Review on Phytoremediation Potential of Floating Aquatic Plants for Heavy Metals: A Promising Approach
Abstract
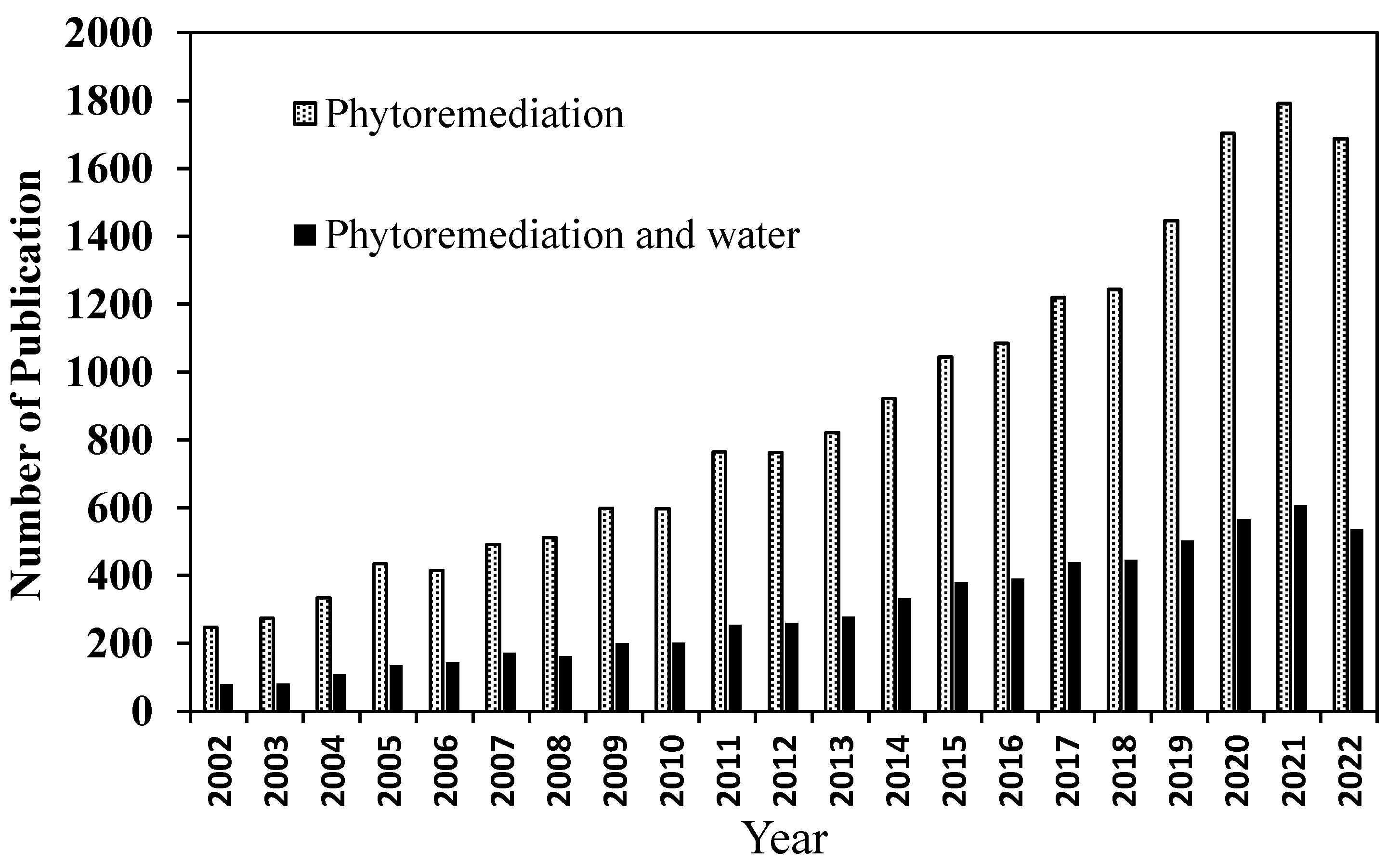
:1. Introduction
2. Types of Phytoremediation
2.1. Soil Remediation
2.2. Water Remediation
3. Classification of Phytoremediation Technique
4. Accumulator Aquatic Plants
4.1. Duckweed (Lemna minor)
4.2. Water Lettuce (Pistia stratiotes)
4.3. Water Hyacinth (Eichhornia crassipes)
4.4. Watermoss (Salvinia)
5. Phytoremediation Mechanisms Using Accumulator Aquatic Plants
5.1. Absorption, Adsorption, and Efflux of Metals by Plants
5.2. Bioconcentration, Translocation, and Distribution of Metals
5.3. Phytotoxicity of Heavy Metals in Plants
6. Phytoremediation Parameters and Kinetic Studies
6.1. Effect of Solution pH
6.2. Effect of Solution Temperature
6.3. Effect of Exposure Duration
6.4. Effect of Water Salinity
6.5. Effect of Initial Metal Concentration
6.6. Effect of Other Metals Concentration
6.7. Effect of Chelating Agent Addition
6.8. Kinetics of Phytoremediation
- = initial concentration of metal in water, mg/L
- = concentration of metal in water at time , mg/L
- = first-order uptake rate constant, day−1
- = sampling time, days
- = metal concentration at time t, mg/L
- = maximum concentration of absorbed metal, mg/L
- = initial metal concentration, mg/L
- = PFO kinetic rate constant, day−1
- = PSO kinetic rate constant, mg/L·day
- = sampling time, day
- = initial sampling time, day
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ali, S.; Abbas, Z.; Rizwan, M.; Zaheer, I.E.; Yavaş, İ.; Ünay, A.; Abdel-DAIM, M.M.; Bin-Jumah, M.; Hasanuzzaman, M.; Kalderis, D. Application of floating aquatic plants in phytoremediation of heavy metals polluted water: A review. Sustainability 2020, 12, 1927. [Google Scholar] [CrossRef] [Green Version]
- Cauberghe, V.; Vazquez-Casaubon, E.; Van de Sompel, D. Perceptions of water as commodity or uniqueness? The role of water value, scarcity concern and moral obligation on conservation behavior. J. Environ. Manag. 2021, 292, 112677. [Google Scholar] [CrossRef]
- Obinnaa, I.B.; Ebere, E.C. A Review: Water pollution by heavy metal and organic pollutants: Brief review of sources, effects and progress on remediation with aquatic plants. Anal. Methods Environ. Chem. J. 2019, 2, 5–38. [Google Scholar] [CrossRef] [Green Version]
- Afroz, R.; Masud, M.M.; Akhtar, R.; Duasa, J.B. Water pollution: Challenges and future direction for water resource management policies in malaysia. Environ. Urban. ASIA 2014, 5, 63–81. [Google Scholar] [CrossRef]
- Fletcher, J.; Willby, N.; Oliver, D.M.; Quilliam, R.S. Phytoremediation using aquatic plants. In Phytoremediation: In-Situ Applications; Shmaefsky, B.R., Ed.; Springer International Publishing: Cham, Switzerland, 2020; pp. 205–260. [Google Scholar]
- Gupta, A. Water Pollution—Sources, Effects and Control; Pointer Publishers: Jaipur, India, 2016. [Google Scholar]
- Anawar, H.; Chowdhury, R. Remediation of polluted river water by biological, chemical, ecological and engineering processes. Sustainability 2020, 12, 7017. [Google Scholar] [CrossRef]
- Rezania, S.; Ponraj, M.; Talaiekhozani, A.; Mohamad, S.E.; Md Din, M.F.; Taib, S.M.; Sabbagh, F.; Sairan, F.M. Perspectives of phytoremediation using water hyacinth for removal of heavy metals, organic and inorganic pollutants in wastewater. J. Environ. Manag. 2015, 163, 125–133. [Google Scholar] [CrossRef]
