Corrosion Mechanisms of 304L NAG in Boiling 9M HNO3 Containing Cr (VI) Ions
Abstract
:1. Introduction
2. Experimental Methodology
2.1. Material Selection and Heat Treatment
2.2. Degree of Sensitisation
2.3. ASTM A 262 Practice C
2.4. End-Grain Corrosion Test
2.5. Measurement of Potential in End-Grain Corrosion Test Solution
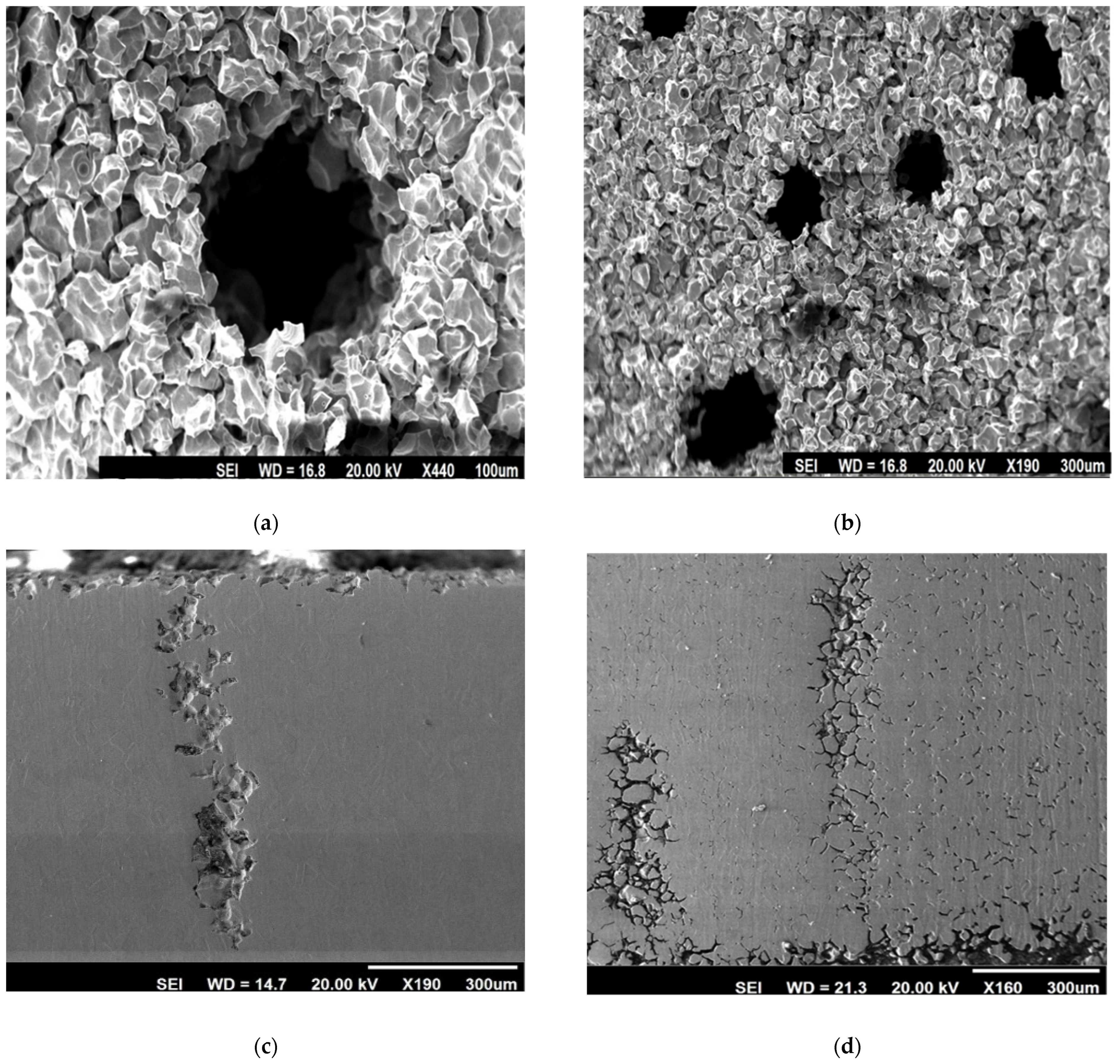
2.6. Study of Morphology of End-Grain Corrosion
3. Results and Discussion
3.1. Degree of Sensitisation
