Recycling of Metallized Plastic as a Case Study for a Continuous Sustainability Improvement Process
Abstract
:1. Introduction
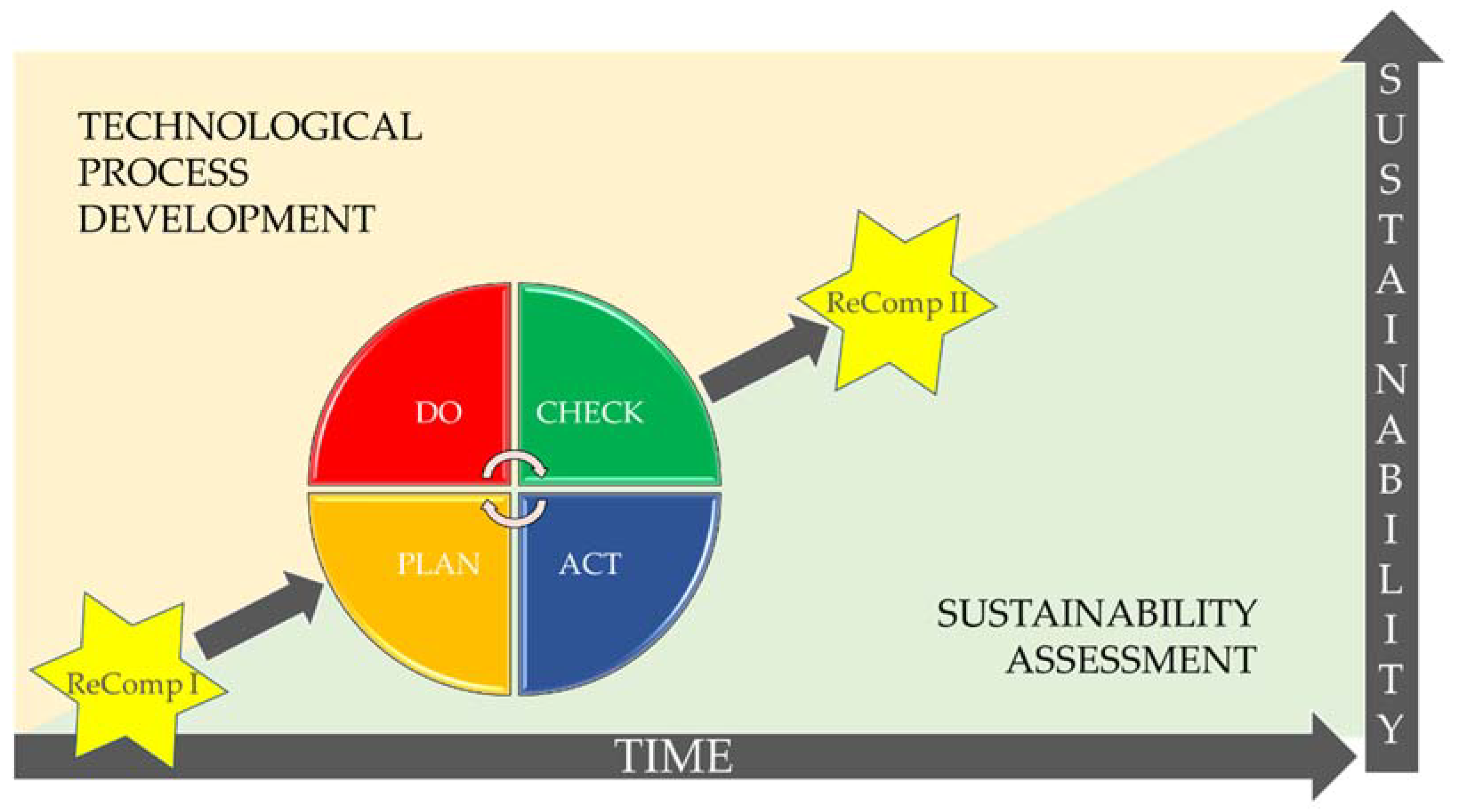
- Plan: establish the objectives, the resources required, and identify and address risks and opportunities in meeting customer requirements.
- Do: implement what was defined in the “plan”.
- Check: monitor and measure the performance against the objectives and policies as defined in the “plan”.
- Act: take actions to improve what was implemented (“do” stage).
- Plan: The researchers/engineers/designers start with engaging stakeholders and defining key technological and sustainability objectives and goals (1).
- Do: Process design (2), research and development experiments in the laboratory (3), and upscaling experiments (4).
- Check: Key technological results are analysed, and interpreted (5) and compared to the technological goals. The sustainability of the technological improvements is then checked (6–8).
- Act: Take actions to improve what was implemented. Identify improvement options (9). Start again at plan (1) and do (2) to (4).
2. Method and Materials