- Prasad, M.N.V. Prospects for manipulation of molecular mechanisms and transgenic approaches in aquatic macrophytes for remediation of toxic metals and metalloids in wastewaters. In Transgenic Plant Technology for Remediation of Toxic Metals and Metalloids; Elsevier: Amsterdam, The Netherlands, 2018; pp. 395–428. [Google Scholar]
- Sarwar, N.; Imran, M.; Shaheen, M.R.; Ishaque, W.; Kamran, M.A.; Matloob, A.; Rehim, A.; Hussain, S. Phytoremediation strategies for soils contaminated with heavy metals: Modifications and future perspectives. Chemosphere 2017, 171, 710–721. [Google Scholar] [CrossRef]
- Rahman, S.U.; Yasin, G.; Nawaz, M.F.; Cheng, H.; Azhar, M.F.; Riaz, L.; Javed, A.; Lu, Y. Evaluation of heavy metal phytoremediation potential of six tree species of Faisalabad city of Pakistan during summer and winter seasons. J. Environ. Manag. 2022, 320, 115801. [Google Scholar] [CrossRef]
- Yasin, G.; Ur Rahman, S.; Yousaf, M.T.B.; Azhar, M.F.; Zahid, D.M.; Imtiaz, M.; Hussain, B. Phytoremediation potential of E. camaldulensis and M. alba for copper, cadmium, and lead absorption in urban areas of Faisalabad City, Pakistan. Int. J. Environ. Res. 2021, 15, 597–612. [Google Scholar] [CrossRef]
- Tangahu, B.V.; Sheikh Abdullah, S.R.; Basri, H.; Idris, M.; Anuar, N.; Mukhlisin, M. A review on heavy metals (As, Pb, and Hg) uptake by plants through phytoremediation. Int. J. Chem. Eng. 2011, 2011, 939161. [Google Scholar] [CrossRef]
- Laghlimi, M.; Baghdad, B.; Hadi, H.E.; Bouabdli, A. Phytoremediation mechanisms of heavy metal contaminated soils: A review. Open J. Ecol. 2015, 5, 14. [Google Scholar] [CrossRef] [Green Version]
- Souza, L.A.; Piotto, F.A.; Nogueirol, R.C.; Azevedo, R.A. Use of non-hyperaccumulator plant species for the phytoextraction of heavy metals using chelating agents. Sci. Agric. 2013, 70, 290–295. [Google Scholar] [CrossRef] [Green Version]
- Shaari, N.E.M.; Tajudin, M.T.F.M.; Khandaker, M.M.; Majrashi, A.; Alenazi, M.M.; Abdullahi, U.A.; Mohd, K.S. Cadmium toxicity symptoms and uptake mechanism in plants: A review. Braz. J. Biol. 2022, 84, e252143. [Google Scholar] [CrossRef]
- Lasat, M.M. Phytoextraction of toxic metals: A review of biological mechanisms. J. Environ. Qual. 2002, 31, 109–120. [Google Scholar] [CrossRef] [Green Version]
- Verma, R.K.; Sankhla, M.S.; Jadhav, E.B.; Parihar, K.; Awasthi, K.K. Phytoremediation of heavy metals extracted from soil and aquatic environments: Current advances as well as emerging trends. Biointerface Res. Appl. Chem. 2022, 12, 5486–5509. [Google Scholar]
- Ansari, A.A.; Naeem, M.; Gill, S.S.; AlZuaibr, F.M. Phytoremediation of contaminated waters: An eco-friendly technology based on aquatic macrophytes application. Egypt. J. Aquat. Res. 2020, 46, 371–376. [Google Scholar] [CrossRef]
- Sharma, S.; Tiwari, S.; Hasan, A.; Saxena, V.; Pandey, L.M. Recent advances in conventional and contemporary methods for remediation of heavy metal-contaminated soils. 3 Biotech 2018, 8, 216. [Google Scholar] [CrossRef]
- Lakshmi, K.S.; Sailaja, V.H.; Reddy, M.A. Phytoremediation—A promising technique in waste water treatment. Int. J. Sci. Res. Manag. 2017, 5, 5480–5489. [Google Scholar] [CrossRef]
- Dixit, A.M.; Dixit, S.; Goswami, C.S. Process and plants for wastewater remediation: A review. Sci. Rev. Chem. Commun. 2011, 1, 71–77. [Google Scholar]
- Jeevanantham, S.; Saravanan, A.; Hemavathy, R.V.; Kumar, P.S.; Yaashikaa, P.R.; Yuvaraj, D. Removal of toxic pollutants from water environment by phytoremediation: A survey on application and future prospects. Environ. Technol. Innov. 2019, 13, 264–276. [Google Scholar] [CrossRef]
