Foam and Antifoam Behavior of PDMS in MDEA-PZ Solution in the Presence of Different Degradation Products for CO2 Absorption Process
Abstract
:1. Introduction
1.1. Foaming Tendency in Amine-Based Solution
1.2. Antifoaming Mechanism
1.3. Antifoaming Behavior Using Silicone-Based Antifoam
2. Materials and Method
2.1. Analysis of Foaming and Antifoaming Behavior in the MDEA+PZ System
2.2. Materials
2.3. Preparation of Test Solution
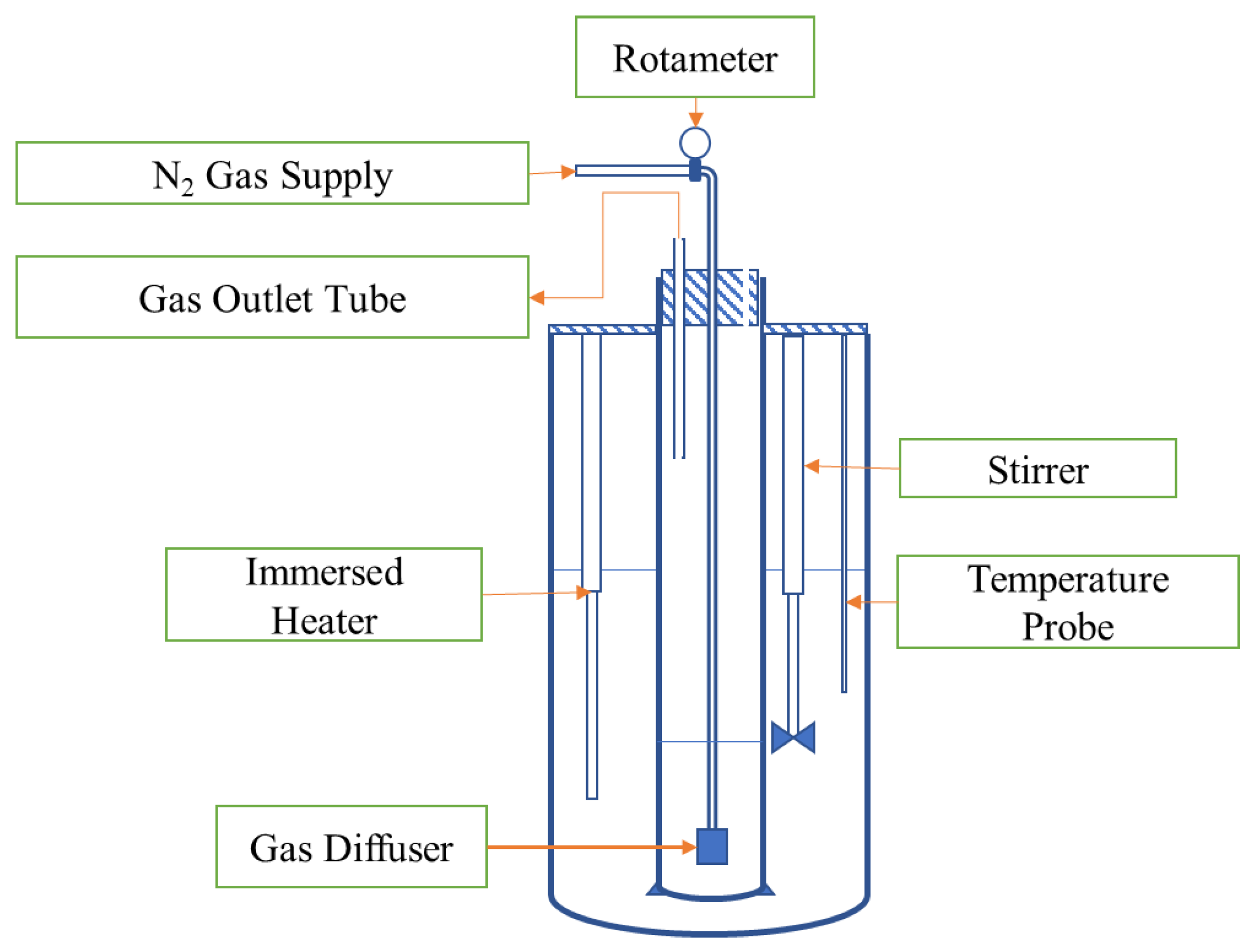
2.4. Experimental Setup
2.5. Experimental Procedure
2.6. Test Parameters
3. Foaming and Antifoaming Behavior in MDEA+PZ Solution
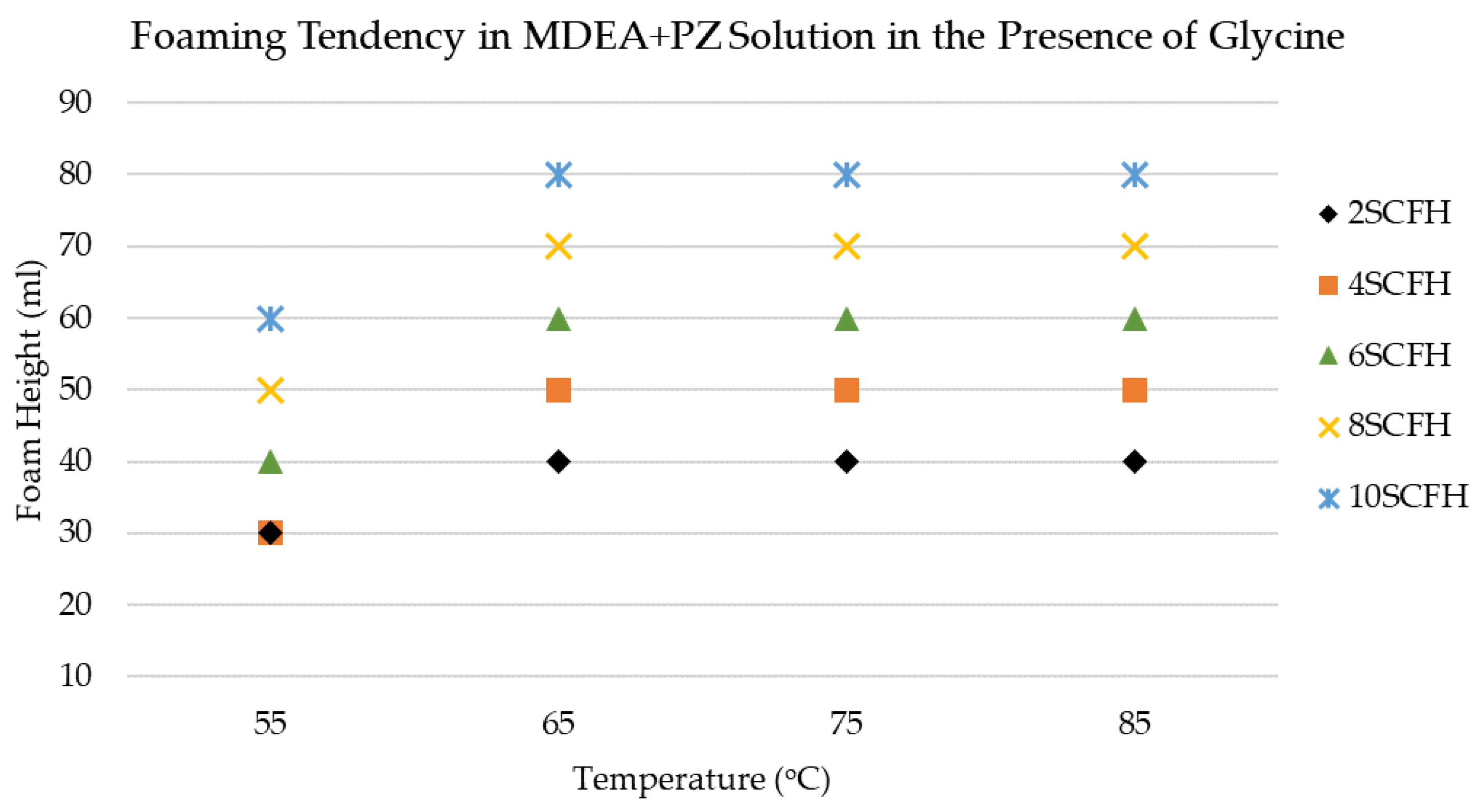
3.1. Foaming Behavior of MDEA+PZ Solution in the Presence of Glycine