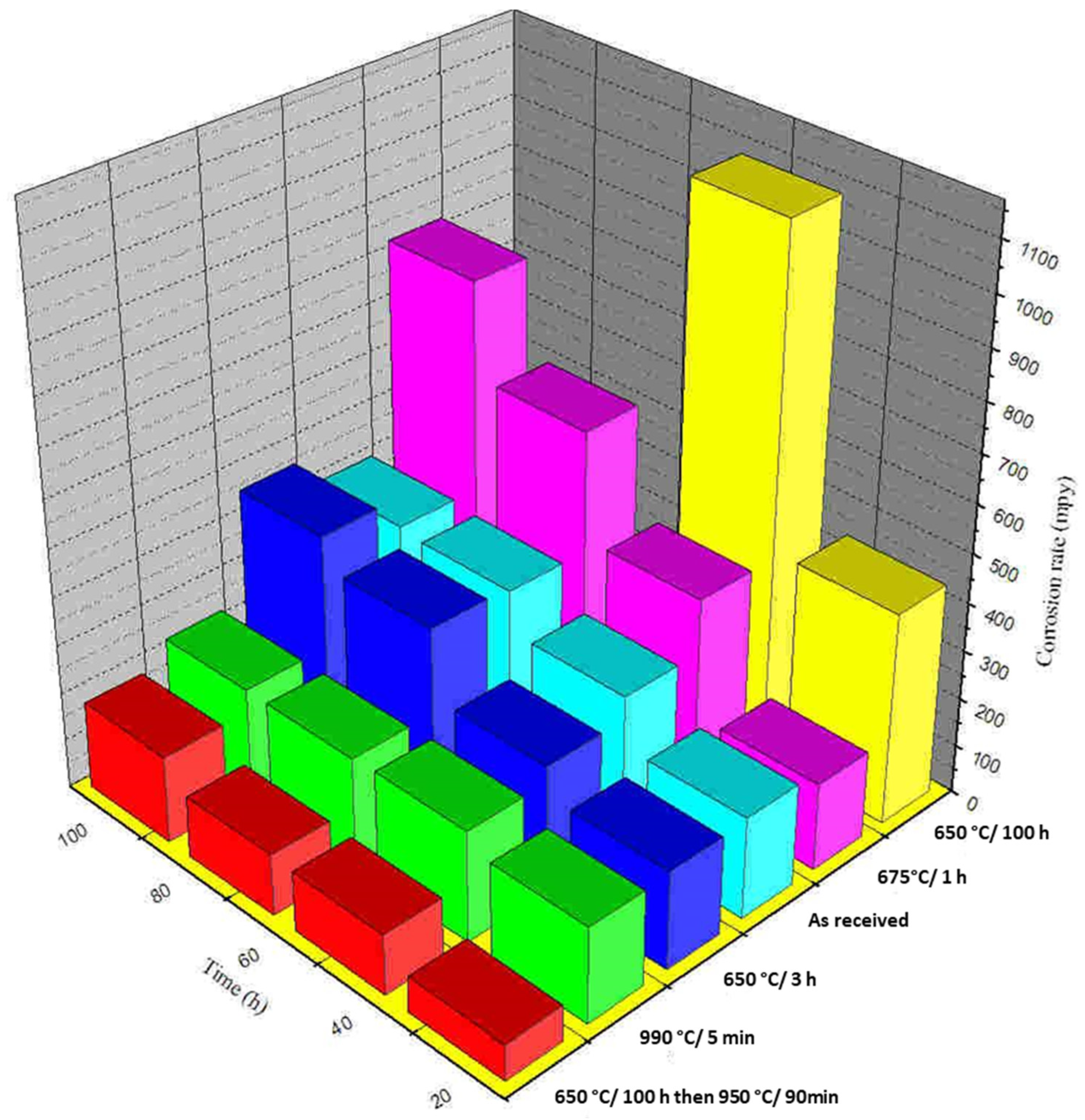
3.2. End-Grain Corrosion at Different Concentrations of Cr6+
3.3. Effect of Various Heat Treatments on End-Grain Corrosion Behaviour of a Type 304L NAG Tube
4. Control of End-Grain Corrosion
- ○
- Using lasers to remelt exposed cross-sectional surfaces was proven to prevent end-grain corrosion. An altered ferritic and cast microstructure were the primary characteristics of the laser-remelted surfaces.