2.1. Technological Route
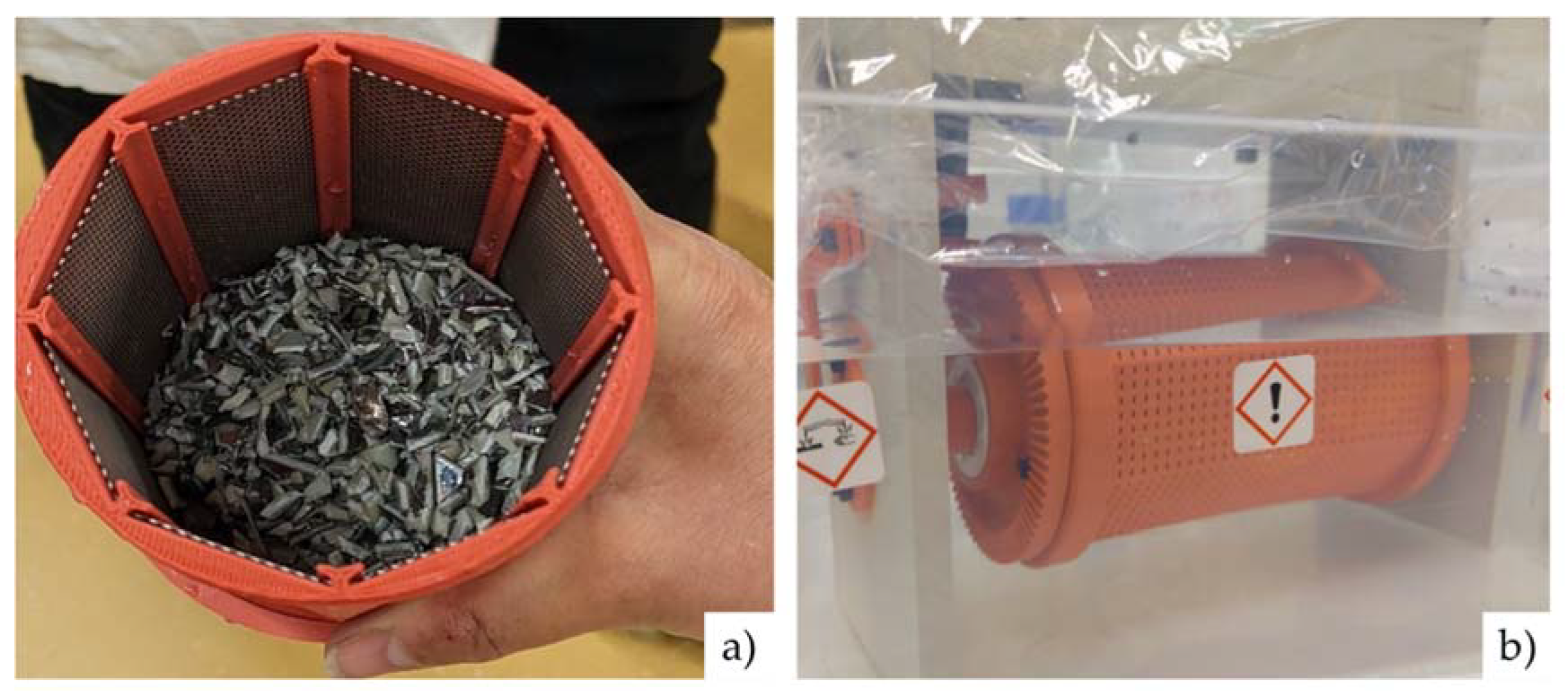
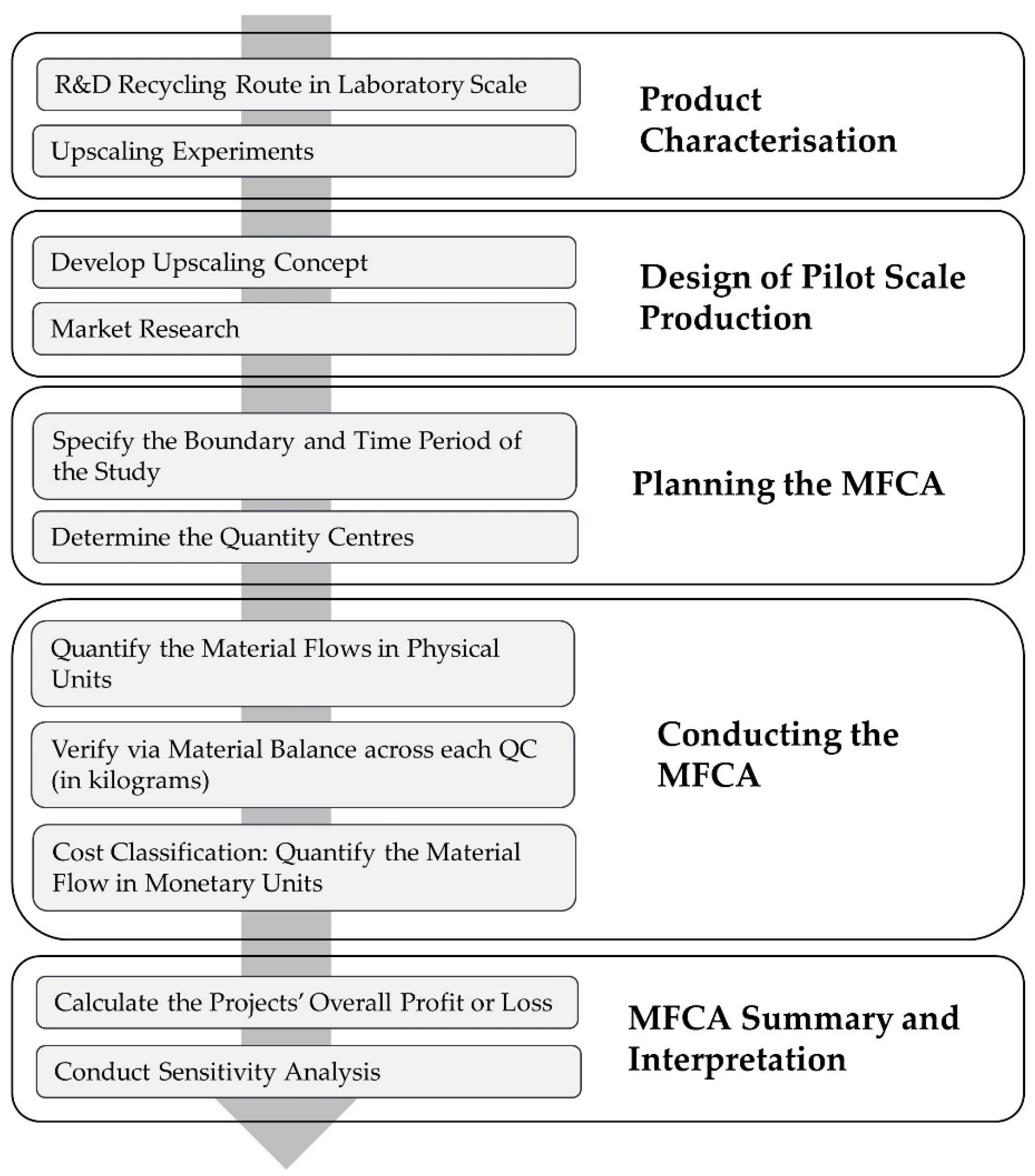
2.1.1. Characterisation
2.1.2. Research and Development in the Laboratory
2.1.3. Upscaling Experiments
2.2. Sustainability Route
2.2.1. Economic
- The machinery cost calculation consisted of three parts: the purchase price of the equipment, salvage value, and depreciation rate. The purchase prices were obtained from vendor quotes. All machinery was given a zero-salvage value, with 5 years of depreciation.
- The waste management costs included all fees associated with the handling and disposal of waste by third parties. The cost of disposal of wastes other than wastewater were from the Berliner Stadtreinigung (BSR) price list for disposal from trade, crafts, commerce, and services with volumes less than 500 kg per producer and year [22]. The costs for disposing chemicals were obtained from waste disposal company quotes. The cost of wastewater was obtained from BWB and priced at 0.0022 EUR/kg [19].
2.2.2. Environmental
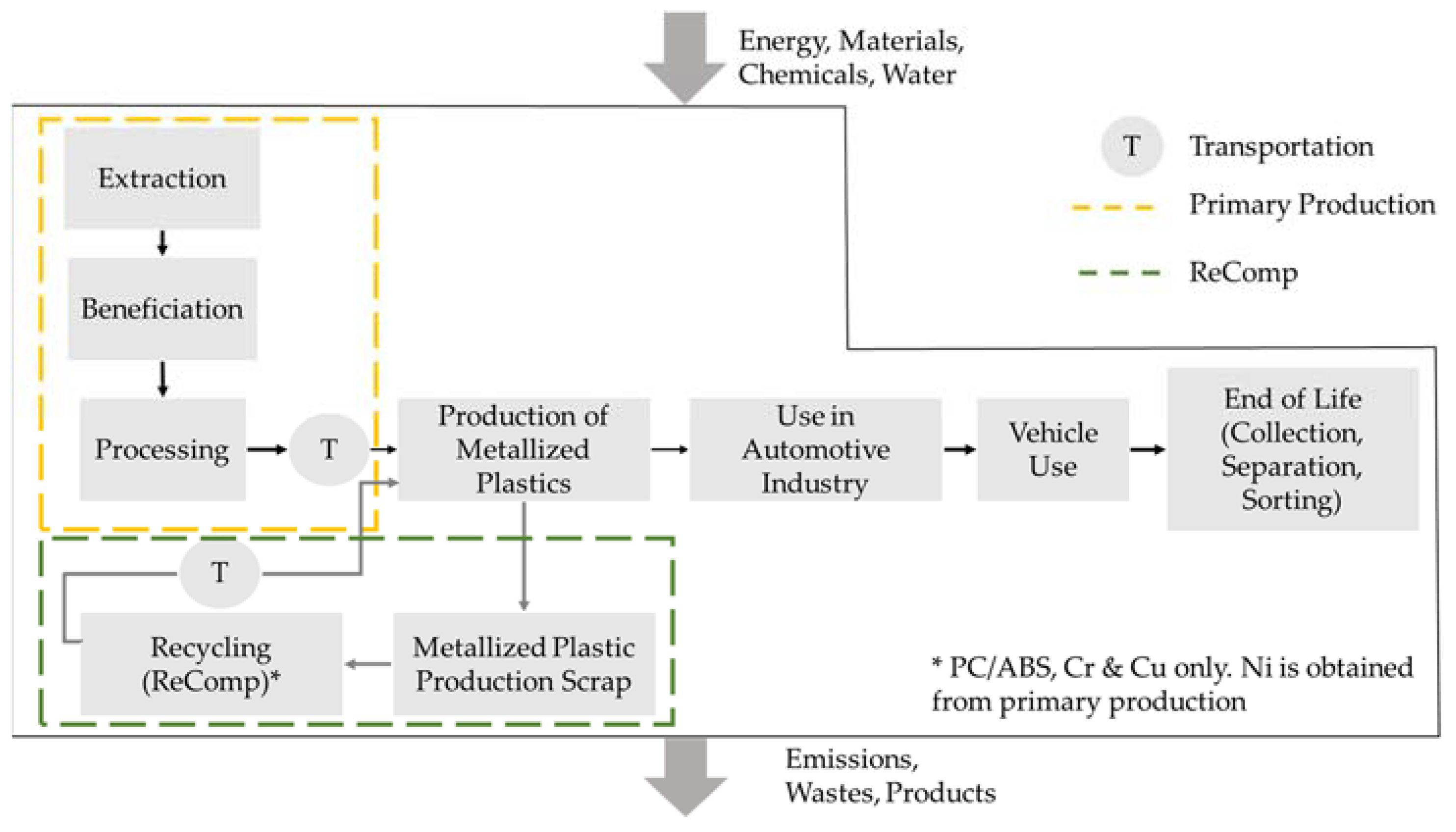
- Processing Country: China (for primary production), Germany (ReComp).