- Shen, X.; Dai, M.; Yang, J.; Sun, L.; Tan, X.; Peng, C.; Ali, I.; Naz, I. A critical review on the phytoremediation of heavy metals from environment: Performance and challenges. Chemosphere 2022, 291, 132979. [Google Scholar] [CrossRef]
- Gunarathne, V.; Gunatilake, S.R.; Wanasinghe, S.T.; Atugoda, T.; Wijekoon, P.; Biswas, J.K.; Vithanage, M. 7—Phytoremediation for E-waste contaminated sites. In Handbook of Electronic Waste Management; Prasad, M.N.V., Vithanage, M., Borthakur, A., Eds.; Butterworth-Heinemann: Oxford, UK, 2020; pp. 141–170. [Google Scholar]
- Ahalya, N.; Ramachandra, T.V. Phytoremediation: Processes and Mechanisms; Academic Press: Cambridge, MA, USA, 2004; Volume 18, pp. 33–38. [Google Scholar]
- Akpor, O.; Otohinoyi, D.; Olaolu, T.; Aderiye, J. Pollutants in wastewater effluents: Impacts and remediation processes. Int. J. Environ. Res. Earth Sci. 2014, 3, 50–59. [Google Scholar]
- Phearkeo, O. A Study on Removal of Heavy Metals from Wastewater by Floating Plants. Master’s Thesis, Master of Science (Engineering and Technology), Sirindhorn International Institute of Technology Thammasat University, Bangkok, Thailand, 2016. [Google Scholar]
- Rezania, S.; Taib, S.M.; Md Din, M.F.; Dahalan, F.A.; Kamyab, H. Comprehensive review on phytotechnology: Heavy metals removal by diverse aquatic plants species from wastewater. J. Hazard. Mater. 2016, 318, 587–599. [Google Scholar] [CrossRef] [PubMed]
- Ubuza, L.J.A.; Padero, P.C.S.; Nacalaban, C.M.N.; Tolentino, J.T.; Alcoran, D.C.; Tolentino, J.C.; Ido, A.L.; Mabayo, V.I.F.; Arazo, R.O. Assessment of the potential of duckweed (Lemna minor L.) in treating lead-contaminated water through phytoremediation in stationary and recirculated set-ups. Environ. Eng. Res. 2020, 25, 977–982. [Google Scholar] [CrossRef] [Green Version]
- Kumar, V.; Singh, J.; Pathak, V.V.; Ahmad, S.; Kothari, R. Experimental and kinetics study for phytoremediation of sugar mill effluent using water lettuce (Pistia stratiotes L.) and its end use for biogas production. 3 Biotech 2017, 7, 330. [Google Scholar] [CrossRef]
- Hua, J.; Zhang, C.; Yin, Y.; Chen, R.; Wang, X. Phytoremediation potential of three aquatic macrophytes in manganese-contaminated water. Water Environ. J. 2012, 26, 335–342. [Google Scholar] [CrossRef]
- Lu, Q.; He, Z.L.; Graetz, D.A.; Stoffella, P.J.; Yang, X. Uptake and distribution of metals by water lettuce (Pistia stratiotes L.). Environ. Sci. Pollut. Res. 2011, 18, 978–986. [Google Scholar] [CrossRef]
- Mishra, V.K.; Tripathi, B.D. Accumulation of chromium and zinc from aqueous solutions using water hyacinth (Eichhornia crassipes). J. Hazard. Mater. 2009, 164, 1059–1063. [Google Scholar] [CrossRef]
- Dhir, B.; Sharmila, P.; Pardha Saradhi, P. Photosynthetic performance of Salvinia natans exposed to chromium and zinc rich wastewater. Braz. J. Plant Physiol. 2008, 20, 61–70. [Google Scholar] [CrossRef]
- Tufaner, F. Post-treatment of effluents from UASB reactor treating industrial wastewater sediment by constructed wetland. Environ. Technol. 2020, 41, 912–920. [Google Scholar] [CrossRef]
- Basile, A.; Sorbo, S.; Conte, B.; Castaldo Cobianchi, R.; Trinchella, F.; Capasso, C.; Carginale, V. Toxicity, accumulation, and removal of heavy metals by three aquatic macrophytes. Int. J. Phytoremediat. 2012, 14, 374–387. [Google Scholar] [CrossRef] [PubMed]
- Aurangzeb, N.; Nisa, S.; Bibi, Y.; Javed, F.; Hussain, F. Phytoremediation potential of aquatic herbs from steel foundry effluent. Braz. J. Chem. Eng. 2014, 31, 881–886. [Google Scholar] [CrossRef] [Green Version]
