A Review of Strategies to Enhance the Water Resistance of Green Wood Adhesives Produced from Sustainable Protein Sources
Abstract
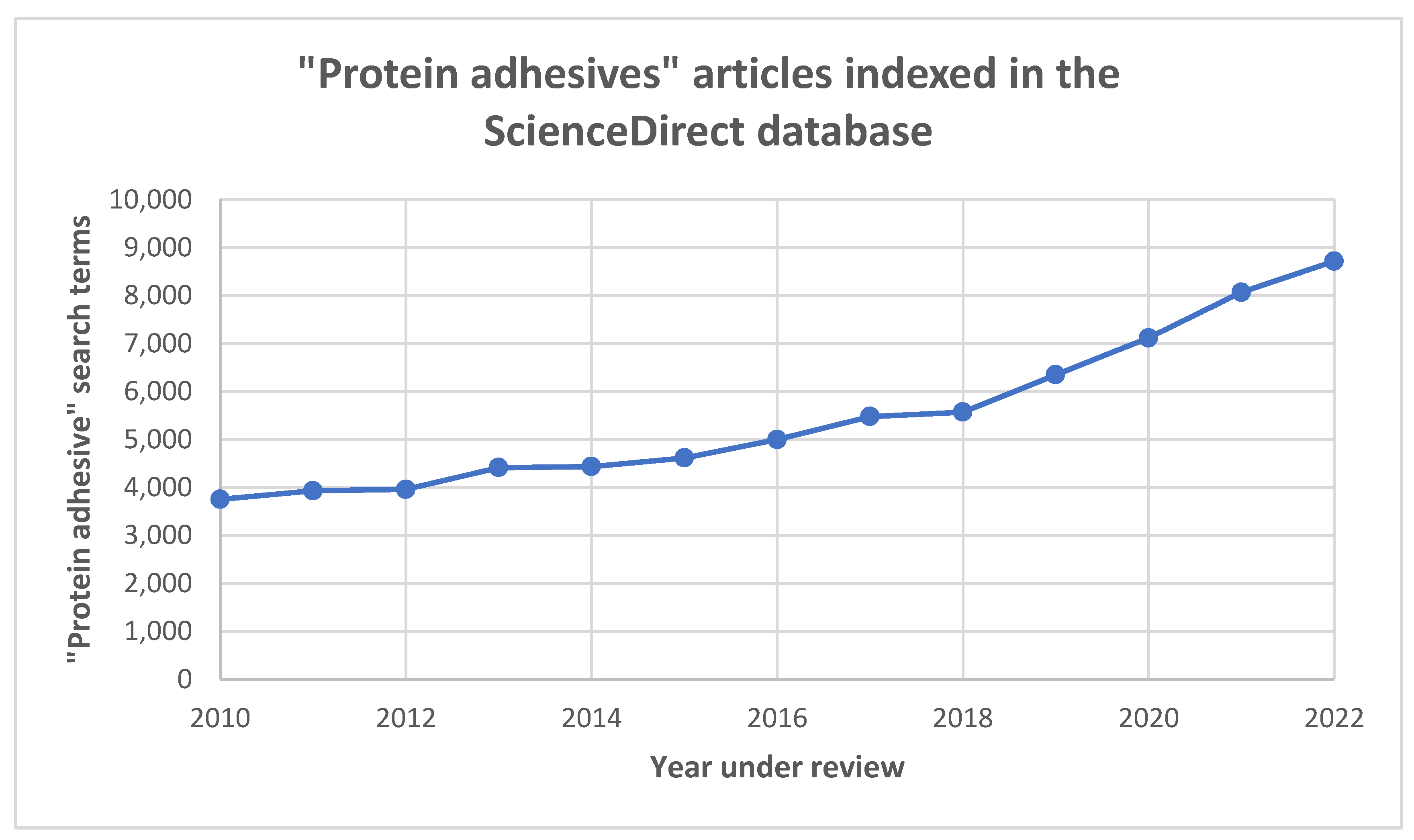
1. Introduction
2. Strategies to Improve Water Resistance in Protein Adhesives
2.1. Cross-Linking Networks
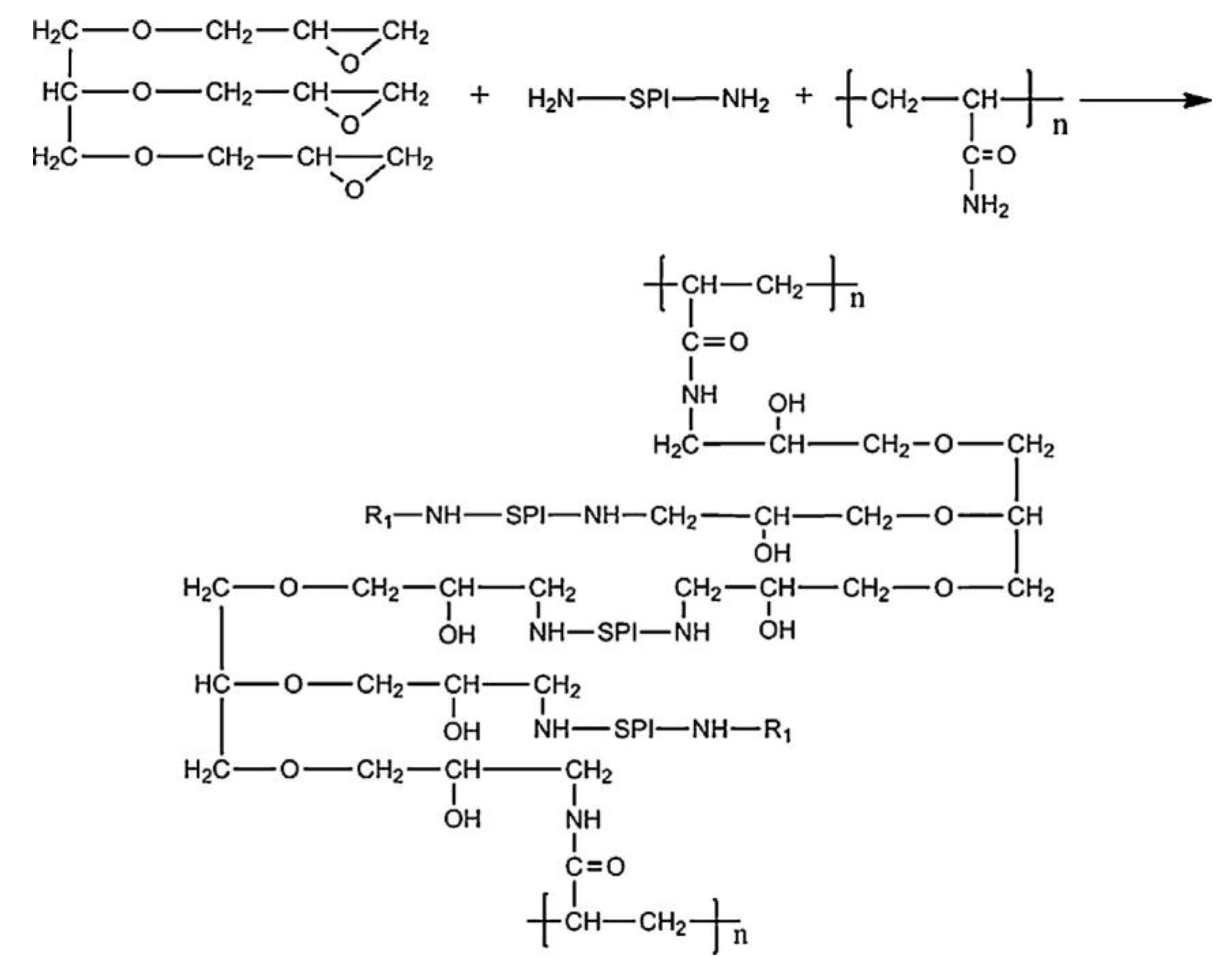
2.1.1. Synthetic Cross-Linking Agent Sources
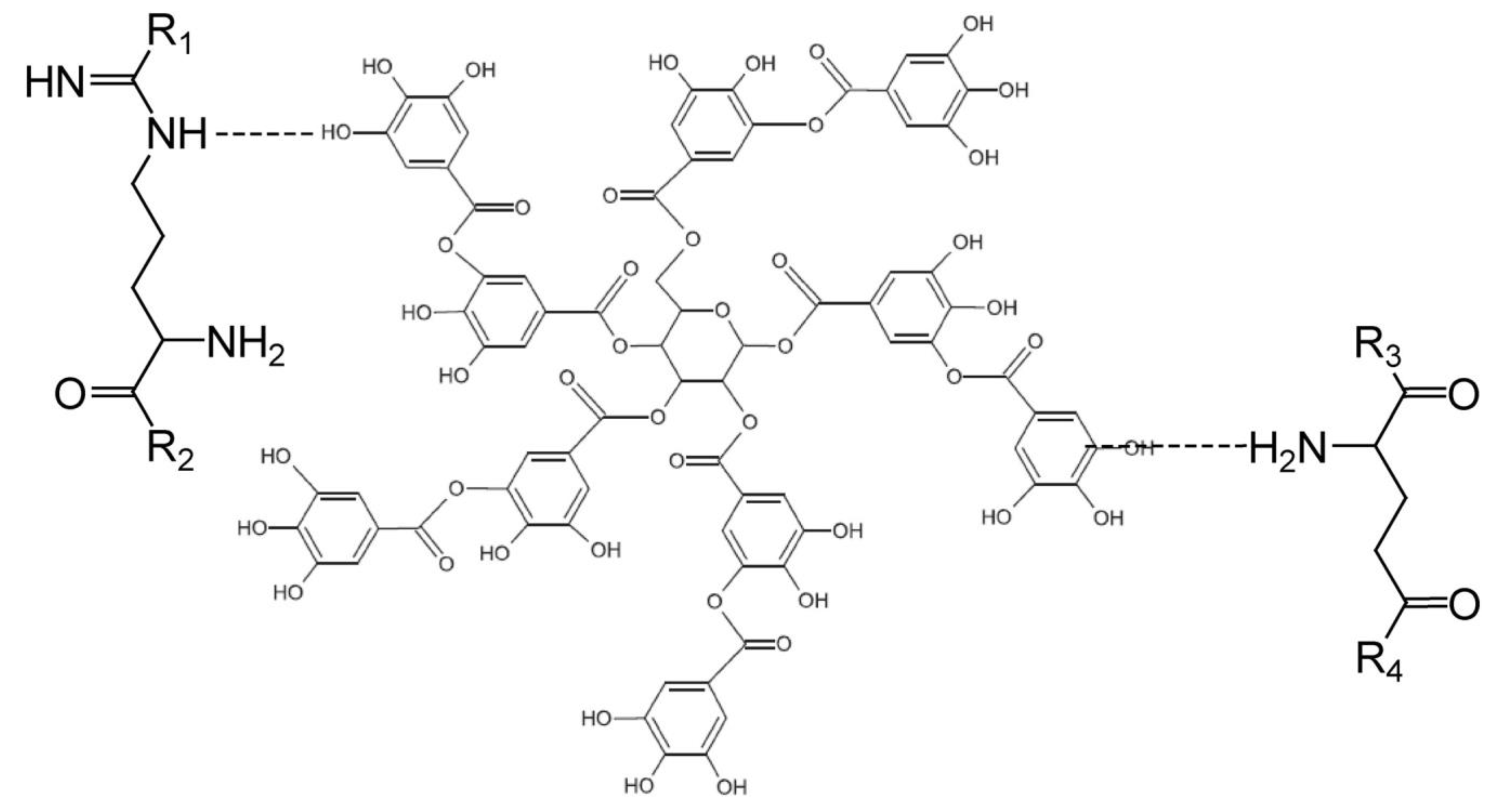
2.1.2. Bio-Based Cross-linking Agent Sources
2.1.3. Synthetic and Bio-Based Cross-linking Sources
2.2. Water Resistance from Modified Fillers
2.3. Removal of Hydrophilic Content
3. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Grand View Research. Wood Adhesives Market Growth & Trends. Grand View Research. Available online: https://www.grandviewresearch.com/press-release/global-wood-adhesives-market (accessed on 21 March 2023).
- Pradyawong, S.; Li, J.; He, Z.; Sun, X.S.; Wang, D.; Cheng, H.N.; Klasson, K.T. Blending Cottonseed Meal Products with Different Protein Contents for Cost-Effective Wood Adhesive Performances. Ind. Crop. Prod. 2018, 126, 31–37. [Google Scholar] [CrossRef]
