Abstract
Ibuprofen (IBU) and ketoprofen (KET) are among the world’s most popular and widely used nonsteroidal anti-inflammatory drugs (NSAIDs). Due to their high usage, these drugs have entered the environment, including the soil, and, like any other chemical compound, can have a negative effect on it. Therefore, an attempt was made to evaluate the effects of these two popular drugs on soil bacteria and fungi, the bivalve crustaceans (Heterocipris incongruens) and the growth and development of spring barley. The tested drugs did not show any negative effects on the total bacterial abundance. Effects were observed on the growth and survival of H. incongruens and on the abundance of fungi in the soil at the highest concentrations tested. The presence of IBU and KET in the soil in which spring barley was cultivated caused an increase in the activity of antioxidant enzymes catalase (CAT), superoxide dismutase (SOD), and guaiacol peroxidase (POD); an increase in the content of proline and ascorbic acid (AsA) in the seedlings of this grain; and a decrease in the yield of fresh plant weight, especially at the application of concentrations of 100 and 1000 mg·kg−1 of soil’s dry weight (DW). Effects on barley seed germination potential and germination capacity, plant dry matter content, assimilation pigment content and malondialdehyde (MDA) were also observed at the highest concentrations.
1. Introduction
Nonsteroidal anti-inflammatory drugs (NSAIDs) comprise a large group of drugs with analgesic, anti-inflammatory and antipyretic effects used in oral form as well as external application (e.g., ointments, gels) by humans and in the veterinary field [1]. Consumption of these drugs worldwide is very high and varies considerably from country to country. NSAIDs include drugs such as ibuprofen, ketoprofen, naproxen, diclofenac, indomethacin, nimesulide, meloxicam and celecoxib. The fact that they are readily available, often without a prescription, is contributing to the high consumption of these drugs [1,2,3]. Medications taken by humans or animals are retained in the body only to a small extent. A significant portion of them is removed from the body in their primary form or as metabolites formed during their metabolism with urine or feces. High intake of NSAIDs results in large amounts of them entering the environment through wastewater treatment plants, where they are disposed of at low rates in rivers, surface runoff or from farm discharges [4,5,6]. Animal excrement, industrial waste or municipal waste can also be a source of NSAIDs in the environment [6]. NSAIDs are most often detected in the environment at low concentrations (from 1 ng·dm−3 to 1 mg·dm−3); however, considering the continuous influx of this pollutant into the environment, a guess could be hazarded that these drugs can have a significant impact on the aquatic and terrestrial ecosystem. The available literature reports make it clear that we can find these compounds not only in water or soil but also in plants, animals or benthos [7,8].
Accordingly, a number of international organizations and research groups, i.e., the Organization for Economic Co-operation and Development, OECD; International Organization for Standardization, ISO; and European Food Safety Authority, EFSA (also European Commission, Chief Inspectorate of Environmental Protection in Poland, Food and Drug Administration (FDA) in the USA, etc.), have developed a number of toxicological standards by which this type of pollution in the environment can be studied and controlled. The European Commission and other similar bodies are trying to adapt existing legislation to best control the pollution of various types of active substances and to counter environmental pollution that could threaten the functioning of individual terrestrial and aquatic biosystems. Examples of such measures are the EU Water Framework Directive and the Directive on Priority Substances, which provide guidelines for identifying substances that could pose a potential threat to surface waters. These directives provide a legal basis under which member states are required to monitor and comply with the environmental quality standards (EQS) set for these substances [1,9].
Unfortunately, despite numerous measures taken, we still know little about the concentrations of pharmaceuticals in soil and water. The situation is also complicated by the fact that the behavior of NSAIDs, like that of other chemical compounds, is influenced by various parameters, such as insolation, pH, temperature and the soil’s physical and chemical properties. Under the influence of these parameters, drugs can be transformed into various types of derivatives, which can be more harmful to the environment than the substances from which they were derived. In addition, it is important to remember that drugs in the environment do not occur individually but in various mixtures [2,6].
Ibuprofen and ketoprofen are among the most popular NSAIDs. They belong to the first-generation NSAIDs, which act by inhibiting the cyclooxygenases COX1 and COX2. Both drugs are propionic acid derivatives that exhibit anti-inflammatory, analgesic and antipyretic effects. These drugs are frequently detected in wastewater and freshwater ecosystems around the world [10]. We know from literature reports that ibuprofen can affect the life and development of aquatic organisms, i.e., fish, mollusks or crustaceans. It can also affect insects. Ketoprofen also can affect, among others, fish in their embryonic stage or the crustacean Daphnia magna [10,11].
NSAIDs, like other chemical compounds, can be adsorbed into particles of solid bottom sludge or soil particles, which can eventually lead to their accumulation and subsequent slow entry into the ecosystem. Agricultural use of sewage sludge, which the drugs have accumulated in, results in their dissemination into the environment. This leads to the introduction of these compounds into the soils where crops are grown. These, in turn, while taking up water and nutrients, can simultaneously take up NSAIDs and accumulate and transform them inside their tissues. NSAIDs, like other drugs, depending on the plant species, type of drug, concentration, length of exposure or developmental stage of the plant, can cause oxidative stress in plants. These plants are then eaten by animals and humans, which can have negative health effects [2,12,13,14].
There are numerous reports in the scientific literature on the negative effects of various types of drugs, including NSAIDs, on plants (i.e., Lemna minor, Raphanus sativus var. sativus, Lettuce sativa), algae (i.e., Acutodesmus obliquus, Scenedesmus obliquus), bacteria (i.e., Alivibrio fisheri), planktonic crustacean (i.e., Daphnia magna), fish (i.e., Cyprinus carpio, Danio rerio) and birds (Gyps) [3,12,14]. The most high-profile case of adverse effects of drugs on the environment was the effect of diclofenac on the decline of several vulture species found in India, Pakistan and Nepal. Studies conducted indicated that these birds consumed dead cattle, in tissues of which diclofenac nephrotoxic effect amounts were found and caused their death [9].
Despite numerous ongoing studies, the mechanism of action of the drugs and the products breakdown in individual organisms has not yet been discovered.
Therefore, in order to broaden the knowledge of the NSAIDs impact on the environment, especially the soil environment, this study attempted to evaluate the impact of ibuprofen, ketoprofen and their mixture on various elements of the trophic chain, i.e., the total abundance of soil bacteria and fungi, H. incongruens crustaceans and spring barley.
2. Materials and Methods
2.1. Materials
The NSAIDs ibuprofen (IBU), (±)-2-(4-isobutylphenyl)propanoic acid (≥98% purity), and ketoprofen (KET), 2-(3-benzoylphenyl)propionic acid (≥98% purity), used in the study were purchased from Sigma–Aldrich Chemical Co. (Poznań, Poland).
2.2. Total Number of Bacteria and Fungi
The study followed the procedure of Cyconia et al. [15] with minor alterations. Soil samples were collected during the establishment of the plant culture (1 day) and during the liquidation of the culture after 14 days. Then, 10 g samples of each of them were placed in Erlenmeyer flasks containing 90 mL of 0.85% sterile NaCl solution and shaken for 10 min. Serial dilutions of the soil suspension were prepared. To determine the total bacterial abundance, samples from the respective dilutions were seeded by surface culture on tryptic soy agar and incubated at 27 °C for 48 h. To determine total fungal abundance, samples from the respective dilutions were seeded by the surface culture method on DRBC Dichloran Rose Bengal Chloramphenicol medium and incubated at 22 °C for 7 days. The total abundance of cultured bacteria and fungi was expressed as CFU (colony forming unit)/g DW of soil [15].
2.3. Ostracodtoxkit F Test
A 6-day chronic toxicity microbiotest for mortality and growth inhibition of Heterocypris incongruens was performed. The tests were conducted using the Ostracodtoxkit F test (MicroBioTests Inc., Ghent, Belgium). The test was also carried out on soil without the IBU, KET and IBU + KET addition to eliminate the effect of the soil on the bivalves. Mortality (%) and growth inhibition (%) of H. incongruens crustaceans were determined.
2.4. Spring Barley Culture
Plants were grown in a vegetation hall. The tests follow the guidelines of the OECD/OCDE 208/2006 guide [16] and the PN-EN ISO 11269-2 standard [17]. The plant material was spring barley (Hordeum vulgare L., cv. Suweren). IBU, KET and mixture IBU + KET were applied at concentrations of 0.1, 1, 10, 50, 100, 1000 mg∙kg−1 dry weight of soil. The content of NSAIDs in the mixture was 1:1. Twenty identical seeds were sown in pots containing soil (loamy sand, pH(KCl) = 6.0, organic carbon—8.5 g∙kg−1, 11% fraction content of <0.02 mm in diameter) with NSAIDs supplementation and control soil (non-NSAIDs content). All tests were performed four times. Throughout the testing period, constant soil moisture (70% ppw), constant temperature of 20 ± 2 °C and constant illumination at 170 μmol∙m−2∙s−1 were maintained on a 16 h day/8 h night schedule. Fourteen days after sowing seeds into the soil, plant photographs were taken, and the obtained plant material was used for detailed analyses.
2.5. Determination of Growth Responses
Seed germination potential (GP) and seed germination rate (GR) were calculated according to the recommendations by Liu et al. [18].
Length inhibition of above-ground plant parts and plant roots was measured as described by Wang et al. [19].
Dry matter was determined using the method described by Kowalska [20]: 1 g fresh weight of spring barley plants was dried at 105 °C until solid. The dry matter content is given in g∙g−1 of the fresh weight (FW).
2.6. Determination of Photosynthetic Pigments Content
The content of chlorophyll a, chlorophyll b and carotenoids were determined by the method presented by Oren et al. [21]. First, 200 mg of fresh weight of spring barley leaves were homogenized with an 80% solution of chilled acetone and centrifuged, and the filtrate was made up to a volume of 25 cm3. The content of individual photosynthetic pigments was determined by measuring absorbance at wavelengths of 470 nm, 647 nm and 664 nm.
2.7. Determination of Malondialdehyde and Hydrogen Peroxide Content
We homogenized 0.5 g of fresh leaves weight with 0.1% trichloroacetic acid solution at 4 °C and then centrifuged the mixture.