- Abbas, Z.; Arooj, F.; Ali, S.; Zaheer, I.E.; Rizwan, M.; Riaz, M.A. Phytoremediation of landfill leachate waste contaminants through floating bed technique using water hyacinth and water lettuce. Int. J. Phytoremediat. 2019, 21, 1356–1367. [Google Scholar] [CrossRef] [PubMed]
- Saha, P.; Shinde, O.; Sarkar, S. Phytoremediation of industrial mines wastewater using water hyacinth. Int. J. Phytoremediat. 2017, 19, 87–96. [Google Scholar] [CrossRef] [Green Version]
- Bennicelli, R.; Stȩpniewska, Z.; Banach, A.; Szajnocha, K.; Ostrowski, J. The ability of Azolla caroliniana to remove heavy metals (Hg(II), Cr(III), Cr(VI)) from municipal waste water. Chemosphere 2004, 55, 141–146. [Google Scholar] [CrossRef]
- Du, Y.; Wu, Q.; Kong, D.; Shi, Y.; Huang, X.; Luo, D.; Chen, Z.; Xiao, T.; Leung, J.Y.S. Accumulation and translocation of heavy metals in water hyacinth: Maximising the use of green resources to remediate sites impacted by e-waste recycling activities. Ecol. Indic. 2020, 115, 106384. [Google Scholar] [CrossRef]
- Greger, M. Metal availability and bioconcentration in plants. In Heavy Metal Stress in Plants: From Molecules to Ecosystems; Prasad, M.N.V., Hagemeyer, J., Eds.; Springer: Berlin/Heidelberg, Germany, 1999; pp. 1–27. [Google Scholar]
- Chaudhary, K.; Jan, S.; Khan, S. Chapter 23—Heavy metal ATPase (HMA2, HMA3, and HMA4) genes in hyperaccumulation mechanism of heavy metals. In Plant Metal Interaction; Ahmad, P., Ed.; Elsevier: Amsterdam, The Netherlands, 2016; pp. 545–556. [Google Scholar]
- Huynh, A.T.; Chen, Y.-C.; Tran, B.N.T. A small-scale study on removal of heavy metals from contaminated water using water hyacinth. Processes 2021, 9, 1802. [Google Scholar] [CrossRef]
- PN, M.; Madhu, G. Removal of heavy metals from waste water using water hyacinth. ACEEE Int. J. Transp. Urban Dev. 2011, 1, 48–52. [Google Scholar]
- Das, S.; Goswami, S.; Talukdar, A.D. A study on cadmium phytoremediation potential of water lettuce, Pistia stratiotes L. Bull. Environ. Contam. Toxicol. 2014, 92, 169–174. [Google Scholar] [CrossRef]
- Vesely, T.; Neuberg, M.; Trakal, L.; Szakova, J.; Tlustoa, P. Water lettuce Pistia stratiotes L. response to lead toxicity. Water Air Soil Pollut. 2012, 223, 1847–1859. [Google Scholar] [CrossRef]
- Buta, E.; Paulette, L.; Mihǎiescu, T.; Buta, M.; Cantor, M. The influence of heavy metals on growth and development of eichhornia crassipes species, cultivated in contaminated water. Not. Bot. Horti Agrobot. 2011, 39, 135–141. [Google Scholar] [CrossRef] [Green Version]
- Emamverdian, A.; Ding, Y.; Mokhberdoran, F.; Xie, Y. Heavy metal stress and some mechanisms of plant defense response. Sci. World J. 2015, 2015, 756120. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hasan, S.H.; Talat, M.; Rai, S. Sorption of cadmium and zinc from aqueous solutions by water hyacinth (Eichchornia crassipes). Bioresour. Technol. 2007, 98, 918–928. [Google Scholar] [CrossRef] [PubMed]
- Miretzky, P.; Saralegui, A.; Cirelli, A.F. Aquatic macrophytes potential for the simultaneous removal of heavy metals (Buenos Aires, Argentina). Chemosphere 2004, 57, 997–1005. [Google Scholar] [CrossRef]
- Singh, K.; Pandey, S.N. Effect of nickel-stresses on uptake, pigments and antioxidative responses of water lettuce, Pistia stratiotes L. J. Environ. Biol. 2011, 32, 391–394. [Google Scholar]
- Hegazy, A.K.; Kabiel, H.F.; Fawzy, M. Duckweed as heavy metal accumulator and pollution indicator in industrial wastewater ponds. Desalinat. Water Treat. 2009, 12, 400–406. [Google Scholar] [CrossRef]
- Wang, A.S.; Angle, J.S.; Chaney, R.L.; Delorme, T.A.; Reeves, R.D. Soil pH effects on uptake of Cd and Zn by Thlaspi caerulescens. Plant Soil 2006, 281, 325–337. [Google Scholar] [CrossRef]