- Antonio Pizzi, K.M. Wood Adhesives, 1st ed.; CRC Press: London, UK, 2011; p. 462. [Google Scholar] [CrossRef]
- Raydan, N.D.V.; Leroyer, L.; Charrier, B.; Robles, E. Recent Advances on the Development of Protein-Based Adhesives for Wood Composite Materials—A Review. Molecules 2021, 26, 7617. [Google Scholar] [CrossRef]
- Wibowo, E.S.; Lubis, M.A.R.; Park, B.D.; Kim, J.S.; Causin, V. Converting Crystalline Thermosetting Urea–Formaldehyde Resins to Amorphous Polymer Using Modified Nanoclay. J. Ind. Eng. Chem. 2020, 87, 78–89. [Google Scholar] [CrossRef]
- He, Z.; Zhang, Y.; Wei, W. Formaldehyde and VOC Emissions at Different Manufacturing Stages of Wood-Based Panels. Build. Environ. 2012, 47, 197–204. [Google Scholar] [CrossRef]
- Kumar, R.N.; Pizzi, A. Environmental Aspects of Adhesives—Emission of Formaldehyde. Adhes. Wood Lignocellul. Mater. 2019, 1, 293–315. [Google Scholar] [CrossRef]
- Congress. Formaldehyde Standards for Composite Wood Products. Public Law, 2010, Title VI (II). Available online: https://www.congress.gov/111/plaws/publ199/PLAW-111publ199.pdf (accessed on 21 March 2023).
- Kristak, L.; Antov, P.; Bekhta, P.; Lubis, M.A.R.; Iswanto, A.H.; Reh, R.; Sedliacik, J.; Savov, V.; Taghiyari, H.R.; Papadopoulos, A.N.; et al. Recent Progress in Ultra-Low Formaldehyde Emitting Adhesive Systems and Formaldehyde Scavengers in Wood-Based Panels: A Review. Wood Mater. Sci. Eng. 2023, 18, 763–782. [Google Scholar] [CrossRef]
- Podlena, M.; Böhm, M.; Saloni, D.; Velarde, G.; Salas, C. Tuning the Adhesive Properties of Soy Protein Wood Adhesives with Different Coadjutant Polymers, Nanocellulose and Lignin. Polymers 2021, 13, 1972. [Google Scholar] [CrossRef] [PubMed]
- Grand View Research. Wood Adhesives Market Size. Grand View Research. Available online: https://www.grandviewresearch.com/industry-analysis/wood-adhesives-market (accessed on 21 March 2023).