Thiobarbituric acid was used as a substrate for the malondialdehyde (MDA) determination according to the procedure described by Hodges et al. [22]. The reaction mixture contained supernatant, thiobarbituric acid and phosphate buffer. The mixture was heated 30 min at 95 °C. It was then cooled in an ice bath, and the absorbance was measured at 532 nm and 600 nm wavelengths. The MDA content was calculated using an extinction coefficient of 155 nm−1∙cm−1. The results are expressed in μmol∙g−1 FW.
The hydrogen peroxide content was determined according to the procedure provided by Singh et al. [23]. A reaction mixture containing supernatant, phosphate buffer and potassium iodide was prepared to determine H2O2 content. The samples were incubated in the dark for 1h, and then the absorbance was measured at 390 nm. H2O2 content was calculated using an extinction coefficient of 155 nm−1∙cm−1 for this purpose. H2O2 content was expressed in μmol∙g−1 FW.
2.8. Determination of AsA Content
Ascorbic acid (AsA) concentration was determined according to the procedure proposed by Law et al. [24]. First, 0.5 g of fresh spring barley leaves was homogenized in 10% trichloroacetic acid and then centrifuged the mixture. The reaction mixture contained supernatant, TCA, H3PO4, bipyridyl in ethanol and FeCl3. After mixing, the samples were incubated at 37 °C for 60 min, and the absorbance was measured at 525 nm. AsA content was read from the standard curve.
2.9. Determination of Free Proline Content
The free proline content was determined using the spectrophotometric method according to the procedure provided by Bates et al. [25]. First, 0.5 g of fresh spring barley plant sample was homogenized with 3% sulfosalicylic acid, then centrifuged. The reaction mixture contained supernatant, glacial acetic acid and ninhydrin acid dissolution. The samples were heated for 1 h at 100 °C, and then the reaction was stopped on an ice bath. Toluene was added to the solution and extracted. Absorbance was measured at 520 nm. The proline content was expressed in mg∙g−1 FW.
2.10. Determination of Superoxide Dismutase Activity
We homogenized 0.5 g of fresh leaf mass with the addition of a chilled (4 °C) extraction mixture containing phosphate buffer, EDTA solution and polyvinylpyrrolidone (PVP) solution. The supernatant obtained after centrifugation was used to determine the activity of the enzymes tested and the protein content.
The spectrophotometric method proposed by Giannopolitis and Ries [26] was used to determine the activity of superoxide dismutase (SOD). The reaction mixture consisted of methionine, riboflavin, NBT, phosphate buffer (pH 7.8) and supernatant. The mixtures were exposed in identical glass tubes. An identical series of samples were prepared at the same time, which were not illuminated and served as reference samples in spectrophotometric measurements. Absorbance was measured at 560 nm. SOD activity was expressed in U∙mg−1 of protein. U—one unit of SOD activity—corresponds to the amount of enzyme causing 50% inhibition of the rate of NBT reduction reaction.
2.11. Determine of Catalase Activity
The spectrophotometric method was used to determine catalase activity (CAT) according to the procedure provided by Liu et al. [27]. The reaction was initiated by mixing enzyme extract, phosphate buffer (pH 7.8) and H2O2. CAT activity was defined as the amount of enzyme that degrades H2O2 in 1 min at 240 nm.
2.12. Determination of Guaiacol Peroxidase Activity
Guaiacol peroxidase (POD) activity was determined using a spectrophotometric method according to the procedure proposed by Abassi et al. [28]. The oxidation rate of guaiacol in the presence of H2O2 for 1 min at 470 nm was measured. POD activity was expressed as U∙mg−1 protein∙min−1.
2.13. Determination of Ascorbate Peroxidase Activity
Ascorbate peroxidase (APX) activity was determined using a spectrophotometric method according to the procedure provided by Nakano and Asada [29]. The reaction mixture contained enzyme extract, ascorbate, H2O2 and phosphate buffer (pH 7.0). The oxidation level of ascorbate was measured at 290 nm.
2.14. Determination of Total Protein Content
The total protein content was determined using the procedure provided by Bradford [30]. The protein content was used to calculate the antioxidant enzyme activity.
2.15. Data Analysis
Obtained experimental data were statistically analyzed using one-way ANOVA followed by Tukey’s post hoc test in STATISTICA 13.3 software. The probability level was determined at p < 0.05. All determinations were performed in 4 replicates. The results are expressed as the arithmetic mean ± SD (standard deviation).
3. Results and Discussion
3.1. Total Number of Bacteria and Fungi
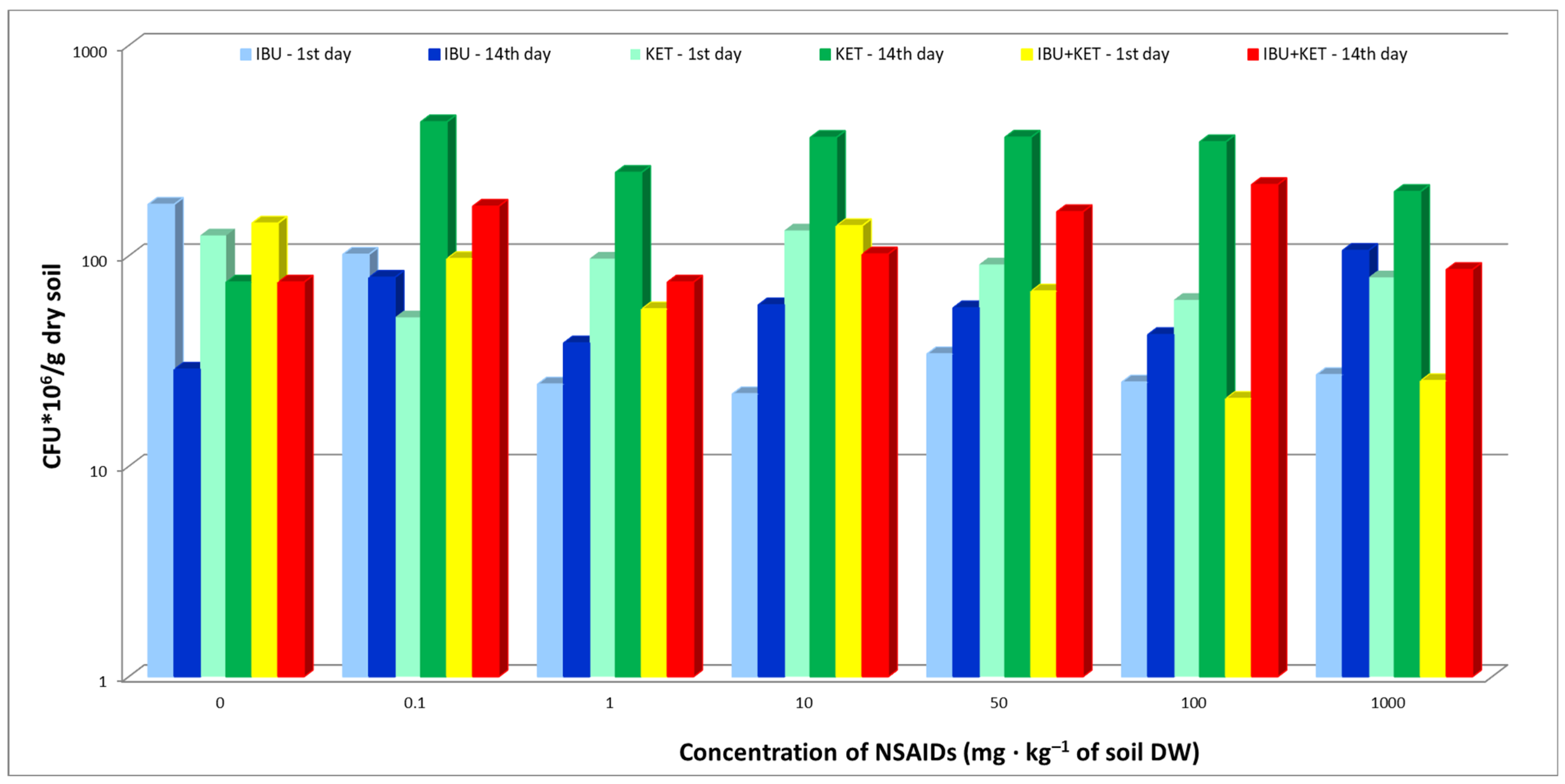
The present study evaluated the effects of IBU, KET and a mixture of IBU and KET on the abundance of bacteria in the soil. The results show an increase in bacterial abundance 14 days after the introduction of the tested NSAIDs into the soil compared to the results obtained for samples from the first day of the study, and this was regardless of the concentration and type of drug used. At the same time, it was observed that the presence of the tested NSAIDs in the soil causes an increase in bacterial counts compared to the control soil. On the first day of IBU tests, a decrease in bacterial counts was observed relative to the control. In contrast, in samples taken on the 14th day of the study, no effect of IBU on bacterial abundance was observed, which may be because bacteria exhibit the ability to biodegrade and biotransform ibuprofen, as confirmed by numerous scientific studies conducted by Chen and Rosazza [31] and Murdoch and Hay [32,33,34], among others. KET showed the greatest effect on the abundance of bacterial cultures (Figure 1).
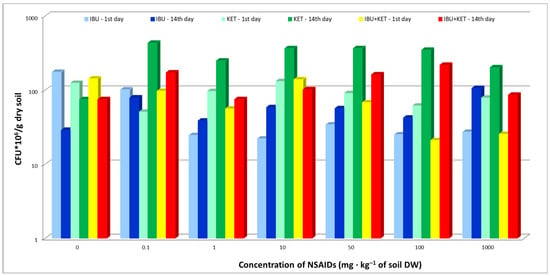
Figure 1.
Total abundance of culturable bacteria in control soil and soil with IBU, KET and IBU + KET mixture. * CFU—(colony forming unit)/g DW of soil.
The slight decrease in bacterial counts at the beginning of the experiment at all applied doses can be explained by the death of the drug-sensitive part of the microbial population. Jiang et al. [35] observed that selected NSAIDs affected not only the activity of individual microorganisms but also their entire community. In response to NSAIDs toxicity, microorganisms produce EPSs (enveloped exopolysaccharides), which promote their survival under stressful environmental conditions. This fact may explain the increase in culturable bacterial abundance in samples taken on day 14 for all concentrations of the NSAIDs used. In the mentioned study, a mixture of two NSAIDs was used, and no increase in toxicity of the mixture of ketoprofen and ibuprofen to bacteria was observed. Different results were obtained in the Jiang et al. study [35], which showed an increase in the toxicity of a three-NSAIDs mixture (naproxen, ibuprofen and diclofenac) compared to single substances. The differences obtained may be due to the type of mixture of drugs used, their concentration and the type of medium in which the bacterial counts were determined.