- Fritioff, Å.; Kautsky, L.; Greger, M. Influence of temperature and salinity on heavy metal uptake by submersed plants. Environ. Pollut. 2005, 133, 265–274. [Google Scholar] [CrossRef]
- Yang, Y.; Liao, J.; Chen, Y.; Tian, Y.; Chen, Q.; Gao, S.; Luo, Z.; Yu, X.; Lei, T.; Jiang, M. Efficiency of heterogeneous chelating agents on the phytoremediation potential and growth of Sasa argenteostriata (Regel) E.G. Camus on Pb-contaminated soil. Ecotoxicol. Environ. Saf. 2022, 238, 113603. [Google Scholar] [CrossRef]
- Yang, Q.; Yang, C.; Yu, H.; Zhao, Z.; Bai, Z. The addition of degradable chelating agents enhances maize phytoremediation efficiency in Cd-contaminated soils. Chemosphere 2021, 269, 129373. [Google Scholar] [CrossRef]
- Soltan, M.E.; Rashed, M.N. Laboratory study on the survival of water hyacinth under several conditions of heavy metal concentrations. Adv. Environ. Res. 2003, 7, 321–334. [Google Scholar] [CrossRef]
- Singh, D.; Gupta, R.; Tiwari, A. Potential of duckweed (Lemna minor) for removal of lead from wastewater by phytoremediation. J. Pharm. Res. 2012, 5, 1578–1582. [Google Scholar]
- Uysal, Y.; Taner, F. Effect of pH, temperature, and lead concentration on the bioremoval of lead from water using Lemna minor. Int. J. Phytoremediat. 2009, 11, 591–608. [Google Scholar] [CrossRef] [PubMed]
- Hardy, J.K.; Raber, N.B. Zinc uptake by the water hyacinth: Effects of solution factors. Chemosphere 1985, 14, 1155–1166. [Google Scholar] [CrossRef]
- Rakhshaee, R.; Khosravi, M.; Ganji, M.T. Kinetic modeling and thermodynamic study to remove Pb(II), Cd(II), Ni(II) and Zn(II) from aqueous solution using dead and living Azolla filiculoides. J. Hazard. Mater. 2006, 134, 120–129. [Google Scholar] [CrossRef]
- Dijoo, Z.K.; Ali, R.; Hameed, M. Role of free-floating aquatic macrophytes in abatement of the disturbed environs. In Bioremediation and Biotechnology, Volume 4: Techniques for Noxious Substances Remediation; Bhat, R.A., Hakeem, K.R., Eds.; Springer International Publishing: Cham, Switerland, 2020; pp. 259–274. [Google Scholar]
- Kumar, S.; Deswal, S. Phytoremediation capabilities of Salvinia molesta, water hyacinth, water lettuce, and duckweed to reduce phosphorus in rice mill wastewater. Int. J. Phytoremed. 2020, 22, 1097–1109. [Google Scholar] [CrossRef]
- Rai, P.K. Heavy metal phytoremediation from aquatic ecosystems with special reference to macrophytes. Crit. Rev. Environ. Sci. Technol. 2009, 39, 697–753. [Google Scholar] [CrossRef]
- Giri, A.K. Removal of Arsenic (III) and Chromium (VI) from the Water Using Phytoremediation and Bioremediation Techniques. Ph.D. Thesis, Degree of Doctor of Philosophy, Department of Chemistry National Institute of Technology Rourkela, Rourkela, India, July 2012. [Google Scholar]
- Shah, M.; Hashmi, H.N.; Ghumman, A.R.; Zeeshan, M. Performance assessment of aquatic macrophytes for treatment of municipal wastewater. J. S. Afr. Inst. Civ. Eng. 2015, 57, 18–25. [Google Scholar] [CrossRef] [Green Version]
- Haller, W.T.; Sutton, D.L.; Barlowe, W.C. Effects of salinity on growth of several aquatic macrophytes. Ecology 1974, 55, 891–894. [Google Scholar] [CrossRef]
- Liang, L.; Liu, W.; Sun, Y.; Huo, X.; Li, S.; Zhou, Q. Phytoremediation of heavy metal-contaminated saline soils using halophytes: Current progress and future perspectives. Environ. Rev. 2016, 25, 269–281. [Google Scholar] [CrossRef] [Green Version]
- Prasad, M.N.V. Phytoremediation of metal-polluted ecosystems: Hype for commercialization. Russ. J. Plant Physiol. 2003, 50, 686–701. [Google Scholar] [CrossRef]