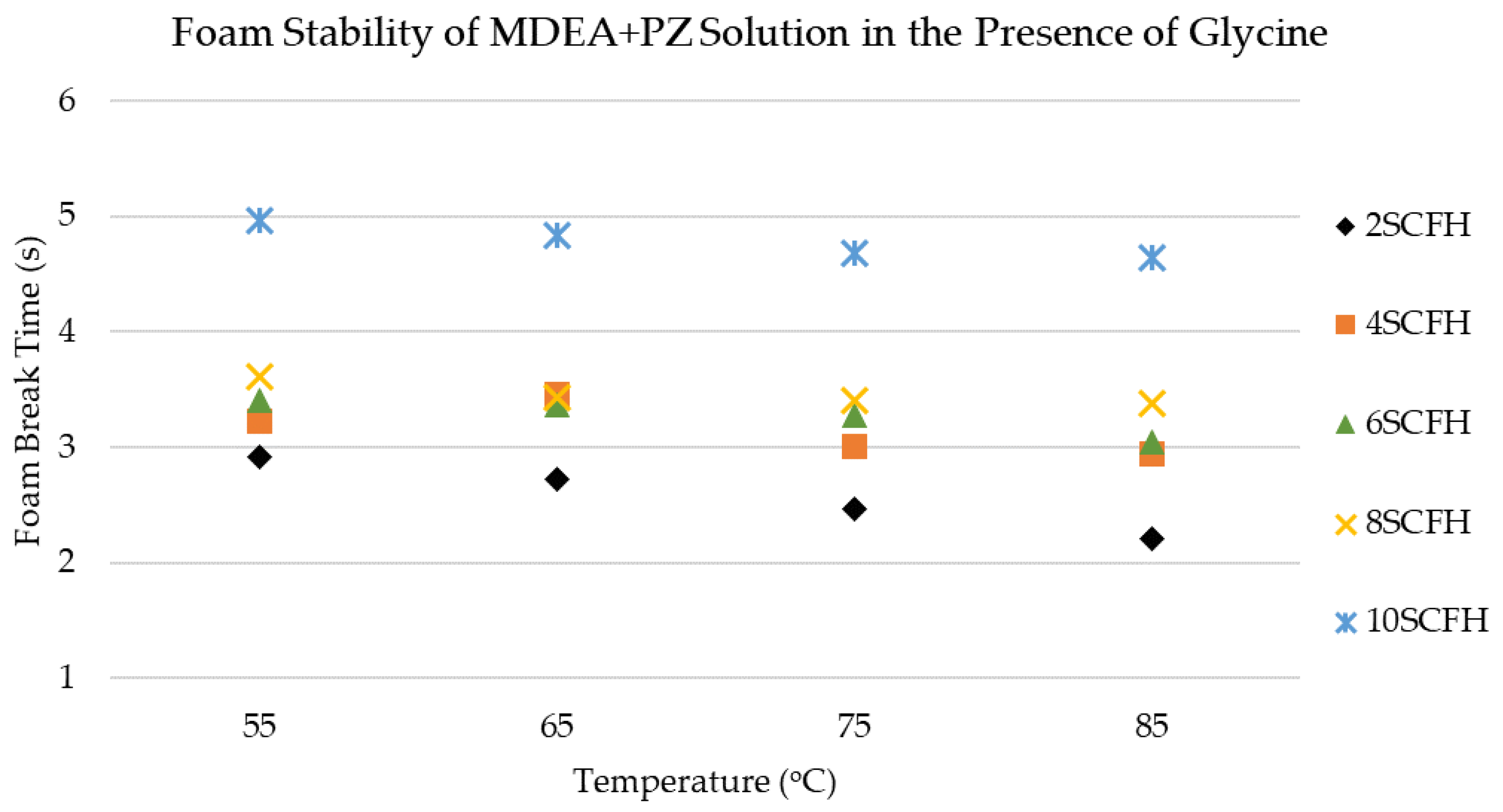
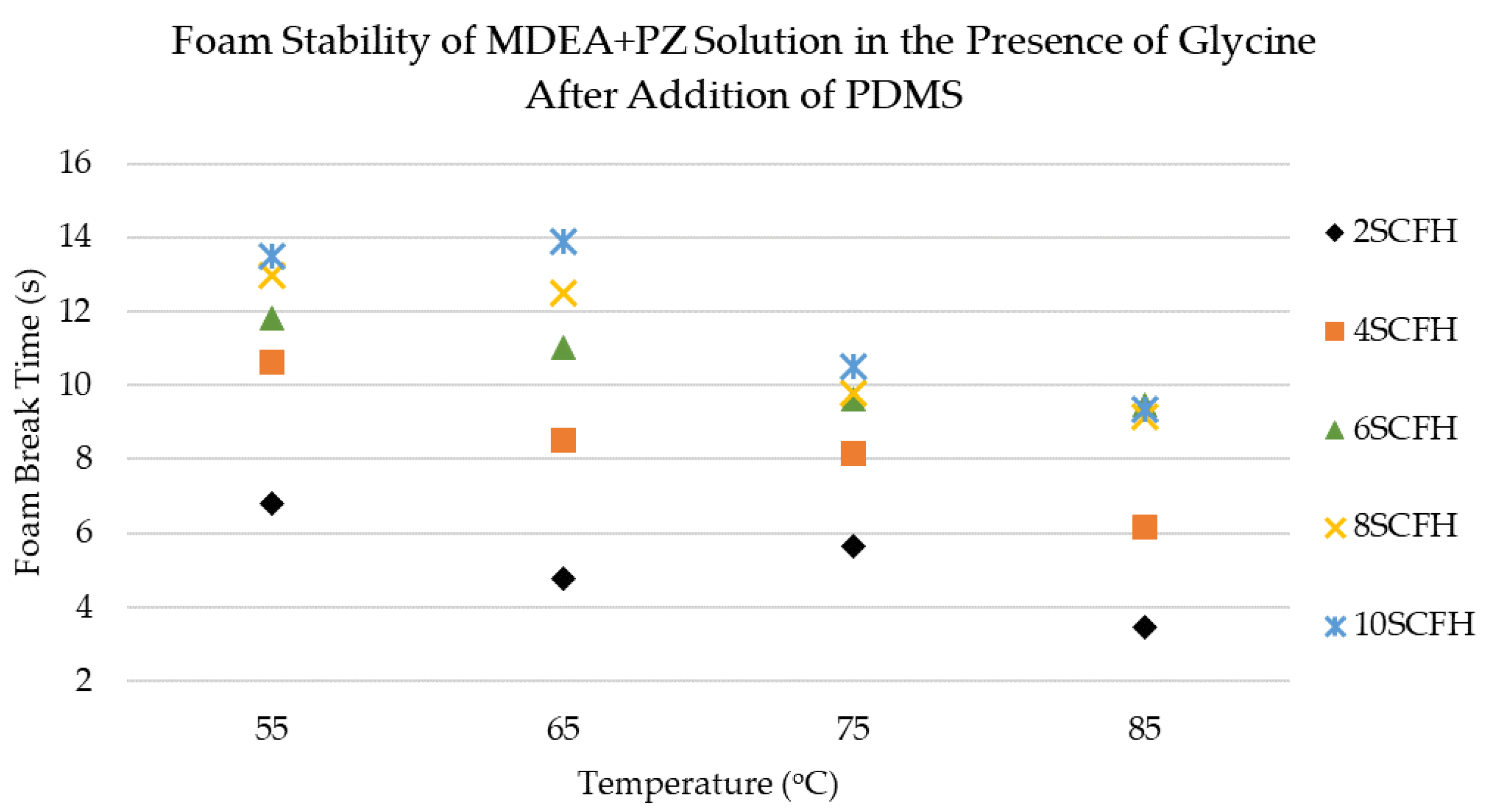
3.2. Antifoaming Performance of PDMS Antifoam in MDEA+PZ Solution in the Presence of Glycine
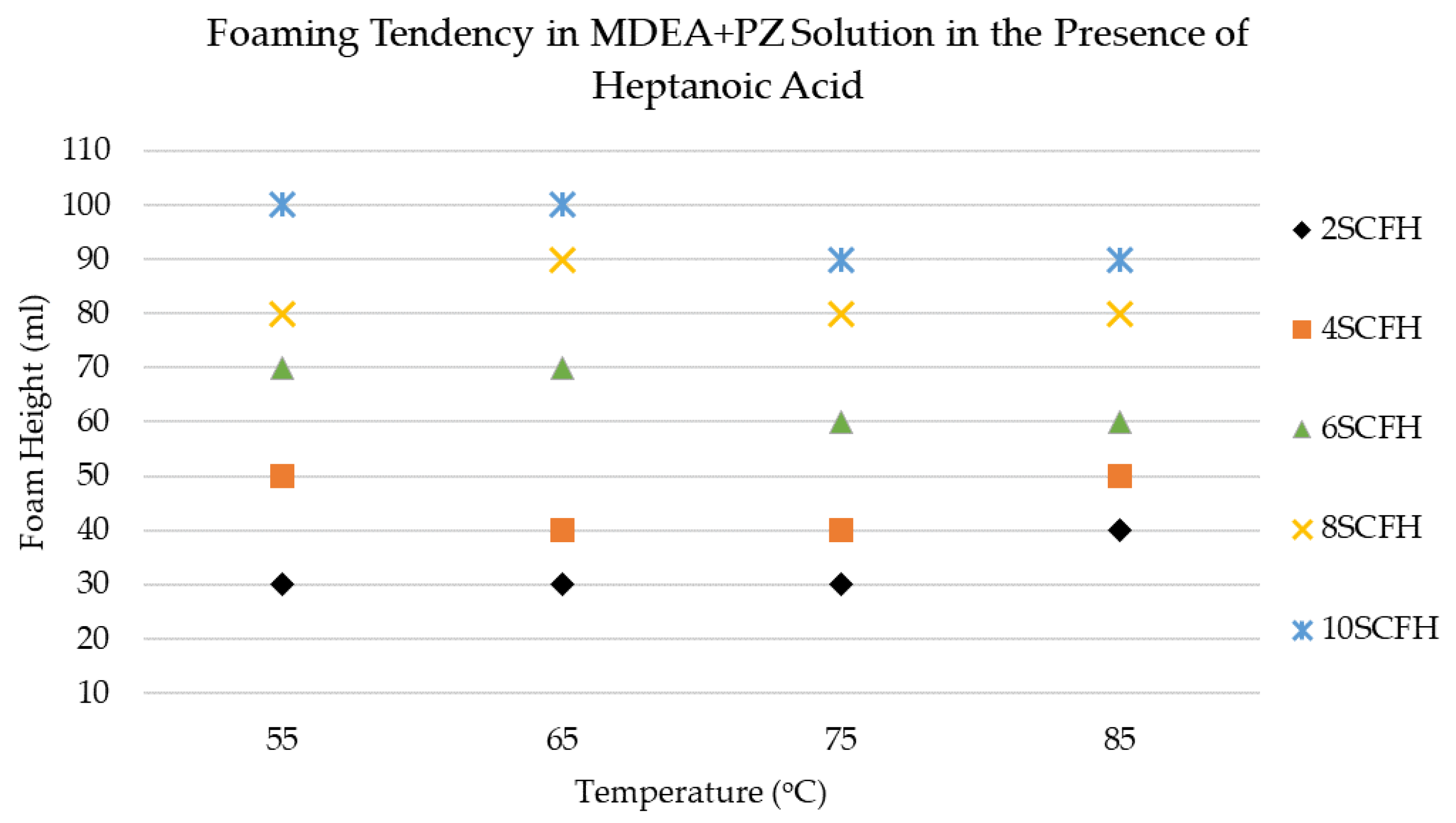
3.3. Foaming Behavior of MDEA+PZ Solution in the Presence of Heptanoic Acid
3.4. Antifoaming Performance of PDMS Antifoam in MDEA+PZ Solution in the Presence of Heptanoic Acid
3.5. Foaming Behavior of MDEA+PZ Solution in the Presence of Hexadecane
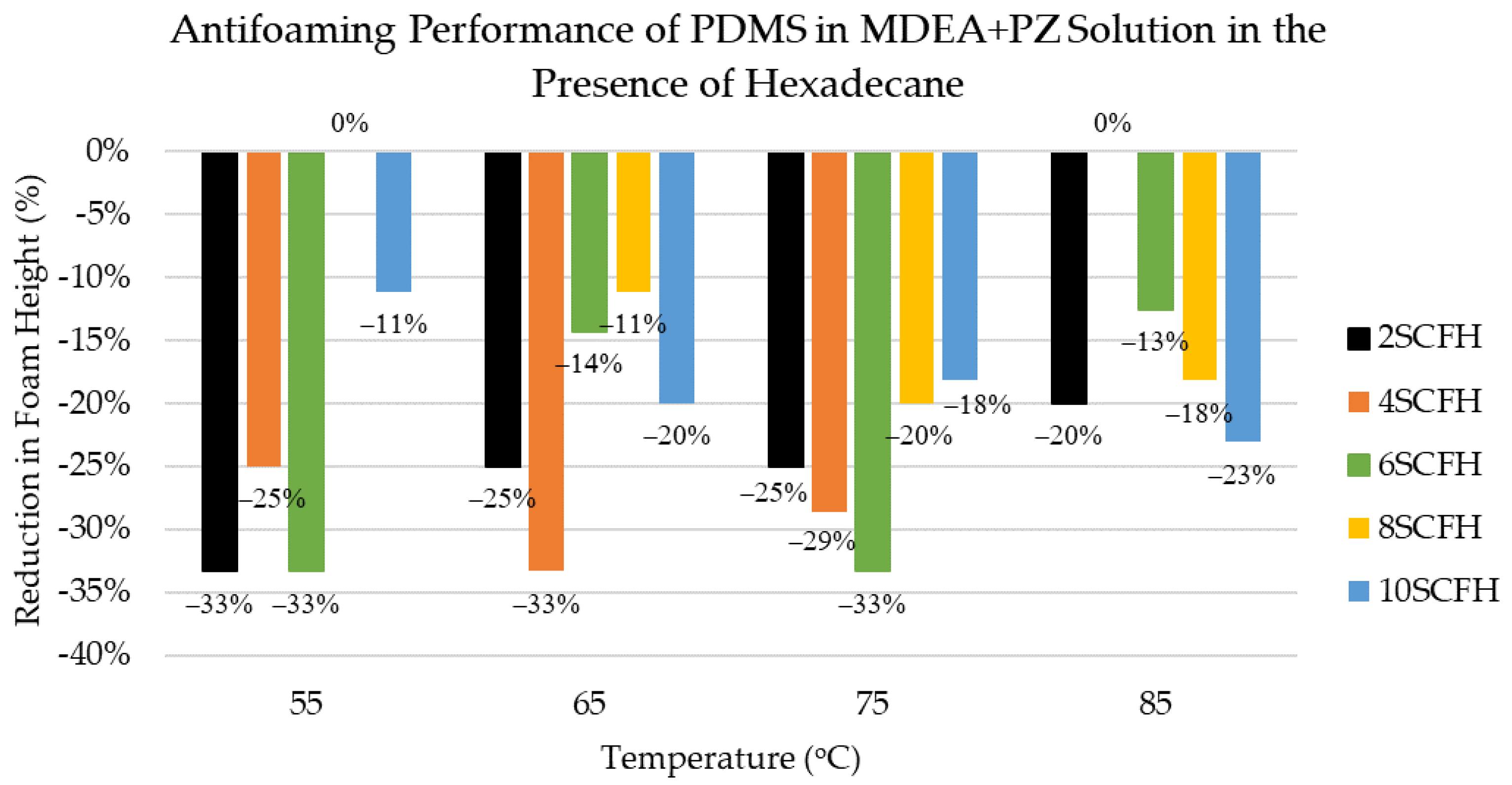
3.6. Antifoaming Performance of PDMS Antifoam in MDEA+PZ Solution in the Presence of Hexadecane
3.7. Foaming Behavior of MDEA+PZ Solution in the Presence of Bicine