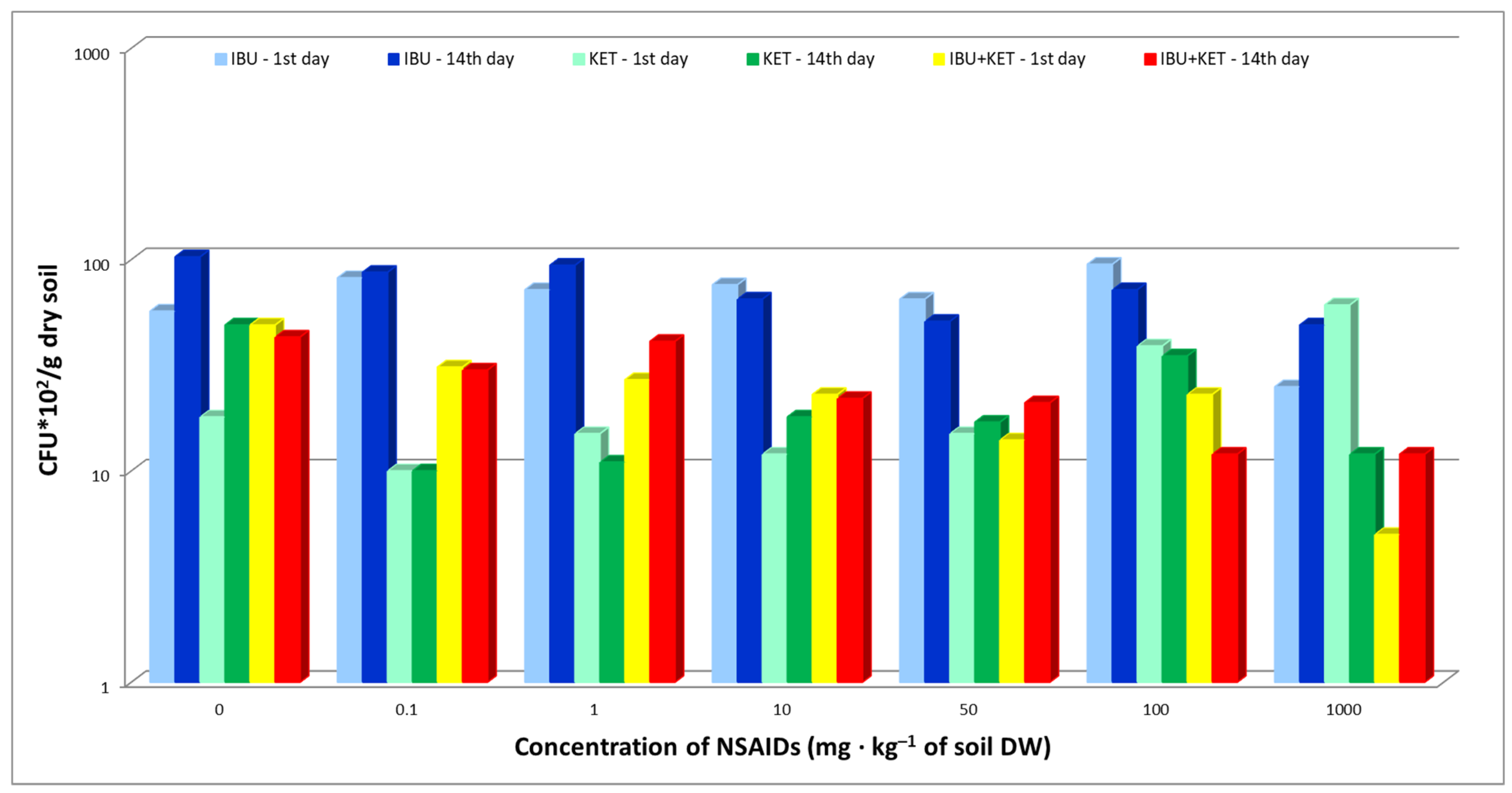
Fungal abundance in the soil after the introduction of IBU, KET and a mixture of these two NSAIDs was also evaluated. The study showed that the presence of the tested NSAIDs in the soil did not have a major effect on the abundance of fungi in the soil. A negative effect of IBU on fungal abundance was observed only at the highest dose of 1000 mg·kg−1 of soil DW, both in samples taken on day 1 and day 14. The use of a mixture of the tested NSAIDs had an adverse effect on the fungal population at a concentration of 1000 mg·kg−1 of soil DW in samples taken on day 1 compared to single drugs. The toxic effect of the IBU + KET mixture was minimized in samples taken on day 14 (Figure 2).
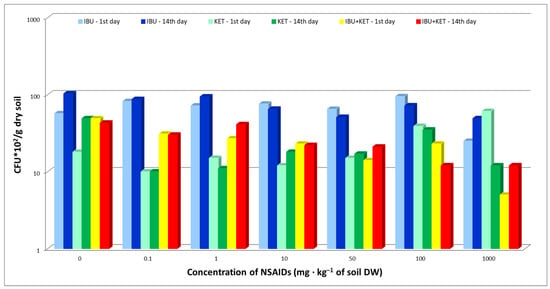
Figure 2.
Total abundance of culturable fungi in control soil and soil with IBU, KET and IBU + KET mixture. * CFU—(colony forming unit)/g DW of soil.
The results indicate a high degradation potential of pharmaceuticals in fungi, which is confirmed by the Tyumin et al. study [36]. Furthermore, in studies by Rodarte-Morales et al. [37], Gonda et al. [38] and Torán et al. [39], the authors indicate that IBU is a compound that is rapidly degraded by the fungi Eurotium amstelodami, Bipolaris tetramera, and Aspergillus nidulans and members of the Polyporales family, the so-called white rot fungi. Biodegradation of IBU will result in 1-hydroxyibuprofen, 2-hydroxyibuprofen and 1,2-dihydroxyibuprofen. Escuder-Gilabert et al. [40], studying the biodegradation of KET and IBU, proved that these compounds biodegrade readily in activated sludge.
3.2. Ostracodtoxkit F Test
Pollution of soils and bottom sediments poses a major threat to both soil and water environments. In the sediment, a huge number of different types of benthic organisms function. Heterocypris incongruens is a very common species found throughout the world. Crustaceans are very sensitive to various types of pollution of the environment in which they live, so they are an excellent bioindicator by which we can assess the impact of pollutants, i.e., NSAIDs on the soil environment [41,42]. Kudlak et al. [43] indicates that Ostracodtoxkit F is at least four times more sensitive (e.g., for 17α-ethinylestradiol) than other bioassays based on organisms such as Daphnia sp., Hyalella azteca or the algae Dunaliella tertiolecta—which are usually considered sensitive organisms. The authors believe that the greater sensitivity of H. incongruens to contaminants is related to the fact that these organisms feed at the boundary between sediment/soil and bottom water, which makes them susceptible to both soluble and insoluble contaminants reaching the organism via the oral route.
The study evaluated the effects of KET, IBU and their mixture on the growth of H. incongruens shellfish in a 6-day Ostracodtoxkit chronic toxicity test. The results obtained indicate that the tested drugs affect the growth of crustaceans H. incongruens and their mortality only at the highest tested concentrations of these compounds. The application of concentrations of 100 mg·kg−1 of soil DW IBU and a mixture of IBU and KET, as well as 50 and 100 mg·kg−1 of soil DW KET, caused inhibition of crustaceans’ growth. One hundred percent mortality of H. incongruens was observed when NSAIDs were applied at a concentration of 1000 mg·kg−1 of soil DW (Table 1).

Table 1.
Effect of IBU, KET, IBU + KET on H. incongruens.
The results obtained in the present study are supported by the literature. Kudłak et al. [43], in their work, showed that NSAIDs, including IBU and KET, affect the growth of H. incongruens and that these organisms are much more sensitive to drugs than other test organisms. In addition, Bownik et al. [10], studying the effects of KET on Daphnia magna, and Ramírez-Morales et al. [44], studying wastewater containing various types of drugs (including IBU and KET) from a wastewater treatment plant in Costa Rica, and Muniz-Gonzales et al. [11], studying the effects of IBU on Chironomus riparius, indicate that the NSAIDs tested affected the growth and development of the test organisms. However, due to the impact complexity and the fact that these drugs do not occur in the environment individually but in various types of mixtures, it should be concluded that further large-scale studies in this direction are necessary.
3.3. Plant Growth and Development
The first stage in the development of any plant is germination. It has a huge impact on the growth and development of plants and, consequently, on the yield and its quality. The correct course of this stage is influenced by many factors, including the presence of various types of stress factors. Germinating grains and emerging seedlings are often unable to cope with stressors present in the environment [45]. Due to the level of environmental pollution, this is one of the significant problems of modern agriculture.
To evaluate the effect of the tested NSAIDs, the potential and germination capacity of spring barley seeds and the inhibition of growth of roots and aboveground parts of this grain, fresh weight yield and dry matter content were determined.
Although the plants did not show visual signs of stress, other stress signals were observed. Analysis of the results obtained in the present study indicates that the greatest effect on seed germination potential was shown by IBU, the effect of which was observed already at a concentration of 10 mg·kg−1 of soil DW. KET and the IBU + KET mixture showed an effect on seed germination potential only at the highest concentrations. None of the tested drugs had a statistically significant effect on seed germination potential (Table 2).

Table 2.
Effect of IBU, KET and IBU + KET on the germination potential (GP) and germination rate (GR) of spring barley. Data shown represent the means of 4 experiments ± standard deviation. Different letters indicate statistically significant differences at p < 0.05.