- Cholewinski, A.; Yang, F.; Zhao, B. Algae-Mussel-Inspired Hydrogel Composite Glue for Underwater Bonding. Mater. Horiz. 2019, 6, 285–293. [Google Scholar] [CrossRef]
- North, M.A.; Del Grosso, C.A.; Wilker, J.J. High Strength Underwater Bonding with Polymer Mimics of Mussel Adhesive Proteins. ACS Appl. Mater. Interfaces 2017, 9, 7866–7872. [Google Scholar] [CrossRef] [PubMed]
- Tsujimoto, Y. Molecular Biology of Cell Death. Jpn. J. Cancer Chemother. 1994, 21, 591–595. [Google Scholar]
- Diani, J.; Gall, K. Finite Strain 3D Thermoviscoelastic Constitutive Model. Polym. Eng. Sci. 2006, 46, 486–492. [Google Scholar] [CrossRef]
- Vnučec, D.; Kutnar, A.; Goršek, A. Soy-Based Adhesives for Wood-Bonding—A Review. J. Adhes. Sci. Technol. 2017, 31, 910–931. [Google Scholar] [CrossRef]
- Chirdon, W.M. Utilization of Biorefi Nery Waste Proteins as Feed, Glues, Composites, and Other Co-Products. In Algal Biorefineries Volume 2: Products and Refinery Design; Springer: Berlin/Heidelberg, Germany, 2015; pp. 367–392. [Google Scholar] [CrossRef]
- Frihart, C.R.; Birkeland, M.J. Soy Properties and Soy Wood Adhesives. ACS Symp. Ser. 2014, 1178, 167–192. [Google Scholar] [CrossRef]
- Shi, Q.; Zhou, Y.; Sun, Y. Influence of PH and Ionic Strength on the Steric Mass-Action Model Parameters around the Isoelectric Point of Protein. Biotechnol. Prog. 2005, 21, 516–523. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.W.; Zhao, X.; Hou, J.L.; Li, Z.T. Aromatic Amide Foldamers: Structures, Properties, and Functions. Chem. Rev. 2012, 112, 5271–5316. [Google Scholar] [CrossRef] [PubMed]
- Abedi, E.; Pourmohammadi, K. Physical Modifications of Wheat Gluten Protein: An Extensive Review. J. Food Process Eng. 2021, 44, e13619. [Google Scholar] [CrossRef]
- Dunky, M. Wood Adhesives Based on Natural Resources: A Critical Review: Part I. Protein-Based Adhesives. Rev. Adhes. Adhes. 2020, 8, 199–332. [Google Scholar] [CrossRef]
- Tavano, O.L. Protein Hydrolysis Using Proteases: An Important Tool for Food Biotechnology. J. Mol. Catal. B Enzym. 2013, 90, 1–11. [Google Scholar] [CrossRef]
- Bekard, I.B.; Asimakis, P.; Bertolini, J.; Dunstan, D.E. The Effects of Shear Flow on Protein Structure and Function. Biopolymers 2011, 95, 733–745. [Google Scholar] [CrossRef] [PubMed]
- Hemmilä, V.; Adamopoulos, S.; Karlsson, O.; Kumar, A. Development of Sustainable Bio-Adhesives for Engineered Wood Panels—A Review. RSC Adv. 2017, 7, 38604–38630. [Google Scholar] [CrossRef]
- Braun, R.; Seebeck, T. RNA Metabolism. In Cell Biology Physarum Didymium; Academic Press: Cambridge, MA, USA, 1982; pp. 393–435. [Google Scholar] [CrossRef]
- Hettiarachchy, N.S.; Kalapathy, U.; Myers, D.J. Alkali-Modified Soy Protein with Improved Adhesive and Hydrophobic Properties. J. Am. Oil Chem. Soc. 1995, 72, 1461–1464. [Google Scholar] [CrossRef]
- Wei, Y.; Yao, J.; Shao, Z.; Chen, X. Water-Resistant Zein-Based Adhesives. ACS Sustain. Chem. Eng. 2020, 8, 7668–7679. [Google Scholar] [CrossRef]
- Chen, N.; Lin, Q.; Rao, J.; Zeng, Q. Water Resistances and Bonding Strengths of Soy-Based Adhesives Containing Different Carbohydrates. Ind. Crop. Prod. 2013, 50, 44–49. [Google Scholar] [CrossRef]
- Gui, C.; Zhu, J.; Zhang, Z.; Liu, X. Research Progress on Formaldehyde-Free Wood Adhesive Derived from Soy Flour. In Adhesives—Applications and Properties; Intechopen: London, UK, 2016. [Google Scholar] [CrossRef]
- Lamaming, S.Z.; Lamaming, J.; Rawi, N.F.M.; Hashim, R.; Kassim, M.H.M.; Hussin, M.H.; Bustami, Y.; Sulaiman, O.; Amini, M.H.M.; Hiziroglu, S. Improvements and Limitation of Soy Protein-Based Adhesive: A Review. Polym. Eng. Sci. 2021, 61, 2393–2405. [Google Scholar] [CrossRef]