- Primary production inventory data were sourced from the Ecoinvent 3.7.1 database, adjusted to suit the specific conditions of this study.
- The ReComp I and II inventory data came from experimental data collected during the technological investigations (primary data).
- Transportation from China to Germany for production of metallized plastics through medium cargo transport vessels with a capacity of 20,000 t.
- Transport of recycled metallized plastics through Germany via Lorry Euro 6.
3. Results and Discussion
3.1. Technological Results
3.1.1. Composition of Input Material
3.1.2. Leaching Experiments
3.1.3. Electrochemical Treatment
3.1.4. Fractionated Distillation
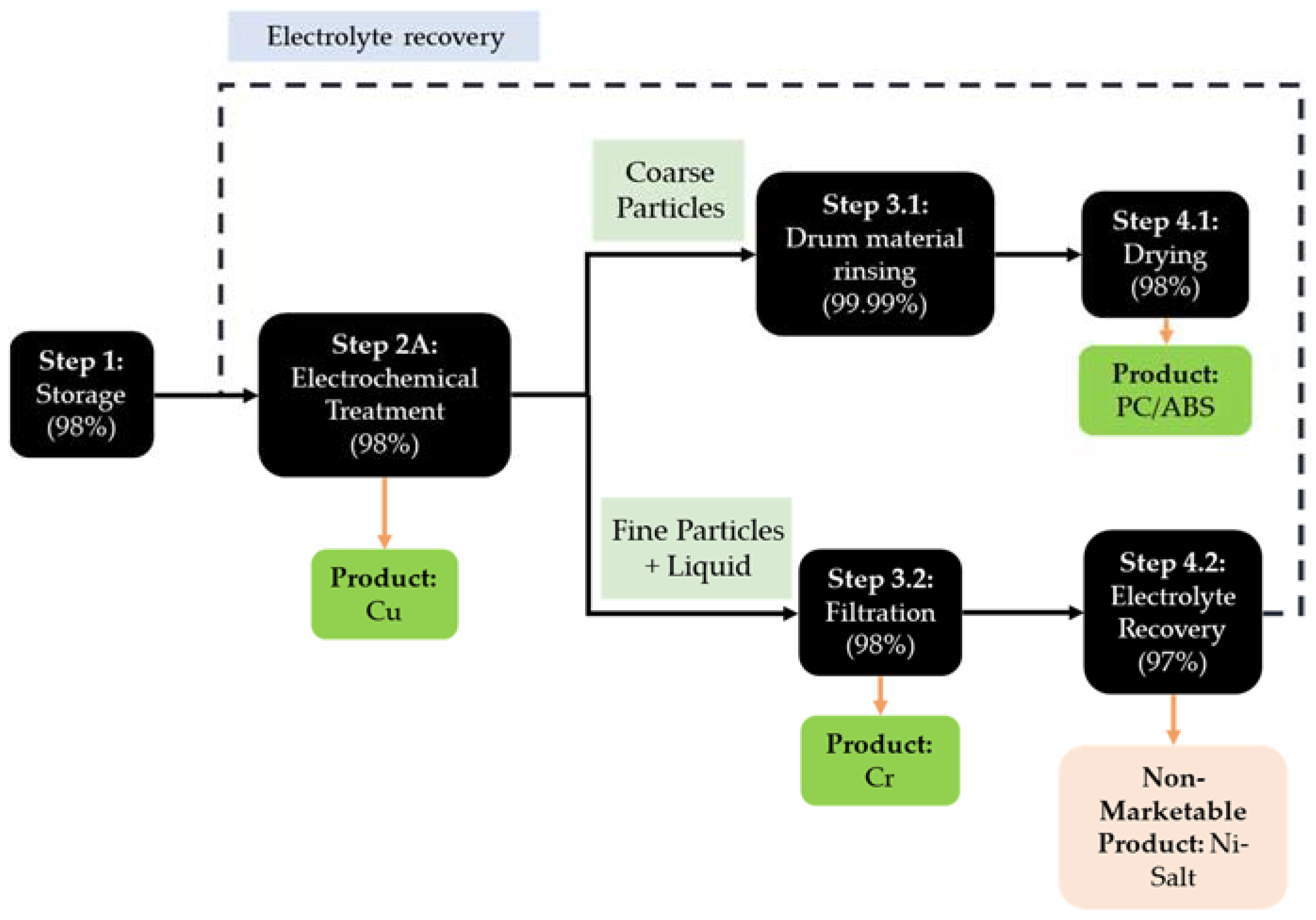
3.2. ReComp I Process Flow
3.3. Sustainability Assessment of ReComp I
3.3.1. Economic Assessment of ReComp I with MFCA
3.3.2. Ecological Assessment of ReComp I with LCA
3.3.3. Identification of Improvement Methods
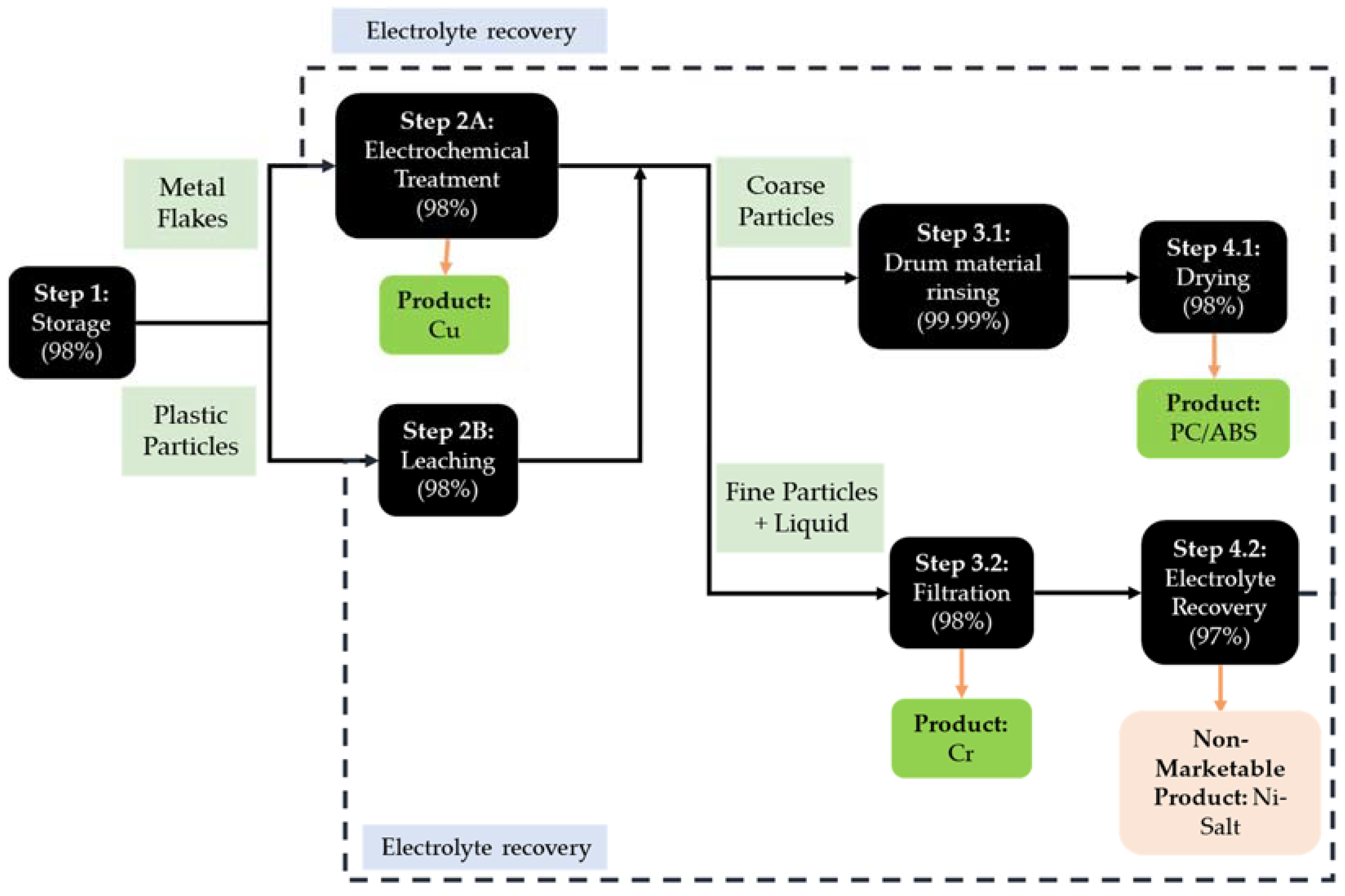
3.4. Process Flow of ReComp II and Comparison to ReComp I
3.5. Sustainability Assessment of ReComp II and Comparison to ReComp I
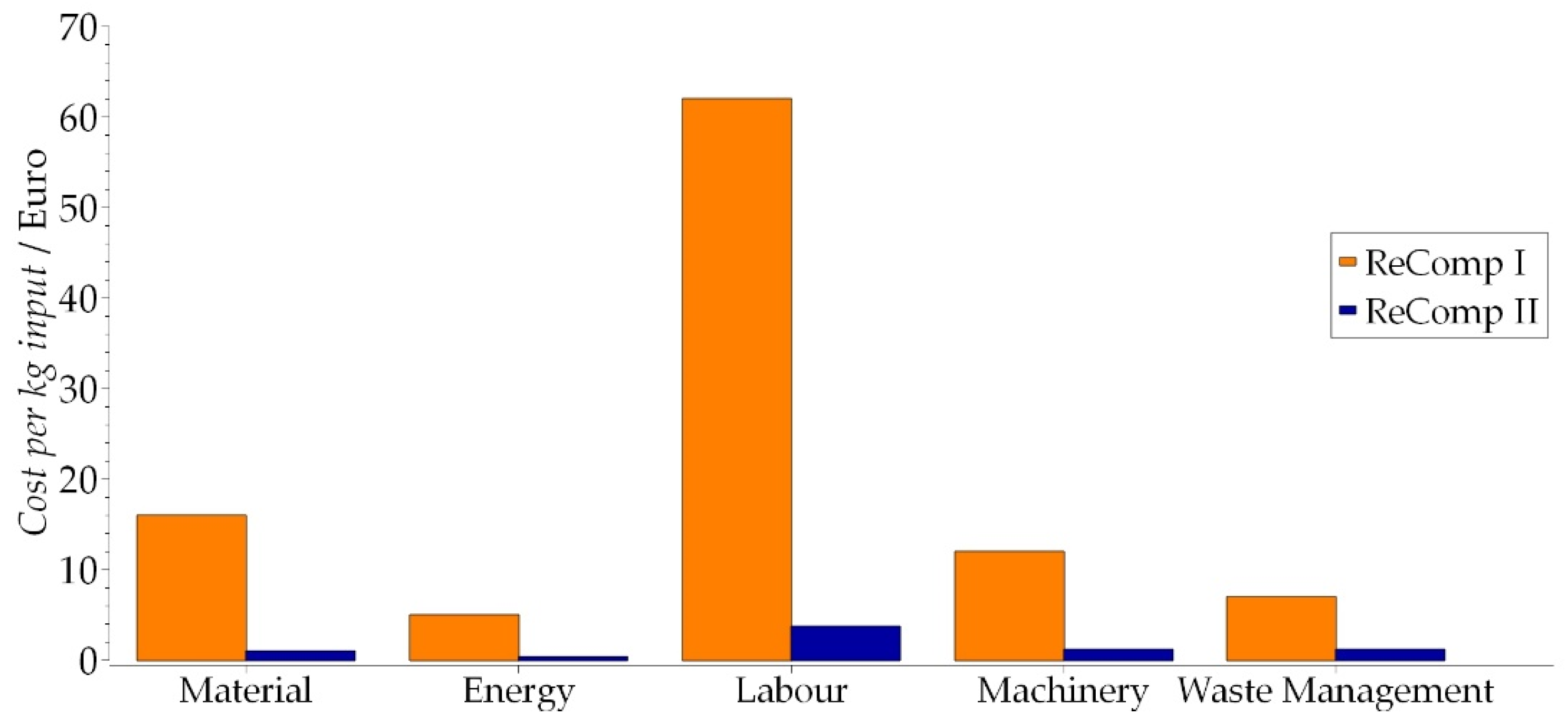
3.5.1. Economic Assessment of ReComp II with MFCA and Comparison
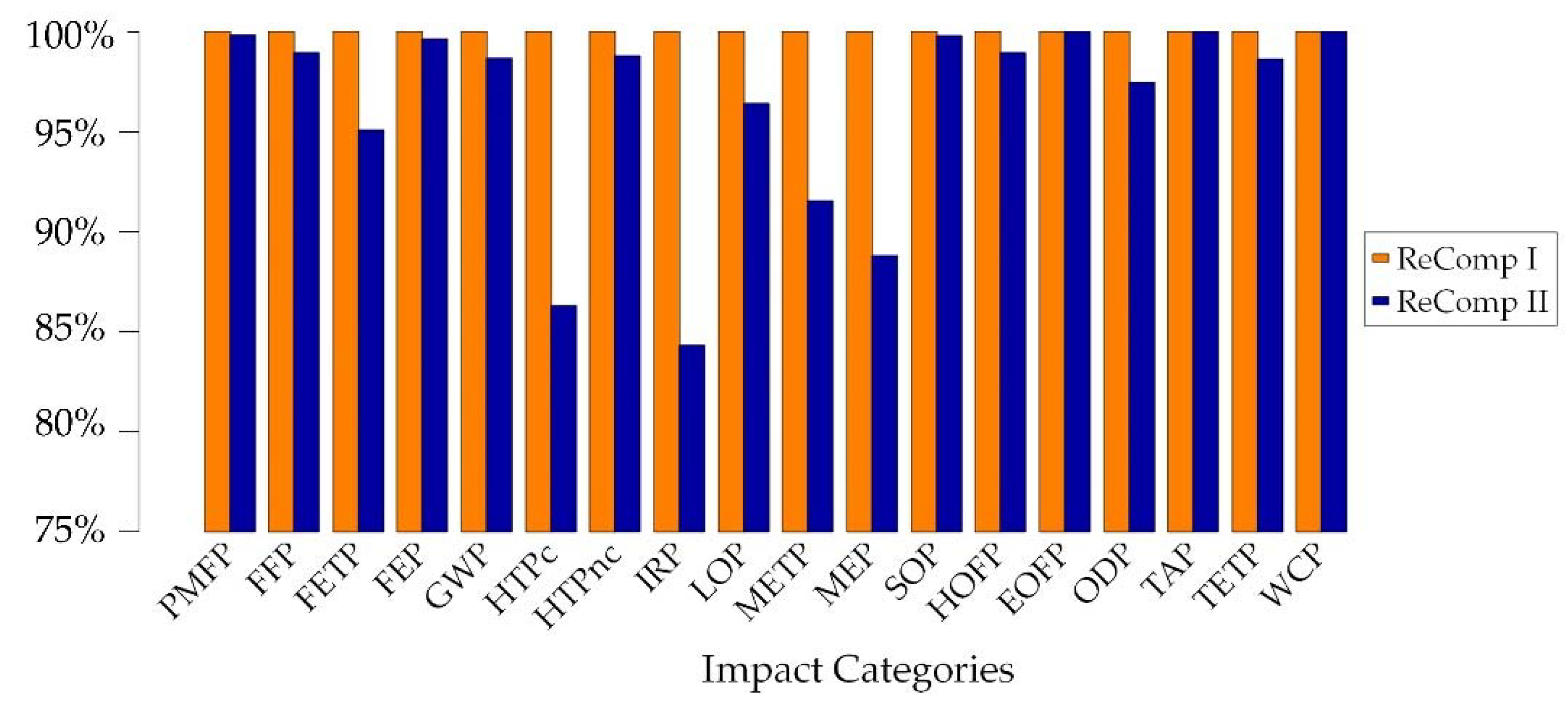
3.5.2. Ecological Assessment of ReComp II with LCA and Comparison to ReComp I
3.5.3. Identification of Improvement Options
4. Summary and Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Melentiev, R.; Yudhanto, A.; Tao, R.; Vuchkov, T.; Lubineau, G. Metallization of polymers and composites: State-of-the-art approaches. Mater. Des. 2022, 221, 110958. [Google Scholar] [CrossRef]