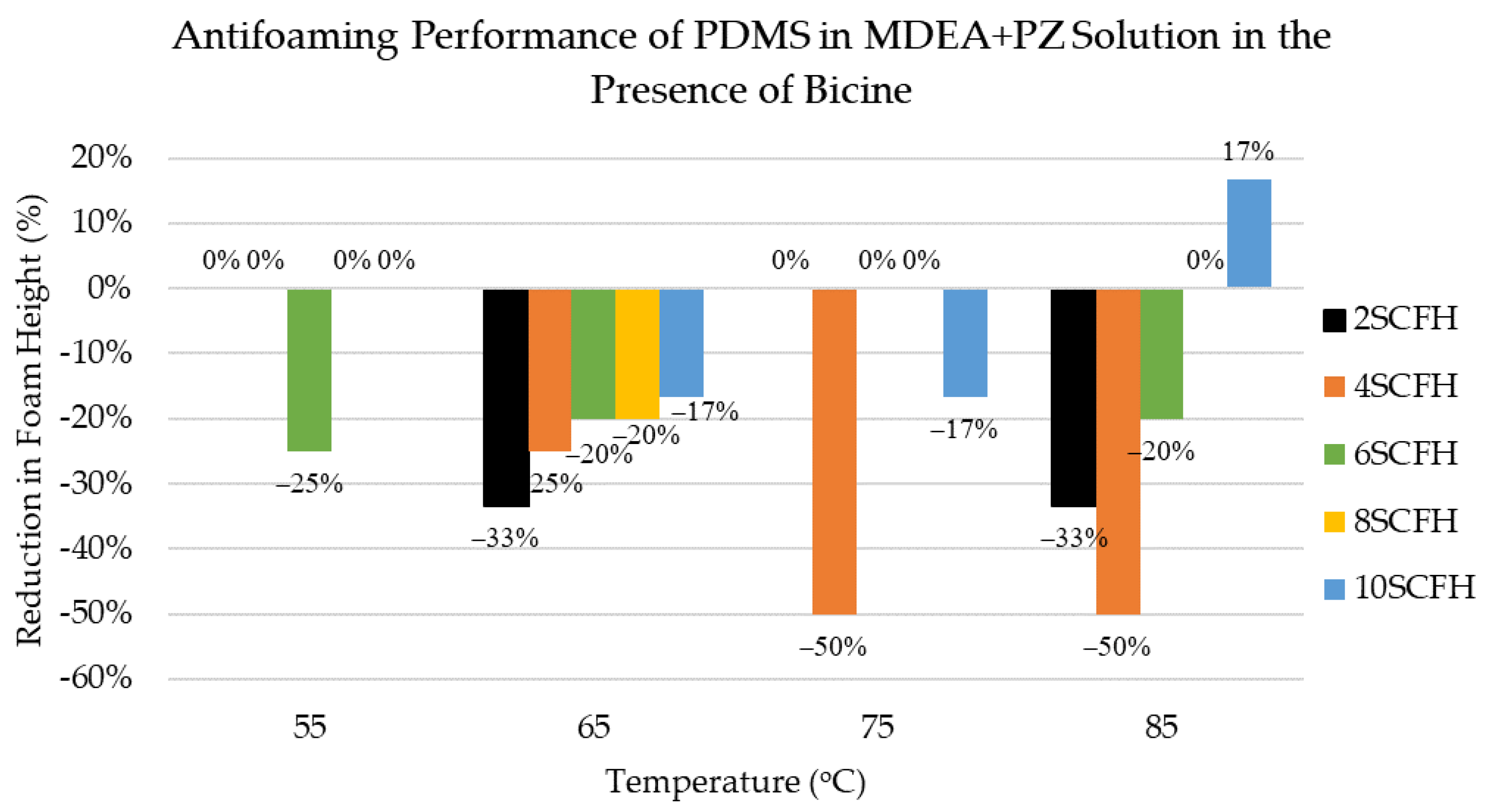
3.8. Antifoaming Performance of PDMS Antifoam in MDEA+PZ Solution in the Presence of Bicine
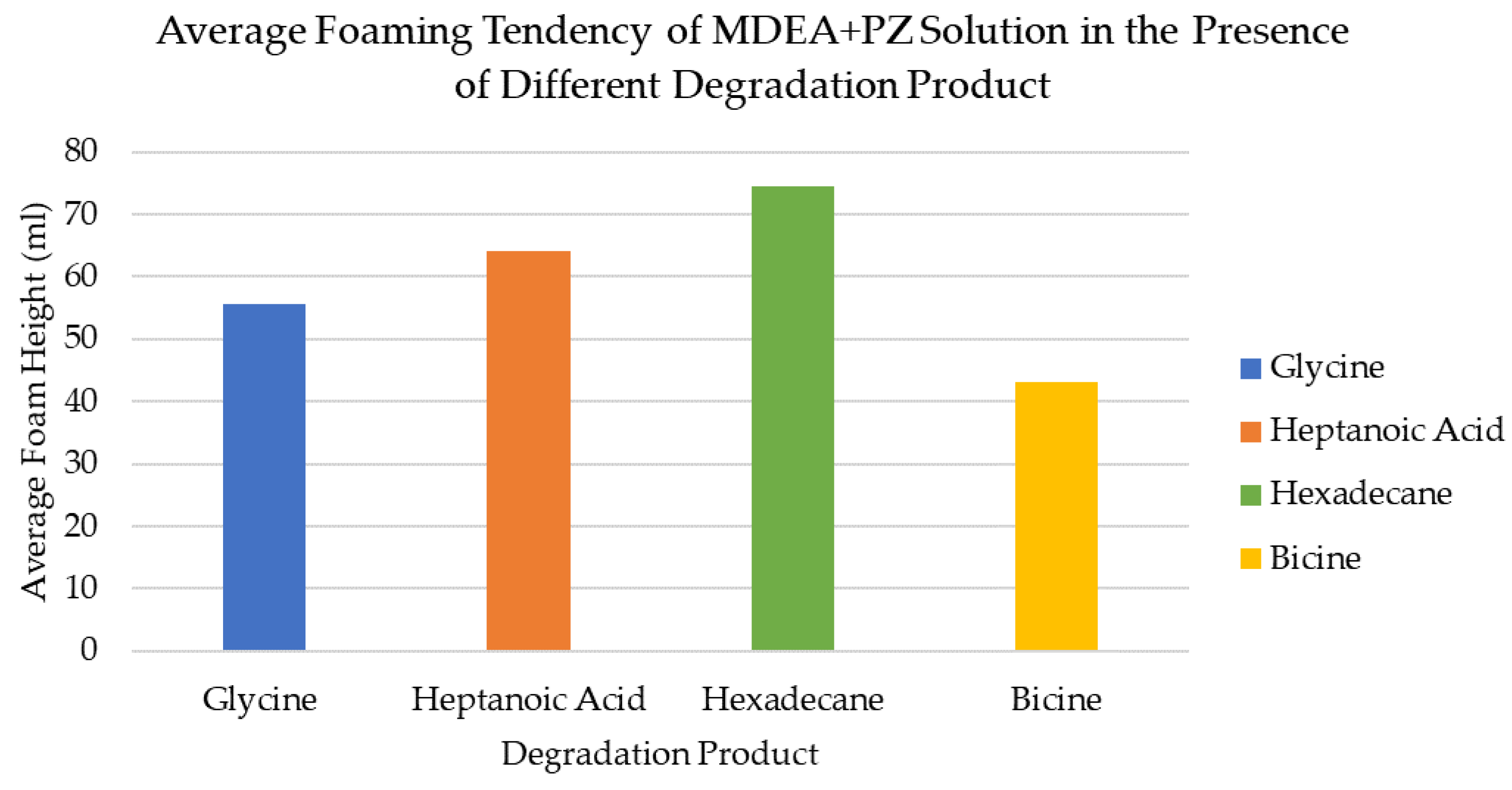
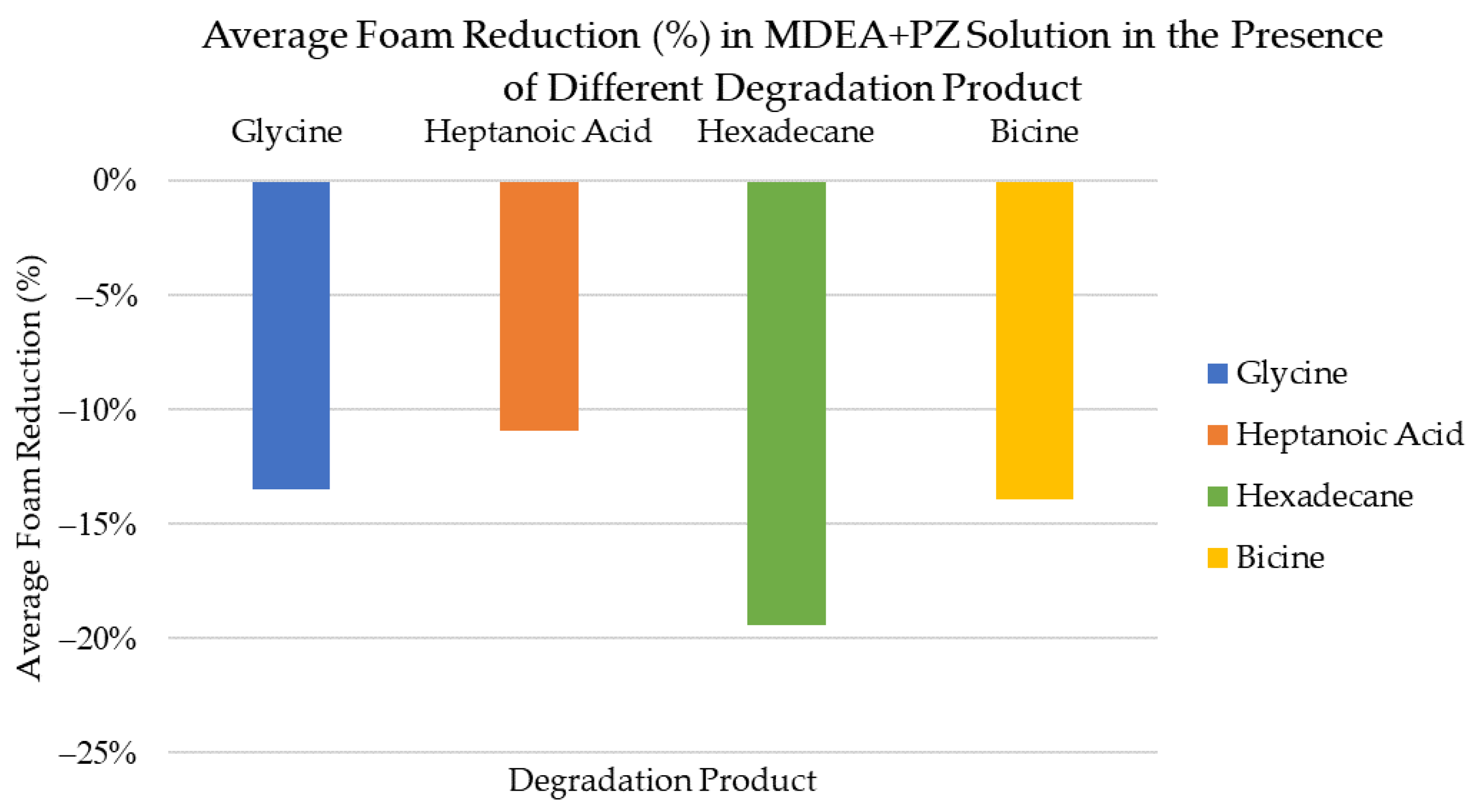
3.9. Comparing Foaming Tendency and Antifoaming Performance
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Vega, F.; Cano, M.; Camino, S.; Fernández, L.M.G.; Portillo, E.; Navarrete, B. Solvents for Carbon Dioxide Capture. In Carbon Dioxide Chemistry, Capture and Oil Recovery; Chapter 8; IntechOpen: London, UK, 2018. [Google Scholar]
- Shoukat, U.; Knuutila, H.K. Effect of Various Parameters on the Thermal Stability and Corrosion of CO2-Loaded Tertiary Amine Blends. Energies 2020, 13, 2626. [Google Scholar] [CrossRef]