- Li, H.; Kang, H.; Zhang, W.; Zhang, S.; Li, J. Physicochemical Properties of Modified Soybean-Flour Adhesives Enhanced by Carboxylated Styrene-Butadiene Rubber Latex. Int. J. Adhes. Adhes. 2016, 66, 59–64. [Google Scholar] [CrossRef]
- Wu, Z.; Xi, X.; Lei, H.; Liang, J.; Liao, J.; Du, G. Study on Soy-Based Adhesives Enhanced by Phenol Formaldehyde Cross-Linker. Polymers 2019, 11, 365. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Han, Y.; Chen, M.; Luo, J.; Shi, S.Q.; Li, J.; Gao, Q. Constructing a Triple Network Structure to Prepare Strong, Tough, and Mildew Resistant Soy Protein Adhesive. Compos. Part B Eng. 2021, 211, 108677. [Google Scholar] [CrossRef]
- Hand, W.G.; Robert Ashurst, W.; Via, B.; Banerjee, S. Curing Behavior of Soy Flour with Phenol-Formaldehyde and Isocyanate Resins. Int. J. Adhes. Adhes. 2018, 87, 105–108. [Google Scholar] [CrossRef]
- Gao, D.; Fan, B.; Zhang, B.; Mi, Y.; Zhang, Y.; Gao, Z. Storage Stability of Polyamidoamine-Epichlorohydrin Resin and Its Effect on the Properties of Defatted Soybean Flour-Based Adhesives. Int. J. Adhes. Adhes. 2019, 91, 92–101. [Google Scholar] [CrossRef]
- Zhao, L.F.; Liu, Y.; Xu, Z.D.; Zhang, Y.Z.; Zhao, F.; Zhang, S.B. State of Research and Trends in Development of Wood Adhesives. For. Stud. China 2011, 13, 321–326. [Google Scholar] [CrossRef]
- Souza, A.M.; Nascimento, M.F.; Almeida, D.H.; Lopes Silva, D.A.; Almeida, T.H.; Christoforo, A.L.; Lahr, F.A.R. Wood-Based Composite Made of Wood Waste and Epoxy Based Ink-Waste as Adhesive: A Cleaner Production Alternative. J. Clean. Prod. 2018, 193, 549–562. [Google Scholar] [CrossRef]
- Jin, S.; Li, K.; Gao, Q.; Zhang, W.; Chen, H.; Li, J.; Shi, S.Q. Multiple Crosslinking Strategy to Achieve High Bonding Strength and Antibacterial Properties of Double-Network Soy Adhesive. J. Clean. Prod. 2020, 254, 120143. [Google Scholar] [CrossRef]
- Bai, Y.Y.; Lei, Y.H.; Shen, X.J.; Luo, J.; Yao, C.L.; Sun, R.C. A Facile Sodium Alginate-Based Approach to Improve the Mechanical Properties of Recycled Fibers. Carbohydr. Polym. 2017, 174, 610–616. [Google Scholar] [CrossRef] [PubMed]
- Li, K.; Peshkova, S.; Geng, X. Investigation of Soy Protein-Kymene® Adhesive Systems for Wood Composites. J. Am. Oil Chem. Soc. 2004, 81, 487–491. [Google Scholar] [CrossRef]
- Kan, Y.; Sun, B.; Bai, Y.; Gao, Z. Double-Network Strategy for a Cost-Effective Soybean Meal-Based Adhesive with Required and Stable Water Resistance for Structural Use. Compos. Part B Eng. 2022, 235, 109744. [Google Scholar] [CrossRef]
- Lei, H.; Du, G.; Wu, Z.; Xi, X.; Dong, Z. Cross-Linked Soy-Based Wood Adhesives for Plywood. Int. J. Adhes. Adhes. 2014, 50, 199–203. [Google Scholar] [CrossRef]
- Xi, X.; Pizzi, A.; Gerardin, C.; Liao, J.; Amirou, S.; Abdalla, S. Glutaraldehyde-Wheat Gluten Protein Adhesives for Wood Bonding. J. Adhes. 2021, 97, 88–100. [Google Scholar] [CrossRef]
- Li, K.; Geng, X. Formaldehyde-Free Wood Adhesives from Decayed Wood. Macromol. Rapid Commun. 2005, 26, 529–532. [Google Scholar] [CrossRef]
- Liu, Y.; Li, K. Development and Characterization of Adhesives from Soy Protein for Bonding Wood. Int. J. Adhes. Adhes. 2007, 27, 59–67. [Google Scholar] [CrossRef]
- Zeng, Y.; Xu, P.; Yang, W.; Chu, H.; Wang, W.; Dong, W.; Chen, M.; Bai, H.; Ma, P. Soy Protein-Based Adhesive with Superior Bonding Strength and Water Resistance by Designing Densely Crosslinking Networks. Eur. Polym. J. 2021, 142, 110128. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, M.; Chen, M.; Luo, J.; Li, X.; Gao, Q.; Li, J. Preparation and Characterization of a Soy Protein-Based High-Performance Adhesive with a Hyperbranched Cross-Linked Structure. Chem. Eng. J. 2018, 354, 1032–1041. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, J.; Chen, M.; Luo, J.; Shi, S.Q.; Gao, Q.; Li, J. A Tough, Water-Resistant, High Bond Strength Adhesive Derived from Soybean Meal and Flexible Hyper-Branched Aminated Starch. Polymers 2019, 11, 1352. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Li, J.; Kan, Y.; Gao, J.; Zhang, Y.; Gao, Z. The Effect of Thermo-Chemical Treatment on the Water Resistance of Defatted Soybean Flour-Based Wood Adhesive. Polymers 2018, 10, 955. [Google Scholar] [CrossRef]