- Knappich, F.; Schlummer, M.; Mäurer, A.; Prestel, H. A new approach to metal- and polymer-recovery from metallized plastic waste using mechanical treatment and subcritical solvents. J. Mater. Cycles Waste Manag. 2018, 20, 1541–1552. [Google Scholar] [CrossRef]
- Federal Statistical Office of Germany. Press Release No. N 035. 7 June 2022. Available online: https://www.destatis.de/EN/Press/2022/06/PE22_N035_51.html (accessed on 23 February 2023).
- Numfor, S.A.; Omosa, G.B.; Zhang, Z.; Matsubae, K. A Review of Challenges and Opportunities for End-of-Life Vehicle Recycling in Developing Countries and Emerging Economies: A SWOT Analysis. Sustainability 2021, 13, 4918. [Google Scholar] [CrossRef]
- Turner, A.; Filella, M. Hazardous metal additives in plastics and their environmental impacts. Environ. Int. 2021, 156, 106622. [Google Scholar] [CrossRef] [PubMed]
- International Energy Agency. The Role of Critical World Energy Outlook Special Report Minerals in Clean Energy Transitions. 2021. Available online: https://www.iea.org/reports/the-role-of-critical-minerals-in-clean-energy-transitions (accessed on 23 February 2023).
- European Commission, Directorate-General for Internal Market, Industry, Entrepreneurship and SMEs; Blengini, G.; El Latunussa, C.; Eynard, U.; Torres De Matos, C.; Wittmer, D.; Georgitzikis, K.; Pavel, C.; Carrara, S.; Mancini, L.; et al. Study on the EU’s List of Critical Raw Materials (2020)—Final Report; Publications Office of the European Union: Luxembourg, 2020; Available online: https://data.europa.eu/doi/10.2873/11619 (accessed on 27 July 2023).