- ○
- End-grain corrosion was avoided by weld-overlaying unprotected end-surfaces, such as tubular cross-sections, with welded filler material such as a 308L type or welding a ring of stainless steel, which would withstand susceptibility to end-grain corrosion over sensitive end/edge surfaces.
- ○
- Annealing with a controlled solution was applied to stainless steel that was susceptible to end-grain corrosion. This can be accomplished by annealing for 90 min at 950°C. Grain coarsening was not a problem with this heat treatment. It was shown that a 950°C heat treatment for 90 min resulted in an adequate homogenisation of alloying elements, thus erasing material segregation.
5. Conclusions
- An increase in Cr6+ concentration in 9M HNO3 increased the 304L NAG tube’s susceptibility to end-grain corrosion. This increase in the predisposition of end-grain corrosion of 304L NAG tubes can be attributed to an increase in electrochemical potential with an increase in Cr6+ concentrations.
- Any heat treatment used in this study did not result in extensive sensitisation and the type 304L NAG tube remained resistant to intergranular corrosion during ASTM A 262 Practices A and C.
- A significant increase in end-grain corrosion for the specimen heat-treated for 100 h at 650 °C was observed, which was taken to be due to the maximum segregation of phosphorus to the grain boundary.
- Heat treatment for 100 h at 650 °C induced a high susceptibility in terms of end-grain corrosion. Therefore, a 90 min heat treatment at 950 °C put an end to this susceptibility as a salvaging measure. This was mainly due to the equilibration of the segregation of phosphorus.
- A heat treatment at 990 °C for 5 min resulted in a high increase in end-grain corrosion. This increase in susceptibility to end-grain corrosion and can be attributed to the segregation of sulphur to the grain boundaries.
- We suggest that end-grain corrosion can be controlled by (a) the laser surface remelting of the exposed end faces, by (b) weld overlaying exposed end faces, and by (c) a controlled solution annealing the heat treatment of the as-received material.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sedriks, A.J. Corrosion of Stainless Steels, 2nd ed.; John Wiley and Sons: New York, NY, USA, 1996. [Google Scholar]
- Cihal, V. Intergranular Corrosion of Stainless Steels and Alloys. Mater. Sci. Monogr. 1984, 18, 196. [Google Scholar]
- Nazir, M.H.; Khan, Z.A.; Saeed, A.; Stokes, K. A predictive model for life assessment of automotive exhaust mufflers subject to internal corrosion failure due to exhaust gas condensation. Eng. Fail. Anal. 2016, 63, 43–60. [Google Scholar] [CrossRef]
- Nazir, M.H.; Khan, Z.A.; Stokes, K. A holistic mathematical modelling and simulation for cathodic delamination mechanism—A novel and an efficient approach. J. Adhes. Sci. Technol. 2015, 29, 2475–2513. [Google Scholar] [CrossRef]
- Nazir, M.H.; Khan, Z.; Stokes, K. Modelling of metal-coating delamination incorporating variable environmental parameters. J. Adhes. Sci. Technol. 2014, 29, 392–423. [Google Scholar] [CrossRef]
- Nazir, M.; Khan, Z.; Saeed, A.; Stokes, K. Modelling the Effect of Residual and Diffusion induced Stresses on Corrosion at the Interface of Coating and Substrate. Corrosion 2015, 72, 500–517. [Google Scholar] [CrossRef] [PubMed]
- Nazir, M.H.; Khan, Z.A.; Stokes, K. Optimisation of Interface Roughness and Coating Thickness to Maximise Coating-Substrate Adhesion—A Failure Prediction and Reliability Assessment Modelling. J. Adhes. Sci. Technol. 2015, 29, 1415–1445. [Google Scholar] [CrossRef] [Green Version]
- Saeed, A.; Khan, Z.A.; Nazir, M.H. Time dependent surface corrosion analysis and modelling of automotive steel under a simplistic model of variations in environmental parameters. Mater. Chem. Phys. 2016, 178, 65–73. [Google Scholar] [CrossRef]