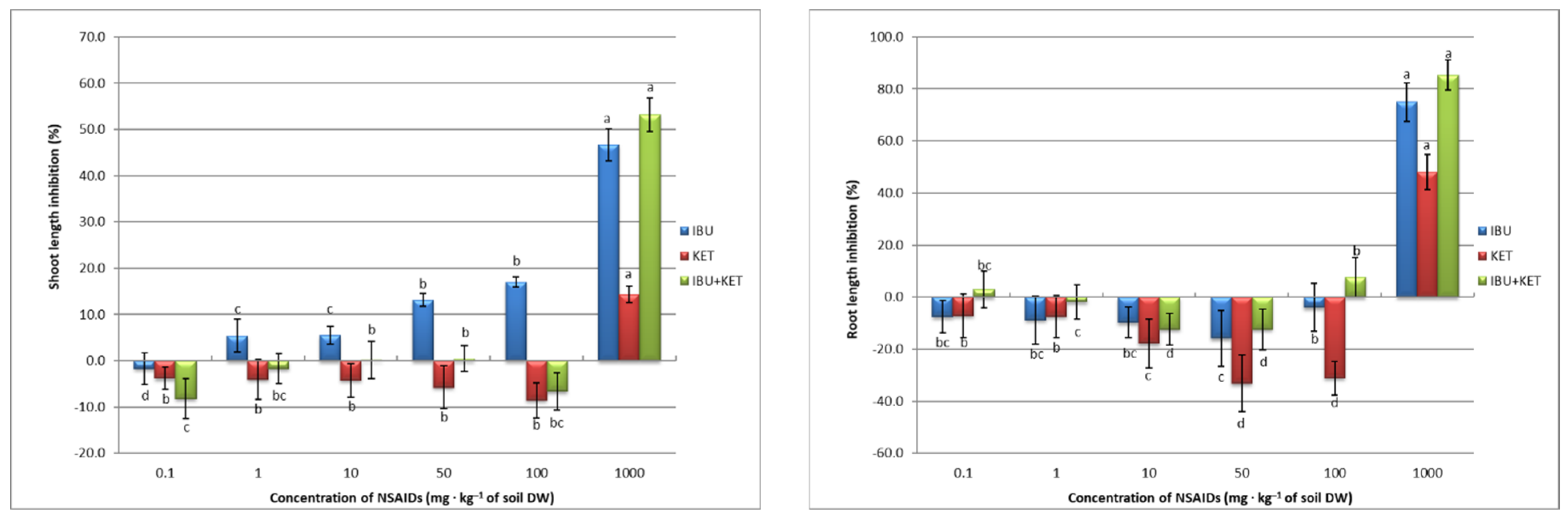
Seed germination has a direct effect on subsequent plant growth. Visible evidence of this is the inhibition of plant shoots and their roots the length, as well as the yield of plant fresh weight obtained. Root elongation is often considered one of the main measures of effect in plant toxicity studies, since the roots are the point of contact with any contaminants present in the soil and are unable to avoid them. In addition, contaminants can enter plants through the roots [46]. An increase in the concentration of IBU in the soil caused an increase in root length inhibition in spring barley. Inhibition was observed in this case with the application of IBU at concentrations of 50 mg·kg−1 of soil DW and higher. KET and the IBU + KET mixture caused root length inhibition only at a concentration of 1000 mg·kg−1 of soil DW. The NSAIDs tested, on the other hand, had a beneficial effect on the length of aboveground plant parts. Application of the tested drugs at concentrations of 10 and 50 mg·kg−1 of soil DW and, in the case of KET, also 100 mg·kg−1 of soil DW, not only did not cause growth inhibition but even accelerated the growth of spring barley seedlings. Only the application of a concentration of 1000 mg·kg−1 of soil DW, for all applied compounds, caused inhibition of shoot growth of spring barley seedlings (Figure 3).
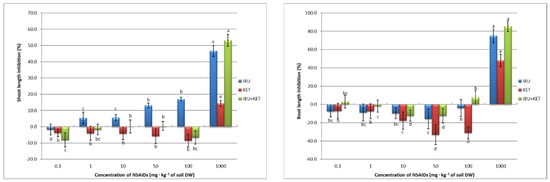
Figure 3.
Growth inhibition (%) of spring barley seedlings as a response to NSAIDs concentrations. Data shown represent the means of 4 experiments ± standard deviation. Different letters indicate statistically significant differences at p < 0.05.
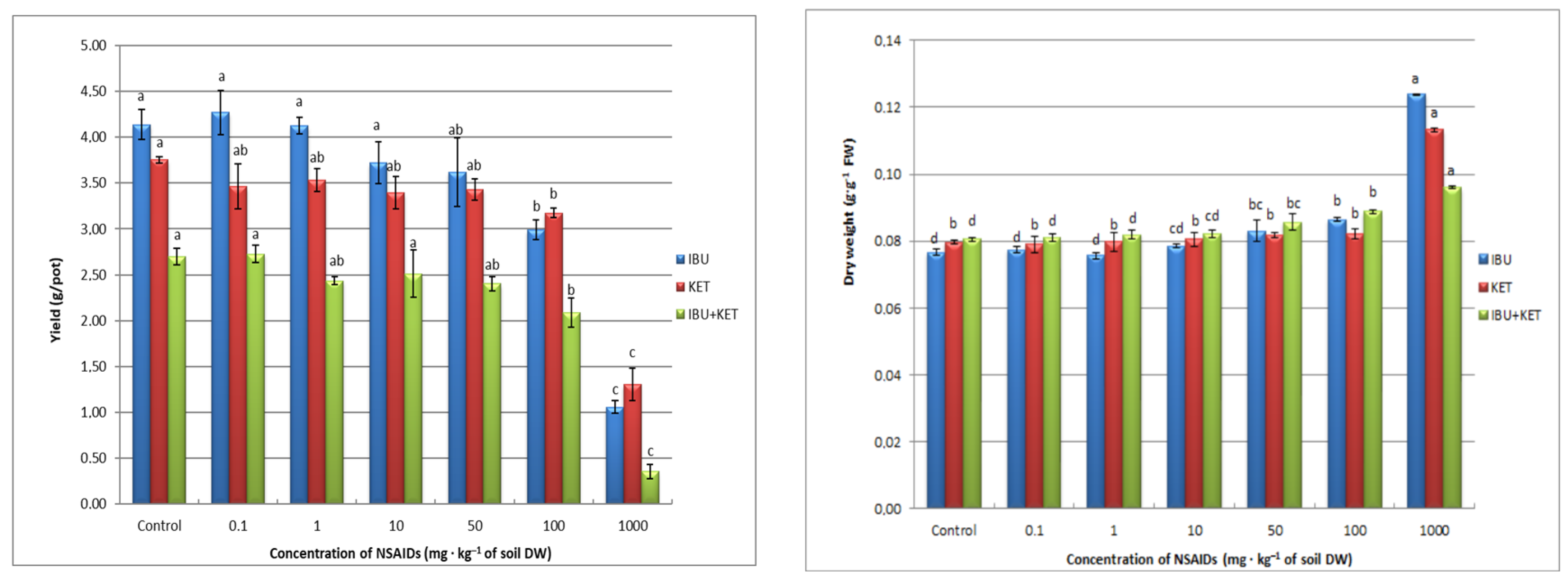
Inhibition of the length of aboveground plant parts has a direct effect on the fresh weight yield of spring barley seedlings. The presence in the soil of all tested NSAIDs causes a decrease in plant fresh weight yield. The application of IBU even causes a linear decrease in plant fresh weight as the concentration of the tested compound in the soil increases. The presence of KET and the drug mixture influences the plant fresh weight yield after applying the highest concentrations of the tested compounds (Figure 4).
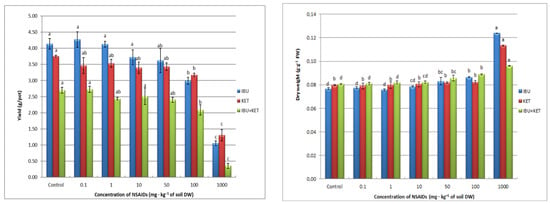
Figure 4.
Biomass production of spring barley seedlings affected by NSAIDs. Data shown represent the means of 4 experiments ± standard deviation. Different letters indicate statistically significant differences at p < 0.05.
A very important biomarker, indicating the effect of chemical compounds including drugs on plant growth, is change in the level of plant dry matter. In our study, we observed an increase in this parameter only with the highest concentration of the tested NSAIDs. The largest increase in dry matter content in spring barley seedlings is caused by IBU, and the smallest by the IBU + KET mixture (Figure 4).
The results obtained in the experiment were confirmed by studies conducted by other researchers. Schmidt et al. [14] studying the effect of NSAIDs on Raphanus sativum and Lactuca sativa indicated that the presence of the tested drugs in the medium in which the plants grow influenced increasing the length of the roots of these plants. In addition, Wang et al. [47] and Svobodnikova et al. [48], examining the effect of KET on rice seedlings and the effect of naproxen on peas, respectively, showed that low concentrations of drugs slightly stimulate plant growth, while high concentrations can significantly inhibit growth by reducing biomass and destroying roots. Moreover, Christou et al. [2], in their study, indicate that PHACs (pharmaceutically active compounds) can have effects on plants that depend on the drug concentration used. At lower concentrations, PHACs can cause hormesis in plants, while at higher concentrations, oxidative stress was observed. According to the authors, the hormonal effects of the influence of such compounds can also be expressed through an increase in root length or, for example, through plant growth disorders. Pino et al. [49], investigating the effects of 15 NSAIDs on lettuce (Lactuca sativa) further demonstrate that the observed effect is dependent on the type of drug and on the species and even variety of the plant which the drugs act on. Zezulka et al. [50] also came to similar conclusions. In their study, they indicate that paracetamol and diclofenac have no effect on the germination of pea, lettuce, onion and tomato seeds, while they affect the germination of corn seeds.
3.4. Effect of NSAIDs on Pigments Content
Photosynthetic pigments, i.e., chlorophylls and carotenoids, are organic chemical compounds capable of absorbing visible light of a certain wavelength. These pigments determine the course of photosynthesis in plants. Photosynthesis in turn is one of the most essential processes that gives plants energy, enabling biomass production and its proper development. Pigments differ in structure, the range of light absorbed and the functions they perform. Abnormalities in the amount of chlorophyll a and b, carotenoids and the ratio of their content to each other are some of the first symptoms of oxidative stress in plants. Only chlorophyll a molecules in plants are capable of releasing electrons when exposed to absorbed light. Chlorophyll b and carotenoids act as auxiliary pigments that, by forming the so-called antenna system, capture light energy and transmit it to the reaction centers of photosystems formed by chlorophyll a molecules. Carotenoids, moreover, have a protective function against photooxidation of the photosynthetic apparatus. If in the PSI or PSII photosystems, in which photosynthesis takes place, the absorption or transport of energy is disturbed, then the excited chlorophyll molecules will react, among other things, with oxygen to form radicals, i.e., singlet oxygen. The resulting oxygen free radicals will damage proteins in photosystems, resulting in cellular dysfunction, which in turn can adversely affect plant growth and development [50,51,52].