- The Organisation for Economic Cooperation and Development (OECD). Global Plastics Outlook: Economic Drivers, Environmental Impacts and Policy Options; OECD: Paris, France, 2022. [Google Scholar] [CrossRef]
- Nagashima, T.; Akiyama, H.; Namihira, T.; Katsuki, K. Recycle of metal-plating on plastics by pulse arc discharge. In Proceedings of the 2007 16th IEEE International Pulsed Power Conference, Albuquerque, NM, USA, 17–22 June 2007; IEEE: Piscataway, NJ, USA, 2007; pp. 1437–1440. [Google Scholar] [CrossRef]
- Elsayed, D.M.; Abdelbasir, S.M.; Abdel-Ghafar, H.M.; Salah, B.A.; Sayed, S.A. Silver and copper nanostructured particles recovered from metalized plastic waste for antibacterial applications. J. Environ. Chem. Eng. 2020, 8, 103826. [Google Scholar] [CrossRef]
- Knappich, F.; Hartl, F.; Schlummer, M.; Mäurer, A. Complete Recycling of Composite Material Comprising Polybutylene Terephthalate and Copper. Recycling 2017, 2, 9. [Google Scholar] [CrossRef]
- Cardenas-Cristancho, D.; Monticolo, D.; Muller, L.; Lhoste, P. CIGI 2021 Continuous Improvement process model: A Knowledge Management approach. In Proceedings of the Conférence Internationale de Génie Industriel—QUALITA, Grenoble, France, 5–7 May 2021; Available online: https://hal.science/hal-03288224 (accessed on 5 June 2023).
- Aleu, F.G.; Van Aken, E.M. Systematic literature review of critical success factors for continuous improvement projects. Int. J. Lean Six Sigma 2016, 7, 214–232. [Google Scholar] [CrossRef]
- ISO 9001:2015; Quality Management Systems—Requirements. International Organisation for Standardisation (ISO): Geneva, Switzerland, 2015.
