Oxidation Enhancement of Gaseous Elemental Mercury Using Waste Steel Slag under Various Experimental Conditions
Abstract
:1. Introduction
2. Materials and Methods
2.1. Samples Selection
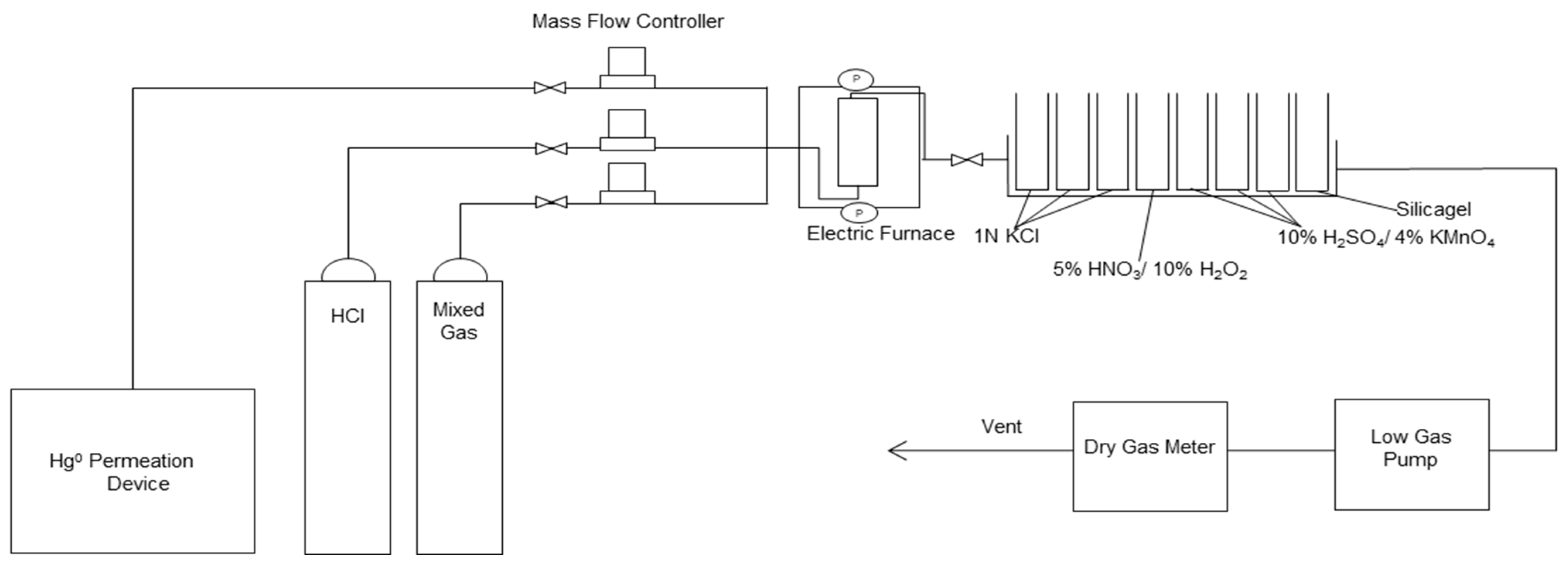
2.2. Experimental Device
2.3. Analysis Method
3. Results and Discussion
3.1. Characteristic Analysis Result in Steel Slag
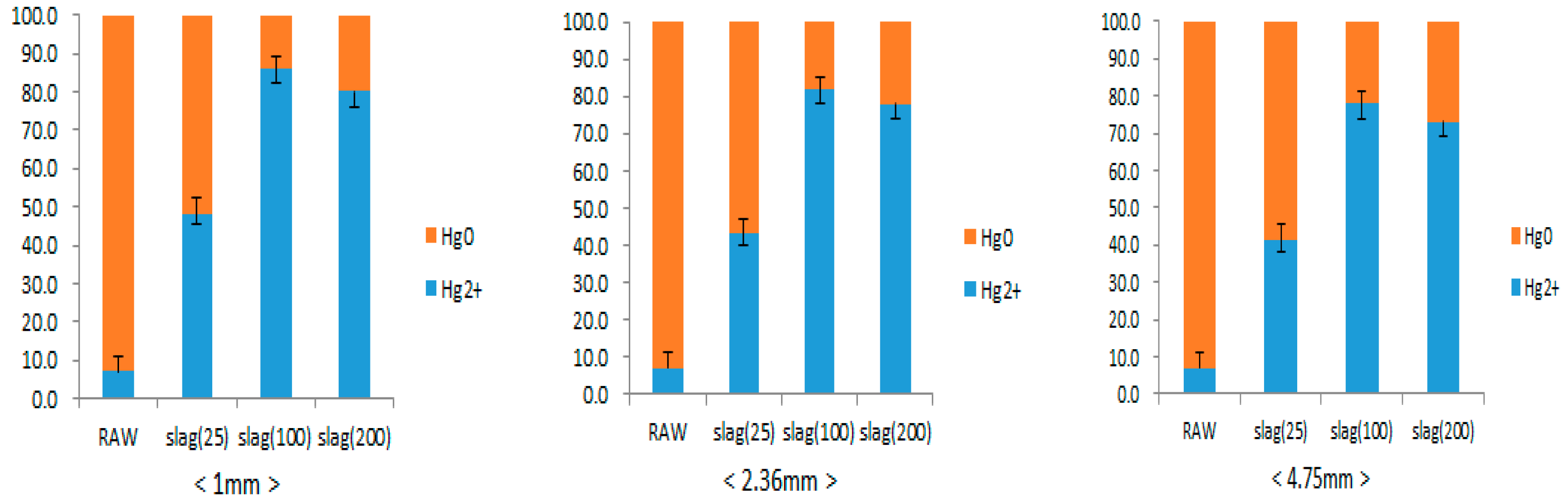
3.2. Elemental Mercury Oxidation Reaction of Steel Slag
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gardner, M.; Jennifer, F.N.; Ellen, K.S. Differential Immunotoxic Effects of Inorganic and Organic Mercury Species in Vitro. Toxicol. Lett. 2010, 198, 182–190. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Risher, J.F. Agency for Toxic Substances and Disease Registry (ATSDR). In Elemental Mercury and Inorganic Mercury Compounds: Human Health Aspects; World Health Organization: Geneva, Switzerland, 2003. [Google Scholar]
- UNEP. Minamata Convention on Mercury; UNEP: Nairobi, Kenya, 2013. [Google Scholar]
- Sung, J.H.; Oh, J.S.; Back, S.K.; Jeong, B.M.; Jang, H.N.; Seo, Y.C.; Kim, S.H. Estimation of Mercury Emission from Major Sources in Annex D of Minamata Convention and Future Trend. J. Korean Soc. Atmos. Environ. 2016, 32, 193–207. [Google Scholar] [CrossRef] [Green Version]
- NIER. Merucy Emissions Survey for Air Emission Facilities; NIER: Incheon, Republic of Korea, 2008. [Google Scholar]
- Park, K.S.; Seo, Y.C.; Lee, S.J.; Lee, J.H. Emission and speciation of mercury from various combustion sources. Powder Technol. 2008, 180, 151–156. [Google Scholar] [CrossRef]
- Kilgroe, J.D.; Sedman, C.B.; Srivastava, R.K.; Ryan, J.V.; Lee, C.W.; Thorneloe, S.A. Control of Mercury Emissions from Coal-Fired Electric Utility Boilers: Interim Report; U.S. Environmental Protection Agency: Washington, DC, USA, 2001.