As a result of the study, an initial increase and then a decrease in the chlorophylls and carotenoids amounts were observed as the concentration of NSAIDs in the soil increased. The greatest changes in the content of assimilatory pigments were observed in plants growing in soil with addition of IBU (Table 3).

Table 3.
Effect of IBU, KET and IBU + KET on the photosynthetic pigment in seedlings of spring barley. Data shown represent the means of 4 experiments ± standard deviation. Different letters indicate statistically significant differences at p < 0.05.
A very important indicator for assessing physiological changes occurring in plants exposed to stress factors is the determination of the reciprocal ratio of chlorophyll a to chlorophyll b (Chl-a/Chl-b). An increase in the value of this parameter is a significant indicator of stress. In contrast, a decrease in the value of this ratio can have various causes. If it is caused solely by an increase in Chl-b content, it is a symptom of favorable changes in the level of assimilatory pigments. A decrease in the Chl-a/Chl-b ratio caused by a decrease in Chl-a content informs about large damage to photosystems and the photoinhibition that follows [53,54].
In the conducted studies, no changes were observed in the ratio of chlorophyll a to chlorophyll b after application of any of the tested NSAIDs, even at the highest concentration. This is because the content of both chlorophylls was reduced to a comparable degree (Table 3).
Another important indicator indicating the amount of oxidative stress in plants exposed to stress factors is the ratio of Chl a + b to carotenoid content (Chl a + b/Car). A decrease in the value of this parameter indicates oxidative stress in the plants under study and at the same time indicates an attempt to defend themselves by increasing the content of carotenoids, which are effective scavengers of ROS [51,53,55].
In the conducted studies only, a slight increase in this parameter was observed with the highest concentrations of IBU, KET and the IBU + KET mixture (Table 3).
As previously mentioned, low concentrations of NSAIDs can stimulate plant growth. More intensive growth requires adequate “nutrition” for the plant, which means a functioning photosynthetic apparatus and more photosynthetic pigments. However, too high concentration of NSAIDs in the substrate has a negative effect on the content of assimilatory pigments and plant growth and development. A similar trend of changes in the content of assimilatory pigments was observed by Opriş et al. [56] studying the effects of diclofenac, ibuprofen and naproxen for Atriplex patula, Spinacia L. and Lactuca sativa. Sun et al. [57], in their study, indicate that the application of PPCPs (pharmaceutical and personal care products) at higher concentrations causes a decrease in chlorophyll a and chlorophyll b levels in cucumber plants. Alkmin et al. [58] demonstrates that the effect of drugs on the number of chlorophylls and carotenoids in Lemna minor and Lemna gibba plants depends on the type of drugs, their concentrations and the species of plant affected by the drug.
3.5. Content of Malondialdehyde and Hydrogen Peroxide
Reactive oxygen species (ROS) are compounds that cause numerous damages in plant cells, i.e., peroxidation of lipids and proteins, nucleic acids and other cellular structures. Malondialdehyde (MDA) is a chemical compound formed by the peroxidation of lipids contained in protein–lipid membranes, mainly linoleic acid. An increase in its content reflects the degree of cell damage and is one of the main biomarkers of oxidative stress [47,59].
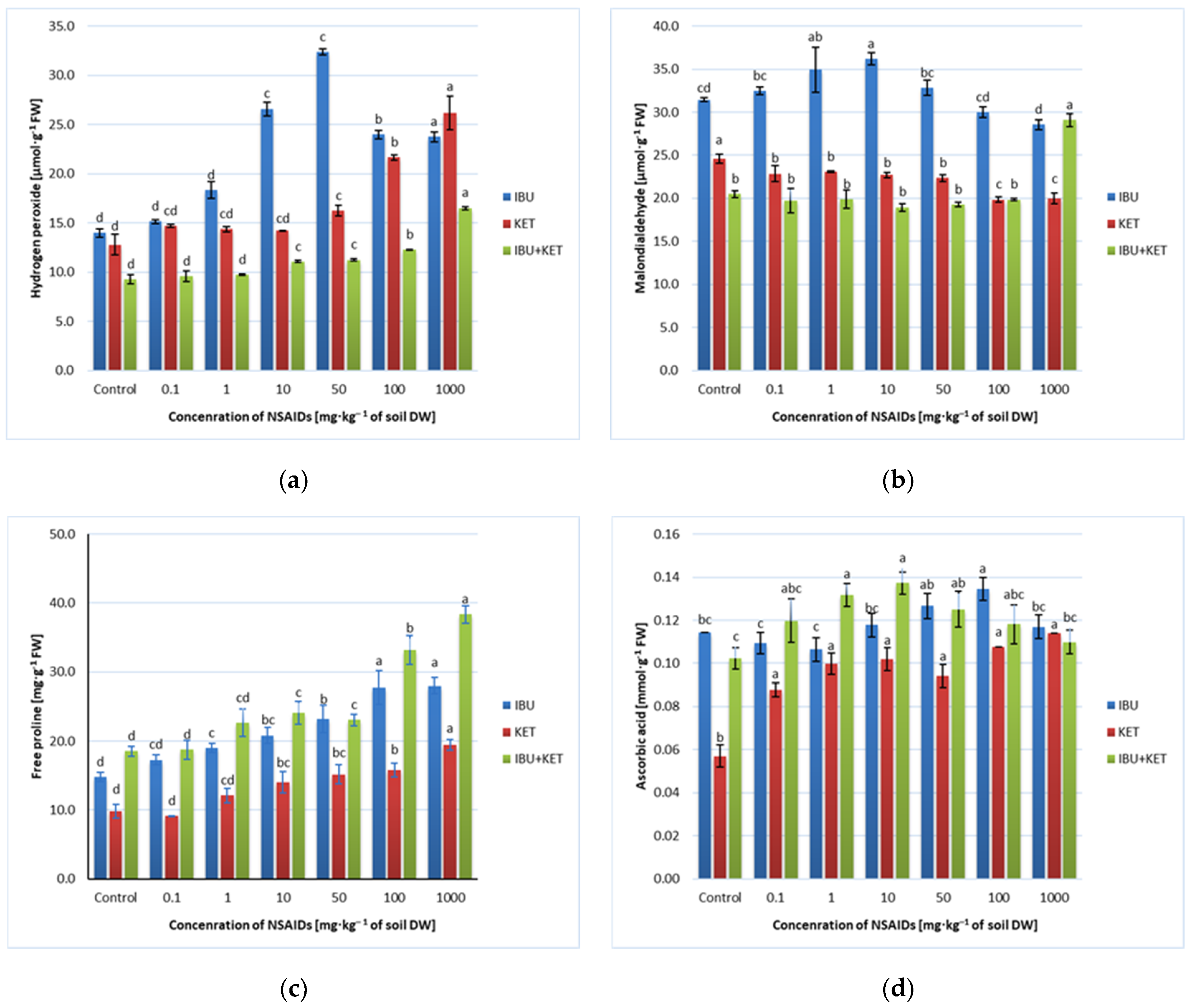
In the present study, spring barley seedlings grown in soil containing IBU, KET and a mixture of IBU and KET with increasing concentrations caused changes in MDA content in plants, and the direction of changes depended on the applied drug and its concentration. The presence of IBU in the soil caused an initial increase and then a slight decrease in MDA content in spring barley seedlings as the concentration of the drug in the soil increased. The presence of KET in the soil caused a slight decrease in MDA content in the plant after applying concentrations of 100 and 1000 mg·kg−1 of soil DW. In contrast, the presence of the IBU + KET mixture in the soil caused an increase in MDA content in spring barley seedlings, but only after application of this mixture at a concentration of 1000 mg·kg−1 of soil DW (Figure 5b).
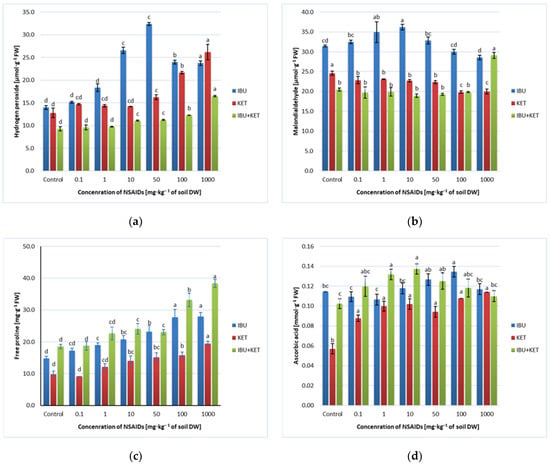
Figure 5.
Effects of IBU, KET and IBU + KET on the content of (a) hydrogen peroxide (μmol·g−1 FW), (b) malondialdehyde (μmol·g−1 FW), (c) free proline (mg·g−1 FW) and (d) ascorbic acid (μg·g−1 FW) in spring barley seedlings. Data shown represent the means of 4 experiments ± standard deviation. Different letters indicate statistically significant differences at p < 0.05.
The increased MDA content indicates that the NSAIDs tested caused some degree of cell damage. However, plants were able to resist the phytotoxicity of pharmaceuticals as evidenced by the decrease in MDA content in spring barley seedlings, and a defense response may be responsible for this result. Few studies on the effect of pharmaceuticals on MDA content in plants have been reported in the literature. Wang et al. [47] investigated MDA content in rice seedlings under the influence of KET, and Zhang et al. [60] observed an increase in MDA in Basella alba L. plants under the influence of carbamazepine and ibuprofen. Similar observations were also made by Iori et al. [61] studying the effect of IBU on willow. The cited authors observed an increase in MDA in willow under the IBU influence.
Another very important biomarker indicating that oxidative stress has occurred is the accumulation of hydrogen peroxide in plant cells. An increase in H2O2 accumulation is observed when there is increased detoxification of superoxide anion radical (O2•−) carried out by superoxide dismutase (SOD) and when enzymatic mechanisms of H2O2 detoxification fail in plants. Note that H2O2 is the most stable chemical molecule of all reactive oxygen species (ROS), able to rapidly cross all cell membranes. H2O2 also acts as a secondary messenger for further signaling, leading to plant responses and various growth and developmental functions [6,62].