- Realyvásquez-Vargas, A.; Arredondo-Soto, K.; Carrillo-Gutiérrez, T.; Ravelo, G. Applying the Plan-Do-Check-Act (PDCA) Cycle to Reduce the Defects in the Manufacturing Industry. A Case Study. Appl. Sci. 2018, 8, 2181. [Google Scholar] [CrossRef]
- Arredondo-Soto, K.C.; Blanco-Fernandez, J.; Miranda-Ackerman, M.A.; Solis-Quinteros, M.M.; Realyvasquez-Vargas, A.; Garcia-Alcaraz, J.L. A Plan-Do-Check-Act Based Process Improvement Intervention for Quality Improvement. IEEE Access 2021, 9, 132779–132790. [Google Scholar] [CrossRef]
- Christ, K.L.; Burritt, R.L. Material flow cost accounting: A review and agenda for future research. J. Clean. Prod. 2015, 108, 1378–1389. [Google Scholar] [CrossRef]
- ISO 14051:2011; Environmental Management—Material Flow Cost Accounting—General Framework. International Organisation for Standardisation (ISO): Geneva, Switzerland, 2011.
- Berlin Wasserbetriebe (BWB). Our Tariffs for Drinking Water and Drainage. 2023. Available online: https://www.bwb.de/en/1720.php (accessed on 26 February 2023).
- Carl Roth—Germany. Methanesulphonic acid, 1 l, CAS No. 75-75-2. Available online: https://www.carlroth.com/de/en/a-to-z/methanesulphonic-acid/p/4090.3 (accessed on 3 March 2023).
- Bundesverband der Energie- und Wasserwirtschaft e.V (BDEW). Industrial Electricity Prices Including Tax Germany 1998–2022|Statista. Available online: https://www.statista.com/statistics/1050448/industrial-electricity-prices-including-tax-germany/?fbclid=IwAR1-dsnrDrKSxUtuzvp_wegPGoQXOoZD_XiMHGoK0kvLePfvfqE9t9PwMVw (accessed on 26 February 2023).
- Berliner Stadtreinigungsbetriebe (BSR). Entgelte Schadstoffe; BSR: Berlin, Germany, 2023; Available online: https://www.bsr.de/assets/downloads/Gebuehren_Recyclinghof_Abfaelle_und_Schadstoffannahme_bf.pdf (accessed on 1 March 2023).
- Plasticker. Plasticker: Market & Prices. Available online: https://plasticker.de/preise/preise_monat_single.php (accessed on 27 February 2023).
- Schrott24. Kupfer schwer, Berry min. 98% Cu. 2023. Available online: https://www.schrott24.de/altmetall-ankauf/kupfer/kupfer-1-a-98-cu-berry/ (accessed on 11 September 2023).
- Strem. Strem Catalog: 93-2456 Chromium › Chromium Pieces (99.3%). 2023. Available online: https://www.strem.com/catalog/v/93-2456/15/chromium_7440-47-3 (accessed on 11 September 2023).
- Mistry, M.; Gediga, J.; Boonzaier, S. Life cycle assessment of nickel products. Int. J. Life Cycle Assess 2016, 21, 1559–1572. [Google Scholar] [CrossRef]
- ISO 14040:2006; Environmental Management—Life Cycle Assessment—Principles and FRAMEWORK. International Organisation for Standardisation (ISO): Geneva, Switzerland, 2006.
- ISO 14044:2006; Environmental Management—Life Cycle Assessment—Requirements and Guidelines. International Organisation for Standardisation (ISO): Geneva, Switzerland, 2006.
- SuboLab GmbH Fine and Industrial Chemicals. Nickel (II) Chloride Nickel Chloride. Available online: https://www.subolab.de/en/nickelchlorid.html (accessed on 11 September 2023).
- Deutsche Rohstoffagentur (DERA) in der Bundesanstalt für Geowissenschaften und Rohstoffe (BGR). Preismonitor Juli 2023. July 2023. Available online: https://www.deutsche-rohstoffagentur.de/DERA/DE/Aktuelles/Monitore/2023/07-23/2023-07-preismonitor.pdf;jsessionid=02A12583C235C11EE7F84104C6AD7A33.internet012?__blob=publicationFile&v=3 (accessed on 11 September 2023).

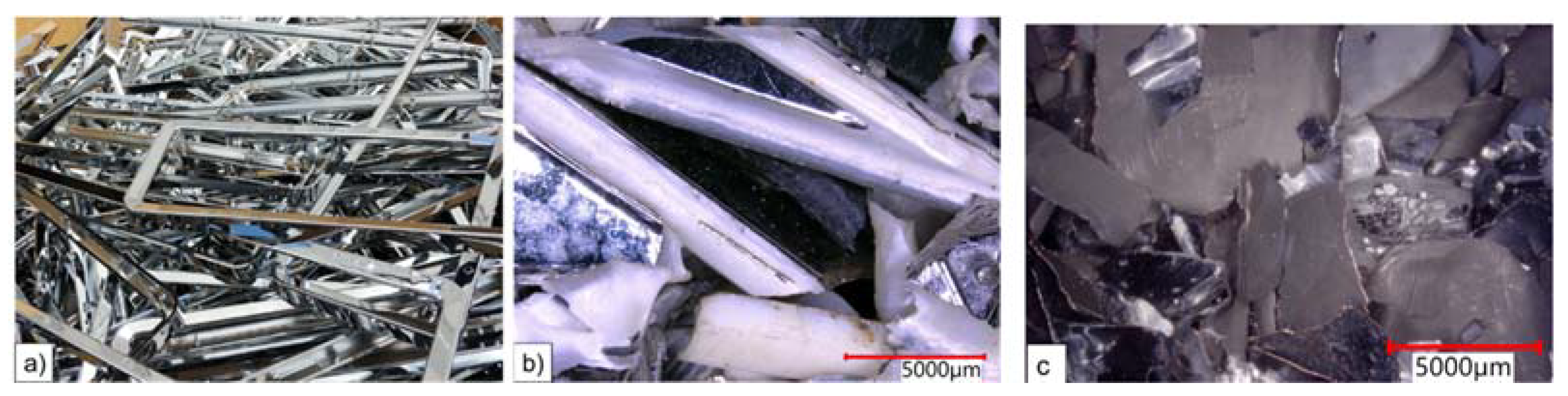
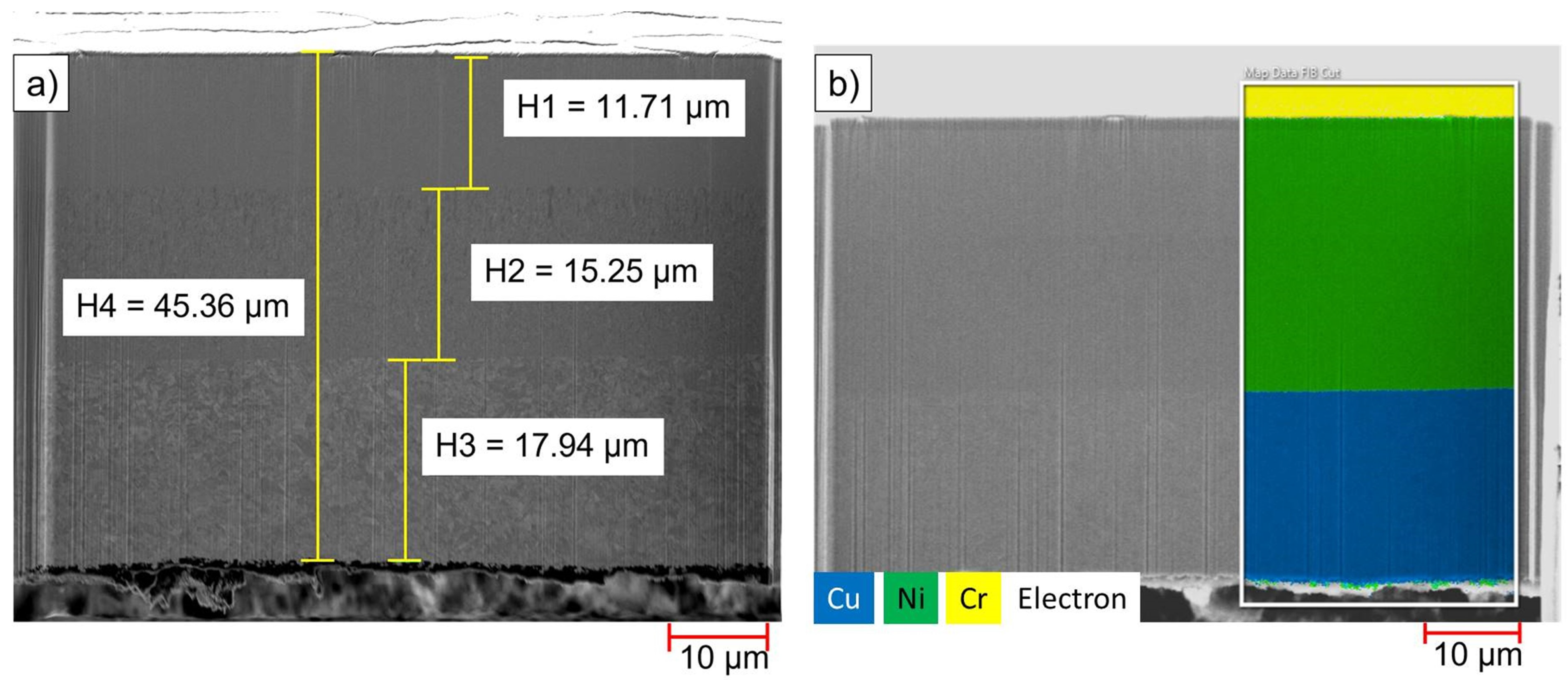





| Energy | Materials (Solids) | Materials (Liquids) | Labour | Machinery | Wastes | Products | Byproducts |
|---|---|---|---|---|---|---|---|
| Kilowatt Hour | Kilogram | Litres | Hours | Number of machines Years (for depreciation) | Kilogram | Kilogram | Kilogram |
| Cost Classification | Description | Price (excl. VAT) | Price Units | Source |
|---|---|---|---|---|
| Direct Materials | Metallized Plastics (ReComp I) | 0 | N/A | Zero cost Industrial production waste streams and a small portion from end-of-life vehicles. |
| Metal Flakes and Plastic Particles (ReComp II) | ||||
| Indirect Materials | Deionised Water | 0.05182 | EUR/L | Berlin Wasserbetriebe (BWB) [19] |
| CH3SO3H (Purity: 99.5% p.a. ACS) | 94.90 | EUR/L | Carl Roth GmbH and Co. KG [20] | |
| Energy | Electricity | 0.27 | EUR/kWh | Average industrial prices including tax in Germany for 2022 [21] |
| Labour Costs | Production Worker | 20 | EUR/h | Company data |
| Engineer | 30 | EUR/h | Company data |
| Material | Nickel/wt.% | Copper/wt.% | Chromium/wt.% | PC/ABS/wt.% |
|---|---|---|---|---|
| Input, ReComp I (Shredded particles of metallized plastic) | 13.9 | 7.9 | 0.2 | 78.0 |
| Input A, ReComp II (Shredded metal flakes) | 42.7 | 52.0 | 0.6 | 4.7 |
| Input B, ReComp II (Shredded plastic particles) | traces | traces | traces | ~100 |
