%) | 59.16 d | 70.60 c | 34.18 g | 49.16 e | 79.91 b | 96.30 a | 46.79 f | 0.42 | <0.0001 |
| Parameter | T1 | T2 | T3 | T4 | T5 | T6 | T7 | SE | p-Value |
|---|---|---|---|---|---|---|---|---|---|
| Smell | 3.00 c | 3.16 c | 2.00 e | 3.00 c | 3.50 b | 4.00 a | 2.50 d | 0.33 | <0.0001 |
| Color | 3.30 d | 3.50 c | 2.00 g | 3.10 e | 3.70 b | 4.00 a | 2.90 f | 0.05 | <0.0001 |
| Moldiness | 3.30 d | 3.50 c | 2.70 g | 3.10 e | 3.70 b | 3.93 a | 2.90 f | 0.06 | <0.0001 |
| Texture | 3.30 d | 3.50 c | 2.00 g | 3.10 e | 3.70 b | 4.00 a | 2.90 f | 0.05 | <0.0001 |
| Parameter | T1 | T2 | T3 | T4 | T5 | T6 | T7 | SE | p |
|---|---|---|---|---|---|---|---|---|---|
| DMi (FW) | 23.91 | 24.76 | 22.96 | 23.19 | 25.37 | 26.7 | 22.96 | ||
| DM (FW) | 21.00 d | 22.00 c | 19.00 f | 20.00 e | 23.00 b | 25.00 a | 21.00 e | 0.01 | <0.0001 |
| Ash | 4.64 d | 3.75 e | 5.72 a | 4.85 c | 3.29 f | 3.2 g | 5.16 b | 0.04 | <0.0001 |
| OM | 95.36 d | 96.25 c | 94.28 g | 95.15 e | 96.71 b | 96.80 a | 94.84 f | 0.04 | <0.0001 |
| EE | 2.72 d | 2.84 c | 2.03 g | 2.69 d | 3.28 b | 3.32 a | 2.35 f | 0.01 | <0.0001 |
| CP | 11.45 d | 12.32 c | 10.03 fg | 14.02 a | 11.25 ef | 13.25 b | 10.17 fg | 0.07 | <0.0001 |
| NDF | 39.70 f | 36.12 g | 49.15 C | 54.31 a | 46.21 d | 40.03 e | 51.15 b | 0.01 | <0.0001 |
| ADF | 17.28 e | 12.84 e | 20.88 c | 25.71 a | 24.74 b | 15.57 f | 19.44 d | 0.01 | <0.0001 |
| ADL | 3.06 e | 2.15 f | 4.06 c | 5.06 a | 4.6 b | 3.50 d | 4.01 c | 0.05 | <0.0001 |
| ME | 9.72 d | 9.89 b | 9.67 e | 9.85 c | 9.85 c | 10.07 a | 9.67 e | 0.005 | <0.0001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wodebo, K.Y.; Ejeta, T.T.; Cherkos, S.D.; Terefe, W.G.; Wamatu, J.N.A.; Equle, M.Z. Fermentation Characteristics and Nutritional Value of Avena sativa Genotypes Ensiled with or without Napier Grass (Pennisetum purpureum). Sustainability 2023, 15, 1260. https://doi.org/10.3390/su15021260
Wodebo KY, Ejeta TT, Cherkos SD, Terefe WG, Wamatu JNA, Equle MZ. Fermentation Characteristics and Nutritional Value of Avena sativa Genotypes Ensiled with or without Napier Grass (Pennisetum purpureum). Sustainability. 2023; 15(2):1260. https://doi.org/10.3390/su15021260
Chicago/Turabian StyleWodebo, Kibreab Yosefe, Taye Tolemariam Ejeta, Solomon Demeke Cherkos, Weyessa Garedew Terefe, Jane Nyaranga Ambuku Wamatu, and Muluken Zeleke Equle. 2023. "Fermentation Characteristics and Nutritional Value of Avena sativa Genotypes Ensiled with or without Napier Grass (Pennisetum purpureum)" Sustainability 15, no. 2: 1260. https://doi.org/10.3390/su15021260
APA StyleWodebo, K. Y., Ejeta, T. T., Cherkos, S. D., Terefe, W. G., Wamatu, J. N. A., & Equle, M. Z. (2023). Fermentation Characteristics and Nutritional Value of Avena sativa Genotypes Ensiled with or without Napier Grass (Pennisetum purpureum). Sustainability, 15(2), 1260. https://doi.org/10.3390/su15021260