In the conducted studies, an increase in H2O2 content was observed in spring barley seedlings growing in soil with the addition of the tested NSAIDs. The highest increase was observed for plants growing in soil with the addition of IBU. An increase in H2O2 content in seedlings growing on IBU-supplemented soil was observed already at a concentration of 1 mg·kg−1 of soil DW. When a concentration of 50 mg·kg−1 of soil DW was applied, the increase in H2O2 content was more than twofold. After the application of higher concentrations of IBU, the H2O2 content decreased slightly, but the H2O2 content of these seedlings was still significantly higher than that of the control plants. The presence of KET and the IBU + KET mixture in the soil causes an increase in H2O2 content in the seedlings when the drug is applied at concentrations of 50, 100 and 1000 mg·kg−1 of soil DW. However, application of KET alone causes much greater changes in H2O2 content than application of the drug mixture (Figure 5a).
The obtained results are confirmed by the available literature. Wang et al. [47], Zhang et al. [60], Sun et al. [57] and Christou et al. [2], in their studies, observed an increase in H2O2 content in the leaves of plants in contact with various pharmaceuticals. This proves conclusively that this biomarker is a very sensitive indicator of changes in plants, and even a small stress causes its accumulation in plants.
3.6. Content of Ascorbic Acid (AsA)
Ascorbate is one of the most important low-molecular antioxidants in plants. It is responsible for scavenging free radicals, i.e., 1O2, OH•, O2•−, superoxide radical and ONOOH; is involved in the regeneration of tocopheroxyl radical; and is one of the cofactors of ROS detoxifying enzymes [6,63].
For each of the tested NSAIDs, the AsA content in spring barley seedlings changed differently depending on the increasing concentration of the tested substance. Only the presence of KET in the soil caused a systematic increase in AsA content. The presence of IBU in the soil caused a slight increase in AsA content only when applying drug concentrations of 50 and 100 mg·kg−1 of soil DW. However, the application of a concentration of 1000 mg·kg−1 of soil DW no longer caused changes in AsA content in spring barley seedlings compared to the control. However, the presence of the IBU + KET drug mixture in the soil caused an increase in AsA content in spring barley seedlings in the concentration range of 0.1-100 mg·kg−1 of soil DW, while the application of a concentration of 1000 mg·kg−1 of soil DW no longer led to changes in AsA levels relative to the control. However, changes in AsA content in barley seedlings growing in soil containing the drug mixture were not linearly correlated with the increase in drug concentration in the soil (Figure 5d).
Ascorbic acid (in various forms) is one of the essential elements of the Halliwell–Asada cycle and the xanthophyll cycle in plants, making it very important in defense against oxygen free radicals. AsA content is closely dependent upon and related to the activity of antioxidant enzymes such as ascorbate peroxidase (APX). Diaz-Vivancos et al. [64], studying salt stress in transgenic plum, indicate that salt stress caused an increase in the content of various forms of ascorbate in these plants. Kummerova et al. [65], studying the effect of diclofenac and paracetamol on Lemna minor, observed changes in the content of various forms of ascorbate in these plants. Opposite results were obtained by Asadi Karam et al. [66] examining the level of oxidative stress in Brassica napus L. under the influence of cadmium. They observed a decrease in AsA content in this plant. Changes in the AsA content indicate the ability of the tested NSAIDs to generate ROS and the involvement of the glutathione–ascorbate cycle in the elimination of H2O2 from spring barley seedlings exposed to IBU, KET and IBU + KET mixture. Comparing the results obtained in the studies presented in this paper to those obtained by other authors, it can be concluded that each stress factor can affect the response of different plant species differently. This indicates the importance of further, thorough research conducted in this direction.
3.7. Content of Free Proline
The free proline content in plants is another important element of the system that is designed to protect the plant against reactive oxygen species. This amino acid can act in several ways. To protect the plant from stress-induced damage, this amino acid can act, for example, as an osmolyte and a metal chelator, as well as a signaling and antioxidant molecule [67].
In this study, we even observed a linear increase in proline content in spring barley seedlings with increasing concentrations of the tested NSAIDs and their mixture in the soil. An increase in proline content was already observed after the application of the tested drugs and their mixture at a concentration of 1 mg·kg−1 of soil DW. The higher the concentration applied, the higher the proline content in spring barley seedlings (Figure 5c).
The obtained results are confirmed in the available literature. Stuchlikova et al. [68] and Sosua et al. [67] observed an increase in proline content in Plantago lanceolata and Solanum lycopersicum L., respectively, under the influence of the drugs flubendazole and fenbendazole and diclofenac, respectively. These authors emphasize the tremendous importance of proline in plant defense against organic pollutants, noting that increased proline accumulation is a common physiological response of plants exposed to various stresses, with proline simultaneously playing an important role in plant stress tolerance.
3.8. Antioxidant Enzymes Activities
To defend against reactive oxygen species that cause oxidative stress, plants have developed a complex defense system that includes enzymes, i.e., catalase, peroxidases, superoxide dismutase and glutathione reductase. The action of these enzymes is closely correlated, as they sequentially convert ROS until they produce compounds that are nontoxic to plant cells.
The first line of defense against ROS is a group of enzymes generally referred to as superoxide dismutases (SODs). These enzymes degrade superoxide anion radical (O2•-) to H2O2 and O2 [69].
In the discussed studies, an increase in SOD activity was observed after the use of IBU at concentrations of 100 and 1000 mg·kg−1 DM. soil. KET and the IBU + KET mixture, in contrast, did not cause major changes in the activity of this enzyme (Table 4).

Table 4.
Effect of IBU, KET and IBU + KET on the activity of catalase (U·mg−1 protein·min−1), superoxide dismutase (U·mg−1 protein), ascorbate peroxidase (U·mg−1 protein·min−1) and guaiacol peroxidase (U·mg−1 protein·min−1) in spring barley seedlings. Data shown represent the means of 4 experiments ± standard deviation. Different letters indicate statistically significant differences at p < 0.05.
A similar lack of major changes in SOD activity in pea plants under the influence of naproxen was observed by Svobodnikova et al. [48]. Wang et al. [47], studying the reaction of rice to KET, and Zhang et al. [60], studying the reaction of Basella alba L. to ibuprofen and carbamazepine, observed an initial increase and then a decrease in SOD activity over the course of the experiments. These differences may be due to the type of experiment conducted and may also depend on the species of plant upon which the drugs under study act. In addition, in our own study, the enzyme activity was determined only once, i.e., on the 14th day after sowing the seeds into the soil, so it is not possible to determine how this activity changed on subsequent days of conducting the study. This may be related to the fact that drugs in the environment are biodegradable, so their effect on plants will depend on the timing of the study.
As a result of SOD activity, H2O2 is produced in plant cells. In order to remove this reactive oxygen form, plants use further enzymes, i.e., catalase and peroxidases. CAT is an enzyme located mainly in peroxisomes and glyoxysomes. It causes the breakdown of hydrogen peroxide and does not need any additional substances for this reaction. In contrast to CAT, peroxidases are found in many cell organelles, and they require phenolic compounds (pyrogallol, benzidine, guaiacol) or certain antioxidants (ascorbic acid) to break down H2O2 [6,47].
In the ongoing studies, it was observed that changes in CAT activity are dependent on the drug used. KET and the IBU + KET mixture caused an initial increase and then a decrease in CAT activity, while IBU did not cause major changes in the activity of this enzyme. However, an increase in POD activity was observed after the application of both single drugs and their mixture. The greatest changes in POD activity were observed under the influence of KET. In contrast, there were no significant changes in APX activity after treatment with KET and the IBU + KET mixture. IBU, however, caused a slight decrease in APX activity in spring barley seedlings (Table 4).
The results of studies on enzyme activity in plants that have been in contact with drugs are inconclusive. The magnitude and direction of changes in enzymatic activity depended on the plant species, the type of drug and its concentration and the length of the study. Sousa et al. [67] observed a decrease in APX and CAT activity under the influence of diclofenac in tomato plants. Svobodnikova et al. [48] and Wang et al. [47], in their studies, found an initial increase and then a decrease in APX and CAT activity under naproxen in pea plants and ketoprofen in rice seedlings, respectively. Zhang et al. [60] also observed an initial increase and then a decrease in POD activity in Basella alba L. under the influence of IBU and carbamazepine. In contrast, Sun et al. [57] observed an increase in POD and APX activity in cucumber shoots and roots under exposure to PPCP, which was linearly correlated with an increase in the concentration of the tested compounds in the medium.
4. Conclusions
The study indicates that the presence of IBU, KET and the IBU + KET mixture in the soil affected the growth and development of spring barley seedlings, especially after the application of higher drug concentrations. The tested NSAIDs caused oxidative stress in the tested plants, which was manifested by an increase in the content of H2O2, proline and AsA; an increase in the activity of antioxidant enzymes CAT, SOD and POD; and a decrease in the yield of plant fresh weight.
The results of the study presented in this paper and the available literature reports indicate the high complexity of the environmental effects of drugs and the need to carry out numerous studies in this direction, since drugs found in the soil environment can show differential effects on individual plant species. Understanding the effects of drugs and the product of their metabolism is essential to explain changes in the growth and development of plants growing in the contaminated environment. This knowledge is necessary because drugs can affect the size and quality of the plant yield obtained, but just as importantly, the consumption of such plants can also have negative effects on humans and animals.
Author Contributions
R.B.: Conceptualization, Supervision, Investigation, Writing—Review and Editing, Funding Acquisition. B.P.: Investigation, Methodology, Resources, Visualization, Writing—Original Draft. A.T.: Funding Acquisition, Statistical Analysis. M.S.: Investigation, Resources, Writing—Review and Editing. A.G.: Investigation, Resources, Writing—Review and Editing. R.Š.: Writing—Review and Editing. All authors have read and agreed to the published version of the manuscript.