| Material | Recovery Yield % | Purity % |
|---|---|---|
| Cathode (Cu) | 23 | 99.8 |
| Filtrate (Cr) | 98 | 99.4 |
| Input | QC | Output |
|---|---|---|
| Metallized plastic (M), technician (L), container (Ma), hand forklift (Ma), IBC tank (Ma) | QC1: Storage | Metallized plastic (OM), loss of metallized plastics (2%) (W) |
| Electricity (E), metallized plastic (M), electrolyte (M), technician (L), galvanic system electrodes (Ma) | QC2: Electrochemical Treatment | Wet and acidic PC/ABS (OM), used electrolyte with Cr particles (OM), loss of metallized plastics (2%) (W), Cu (P) |
| Electricity (E), wet and acidic PC/ABS (M), technician (L), water bath (Ma) | QC3.1: Drum Material Rinsing | Wet PC/ABS (OM), acidic water (W), loss of PC/ABS (0.01%) (W) |
| Electricity (E), wet PC/ABS (M), technician (L), dewatering machine (Ma) | QC4.1: Plastic Dewatering | Water (W), loss of PC/ABS (2%) (W), PC/ABS (P) |
| Electricity (E), used electrolyte with Cr particles (M), technician (L), filter bags (Ma), IBC container (Ma), pump set (Ma) | QC3.2: Filtration | Used electrolyte (OM), loss of Cr particles (2%) (W), Cr particles (P) |
| Electricity (E), used electrolyte (M), technician (L), distillation apparatus (Ma), pump set (Ma) | QC4.2: Electrolyte Recovery | Distillation residue with Ni/Cu precipitate (W), loss of electrolyte (3%), Purified Electrolyte (BP) |
| Quantity/kg/Annum | Revenue/EUR/Annum | |
|---|---|---|
| Copper | 13.0 | 90 |
| PC/ABS | 130.0 | 300 |
| Chromium | 0.3 | 55 |
| SUM | 143.3 | 445 |
| No. | Impact Category | Unit | Primary Production | ReComp I |
|---|---|---|---|---|
| 1 | Fine particulate matter formation (PMFP) | kg PM2.5 eq | ||
| 2 | Fossil resource scarcity (FFP) | kg oil eq | ||
| 3 | Freshwater ecotoxicity (FETP) | kg 1,4-DCB | ||
| 4 | Freshwater eutrophication (FEP) | kg P eq | ||
| 5 | Global warming (GWP) | kg CO2 eq | 2.24 | |
| 6 | Human carcinogenic toxicity (HTPc) | kg 1,4-DCB | ||
| 7 | Human non-carcinogenic toxicity (HTPnc) | kg 1,4-DCB | ||
| 8 | Ionizing radiation (IRP) | kBq Co-60 eq | ||
| 9 | Land use (LOP) | m2a crop eq | ||
| 10 | Marine ecotoxicity (METP) | kg 1,4-DCB | ||
| 11 | Marine eutrophication (MEP) | kg N eq | ||
| 12 | Mineral resource scarcity (SOP) | kg Cu eq | ||
| 13 | Ozone formation, human health (HOFP) | kg NOx eq | ||
| 14 | Ozone formation, terrestrial ecosystems (EOFP) | kg NOx eq | ||
| 15 | Stratospheric ozone depletion (ODP) | kg CFC-11 eq | ||
| 16 | Terrestrial acidification (TAP) | kg SO2 eq | ||
| 17 | Terrestrial ecotoxicity (TETP) | kg 1,4-DCB | ||
| 18 | Water consumption (WCP) | m3 |
| Process Step | Process Description (ReComp I) | Changes in Process (ReComp II) |
|---|---|---|
| Step 1: Storage | The shredded metallized plastics are stored and prepared for the next batch in this quantity centre. | Shredding produces two different material streams—plastic particles and metal flakes. These are stored separately. |
| Step 2A: Electrochemical Treatment (including Cu Deposition and Recovery of Cr as Flakes) | The pre-processed metallized plastic particles are filled in a rotary drum electrode. The drum is placed in a bath with an electrolyte (methanesulfonic acid, CH3SO3H) in a three-electrode setup (the drum is the working electrode, Cu foil as counter, and standard hydrogen as reference electrode). An electrical voltage is used to dissolve copper and nickel from the plastic (PC/ABS). The voltage is applied in a way that copper is deposited, and chromium is not dissolved but instead separated as metal flakes. | Similar to ReComp I, except that now only metal flakes are processed in the electrochemical treatment. This reduces the overall cycle time. |
| Step 2B: Leaching | This process step only exists in ReComp II. | This is a new step for ReComp II. The plastic flakes are held in a leaching reactor, filled with an electrolyte (methane sulfonic acid, CH3SO3H). The bath is heated and any remaining nickel and copper on the plastic flakes is dissolved. |
| Step 3.1: Drum Material Rinsing | PC/ABS is cleaned by immersing the drum from the QC2A in water. Thereby, the leftover acid is neutralised and the plastic cleaned. This process can clean large volumes of plastic very effectively. | Similar to ReComp I, except that the PC/ABS is sourced from Step 2B, leaching for the plastic particles only. |
| Step 4.1: Drying | Here, the water is removed from the plastic fraction. This process includes vacuum, centrifugation, filtration, solid–liquid separation processes, and removal of residual liquids with a filter press. | No change from ReComp I |