- Cao, Y.; Rehman, Z.U.; Ghasem, N.; Al-Marzouqi, M.; Abdullatif, N.; Nakhjiri, A.T.; Ghadiri, M.; Rezakazemi, M.; Marjani, A.; Pishnamazi, M.; et al. Intensification of CO2 absorption using MDEA-based nanofluid in a hollow fibre membrane contactor. Sci. Rep. 2021, 11, 2649. [Google Scholar] [CrossRef] [PubMed]
- Alhseinat, E.; Pal, P.; Ganesan, A.; Banat, F. Effect of MDEA degradation products on foaming behavior and physical properties of aqueous MDEA solutions. Int. J. Greenh. Gas Control. 2015, 37, 280–286. [Google Scholar] [CrossRef]
- Alhseinat, E.; Pal, P.; Keewan, M.; Banat, F. Foaming study combined with physical characterization of aqueous MDEA gas sweetening solutions. J. Nat. Gas Sci. Eng. 2014, 17, 49–57. [Google Scholar] [CrossRef]
- Bahadori, A. Natural Gas Sweetening. In Natural Gas Processing; Bahadori, A., Ed.; Gulf Professional Publishing: Boston, MA, USA, 2014; Chapter 10; pp. 483–518. [Google Scholar]
- Rohani, M.; Jazayeri-Rad, H.; Behbahani, R.M. Continuous Prediction of the Gas Dew Point Temperature for the Prevention of the Foaming Phenomenon in Acid Gas Removal Units Using Artificial Intelligence Models. Int. J. Comput. Intell. Syst. 2017, 10, 165–175. [Google Scholar] [CrossRef] [Green Version]
- Jameh, A.A.; Mokhatab, S.; Gharaghoosh, A.Z.; Shazadeh, A.G. Hydrocarbon Processing: Is Your Antifoam Compatible with the Amine System? Sarkhoon & Qeshm Gas treating Company: Bandar Abbas, Iran, 2011. [Google Scholar]
- Heldebrant, D.J.; Koech, P.K.; Glezakou, V.A.; Rousseau, R.; Malhotra, D.; Cantu, D.C. Water-Lean Solvents for Post-Combustion CO2 Capture: Fundamentals, Uncertainties, Opportunities, and Outlook. Chem. Rev. 2017, 117, 9594–9624. [Google Scholar] [CrossRef]
- Butwell, K.F.; Kroop, L. Fundamentals of Gas Sweetening. 1983, pp. 1–32. Available online: https://pacs.ou.edu/media/filer_public/2e/c3/2ec3ca43-586d-4b30-ad64-a8dfcfd87a4d/4_fundamentals_of_gas_sweetening_by_k_f_butwell_and_l_kroop.pdf (accessed on 1 December 2022).