- Nolan, P.S.; Redinger, K.E.; Amrheim, G.T.; Kudlac, G.A. Demonstration of additive use for enhanced mercury emission control in wet FGD systems. Fuel Proc. Technol. 2004, 85, 587–600. [Google Scholar] [CrossRef]
- Kang, S.W.; Shim, S.H.; Jeong, S.H.; Jung, J.H.; Lee, S.S. Mercury Emission Characteristics from Co-combustion of Coal and Sludge. J. Korea Soc. Atmos. Environ. 2012, 28, 182–189. [Google Scholar] [CrossRef] [Green Version]
- Kolker, A.; Senior, C.L.; Quick, J.C. Mercury in Coal and the Impact of Coal Quality on Mercury Emissions from Combustion Systems. Appl. Geochem. 2006, 21, 1821–1836. [Google Scholar] [CrossRef]
- Serre, S.D.; Silcox, G.D. Adsorption of Elemental Mercury on Residual Carbon in Coal Fly Combustion Fly ash. Ind. Eng. Chem. Res. 2000, 39, 1723–1730. [Google Scholar] [CrossRef]
- Dunham, G.E.; Dewall, R.A.; Senior, C.L. Fixed-bed Studies of the Interaction between Mercury and Coal Combustion Fly. Ash. Fuel Proc. Technol. 2003, 82, 197–213. [Google Scholar] [CrossRef]
- Futsaeter, G.; Wilson, S. The UNEP Global Atmospheric Mercury Assessment: Sources, Emissions and Transport. In Proceedings of the 16th International Conference on Heavy Metals in the Environment UNEP Chemicals Branch, Rome, Italy, 23–27 September 2012. [Google Scholar]
- UNEP. Global Mercury Assessment 2013: Sources, Emissions, Releases and Environmental Transport; UNEP Chemicals Branch: Geneva, Switzerland, 2013. [Google Scholar]
- Wang, F.; Wang, S.; Zhang, L.; Yang, H.; Gao, W.; Wu, Q.; Hao, J. Mercury mass flow in iron and steel production process and its implications for mercury emission control. J. Environ. Sci. 2016, 43, 293–301. [Google Scholar] [CrossRef] [PubMed]
- World Steel Recycling in Figures 2015–2019. Available online: https://www.bir.org/publications/facts-figures/download/643/175/36?method=view (accessed on 26 December 2022).