- Li, J.; Zhang, B.; Li, X.; Yi, Y.; Shi, F.; Guo, J.; Gao, Z. Effects of Typical Soybean Meal Type on the Properties of Soybean-Based Adhesive. Int. J. Adhes. Adhes. 2019, 90, 15–21. [Google Scholar] [CrossRef]
- Remya, R.; Jyothi, A.N.; Sreekumar, J. Effect of Chemical Modification with Citric Acid on the Physicochemical Properties and Resistant Starch Formation in Different Starches. Carbohydr. Polym. 2018, 202, 29–38. [Google Scholar] [CrossRef] [PubMed]
- Zheng, X.; Cheng, L.; Gu, Z.; Hong, Y.; Li, Z.; Li, C. Effects of Heat Pretreatment of Starch on Graft Copolymerization Reaction and Performance of Resulting Starch-Based Wood Adhesive. Int. J. Biol. Macromol. 2017, 96, 11–18. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Xiong, Z.; Din, Z.-U.; Nawaz, A.; Xiong, H.; Cai, J. Interfacial Modification of Starch at High Concentration by Sodium Dodecylsulfate as Revealed by Experiments and Molecular Simulation. J. Mol. Liq. 2020, 310, 113190. [Google Scholar] [CrossRef]
- Bai, Y.; Zhao, F.; Shen, J.; Zhang, Y. Improvement of Water Resistance of Wheat Flour-Based Adhesives by Thermal–Chemical Treatment and Chemical Crosslinking. J. Appl. Polym. Sci. 2021, 138, 50458. [Google Scholar] [CrossRef]
- Wang, Y.; Fan, Y.; Deng, L.; Li, Z.; Chen, Z. Properties of Soy-Based Wood Adhesives Enhanced by Waterborne Polyurethane Modification. J. Biobased Mater. Bioenergy 2017, 11, 330–335. [Google Scholar] [CrossRef]
- Zia, F.; Zia, K.M.; Zuber, M.; Ahmad, H.B.; Muneer, M.I. Glucomannan Based Polyurethanes: A Critical Short Review of Recent Advances and Future Perspectives. Int. J. Biol. Macromol. 2016, 87, 229–236. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Luo, Z.; Luo, L.; Yin, X.; Li, Q.; Mao, A. Study on Protein Based Adhesives Blending-Modified by Isocyanate. Am. J. Environ. Sci. Eng. 2022, 6, 62. [Google Scholar] [CrossRef]
- Liu, C.; Zhang, Y.; Li, X.; Luo, J.; Gao, Q.; Li, J. A High-Performance Bio-Adhesive Derived from Soy Protein Isolate and Condensed Tannins. RSC Adv. 2017, 7, 21226–21233. [Google Scholar] [CrossRef]
- Xu, F.; Dong, Y.; Zhang, W.; Zhang, S.; Li, L.; Li, J. Preparation of Cross-Linked Soy Protein Isolate-Based Environmentally-Friendly Films Enhanced by PTGE and PAM. Ind. Crop. Prod. 2015, 67, 373–380. [Google Scholar] [CrossRef]
- Lee, H.; Lee, B.P.; Messersmith, P.B. A Reversible Wet/Dry Adhesive Inspired by Mussels and Geckos. Nature 2007, 448, 338–341. [Google Scholar] [CrossRef]
- Al Loman, A.; Ju, L.K. Towards Complete Hydrolysis of Soy Flour Carbohydrates by Enzyme Mixtures for Protein Enrichment: A Modeling Approach. Enzym. Microb. Technol. 2016, 86, 25–33. [Google Scholar] [CrossRef]
- Islam, S.M.M.; Loman, A.A.; Ju, L.K. High Monomeric Sugar Yields from Enzymatic Hydrolysis of Soybean Meal and Effects of Mild Heat Pretreatments with Chelators. Bioresour. Technol. 2018, 256, 438–445. [Google Scholar] [CrossRef] [PubMed]
- Zheng, P.; Chen, N.; Mahfuzul Islam, S.M.; Ju, L.K.; Liu, J.; Zhou, J.; Chen, L.; Zeng, H.; Lin, Q. Development of Self-Cross-Linked Soy Adhesive by Enzyme Complex from Aspergillus Niger for Production of All-Biomass Composite Materials. ACS Sustain. Chem. Eng. 2019, 7, 3909–3916. [Google Scholar] [CrossRef]
- Lo Presti, M.; Rizzo, G.; Farinola, G.M.; Omenetto, F.G. Bioinspired Biomaterial Composite for All-Water-Based High-Performance Adhesives. Adv. Sci. 2021, 8, 2004786. [Google Scholar] [CrossRef]