Funding
The work was financed by a statutory activity subsidy from the Polish Ministry of Science and Higher Education for The Faculty of Science and Technology of Jan Długosz University in Czestochowa (SBR/WNSPT/KBBE/18/2020) and The Faculty of Environmental Management and Agriculture, West Pomeranian University of Technology in Szczecin.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The dataset used during the current research is available from the corresponding author on reasonable request.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Christou, A.; Michael, C.; Fatta-Kassinosb, D.; Fotopoulos, V. Can the pharmaceutically active compounds released in agroecosystems be considered as emerging plant stressors? Environ. Int. 2018, 114, 360–364. [Google Scholar] [CrossRef] [PubMed]
- Austin, T.; Bregoli, F.; Höhne, D.; Hendriks, A.J.; Ragas, A.M.J. Ibuprofen exposure in Europe; ePiE as an alternative to costly environmental monitoring. Environ. Res. 2022, 209, 112777. [Google Scholar] [CrossRef]
- Maculewicz, J.; Kowalska, D.; Świacka, K.; Toński, M.; Stepnowski, P.; Białk-Bielińska, A.; Dołżonek, J. Transformation products of pharmaceuticals in the environment: Their fate, (eco)toxicity and bioaccumulation potential. Sci. Total Environ. 2022, 802, 149916. [Google Scholar] [CrossRef] [PubMed]
- Bonnefille, B.; Gomez, E.; Courant, F.; Escande, A.; Fenet, H. Diclofenac in themarine environment: A review of its occurrence and effects. Mar. Pollut. Bull. 2018, 131, 496–506. [Google Scholar] [CrossRef] [PubMed]
- Nikolaou, A.; Meric, S.; Fatta, D. Occurrence patterns of pharmaceuticals in water and wastewater environments. Anal. Bioanal. Chem. 2007, 387, 1225–1234. [Google Scholar] [CrossRef]
- Pawłowska, B.; Telesiński, A.; Biczak, R. Effect of diclofenac and naproxen and their mixture on spring barley seedlings and Heterocypris incongruens. Environ. Toxicol. Pharmacol. 2021, 88, 103746. [Google Scholar] [CrossRef]
- Brain, R.A.; Hanson, M.L.; Solomon, K.R.; Brooks, B.W. Aquatic Plants Exposed to Pharmaceuticals: Effects and Risks. In Reviews of Environmental Contamination and Toxicology; Springer: Berlin/Heidelberg, Germany, 2008; pp. 67–115. [Google Scholar]
- Batucan, N.S.P.; Tremblay, L.A.; Northcott, G.L.; Matthaei, C.D. Medicating the environment? A critical review on the risks of carbamazepine, diclofenac and ibuprofen to aquatic organisms. Environ. Adv. 2022, 7, 100164. [Google Scholar] [CrossRef]
- Harshkova, D.; Aksmann, A. Environmental pollution by non-steroidal anti-inflammatory drugs—Diclofenac as an example. Kosmos 2019, 68, 185–194. (In Polish) [Google Scholar] [CrossRef]
- Bownik, A.; Jasieczek, M.; Kosztowny, E. Ketoprofen affects swimming behavior and impairs physiological endpoints of Daphnia magna. Sci. Total Environ. 2020, 725, 138312. [Google Scholar] [CrossRef]
- Muñiz-González, A.-B. Ibuprofen as an emerging pollutant on non-target aquatic invertebrates: Effects on Chironomus riparius. Environ. Toxicol. Pharmacol. 2021, 81, 103537. [Google Scholar] [CrossRef]
- Hájková, M.; Kummerová, M.; Zezulka, Š.; Babula, P.; Váczi, P. Diclofenac as an environmental threat: Impact on the photosynthetic processes of Lemna minor chloroplasts. Chemosphere 2019, 224, 892–899. [Google Scholar] [CrossRef] [PubMed]
- Cortés, J.M.; Larsson, E.; Jönsson, J.A. Study of the uptake of non-steroid anti-inflammatory drugs in wheat and soybean after application of sewage sludge as a fertilizer. Sci. Total Environ. 2013, 449, 385–389. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, W.; Redshaw, C.H. Evaluation of biological endpoints in crop plants after exposure to non-steroidal anti-inflammatory drugs (NSAIDs): Implications for phytotoxicological assessment of novel contaminants. Ecotox. Environ. Saf. 2015, 112, 212–222. [Google Scholar] [CrossRef] [PubMed]
- Cycoń, M.; Borymski, S.; Żołnierczyk, B.; Piotrowska-Seget, Z. Variable Effects of Non-steroidal Anti-inflammatory Drugs (NSAIDs) on Selected Biochemical Processes Mediated by Soil Microorganisms. Front. Microbiol. 2016, 7, 1969. [Google Scholar] [CrossRef]
- OECD/OCDE 208 Guidelines for the Testing of Chemical. Terrestrial Plant: Seedling Test: Seedlings Emergence and Seedling Growth Test. 2006. Available online: https://www.oecd.org/chemicalsafety/testing/33653757.pdf (accessed on 30 June 2022).
- PN-EN ISO 11269:2; Determination of the effects of pollutants on soil flora—Part 2: Effects of contaminated soil on the emergence and early growth of higher plants. ISO: Geneva, Switzerland, 2012.
- Liu, T.; Zhu, L.; Xie, H.; Wang, J.; Wang, J.; Sun, F.; Wang, F. Effects of the ionic liquid 1-octyl-3-methylimidazolium hexafluorophosphate on the growth of wheat seedlings. Environ. Sci. Pollut. Res. 2014, 21, 3936–3945. [Google Scholar] [CrossRef]
- Wang, L.-S.; Wang, L.; Wang, L.; Wang, G.; Li, Z.-H.; Wang, J.-J. Effect of 1-butyl- 3-methylimidazolium tetrafluoroborate on the wheat (Triticum aestivum L.) seedlings. Environ. Toxicol. 2009, 24, 296–303. [Google Scholar] [CrossRef]
- Kowalska, I. Zawartość Wybranych Składników Szpinaku (Spinacia oleraceae L.) Uprawianym Przy Zróżnicowanej Zawartości Wapnia. Rocz. AR Pozn. 2004, 38, 105–110. (In Polish) [Google Scholar]
- Oren, R.; Werk, K.S.; Buchmann, N.; Zimmermann, R. Chlorophyll-nutrient relationships identify nutritionally caused decline in Picea abies stands. Can. J. For. Res. 1993, 23, 1187–1195. [Google Scholar] [CrossRef]
- Hodges, D.M.; DeLong, J.M.; Forney, C.F.; Prange, R.K. Improving the thiobarbituric acid-reactive-substances assay for estimating lipid peroxidation in plant tissues containing anthocyanin and other interfering compounds. Planta 1999, 207, 604–611. [Google Scholar] [CrossRef]
- Singh, H.P.; Batish, D.R.; Kohli, R.K.; Arora, K. Arsenic induced root growth inhibition in mung bean (Phaseolus aureus Roxb.) is due to oxidative stress resulting from enhanced lipid peroxidation. Plant Growth Regul. 2007, 53, 65–73. [Google Scholar] [CrossRef]
- Law, M.Y.; Charles, S.A.; Halliwell, B. Glutathione and ascorbic acid in spinach (Spinacia oleracea) chloroplasts. The effect of hydrogen peroxide and of Paraquat. Biochem. J. 1983, 210, 899–903. [Google Scholar] [CrossRef] [PubMed]
- Bates, L.S. Rapid determination of free proline for water-stress studies. Plant Soil 1973, 39, 205–207. [Google Scholar] [CrossRef]
- Giannopolitis, C.N.; Ries, S.K. Superoxide dismutase. I. Occurrence in higher plants. Plant Physiol. 1977, 59, 309–314. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Wu, J.; Zhang, X.; Xia, Y.; Li, Y.; Du, S. Enantioselective oxidative stress caused by chiral ionic liquids forms of 1-alkyl-3-methyl imidazolium tartrate on Scenedesmus obliquus. Sci. Total. Environ. 2017, 595, 819–827. [Google Scholar] [CrossRef] [PubMed]
- Abassi, N.A.; Kushad, M.M.; Endress, A.G. Active oxygen scavenging enzymes activities in developing apple flowers and fruits. Sci. Hortic. 1998, 74, 183–194. [Google Scholar] [CrossRef]
- Nakano, Y.; Asada, K. Hydrogen peroxide is scavenged by ascorbate-specific peroxidase in spinach chloroplasts. Plant Cell. Physiol. 1981, 22, 867–880. [Google Scholar]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities utilizing the principle of protein dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Chen, Y.; Rosazza, J.P.N. Microbial transformation of ibuprofen by a Nocardia species. Appl. Environ. Microbiol. 1994, 60, 1292–1296. [Google Scholar] [CrossRef]
- Murdoch, R.W.; Hay, A.G. Formation of catechols via removal of acid side chains from ibuprofen and related aromatic acids. Appl. Environ. Microbiol. 2005, 71, 6121–6125. [Google Scholar] [CrossRef]
- Murdoch, R.W.; Hay, A.G. Genetic and chemical characterization of ibuprofen degradation by Sphingomonas Ibu-2. Microbiology 2013, 159, 621–632. [Google Scholar] [CrossRef]
- Murdoch, R.W.; Hay, A.G. The biotransformation of ibuprofen to trihydroxyibuprofen in activated sludge and by Variovorax Ibu-1. Biodegradation 2015, 26, 105–113. [Google Scholar] [CrossRef] [PubMed]
- Jiang, C.; Geng, J.; Hu, H.; Ma, H.; Gao, X.; Hongqiang, R. Impact of selected non-steroidal anti-inflammatory pharmaceuticals on microbial community assembly and activity in sequencing batch reactors. PLoS ONE 2017, 12, 0179236. [Google Scholar] [CrossRef] [PubMed]
- Tyumin, E.A.; Bazhutin, G.A.; Cartagena Gómez, A.; Ivshina, I.B. Nonsteroidal Anti-inflammatory Drugs as Emerging Contaminants. Microbiology 2020, 89, 148–163. [Google Scholar] [CrossRef]
- Rodarte-Morales, A.I.; Feijoo, G.; Moreira, M.T.; Lema, J.M. Biotransformation of three pharmaceutical active compounds by the fungus Phanerochaete chrysosporium in a fed batch stirred reactor under air and oxygen supply. Biodegradation 2012, 23, 145–156. [Google Scholar] [CrossRef]