- POSCO Eco Report 4, 98% Recycled Steel By-Products, How Are They Used. 2019. Available online: https://newsroom.posco.com/kr/%ED%8F%AC%EC%8A%A4%EC%BD%94-%EC%97%90%EC%BD%94-%EB%A6%AC%ED%8F%AC%ED%8A%B8-4/ (accessed on 26 December 2022).
- United States Environmental Protection Agency (U.S. EPA). Method 7471A, Hg in Solid or Semisolid Waste; U.S. EPA: Washington, DC, USA, 1994.
- ASTM D6784-02; Standard Test Method for Elemental, Oxidized, Particle-Bound and Totalmercury in Flue Gas Generated Fromcoal-Fired Stationary Sources (Ontario Hydro Method). USEPA: Washington, DC, USA, 2008.
- Lee, S.S.; Kim, K.Y.; Oh, K.J.; Jeon, J.M.; Kang, D.C. Reaction Characteristics of Elemental and Oxidized Mercury with Fly Ash Components. Clean Technol. 2013, 19, 453–458. [Google Scholar] [CrossRef]
- Ghorishi, S.B.; Lee, C.W.; Jozewicz, W.S.; Kilgroe, J.D. Effects of Fly Ash Transition Metal Content and Flue gas HCl/SO2 Ratio on Mercury Speciation in Waste Combustion. Environ. Eng. Sci. 2005, 22, 221–231. [Google Scholar] [CrossRef]
- Bhardwaj, R.; Chen, X.; Vidic, R.D. Impact of Fly Ash Composition on Mercury Speciation in Simulated Flue Gas. J. Air Waste Manag. Assoc. 2009, 59, 1331–1338. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Liu, J.; Yang, Y.; Chang, M. Oxidation mechanism of elemental mercury by HCl over MnO2 catalyst: Insights from first principles. Chem. Eng. J. 2015, 280, 354–362. [Google Scholar] [CrossRef]
- Li, H.; Li, Y.; Wu, C.Y.; Zhang, J. Oxidation and Capture of Elemental Mercury over SiO2–TiO2–V2O5 Catalysts in Simulated Low-rank Coal Combustion Flue gas. Chem. Eng. J. 2011, 169, 186–193. [Google Scholar] [CrossRef]
- Zhang, Y.; Laumb, J.; Liggett, R.; Holmes, M.; Pavlish, J. Impact of acid gases on mercury oxidation across SCR catalyst. Fuel Proc. Technol. 2007, 88, 929–934. [Google Scholar] [CrossRef]
- Hong, H.J.; Ham, S.W.; Kim, M.H.; Lee, S.M.; Lee, J.B. Characteristics of Commercial Selective Catalytic Reduction Catalyst for the Oxidation of Gaseous Elemental Mercury with Respect to Reaction Conditions. Korean J. Chem. Eng. 2010, 27, 1117–1122. [Google Scholar] [CrossRef]





| Large Category | Middle Category | Emission Source |
|---|---|---|
| Anthropogenic use and emission facilities | Fuel and energy-related facilities | Coal and combustion facilities, oil extraction, refining facilities, other fossil fuel extraction facilities, utilization facilities, biomass power generation, and heat power generation facilities, geothermal power plant |
| Primary metal production facilities | Mercury extraction, processing facility, gold silver extraction, processing facilities, zinc, copper, lead, aluminum, steel extraction, processing facilities | |
| Metal recycling facility | Mercury recovery, iron recovery, and other metal recovery facilities | |
| Other pollution and material production facilities | Cement production facilities, pulp paper production facilities, lime production facilities, carbon black production facilities, coke production facilities | |
| Crematorium and graveyard | Crematorium and graveyard | |
| Other anthropogenic emissions | Mobile pollutant | |
| Waste facilities | Waste incineration facilities | Household wastes incineration facility, designated waste, incineration facility, hazardous waste incineration facility, sewage incineration facility, and other waste incineration facilities |