- Qian, Y.; Zhou, Y.; Lu, M.; Guo, X.; Yang, D.; Lou, H.; Qiu, X.; Guo, C.F. Direct Construction of Catechol Lignin for Engineering Long-Acting Conductive, Adhesive, and UV-Blocking Hydrogel Bioelectronics. Small Methods 2021, 5, 2001311. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Liu, T.; Li, Y.; Zhang, X.; Xu, Y.; Li, J.; Gao, Q. Performance of Soybean Protein Adhesive Cross-Linked by Lignin and Cuprum. J. Clean. Prod. 2022, 366, 132906. [Google Scholar] [CrossRef]
- Dyer, J.M.; Chapital, D.C.; Kuan, J.C.W.; Shepherd, H.S.; Tang, F.; Pepperman, A.B. Production of Linolenic Acid in Yeast Cells Expressing an Omega-3 Desaturase from Tung (Aleurites Fordii). JAOCS J. Am. Oil Chem. Soc. 2004, 81, 647–651. [Google Scholar] [CrossRef]
- Zappi, M.E.; Zappi, A.; Revellame, E.; Sharp, W.; Fortela, D.L.; Hernandez, R.; Chambers, T.; Ritter, K.; Gang, D. An Assessment of the Potential to Produce Commercially Valuable Lipids on Highway Right-of-Way Land Areas Located within the Southeastern United States. Sustainability 2020, 12, 5225. [Google Scholar] [CrossRef]
- Huang, Y.; Pang, L.; Wang, H.; Zhong, R.; Zeng, Z.; Yang, J. Synthesis and Properties of UV-Curable Tung Oil Based Resins via Modification of Diels-Alder Reaction, Nonisocyanate Polyurethane and Acrylates. Prog. Org. Coat. 2013, 76, 654–661. [Google Scholar] [CrossRef]
- Thanamongkollit, N.; Miller, K.R.; Soucek, M.D. Synthesis of UV-Curable Tung Oil and UV-Curable Tung Oil Based Alkyd. Prog. Org. Coat. 2012, 73, 425–434. [Google Scholar] [CrossRef]
- He, Z.; Chapital, D.C.; Cheng, H.N.; Thomas Klasson, K.; Olanya, O.M.; Uknalis, J. Application of Tung Oil to Improve Adhesion Strength and Water Resistance of Cottonseed Meal and Protein Adhesives on Maple Veneer. Ind. Crop. Prod. 2014, 61, 398–402. [Google Scholar] [CrossRef]
- Božič, M.; Gorgieva, S.; Kokol, V. Homogeneous and Heterogeneous Methods for Laccase-Mediated Functionalization of Chitosan by Tannic Acid and Quercetin. Carbohydr. Polym. 2012, 89, 854–864. [Google Scholar] [CrossRef]
- Koupantsis, T.; Pavlidou, E.; Paraskevopoulou, A. Glycerol and Tannic Acid as Applied in the Preparation of Milk Proteins—CMC Complex Coavervates for Flavour Encapsulation. Food Hydrocoll. 2016, 57, 62–71. [Google Scholar] [CrossRef]
- Xu, F.; Weng, B.; Gilkerson, R.; Materon, L.A.; Lozano, K. Development of Tannic Acid/Chitosan/Pullulan Composite Nanofibers from Aqueous Solution for Potential Applications as Wound Dressing. Carbohydr. Polym. 2015, 115, 16–24. [Google Scholar] [CrossRef]
- Rubentheren, V.; Ward, T.A.; Chee, C.Y.; Tang, C.K. Processing and Analysis of Chitosan Nanocomposites Reinforced with Chitin Whiskers and Tannic Acid as a Crosslinker. Carbohydr. Polym. 2015, 115, 379–387. [Google Scholar] [CrossRef] [PubMed]
- Sionkowska, A.; Kaczmarek, B.; Lewandowska, K. Modification of Collagen and Chitosan Mixtures by the Addition of Tannic Acid. J. Mol. Liq. 2014, 199, 318–323. [Google Scholar] [CrossRef]
- Oh, H.; Hoff, J.E.; Armstrong, G.S.; Haff, L.A. Hydrophobic Interaction in Tannin-Protein Complexes. J. Agric. Food Chem. 1980, 28, 394–398. [Google Scholar] [CrossRef]
- Van Buren, J.P.; Robinson, W.B. Formation of Complexes between Protein and Tannic Acid. J. Agric. Food Chem. 1969, 17, 772–777. [Google Scholar] [CrossRef]
- Ghahri, S.; Mohebby, B.; Pizzi, A.; Mirshokraie, A.; Mansouri, H.R. Improving Water Resistance of Soy-Based Adhesive by Vegetable Tannin. J. Polym. Environ. 2018, 26, 1881–1890. [Google Scholar] [CrossRef]
- Yang, S.; Yuan, T.Q.; Li, M.F.; Sun, R.C. Hydrothermal Degradation of Lignin: Products Analysis for Phenol Formaldehyde Adhesive Synthesis. Int. J. Biol. Macromol. 2015, 72, 54–62. [Google Scholar] [CrossRef]