- Nazir, M.; Khan, Z.A.; Stokes, K. A unified mathematical modelling and simulation for cathodic blistering mechanism incorporating diffusion and fracture mechanics concepts. J. Adhes. Sci. Technol. 2015, 29, 1200–1228. [Google Scholar] [CrossRef] [Green Version]
- Nazir, M.H.; Khan, Z.A.; Stokes, K. Analysing the coupled effects of compressive and diffusion induced stresses on the nucleation and propagation of circular coating blisters in the presence of micro-cracks. Eng. Fail. Anal. 2016, 70, 1–15. [Google Scholar] [CrossRef]
- Nazir, M.H.; Saeed, A.; Khan, Z. A comprehensive predictive corrosion model incorporating varying environmental gas pollutants applied to wider steel applications. Mater. Chem. Phys. 2017, 193, 19–34. [Google Scholar] [CrossRef]
- Nazir, M.; Khan, Z. A review of theoretical analysis techniques for cracking and corrosive degradation of film-substrate systems. Eng. Fail. Anal. 2016, 82, 80–113. [Google Scholar] [CrossRef]
- Bajwa, R.; Khan, Z.; Nazir, H.; Chacko, V.; Saeed, A. Wear and Friction Properties of Electrodeposited Ni-Based Coatings Subject to Nano-Enhanced Lubricant and Composite Coating. Acta Metall. Sin. Engl. Lett. 2016, 29, 902–910. [Google Scholar] [CrossRef] [Green Version]
- Nazir, M.H.; Khan, Z. Maximising the interfacial toughness of thin coatings and substrate through optimisation of defined parameters. Int. J. Comput. Methods Exp. Meas. 2015, 3, 316–328. [Google Scholar] [CrossRef]
- Bajwa, R.S.; Khan, Z.; Bakolas, V.; Braun, W. Effect of bath ionic strength on adhesion and tribological properties of pure nickel and Ni-based nanocomposite coatings. J. Adhes. Sci. Technol. 2016, 30, 653–665. [Google Scholar] [CrossRef]
- Bajwa, R.S.; Khan, Z.; Bakolas, V.; Braun, W. Water-Lubricated Ni-Based Composite (Ni–Al2O3, Ni–SiC and Ni–ZrO2) Thin Film Coatings for Industrial Applications. Acta Metall. Sin. Engl. Lett. 2015, 29, 8–16. [Google Scholar] [CrossRef] [Green Version]
- Nazir, M.H.; Khan, Z.A.; Saeed, A.; Bakolas, V.; Braun, W.; Bajwa, R.; Rafique, S. Analyzing and modelling the corrosion behavior of Ni/Al2O3, Ni/SiC, Ni/ZrO2 and Ni/Graphene nanocomposite coatings. Materials 2017, 10, 1225. [Google Scholar] [CrossRef] [Green Version]
- Khan, Z.A.; Grover, M.; Nazir, M.H. The implications of wet and dry turning on the surface quality of EN8 steel. In Transactions on Engineering Technologies; Springer: Dordrecht, The Netherlands, 2015; pp. 413–423. [Google Scholar]
- Khan, Z.A.; Chacko, V.; Nazir, H. A review of friction models in interacting joints for durability design. Friction 2017, 5, 1–22. [Google Scholar] [CrossRef] [Green Version]
- Khan, Z.A.; Nazir, H.; Saeed, A. Corrosion Measurement Device. Google Patents Patent Number S 11,486,816 B2, 1 November 2022. [Google Scholar]
- Latif, J.; Khan, Z.A.; Nazir, M.H.; Stokes, K.; Smith, R. An optimal condition based maintenance scheduling for metal structures based on a multidisciplinary research approach. Struct. Infrastruct. Eng. 2019, 15, 1366–1381. [Google Scholar] [CrossRef]
- Latif, J.; Khan, Z.A.; Nazir, M.H.; Stokes, K.; Plummer, J. Condition monitoring and predictive modelling of coating delamination applied to remote stationary and mobile assets. Struct. Health Monit. 2019, 18, 1056–1073. [Google Scholar] [CrossRef]
- Todoroki, A.; Tanaka, M.; Shimamura, Y. Electrical resistance change method for monitoring delaminations of CFRP laminates: Effect of spacing between electrodes. Compos. Sci. Technol. 2005, 65, 37–46. [Google Scholar] [CrossRef]
- Nazir, M.; Saeed, A.; Khan, Z.A. Electrochemical corrosion failure analysis of large complex engineering structures by using micro-LPR sensors. Sens. Actuators B Chem. 2018, 268, 232–244. [Google Scholar] [CrossRef]
- Nazir, M.H.; Khan, Z.A.; Saeed, A. A novel non-destructive sensing technology for on-site corrosion failure evaluation of coatings. IEEE Access 2017, 6, 1042–1054. [Google Scholar] [CrossRef]