| Waste landfill, wastewater facilities | Management type landfill facility, industrial waste dumping, general waste dumping, wastewater facilities |
| Facilities | Air Pollution Control Device | Control Efficiency (%) | |
|---|---|---|---|
| Coal-fired power plant | Bituminous | SCR-ESP-FGD-Stack | 77.12 |
| ESP-Stack | 68.57 | ||
| ESP-FGD-Stack | 83.51 | ||
| Anthracite | SCR-ESP-FGD-Stack | 73.17 | |
| Oil-fired power plant | ESP-Stack | - | |
| SCR-ESP-FGD-stack | - | ||
| Non-ferrous metal smelting | Zinc | ESP-Venturi Scrubber-ESP-Boliden Norzink-Dry tower-SO2 1st adsorption tower-SO2 2nd adsorption tower-Scrubber-Stack | 99.99 |
| Cupper | Wet scrubber-ESP-dry tower-SO2 1st adsorption tower-SO2 2nd adsorption tower-FGD-Stack | 99.99 | |
| Lead | ESP-Venturi Scrubber-ESP-Boliden Norzink-Dry tower-SO2 1st adsorption tower-SO2 2nd adsorption tower-Scrubber-Stack | 99.99 | |
| Cement production | Spray tower-BF-Stack | ||
| Iron manufacturing plant | Sintering furnace | ESP-Stack | 22.42 |
| ESP-BF-SCR-Stack | - | ||
| Electric furnace | BF-Stack | - | |
| Waste incineration | Municipal solid waste | SDR-SCR-SNCR-BF-Stack | 98.14 |
| SDA-ACI-BF-SCR-Stack | 92.92 | ||
| ESP-WS-ACI-BF-SCR-Stack | 71.01 | ||
| SDA-ACI-BF-Stack | 86.95 | ||
| SDA-BF-SCR-Stack | 93.95 | ||
| SDA-BF-Stack | 68.56 | ||
| Industrial solid waste | Cy-BF-Stack | 41.79 | |
| Cy-BF-WS-Stack | 60.52 | ||
| SNCR-SDR-BF-WS-Stack | 43.41 | ||
| Hospital waste | SDR-BF-WS-Stack | - | |
| Sewage sludge | BF-SDR-WS-Stack | - | |
| Sieve Range | Weight (g) | Weight Fraction (wt.%) |
|---|---|---|
| >8.65 mm | 77.1 | 25.70 |
| 4.75–8.65 mm | 75.91 | 25.30 |
| 2.36–4.75 mm | 58.25 | 19.42 |
| 1–2.36 mm | 53.05 | 17.68 |
| 850 µm–1 mm | 11.28 | 3.76 |
| 150–850 µm | 12.9 | 4.30 |
| <150 µm | 11.51 | 3.84 |
| Total | 300 | 100 |
| Parameter | Value | |
|---|---|---|
| Size of Steel Slag (mm) | 4.75, 2.36, 1 | |
| Temperature (°C) | 25, 100, 200 | |
| Gas (mL/min) | N2 basis | Permeation Device: 0.8 HCl: 0.1 Mixed gas: 0.1 |
| Air | Permeation Device: 0.8 Air: 0.2 | |
| ※ Mixed Gas Composition: CO2 30%, SO2 0.1%, NO 0.01% | ||
| Steel Slag Composition and Distribution | Fly Ash Composition and Distribution | ||
|---|---|---|---|
| Compounds | Distribution (wt.%) | Compounds | Distribution (wt.%) |
| Fe2O3 | 36.4 | SiO2 | 44.6 |
| CaO | 31.9 | Al2O3 | 22.4 |
| MgO | 11.1 | Fe2O3 | 8.1 |
| SiO2 | 9.5 | CaO | 7.3 |
| MnO | 4.0 | MgO | 1.3 |
| Al2O3 | 3.5 | TiO2 | 1.4 |
| P2O5 | 1.3 | CuO | 0.0 |
| K2O, TiO2, SO3, V2O5, Cr2O3, Cl | <1 | - | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, J.C.; Park, S.-W.; Kim, H.S.; Alam, T.; Lee, S.Y. Oxidation Enhancement of Gaseous Elemental Mercury Using Waste Steel Slag under Various Experimental Conditions. Sustainability 2023, 15, 1406. https://doi.org/10.3390/su15021406
Lee JC, Park S-W, Kim HS, Alam T, Lee SY. Oxidation Enhancement of Gaseous Elemental Mercury Using Waste Steel Slag under Various Experimental Conditions. Sustainability. 2023; 15(2):1406. https://doi.org/10.3390/su15021406
Chicago/Turabian StyleLee, Joo Chan, Se-Won Park, Hyun Sub Kim, Tanvir Alam, and Sang Yeop Lee. 2023. "Oxidation Enhancement of Gaseous Elemental Mercury Using Waste Steel Slag under Various Experimental Conditions" Sustainability 15, no. 2: 1406. https://doi.org/10.3390/su15021406