- Wuana, R.A.; Okieimen, F.E. Heavy metals in contaminated soils: A review of sources, chemistry, risks, and best available strategies for remediation. In Heavy Metal Contamination of Water and Soil: Analysis, Assessment, and Remediation Strategies; International Scholarly Research Network: Londoin, UK, 2014; pp. 1–50. [Google Scholar]
- Oladoye, P.O.; Olowe, O.M.; Asemoloye, M.D. Phytoremediation technology and food security impacts of heavy metal contaminated soils: A review of literature. Chemosphere 2022, 288, 132555. [Google Scholar] [CrossRef] [PubMed]
- Dhaliwal, S.S.; Sharma, V.; Taneja, P.K.; Shukla, A.K.; Kaur, L.; Verma, G.; Verma, V.; Singh, J. Effect of cadmium and ethylenediamine tetraacetic acid supplementation on cadmium accumulation by roots of Brassica species in Cd spiked soil. Environ. Sci. Pollut. Res. 2022, 29, 6000–6009. [Google Scholar] [CrossRef]
- Shinta, Y.C.; Zaman, B.; Sumiyati, S. Citric Acid and EDTA as Chelating Agents in Phytoremediation of Heavy Metal in Polluted Soil: A Review. IOP Conf. Ser. Earth Environ. Sci. 2021, 896, 012023. [Google Scholar] [CrossRef]
- Lee, J.; Sung, K. Effects of chelates on soil microbial properties, plant growth and heavy metal accumulation in plants. Ecol. Eng. 2014, 73, 386–394. [Google Scholar] [CrossRef]
- Singh, J.; Kumar, V.; Kumar, P.; Kumar, P. Kinetics and prediction modeling of heavy metal phytoremediation from glass industry effluent by water hyacinth (Eichhornia crassipes). Int. J. Environ. Sci. Technol. 2022, 19, 5481–5492. [Google Scholar] [CrossRef]
- Naaz, M.; Dutta, A.; Kumari, S.; Farooqui, S. Bioaccumulation, phytoremediation and kinetics of uptake of heavy metals (Copper and Zinc) by Eichhornia crassipes. Res. Rev. J. Ecol. 2013, 2, 2278. [Google Scholar]
- Ingole, N.W.; Bhole, A.G. Removal of heavy metals from aqueous solution by water hyacinth (Eichhornia crassipes). J. Water Supply Res. Technol. AQUA 2003, 52, 119–128. [Google Scholar] [CrossRef]
- Go, J.L.C.; Madrazo, C.F.; Orbecido, A.H.; de Castro, M.E.G.; Deocaris, C.C.; Belo, L.P. Analysis of the copper removal kinetics of the Philippine giant bamboo (Dendrocalamus asper) in hydroponics. Heliyon 2021, 7, e06208. [Google Scholar] [CrossRef]
- Chua, J.; Banua, J.M.; Arcilla, I.; Orbecido, A.; de Castro, M.E.; Ledesma, N.; Deocaris, C.; Madrazo, C.; Belo, L. Phytoremediation potential and copper uptake kinetics of Philippine bamboo species in copper contaminated substrate. Heliyon 2019, 5, e02440. [Google Scholar] [CrossRef] [Green Version]
- Mondal, N.K.; Nayek, P. Hexavalent chromium accumulation kinetics and physiological responses exhibited by Eichhornia sp. and Pistia sp. Int. J. Environ. Sci. Technol. 2020, 17, 1397–1410. [Google Scholar] [CrossRef]
- Cleide Barbieri de, S.; Gabriel Rodrigues, S. Phytoremediation of effluents contaminated with heavy metals by floating aquatic macrophytes species. In Biotechnology and Bioengineering; Eduardo Jacob, L., Leila Queiroz, Z., Eds.; IntechOpen: Rijeka, Croatia, 2019; Chapter 10. [Google Scholar]
- Kamalu, C.I.O.; Okere, P.C.; Egbufor, U.C.; Nwandikom, G.I.; Obijiaku, J.C.; Asomugha, C.C. Modeling and optimization of phytoremediation kinetics of metals in soil by a plant hyperacumulator. Am. J. Eng. Res. 2017, 6, 196–207. [Google Scholar]

| Pollutants | Mechanisms | Descriptions |
|---|---|---|
| Inorganic | Phytoextraction | Eliminate the contaminants in the form of harvestable plant biomass. |
| Phytostabilization | Minimize the contaminants mobility. | |