- Nazir, M.H.; Khan, Z.A.; Saeed, A.; Bakolas, V.; Braun, W.; Bajwa, R. Experimental analysis and modelling for reciprocating wear behaviour of nanocomposite coatings. Wear 2018, 416, 89–102. [Google Scholar] [CrossRef]
- Nazir, M.H.; Khan, Z.A.; Saeed, A. Experimental analysis and modelling of c-crack propagation in silicon nitride ball bearing element under rolling contact fatigue. Tribol. Int. 2018, 126, 386–401. [Google Scholar] [CrossRef]
- Latif, J.; Khan, Z.A.; Nazir, M.H.; Stokes, K.; Plummer, J. Life assessment prognostic modelling for multi-layered coating systems using a multidisciplinary approach. Mater. Sci. Technol. 2018, 34, 664–678. [Google Scholar] [CrossRef]
- Khan, Z.A.; Latif, J.; Nazir, M.H.; Stokes, K.; Plummer, J. Sensor Based Corrosion Condition Monitoring of Coating, Paper No. 2017-600147. In Proceedings of the Department of Defense-Allied Nations Technical Corrosion Conference, Tucson, AZ, USA, 9–12 August 2021. [Google Scholar]
- Saeed, A.; Khan, Z.A.; Nazir, H.; Hadfield, M.; Smith, R. Research impact of conserving large military vehicles through a sustainable methodology. Int. J. Herit. Archit. 2017, 1, 267–274. [Google Scholar] [CrossRef] [Green Version]
- ASTM International. ASTM Designation: A967-05 Standard specifications for chemical passivation treatments for stainless steel parts. In Annual Book of ASTM Standards; ASTM International: West Conshohocken, PA, USA, 2005. [Google Scholar]
- Shaw, R.D. Corrosion prevention and control at Sellafield nuclear fuel reprocessing plant. Br. Corros. J. 1992, 25, 97–107. [Google Scholar] [CrossRef]
- Khan, S.; Kain, V.; Reddy, A.V.R. Corrosion in transpassive potential regime: Effect of composition and microstructure of austenitic stainless steel. Corrosion 2014, 70, 19–28. [Google Scholar] [CrossRef]
- Kain, V.; Khan, S.; Reddy, A.V.R.; Wattal, P.K. Corrosion of non-sensitized austenitic stainless steels in nitric acid environment: An electrochemical approach. Adv. Mater. Res. 2013, 794, 517–529. [Google Scholar] [CrossRef]
- Ketcham, S.J.; Shaffer, I.S. Exfoliation Corrosion of Aluminum Alloys. In Localized Corrosion-Cause of Metal Failure; ASTM Special Technical Publication 516; ASTM International: West Conshohocken, PA, USA, 1972; p. 3. [Google Scholar]
- Kain, V.; Prasad, R.C.; De, P.K. Detection of sensitization and intergranular corrosion of Fe-Cr-Ni alloys. High Temp. Mater. Proc. 1997, 16, 183–200. [Google Scholar] [CrossRef]
- Kain, V.; De, P.K. Developments in austenitic stainless steels for the back end of nuclear fuel cycle. Trans. Indian Inst. Met. 2003, 56, 31. [Google Scholar]
- Kain, V.; Shinde, S.S.; Gadiyar, H.S. Mechanism of improved corrosion resistance of Type 304L stainless steel, nitric acid grade in nitric acid environment. J. Mater. Eng. Perform. 1994, 3, 699–705. [Google Scholar] [CrossRef]
- Kain, V.; De, P.K. Controlling corrosion in the back end of fuel cycle using nitric acid grade stainless steels. Int. J. Nucl. Energy Sci. Technol. 2005, 1, 220. [Google Scholar] [CrossRef]
- Kain, V.; Sengupta, P.; De, P.K.; Banerjee, S. Case reviews on the effect of microstructure on the corrosion behavior of austenitic alloys for processing and storage of nuclear waste. Metall. Mater. Trans. A 2005, 36, 1075–1084. [Google Scholar] [CrossRef]
- Kain, V.; Chouthai, S.S.; Gadiyar, H.S. Performance of AISI 304L stainless steel with exposed end grain in intergranular corrosion tests. Br. Corros. J. 1992, 27, 59–65. [Google Scholar] [CrossRef]
- ASTM International. ASTM Designation A 262-02a: Standard practice for detecting susceptibility to intergranular attack in austenitic stainless steels. In Annual Book of ASTM Standards; ASTM International: West Conshohocken, PA, USA, 2002. [Google Scholar]
- ISO 12732:2006; Corrosion of Metals and Alloys—Electrochemical Potentiokinetic Reactivation Measurement Using the Double Loop Method. International Organization of Standardization: Geneva, Switzerland, 2006; pp. 584–593.