- Gonda, S.; Kiss-Szikszai, A.; Szucs, Z.; Balla, B.; Vasas, G. Efficient biotransformation of non-steroid anti-inflammatory drugs by endophytic and epiphytic fungi from dried leaves of a medicinal plant, Plantago lanceolata L. Int. Biodeterior. Biodegrad. 2016, 108, 115–121. [Google Scholar] [CrossRef]
- Torán, J.; Blánquez, P.; Caminal, G. Comparison between several reactors with Trametes versicolor immobilized on lignocellulosic support for the continuous treatments of hospital wastewater. Bioresour. Technol. 2017, 243, 966–974. [Google Scholar] [CrossRef]
- Escuder-Gilabert, L.; Martín-Biosca, Y.; Perez-Baeza, M.; Sagradoa, S.; Medina-Hernández, M.J. Direct chromatographic study of the enantioselective biodegradation of ibuprofen and ketoprofen by an activated sludge. J. Chromatogr. A 2018, 1568, 140–148. [Google Scholar] [CrossRef]
- Gu, Y.; Tobino, T.; Nakajima, F. Effect of calcite saturation state on the growth and mortality of Heterocypris incongruens and a proposal for an reference artificial sediment in the sediment toxicity test ISO14371. Sci. Total Environ. 2020, 702, 134993. [Google Scholar] [CrossRef]
- Niyommaneerat, W.; Nakajima, F.; Tobino, T.; Yamamoto, K. Development of a chronic sediment toxicity test using the benthic ostracod Heterocypris incongruens and their application to toxicity assessments of urban road dust. Ecotoxicol. Environ. Saf. 2017, 143, 266–274. [Google Scholar] [CrossRef]
- Kudłak, B.; Wieczerzak, M.; Namiesnik, J. Determination of toxicological parameters of selected bioactive organic chemicals using the Ostracodtoxkit FTM. Chem. Didact. Ecol. Metrol. 2018, 23, 113–126. [Google Scholar] [CrossRef]
- Ramírez-Morales, D.; Masís-Mora, M.; Montiel-Mora, J.R.; Cambronero-Heinrichs, J.C.; Briceño-Guevara, S.; Rojas-Sánchez, C.E.; Méndez-Rivera, M.; Arias-Mora, V.; Tormo-Budowski, R.; Brenes-Alfaro, L.; et al. Occurrence of pharmaceuticals, hazard assessment and ecotoxicological evaluation of wastewater treatment plants in Costa Rica. Sci. Total Environ. 2020, 746, 141200. [Google Scholar] [CrossRef] [PubMed]
- Forsberg, G.; Kristensen, L.; Eibel, P.; Titone, P.; Haiti, W. Sensitivity of cereal seeds to hort duration treatment with hot, humid air. J. Plant Dis. Prot. 2003, 110, 1–16. [Google Scholar]
- Sharma, H.R.; Garg, S.; Malan, A. Paracetamol and Ibuprofen effect on seed quality attributes of Triticum aestivum (wheat). Int. Res. J. Environ. Sci. 2018, 7, 44–48. [Google Scholar]
- Wang, H.; Jin, M.; Xu, L.; Xi, H.; Wang, B.; Du, S.; Liu, H.; Wen, Y. Effects of ketoprofen on rice seedlings: Insights from photosynthesis, antioxidative stress, gene expression patterns, and integrated biomarker response analysis. Environ. Pollut. 2020, 263, 114533. [Google Scholar] [CrossRef] [PubMed]
- Svobodníková, L.; Kemerova, M.; Zezulka, S.; Babula, P.; Sendecká, K. Root response in Pisum sativum under naproxen stress: Morphoanatomical, cytological, and biochemical traits. Chemosphere 2020, 258, 127411. [Google Scholar] [CrossRef] [PubMed]
- Pino, M.R.; Muñiz, S.; Val, J.; Navarro, E. Phytotoxicity of 15 common pharmaceuticals on the germination of Lactuca sativa and photosynthesis of Chlamydomonas reinhardtii. Environ. Sci. Pollut. Res. 2016, 23, 2253–22541. [Google Scholar] [CrossRef]
- Zezulka, S.; Kummerová, M.; Babula, P.; Hájková, M.; Oravec, M. Sensitivity of physiological and biochemical endpoints in early ontogenetic stages of crops under diclofenac and paracetamol treatments. Environ. Sci. Pollut. Res. 2019, 26, 3965–3979. [Google Scholar] [CrossRef]
- Jbir-Koubaa, R.; Charfeddine, S.; Ellouz, W.; Saidi, M.N.; Gargouri-Bouzid, R.; Nouri-Ellouz, O. Investigation of the response to salinity and to oxidative stress of interspecific potato somatic hybrids grown in a greenhouse. Plant Cell Tissue Organ Cult. 2015, 120, 933–947. [Google Scholar] [CrossRef]
- Pawłowska, B.; Telesiński, A.; Płatkowski, M.; Stręk, M.; Śnioszek, M.; Biczak, R. Reaction of Spring Barley and Common Radish on the Introduction of Ionic Liquids Containing Asymmetric Cations to the Soil. J. Agric. Food Chem. 2017, 65, 4562–4571. [Google Scholar] [CrossRef]
- Chen, Y.; Lin, F.; Yang, H.; Yue, L.; Hu, F.; Wang, J.; Luo, Y.; Cao, F. Effect of varying NaCl doses on flavonoid production in suspension cells of Ginkgo biloba: Relationship to chlorophyll fluorescence, ion homeostasis, antioxidant system and ultrastructure. Acta Physiol. Plant. 2014, 36, 3173–3187. [Google Scholar] [CrossRef]
- Wang, Q.; Que, X.; Zheng, R.; Pang, Z.; Li, C.; Xiao, B. Phytotoxicity assessment of atrazine on growth and physiology of three emergent plants. Environ. Sci. Pollut. Res. 2015, 22, 9646–9657. [Google Scholar] [CrossRef] [PubMed]
- Arias-Baldrich, C.; Bosch, N.; Begines, D.; Feria, A.B.; Monreal, J.A.; García-Mauriño, S. Proline synthesis in barley under iron deficiency and salinity. J. Plant Physiol. 2015, 183, 121–129. [Google Scholar] [CrossRef]
- Opriş, O.; Ciorîţă, A.; Soran, M.-L.; Lung, I.; Copolovici, D.; Copolovici, L. Evaluation of the photosynthetic parameters, emission of volatile organic compounds and ultrastructure of common green leafy vegetables after exposure to non-steroidal anti-inflammatory drugs (NSAIDs). Ecotoxicology 2019, 28, 631–642. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Liu, J.; Lu, G. Influence of aquatic colloids on the bioaccumulation and biological effects of diclofenac in zebrafish (Danio rerio). Ecotoxicol. Environ. Saf. 2018, 195, 110470. [Google Scholar] [CrossRef] [PubMed]
- Alkimin, G.D.; Daniel, D.S.; Frankenbach, S.; Serôdio, J.; Soares, A.M.V.M.; Barata, C.; Nunes, B. Evaluation of pharmaceutical toxic effects of non-standard endpoints on the macrophyte species Lemna minor and Lemna gibba. Sci. Total Environ. 2019, 657, 926–937. [Google Scholar] [CrossRef] [PubMed]
- Biczak, R. Changes in growth and physiological parameters of spring barley and common radish under the influence of 1-butyl-2,3- dimethylimidazolium tetrafluoroborate. Plant Physiol. Biochem. 2017, 115, 259–268. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Li, N.; Chen, G.; Xu, J.; Ouyang, G.; Zhu, F. Stress symptoms and plant hormone-modulated defense response induced by the uptake of carbamazepine and ibuprofen in Malabar spinach (Basella alba L.). Sci. Total Environ. 2021, 793, 148628. [Google Scholar] [CrossRef]
- Iori, V.; Zacchini, M.; Pietrini, F. Growth, physiological response and phytoremoval capability of two willow clones exposed to ibuprofen under hydroponic culture. J. Hazard. Mater. 2013, 262, 796–804. [Google Scholar] [CrossRef]
- Bigott, Y.; Chowdhury, S.P.; Pérez, S.; Montemurro, N.; Manasfi, R.; Schröder, P. Effect of the pharmaceuticals diclofenac and lamotrigine on stress responses and stress gene expression in lettuce (Lactuca sativa) at environmentally relevant concentrations. J. Hazard. Mater. 2021, 403, 123881. [Google Scholar] [CrossRef]
- Nowicka, B.; Kruk, J. Reactive oxygen species in plants—Far more than just a poison. Kosmos 2013, 62, 583–596. (In Polish) [Google Scholar]
- Diaz-Vivancos, D.; Faize, M.; Barba-Espin, G.; Faize, L.; Petri, C.; Hernández, J.A.; Burgos, L. Ectopic expression of cytosolic superoxide dismutase and ascorbate peroxidase leads to salt stress tolerance in transgenic plums. Plant Biotechnol. J. 2013, 11, 976–985. [Google Scholar] [CrossRef] [PubMed]
- Kummerová, M.; Zezulka, S.; Babula, P.; Trískaca, J. (Possible ecological risk of two pharmaceuticals diclofenac and paracetamol demonstrated on a model plant Lemna minor. J. Hazard. Mater. 2016, 302, 351–361. [Google Scholar] [CrossRef] [PubMed]
- Asadi Karam, E.; Maresca, V.; Sorbo, S.; Keramat, B.; Basil, A. Effects of triacontanol on ascorbate-glutathione cycle in Brassica napus L. exposed to cadmium induced oxidative stress. Ecotoxicol. Environ. Saf. 2017, 144, 268–274. [Google Scholar] [CrossRef] [PubMed]
- Sousa, B.; Lopes, J.; Leal, A.; Martins, M.; Soares, C.; Valente, I.M.; Rodrigues, J.A.; Fidalgo, F.; Teixeira, J. Response of Solanum lycopersicum L. to diclofenac e impacts on the plant’s antioxidant mechanisms. Environ. Pollut. 2020, 258, 113762. [Google Scholar] [CrossRef]
- Stuchlíková, L.R.; Skálová, L.; Szotáková, B.; Syslová, E.; Vokřál, I.; Vaněk, T.; Podlipná, R. Biotransformation of flubendazole and fenbendazole and their effects in the ribwort plantain (Plantago lanceolata). Ecotoxicol. Environ. Saf. 2018, 147, 681–687. [Google Scholar] [CrossRef]
- Biczak, R.; Pawłowska, B. Reaction of spring barley seedlings and H. incongruens crustaceans to the presence of acetylsalicylic acid in soil. J. Environ. Manag. 2022, 302, 113936. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).