- Vardar-Sukan, F. Foaming: Consequences, Prevention and Destruction. Biotechnol. Adv. 1998, 16, 913–948. [Google Scholar] [CrossRef]
- Koczo, K.; Quinn, D.G. Low-Foaming Gas Processing Compositions And Uses Thereof. U.S. Patent 2007/0244205 A1, 18 October 2007. [Google Scholar]
- Quinn, D.G. Silicone Antifoam Composition And Method Using Same. U.S. Patent 20080121104A1, 29 May 2008. [Google Scholar]
- Keewan, M.; Banat, F.; Alhseinat, E.; Zain, J.; Pal, P. Effect of operating parameters and corrosion inhibitors on foaming behavior of aqueous methyldiethanolamine solutions. J. Pet. Sci. Eng. 2018, 165, 358–364. [Google Scholar] [CrossRef]
- Ratman, I.; Kusworo, T.D.; Ismail, A.F. Foam Behaviour of An Aqueous Solution of Piperazine- N-Methyldiethanolamine (MDEA) Blend as A Function of The Type of Impurities and Concentrations. Internat. J. Waste Resour. 2011, 1, 7–12. [Google Scholar] [CrossRef]
- Alhseinat, E.; Keewan, M.; Banat, F. Impact of dissolved and undissolved organics on foaming of industrial amine. Int. J. Greenh. Gas Control. 2017, 60, 156–161. [Google Scholar] [CrossRef]
- Thitakamol, B.; Veawab, A.; Aroonwilas, A. Foaming in amine-based CO2 capture process: Experiment, modeling and simulation. Energy Procedia 2009, 1, 1381–1386. [Google Scholar] [CrossRef] [Green Version]
- Fatemi, M.; Shahraki, B.H. An Experimental and Theoretical Analysis of Foam Formation in the Sour Gas Sweetening Process. Iran. J. Oil Gas Sci. Technol. 2018, 7, 79–89. [Google Scholar]
- Karakashev, S.I.; Grozdanova, M.V. Foams and antifoams. Adv. Colloid Interface Sci. 2012, 176–177, 1–17. [Google Scholar] [CrossRef]
- Frank, A.; Scholz, W. Defoamers in the Coatings Industry. CHIMIA 2002, 56, 177–183. [Google Scholar] [CrossRef]
- Owen, M.J. Foam Control. In Advances in Silicones and Silicone-Modified Materials; ACS Symposium Series; American Chemical Society: Washington, DC, USA, 2010; Volume 1051, pp. 269–283. [Google Scholar]
- Fink, J. Defoamers. In Petroleum Engineer’s Guide to Oil Field Chemicals and Fluids, 2nd ed.; Fink, J., Ed.; Chapter 22; Gulf Professional Publishing: Boston, MA, USA, 2015; pp. 775–785. [Google Scholar]
- Onoghwarite, O.E.; Okeoghene, E.A.; Ovonomo, O.; Ufuoma, O.B.; Ikechukwu, O.P. Performance Evaluation of Polydimethylsiloxane-Solvent Blends as Defoamer for Crude Oil Foam. In Proceedings of the IOP Conference Series: Materials Science and Engineering, Ota, Nigeria, 9–13 July 2018; pp. 1–6. [Google Scholar]
- Chen, X.; Freeman, S.A.; Rochelle, G.T. Foaming of aqueous piperazine and monoethanolamine for CO2 capture. Int. J. Greenh. Gas Control 2011, 5, 381–386. [Google Scholar] [CrossRef]
- Kikhavani, T.; Sademirinejad, M.; Tavakolmoghadam, M. Investigating the Foaming Phenomenon in the Absorption Tower of Gas Refinery Using Response Surface Methodology and Determining the Effective Antifoam. Pet Sci. 2018, 27, 116–127. [Google Scholar] [CrossRef]
- Mota-Martinez, M.T.; Samdani, S.; Berrouk, A.S.; Kroon, M.C.; Peters, C.J. Effect of Additives on the CO2 Absorption in Aqueous MDEA Solutions. Ind. Eng. Chem. Res. 2014, 53, 20032–20035. [Google Scholar] [CrossRef] [Green Version]
- Ohta, K. Method of Defoaming Amine Solutions. U.S. Patent 4313917, 2 February 1982. [Google Scholar]
- Mudhasakul, S.; Ku, H.-m.; Douglas, P.L. A simulation model of a CO2 absorption process with methyldiethanolamine solvent and piperazine as an activator. Int. J. Greenh. Gas Control 2013, 15, 134–141. [Google Scholar] [CrossRef]
- Nwaoha, C.; Tontiwachwuthikul, P.; Benamor, A. CO2 capture from water-gas shift process plant: Comparative bench-scale pilot plant investigation of MDEA-PZ blend vs novel MDEA activated by 1,5-diamino-2-methylpentane. Int. J. Greenh. Gas Control 2019, 82, 218–228. [Google Scholar] [CrossRef]
- Khan, A.A.; Halder, G.N.; Saha, A.K. Experimental investigation on efficient carbon dioxide capture using piperazine (PZ) activated aqueous methyldiethanolamine (MDEA) solution in a packed column. Int. J. Greenh. Gas Control 2017, 64, 163–173. [Google Scholar] [CrossRef]
- Thitakamol, B.; Veawab, A. Foaming Behavior in CO2 Absorption Process Using Aqueous Solutions of Single and Blended Alkanolamines. Ind. Eng. Chem. Res. 2008, 47, 216–225. [Google Scholar] [CrossRef]
- Fredriksen, S.B.; Jens, K.-J. Oxidative Degradation of Aqueous Amine Solutions of MEA, AMP, MDEA, Pz: A Review. Energy Procedia 2013, 37, 1770–1777. [Google Scholar] [CrossRef] [Green Version]
- ASTM D 892-03; Standard Test Method for Foaming Characteristics of Lubricating Oils. ASTM International: West Conshohocken, PA, USA, 2003.