- Cíhal, V.; Shoji, T.; Kain, V.; Watanabe, Y.; Stefec, R. EPR—A Comprehensive Review; FRRI Publication: Sendai, Japan, 2004. [Google Scholar]
- Tedmon, C.S.; Vermilyea, D.A.; Broecker, D.E. Effect of coldwork on intergranular corrosion of sensitized stainless steel. Corrosion 1871, 27, 104–106. [Google Scholar] [CrossRef]
- Bhise, S.; Kain, V. Methodology based on potential measurement for predicting corrosion behaviour of SS 304L in boiling nitric acid containing oxidising ions. Corros. Eng. Sci. Technol. 2011, 47, 61. [Google Scholar] [CrossRef]
- Striecher, M.A. General and Intergranular Corrosion of Austenitic Stainless Steels in Acids. J. Electrochem. Soc. 1959, 106, 161–180. [Google Scholar] [CrossRef]
- Briant, C.L.; Hall, E.L. Heat-to-Heat Variability in the Corrosion Resistance and Microstructure of Low Carbon AISI 316 Nuclear Grade Stainless Steel. Corrosion 1987, 43, 525–533. [Google Scholar] [CrossRef]
- Tvrdy, M.; Seidl, R.; Hyspecka, L.; Mazanec, K. Evaluation of the segregation of impurity elements on free surfaces of steel specimens. Scr. Metall. 1985, 19, 51–55. [Google Scholar] [CrossRef]
- Chandra, K.; Kain, V.; Ganesh, P. Controlling end-grain corrosion of austenitic stainless steels. J. Mater. Eng. Perform. 2008, 17, 115–122. [Google Scholar] [CrossRef]
- Watanabe, Y.; Ballinger, R.G.; Harling, O.K.; Kohs, G.E. Effects of neutron irradiation on transpassive corrosion behavior of austenitic stainless steels. Corrosion 1995, 51, 651–659. [Google Scholar] [CrossRef]



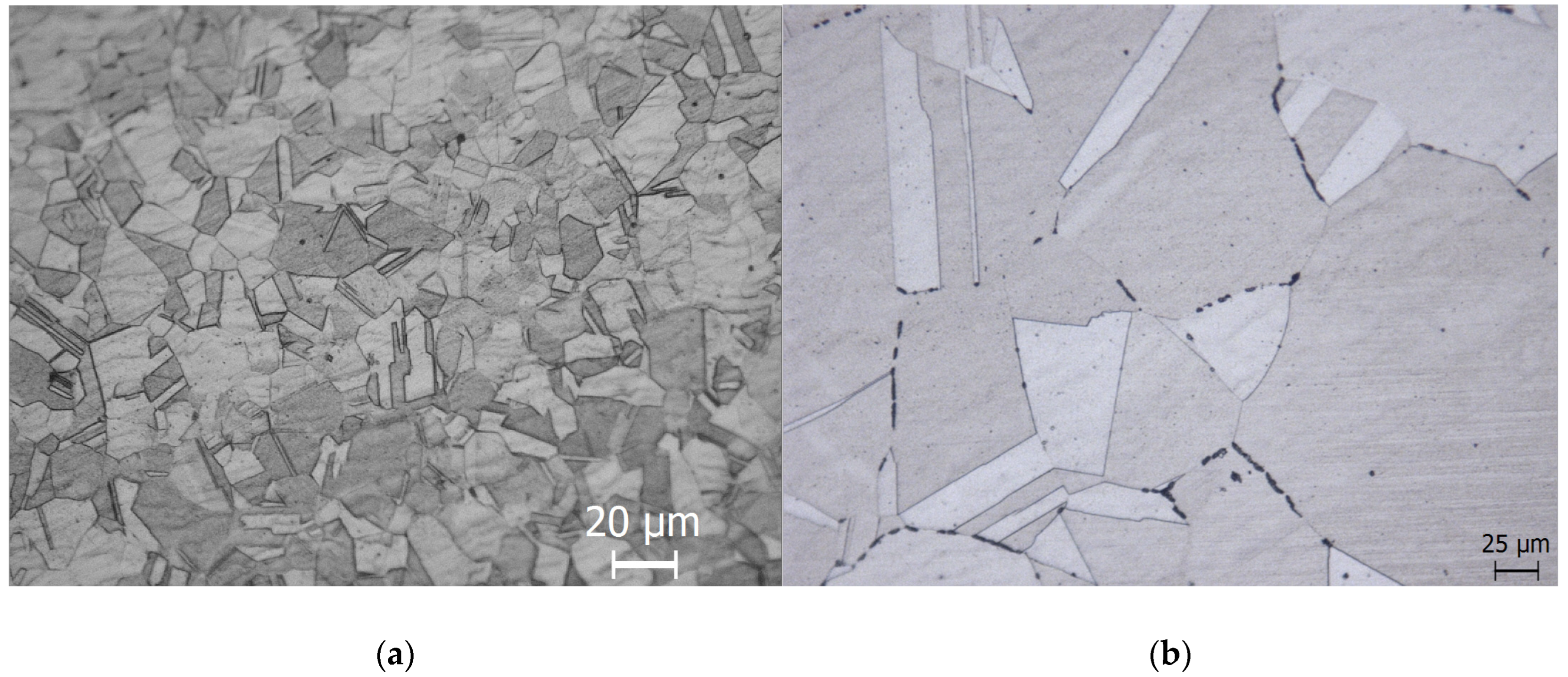