| Phytoaccumulation | Hyperaccumulation due to hypertolerance. | |
| Rhizofiltration | Roots filter water via absorption or adsorption. | |
| Organic | Phytodegradation | Degrade the contaminants in the plant. |
| Phytostimulation | Stimulate the microbial activity to degrade the contaminants. | |
| Phytoassimilation | Transport and metabolize the contaminants in plants. | |
| Phytovolatilization | Extract the contaminants from media and liberate them through air. | |
| Phytotransformation | Degrade contaminants into a simpler form. |
| Aquatic Plants | Conditions | Heavy Metals Removal Efficiency | References |
|---|---|---|---|
| Duckweed (Lemna minor) | Sampling time: 25 days Temperature: 7 to 20 °C Initial concentration (ppb): 16.31 As, 1.47 Cd, 67.37 Cr, 25.84 Cu, 0.36 Hg, 347.8 Ni, 23.37 Pb, 49.59 Zn Framework: industrial wastewater | 90.95% As, 97.79% Cd, 90.25% Cr, 98.46% Cu, 82.84% Hg, 98.08% Ni, 99.91% Pb, 98.00% Zn | [36] |
| Sampling time: 7 days Temperature: 13 to 20 °C Relative humidity: 70% Photoperiod: 16 h light, 8 h dark Concentration: 10−6 mol/L metal solutions | 95% Cd, 93% Pb, 81.2% Zn, 86.5% Cu | [36] | |
| Water lettuce (Pistia stratiotes) | Sampling time: 15 days Initial concentration (mg/L): 0.08–0.46 Cu, 0.03–1.36 Ni, 0.09–0.86 Pb, 0.26–1.31 Zn Framework: field | 39.72–72.58% Cu, 28.96–68.79% Ni, 43.02–76.66% Pb, 26.99–79.57% Zn | [3] |
| Sampling time: 30 days Initial concentration (mg/L): 22.17 Al, 5.03 As, 0.028 Cd, 2.84 Cr, 0.16 Cu, 14.70 Fe, 20.37 Mn, 5.25 Pb, 2.01 Zn Framework: steel industry effluent | 73% Al, 74% As, 82.8% Cd, 62.8% Cr, 78.6% Cu, 61% Fe, 39.5% Mn, 73% Pb, 65.2% Zn | [37] | |
| Water hyacinth (Eichhornia crassipes) | Sampling time: 15 days Temperature: 25 ± 5 °C Humidity: 72 ± 15% Initial concentration (mg/L): 1.12 Fe, 0.62 Cu, 1.41 Ni, 0.77 Pb, 1.42 Zn Framework: landfill leachate | 87.56% Fe, 87.09% Cu, 81.56% Ni, 84.41% Pb, 90.18% Zn | [38,39] |
| Initial concentration (mg/L): 0.24 Pb, 1.20 Pb, 4.97 Hg, 3.34 Ni Framework: industrial wastewater | 97.50% Cd, 95.10% Ni, 99.90% Hg, 83.40% Pb | [29] | |
| Watermoss (Salvinia) | Sampling time: 28 days Initial concentration (mg/L): 0–12.39 Framework: field | 72–91% Cd, 80% Cu, 72–91% Ni, 72–91% Zn | [3] |
| Sampling time: 12 days Temperature: 25 °C Humidity: 70–75% Photoperiod: 16 h light, 8 h dark Initial concentration: 1.0 mg/dm3 Cr, 1.0 mg/dm3 Hg | 74% Cr, 93% Hg | [40] |
| Plants | Heavy Metals | Concentration | Experimental Layout and Duration | Phytotoxic Responses | References |
|---|---|---|---|---|---|
| Water hyacinth | Cr | 10.0 to 20.0 mg/L | 15 L experimental tanks filled with 10 L of tap water and investigated up to day 11 | Yellowing of leaves, leaf chlorosis, and growth retardation. | [34] |
| Zn | 2.0 to 12.0 ppm | 2 L container filled with 1 L tap water and investigated up to day 16 | Growth reduction, leaf chlorosis, metabolism disruption. | [51] | |
| Cd | 1.0 to 4.0 ppm | Growth reduction, growth retardation, new root growth inhibition, root function disruption, leaf chlorosis. | |||
| Duckweed | Cd | >10 mM | 10 L plastic reactors with 5 L of lake water and investigated up to day 15 | Pigment degradation and photosynthesis restriction. | [52] |
| Cu | >50 μM | ||||
| Water lettuce | Pb | 1 to 2 mmol/L | 60 L PE containers filled with 10 L of Hoagland nutrient solution and investigated up to day 8 | Chlorophyll synthesis inhibition, chlorophyll reduction, loss of photosynthesis activity. | [48] |