- Pal, P.; AbuKashabeh, A.; Al-Asheh, S.; Banat, F. Role of aqueous methyldiethanolamine (MDEA) as solvent in natural gas sweetening unit and process contaminants with probable reaction pathway. J. Nat. Gas Sci. Eng. 2015, 24, 124–131. [Google Scholar] [CrossRef]
- Mao, J.; Chen, T.; Guo, L.; Yang, S.; Xu, X.; Ma, J.; Hu, J. Effect of Additives on the Foam Behavior of Aviation Coolants: Tendency, Stability, and Defoaming. ACS Omega 2020, 5, 17686–17691. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Huang, X.; He, L.; Luo, X. Foaming of Oils: Effect of Poly(dimethylsiloxanes) and Silica Nanoparticles. ACS Omega 2019, 4, 6502–6510. [Google Scholar] [CrossRef] [Green Version]
- Jha, B.K.; Christiano, S.P.; Shah, D.O. Silicone Antifoam Performance: Correlation with Spreading and Surfactant Monolayer Packing. Langmuir 2000, 16, 9947–9954. [Google Scholar] [CrossRef]
- Renpu, W. Measures for Putting a Well into Production. In Advanced Well Completion Engineering, 3rd ed.; Gulf Professional Publishing: Oxford, UK, 2011; pp. 417–523. [Google Scholar]
- Al Rumaih, M. Acid Gas Removal Unit Successful Switch from Silicon to Polyglycol Antifoam to Eliminate Foaming. In Proceedings of the Abu Dhabi International Petroleum Exhibition & Conference, Abu Dhabi, United Arab Emirates, 11–14 November 2019; pp. 1–16. [Google Scholar]
- Denkov, N.D. Mechanisms of Foam Destruction by Oil-Based Antifoams. Langmuir 2004, 20, 9463–9505. [Google Scholar] [CrossRef]
- Golubeva, I.A.; Dashkina, A.V.; Shulga, I.V. Demanding Problems of Amine Treating of Natural Gas: Analysis and Ways of Solution. Pet. Chem. 2020, 60, 45–50. [Google Scholar] [CrossRef]
- Chang, Q. Emulsion, Foam, and Gel. In Colloid and Interface Chemistry for Water Quality Control; Chemical Industry Press: Beijing, China, 2016; pp. 227–245. [Google Scholar]










| Classification | Types of Antifoams | Ref. |
|---|---|---|
| Non-polar Antifoam |
| [8] |
| Polar Antifoam |
| [8] |
| [12] [10] | |
| Solid Antifoam |
| [13] |
| [8] |
| Purpose | Details | Ref. |
|---|---|---|
| To investigate the tendency of foaming in aqueous MDEA with the presence of BHCL and HCB corrosion inhibitors. |
| [14] |
| To investigate the behavior of foam in MDEA in the presence of THEED, HEED, bHEP, bicine, acetic acid and octanoic acid as a degradation product. |
| [4] |
| To evaluate the foaming behavior of MDEA when subjected to various degradation products and contaminants. |
| [5] |
| To study the effect of impurities in the natural gas stream on the characteristic of foam behavior in MDEA and PZ solution. |
| [15] |
| To investigate the effect of dissolved and undissolved organics in MDEA solution. |
| [16] |
| To investigate foaming behavior in MEA solution in the presence of various degradation product. |
| [17] |
| To investigate the foaming behavior on the removal of CO2 in DEA solution. |
| [18] |
| Silicone Antifoam | Solvent Used | Details | Ref. |
|---|---|---|---|
| Dow Corning Q2-3183 (Mixture of silicone and polyether glycol) | PZ |
| [24] |
| MDEA, MEA |
| [25,26] |
| MDEA, MEA |
| [26] |
| Polydimethylsiloxane (100 cst and 300 sct) | DIPA, DEA |
| [27] |
| Temperature (°C) | Density (g/cm3) | Surface Tension (mN/m) |
|---|---|---|
| 55 | 1.039 | 47.95 |
| 65 | 1.033 | 46.57 |
| 75 | 1.026 | 44.94 |
| 85 | 1.019 | 43.96 |
| Temperature (°C) | Density (g/cm3) | Surface Tension (mN/m) |
|---|---|---|
| 55 | 1.012 | 38.68 |
| 65 | 1.006 | 37.83 |
| 75 | 0.997 | 37.35 |
| 85 | 0.987 | 36.54 |
| Temperature (°C) | Density (g/cm3) | Surface Tension (mN/m) |
|---|---|---|
| 55 | 1.016 | 44.95 |
| 65 | 1.009 | 43.10 |
| 75 | 1.002 | 42.16 |
| 85 | 0.994 | 40.69 |
| Temperature (°C) | Density (g/cm3) | Surface Tension (mN/m) |
|---|---|---|
| 55 | 1.034 | 46.83 |
| 65 | 1.026 | 45.59 |
| 75 | 1.019 | 43.69 |
| 85 | 1.009 | 42.7 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ng, E.L.S.; Lau, K.K.; Chin, S.Y.; Lim, S.F. Foam and Antifoam Behavior of PDMS in MDEA-PZ Solution in the Presence of Different Degradation Products for CO2 Absorption Process. Sustainability 2023, 15, 1608. https://doi.org/10.3390/su15021608
Ng ELS, Lau KK, Chin SY, Lim SF. Foam and Antifoam Behavior of PDMS in MDEA-PZ Solution in the Presence of Different Degradation Products for CO2 Absorption Process. Sustainability. 2023; 15(2):1608. https://doi.org/10.3390/su15021608
Chicago/Turabian StyleNg, Eileen Li Shien, Kok Keong Lau, Sim Yee Chin, and Soh Fong Lim. 2023. "Foam and Antifoam Behavior of PDMS in MDEA-PZ Solution in the Presence of Different Degradation Products for CO2 Absorption Process" Sustainability 15, no. 2: 1608. https://doi.org/10.3390/su15021608
APA StyleNg, E. L. S., Lau, K. K., Chin, S. Y., & Lim, S. F. (2023). Foam and Antifoam Behavior of PDMS in MDEA-PZ Solution in the Presence of Different Degradation Products for CO2 Absorption Process. Sustainability, 15(2), 1608. https://doi.org/10.3390/su15021608