| Heat Treatment | Time | Influence on Microstructural Features | DOS (%) | Microstructure after DL-EPR |
|---|---|---|---|---|
| As-received | -- | 0.11 | Step | |
| 675 °C for 1 h | 1 h | Simulate critical weldment’s microstructure [42] | 0.17 | Dual |
| 650 °C for 3 h | 3 h | Cr and P segregation [48] | 0.18 | Dual |
| 650 °C for 100 h | 100 h | P segregation [48] | 1.4 | Dual |
| 990 °C for 5 min | 5 min | S segregation [49] | 0.13 | Step |
| 650 °C for 100 h then 950 °C for 90 min | -- | Equilibrate segregation without altering grain size [50] | 0.06 | Step |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khan, S.; Saeed, A.; Nazir, M.H.; Abdullah, M.U.; Khan, Z.A. Corrosion Mechanisms of 304L NAG in Boiling 9M HNO3 Containing Cr (VI) Ions. Sustainability 2023, 15, 916. https://doi.org/10.3390/su15020916
Khan S, Saeed A, Nazir MH, Abdullah MU, Khan ZA. Corrosion Mechanisms of 304L NAG in Boiling 9M HNO3 Containing Cr (VI) Ions. Sustainability. 2023; 15(2):916. https://doi.org/10.3390/su15020916
Chicago/Turabian StyleKhan, Shagufta, Adil Saeed, Mian Hammad Nazir, Muhammad Usman Abdullah, and Zulfiqar Ahmad Khan. 2023. "Corrosion Mechanisms of 304L NAG in Boiling 9M HNO3 Containing Cr (VI) Ions" Sustainability 15, no. 2: 916. https://doi.org/10.3390/su15020916
APA StyleKhan, S., Saeed, A., Nazir, M. H., Abdullah, M. U., & Khan, Z. A. (2023). Corrosion Mechanisms of 304L NAG in Boiling 9M HNO3 Containing Cr (VI) Ions. Sustainability, 15(2), 916. https://doi.org/10.3390/su15020916