| Ni | 1.0 and 10.0 ppm | Unknown size for hydroponic tubs filled with 10% Hoagland’s solution and investigated up to day 6 | Plant wilting, chlorosis in leaves, chlorophyll reduction, carotenoid reduction, water loss, browning of root tips, and root damage. | [53] |
| Plant Species | Heavy Metals | Influence/Enhancement Factor and Details | Significant Results | Reference |
|---|---|---|---|---|
| Thlaspi caerulescens | Cd, Zn | pH | The soluble metal form of both Cd and Zn was greatly increased with decreasing pH. | [55] |
| Eichhornia crassipes | Cd, As, Pb, Zn, and Cu | Temperature | The ideal water temperature for growth is between 28 °C and 30 °C. Temperatures exceeding 33 °C stifle further development. | [45] |
| Elodea canadensis, Potamogeton natans | Cu, Zn, Cd, Pb | The metal concentration and accumulations increased with increasing water temperature. | [56] | |
| Salinity | The metal concentration increased with decreasing salinity. | [56] | ||
| Eichchornia crassipes | Zn, Cd | Exposure duration | The overall metal uptake by the plant increased with the duration of the exposure time. | [51] |
| Initial solution concentration | The uptake of heavy metals increased with an increase in the initial solution concentration. | [51] | ||
| Sasa argenteostriata (Regel) E.G. Camus | Pb | Chelating agents (Ethylenediaminetetraacetic acid (EDTA) and nitrilotriacetic acid (NTA)) | The combined application of EDTA and NTA brought the accumulation of Pb availability to a more reasonable level than EDTA alone. | [57] |
| Zea mays L. | Cd | Chelating agents (ethylenediamine tetraacetic acid (EDTA), diethylenetriacetic acid (NTA), tetrasodium N, N-diacetate (GLDA), aspartate dibutyric acid ether (AES), and iminodisuccinic acid (IDSA)) | Total Cd extraction followed the order AES (6 mmol kg−1) > GLDA > NTA > EDTA > IDSA (3 mmol kg−1) | [58] |
| Plant | Heavy Metal | Research Highlight | References |
|---|---|---|---|
| Dendrocalamus asper | Cu | The removal rate of Cu from the contaminated source had an order of 2.71 and a kinetic constant of 0.0013 ppm−1.71 day−1 | [80] |
| Bambusa merilliana, Bambusa blumeana, Dendrocalamus asper | Cu | The zero-order model has well described the uptake of metal ions per mass of plant with a correlation value R2 of 0.954 and a rate constant of 3.136 mg/(kg∙day). | [81] |
| Eichhornia sp., Pistia sp. | Cr | Pseudo-first-order (0.910) and pseudo-second model (0.665) are more suitable for bioaccumulation kinetic in Pistia sp. rather than Eichhornia sp. | [82] |
| Eichhornia crassipes, Lemma valdniana | As | Pseudo-first-order gave a good correlation for both plants, with a correlation value R2 > 0.8 for all the concentrations involved. | [83] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pang, Y.L.; Quek, Y.Y.; Lim, S.; Shuit, S.H. Review on Phytoremediation Potential of Floating Aquatic Plants for Heavy Metals: A Promising Approach. Sustainability 2023, 15, 1290. https://doi.org/10.3390/su15021290
Pang YL, Quek YY, Lim S, Shuit SH. Review on Phytoremediation Potential of Floating Aquatic Plants for Heavy Metals: A Promising Approach. Sustainability. 2023; 15(2):1290. https://doi.org/10.3390/su15021290
Chicago/Turabian StylePang, Yean Ling, Yen Ying Quek, Steven Lim, and Siew Hoong Shuit. 2023. "Review on Phytoremediation Potential of Floating Aquatic Plants for Heavy Metals: A Promising Approach" Sustainability 15, no. 2: 1290. https://doi.org/10.3390/su15021290
APA StylePang, Y. L., Quek, Y. Y., Lim, S., & Shuit, S. H. (2023). Review on Phytoremediation Potential of Floating Aquatic Plants for Heavy Metals: A Promising Approach. Sustainability, 15(2), 1290. https://doi.org/10.3390/su15021290