| Step 3.2: Filtration | A ceramic filter is used to separate the chromium flakes from the electrolyte. The solid (chromium) parts remain on the filter, and the liquid passes through the pores. | No change from ReComp I. Metal flakes are sourced from QC2B. |
| QC 4.2: Electrolyte Recovery | A distillation system designed to recover electrolyte with distillation. Nickel salt contaminated with the non-deposited copper is produced as a waste. The distillation system can purify and concentrate various acids and mixed acid solutions. | Unlike ReComp I, where only the electrolyte from Step 2B is treated, the electrolyte recovery system in ReComp II recovers electrolyte from both processes Step 2A and 2B. |
| Input | QC | Output |
|---|---|---|
| Metal flakes (M), plastic particles (M), technician (L), container (Ma), hand forklift (Ma), IBC tank (Ma) | QC1: Storage | Metal flakes (OM), plastic particles (OM), loss of metal flakes and plastic particles (2%) (W) |
| Electricity (E), metal flakes (M), electrolyte (M), technician (L), galvanic system (Ma), electrodes (Ma) | QC2A: Electrochemical Treatment | Wet and acidic PC/ABS (OM), used electrolyte with Cr particles (OM), loss of metallized plastic (2%) (W), Cu (P) |
| Electricity (E), plastic flakes (M), electrolyte (M), technician (L), leaching reactor (Ma), cooling device (Ma), filter bag/collection vessel (Ma) | QC2B: Leaching | Wet and acidic PC/ABS (OM), electrolyte with dissolved Ni and Cu (OM), Ni and Cr flakes (W), loss of electrolyte and I/ABS flakes (2%) (W) |
| Electricity (E), wet and acidic PC/ABS (M), technician (L), water bath (Ma) | QC3.1: Drum Material Rinsing | Wet PC/ABS (OM), acidic water (W), loss of PC/ABS (0.01%) (W) |
| Electricity (E), wet PC/ABS (M), technician (L), dewatering machine (Ma) | QC4.1: Plastic Dewatering | Water (W), loss of IC/ABS (2%) (W), PC/ABS (P) |
| Electricity (E), used electrolyte with Cr particles (M), technician (L), filter bags (Ma), IBC container (Ma), pump set (Ma) | QC3.2: Filtration | Used electrolyte (OM), loss of Cr particles (2%) (W), Cr particles (P) |
| Electricity (E), used electrolyte (M), technician (L), distillation apparatus (Ma), pump set (Ma) | QC4.2: Electrolyte Recovery | Distillation residue with Ni/Cu precipitate (W), loss of electrolyte (3%), Purified Electrolyte (BP) |
| Quantity kg/Annum | Revenue EUR/Annum | |
|---|---|---|
| Copper | 760 | 5300 |
| PC/ABS | 4500 | 11,000 |
| Chromium | 14 | 2400 |
| SUM | 5274 | 18,700 |
| No. | Impact Category | Unit | ReComp 1 | ReComp 2 |
|---|---|---|---|---|
| 1 | Fine particulate matter formation (PMFP) | kg PM2.5 eq | ||
| 2 | Fossil resource scarcity (FFP) | kg oil eq | ||
| 3 | Freshwater ecotoxicity (FETP) | kg 1,4-DCB | ||
| 4 | Freshwater eutrophication (FEP) | kg P eq | ||
| 5 | Global warming (GWP) | kg CO2 eq | 2.24 | |
| 6 | Human carcinogenic toxicity (HTPc) | kg 1,4-DCB | ||
| 7 | Human non-carcinogenic toxicity (HTPnc) | kg 1,4-DCB | ||
| 8 | Ionizing radiation (IRP) | kBq Co-60 eq | ||
| 9 | Land use (LOP) | m2a crop eq | ||
| 10 | Marine ecotoxicity (METP) | kg 1,4-DCB | ||
| 11 | Marine eutrophication (MEP) | kg N eq | ||
| 12 | Mineral resource scarcity (SOP) | kg Cu eq | ||
| 13 | Ozone formation, human health (HOFP) | kg NOx eq | ||
| 14 | Ozone formation, terrestrial ecosystems (EOFP) | kg NOx eq | ||
| 15 | Stratospheric ozone depletion (ODP) | kg CFC-11 eq | ||
| 16 | Terrestrial acidification (TAP) | kg SO2 eq | ||
| 17 | Terrestrial ecotoxicity (TETP) | kg 1,4-DCB | ||
| 18 | Water consumption (WCP) | m3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Walls, C.; Choi, B.-K.; Putri, A.R.K.; Bernal-Osorio, A.; D’Souza, A.; Khadse, H.; Ghori, M.; Rossa, J.; Varute, S.; Beck, G. Recycling of Metallized Plastic as a Case Study for a Continuous Sustainability Improvement Process. Sustainability 2023, 15, 14737. https://doi.org/10.3390/su152014737
Walls C, Choi B-K, Putri ARK, Bernal-Osorio A, D’Souza A, Khadse H, Ghori M, Rossa J, Varute S, Beck G. Recycling of Metallized Plastic as a Case Study for a Continuous Sustainability Improvement Process. Sustainability. 2023; 15(20):14737. https://doi.org/10.3390/su152014737
Chicago/Turabian StyleWalls, Caitlin, Bum-Ki Choi, Almy Ruzni Keumala Putri, Adriana Bernal-Osorio, Alan D’Souza, Himanshu Khadse, Monark Ghori, Jürgen Rossa, Siddharth Varute, and Gesa Beck. 2023. "Recycling of Metallized Plastic as a Case Study for a Continuous Sustainability Improvement Process" Sustainability 15, no. 20: 14737. https://doi.org/10.3390/su152014737
APA StyleWalls, C., Choi, B.-K., Putri, A. R. K., Bernal-Osorio, A., D’Souza, A., Khadse, H., Ghori, M., Rossa, J., Varute, S., & Beck, G. (2023). Recycling of Metallized Plastic as a Case Study for a Continuous Sustainability Improvement Process. Sustainability, 15(20), 14737. https://doi.org/10.3390/su152014737