- Zhang, W.; Ma, Y.; Wang, C.; Li, S.; Zhang, M.; Chu, F. Preparation and Properties of Lignin-Phenol-Formaldehyde Resins Based on Different Biorefinery Residues of Agricultural Biomass. Ind. Crop. Prod. 2013, 43, 326–333. [Google Scholar] [CrossRef]
- Xiao, Z.; Li, Y.; Wu, X.; Qi, G.; Li, N.; Zhang, K.; Wang, D.; Sun, X.S. Utilization of Sorghum Lignin to Improve Adhesion Strength of Soy Protein Adhesives on Wood Veneer. Ind. Crop. Prod. 2013, 50, 501–509. [Google Scholar] [CrossRef]
- Luo, J.; Luo, J.; Yuan, C.; Zhang, W.; Li, J.; Gao, Q.; Chen, H. An Eco-Friendly Wood Adhesive from Soy Protein and Lignin: Performance Properties. RSC Adv. 2015, 5, 100849–100855. [Google Scholar] [CrossRef]
- Pradyawong, S.; Qi, G.; Li, N.; Sun, X.S.; Wang, D. Adhesion Properties of Soy Protein Adhesives Enhanced by Biomass Lignin. Int. J. Adhes. Adhes. 2017, 75, 66–73. [Google Scholar] [CrossRef]
- Doherty, W.O.S.; Mousavioun, P.; Fellows, C.M. Value-Adding to Cellulosic Ethanol: Lignin Polymers. Ind. Crop. Prod. 2011, 33, 259–276. [Google Scholar] [CrossRef]
- El Mansouri, N.E.; Salvadó, J. Structural Characterization of Technical Lignins for the Production of Adhesives: Application to Lignosulfonate, Kraft, Soda-Anthraquinone, Organosolv and Ethanol Process Lignins. Ind. Crop. Prod. 2006, 24, 8–16. [Google Scholar] [CrossRef]
- Pradyawong, S.; Qi, G.; Sun, X.S.; Wang, D. Laccase/TEMPO-Modified Lignin Improved Soy-Protein-Based Adhesives: Adhesion Performance and Properties. Int. J. Adhes. Adhes. 2019, 91, 116–122. [Google Scholar] [CrossRef]
- Zhu, X.; Wang, D.; Li, N.; Sun, X.S. Bio-Based Wood Adhesive from Camelina Protein (a Biodiesel Residue) and Depolymerized Lignin with Improved Water Resistance. ACS Omega 2017, 2, 7996–8004. [Google Scholar] [CrossRef] [PubMed]
- Xin, J.; Zhang, P.; Wolcott, M.P.; Zhang, J.; Hiscox, W.C.; Zhang, X. A Novel and Formaldehyde-Free Preparation Method for Lignin Amine and Its Enhancement for Soy Protein Adhesive. J. Polym. Environ. 2017, 25, 599–605. [Google Scholar] [CrossRef]
- Ma, R.; Guo, M.; Zhang, X. Selective Conversion of Biorefinery Lignin into Dicarboxylic Acids. ChemSusChem 2014, 7, 412–415. [Google Scholar] [CrossRef] [PubMed]
- Passauer, L.; Fischer, K.; Liebner, F. Activation of Pine Kraft Lignin by Fenton-Type Oxidation for Cross-Linking with Oligo(Oxyethylene) Diglycidyl Ether. Holzforschung 2011, 65, 319–326. [Google Scholar] [CrossRef]
- Shokrolahi, A.; Zali, A.; Keshavarz, M.H. Reductive Amination of Aldehydes and Ketones by NaBH 4 Using Carbon-Based Solid Acid (CBSA) as Catalyst. Green Chem. Lett. Rev. 2011, 4, 195–203. [Google Scholar] [CrossRef][Green Version]
- Song, F.; Zhang, L.M. Gelation Modification of Soy Protein Isolate by a Naturally Occurring Cross-Linking Agent and Its Potential Biomedical Application. Ind. Eng. Chem. Res. 2009, 48, 7077–7083. [Google Scholar] [CrossRef]
- Xu, Y.; Han, Y.; Shi, S.Q.; Gao, Q.; Li, J. Preparation of a Moderate Viscosity, High Performance and Adequately-Stabilized Soy Protein-Based Adhesive via Recombination of Protein Molecules. J. Clean. Prod. 2020, 255, 120303. [Google Scholar] [CrossRef]
- Wu, F.; Yang, Z.; Su, X.; Gong, Y.; Kuang, T. Molecular Reorganization Induced by Ca2+ of Plant Photosystem I Reconstituted into Phosphatidylglycerol Liposomes. Chem. Phys. Lipids 2005, 136, 73–82. [Google Scholar] [CrossRef] [PubMed]
- Lyu, Q.; Hsueh, N.; Chai, C.L.L. Direct Evidence for the Critical Role of 5,6-Dihydroxyindole in Polydopamine Deposition and Aggregation. Langmuir 2019, 35, 5191–5201. [Google Scholar] [CrossRef] [PubMed]
- Hu, W.; Lu, S.; Zhang, Z.; Zhu, L.; Wen, Y.; Zhang, T.; Ji, Z. Mussel-Inspired Copolymer-Coated Polypropylene Mesh with Anti-Adhesion Efficiency for Abdominal Wall Defect Repair. Biomater. Sci. 2019, 7, 1323–1334. [Google Scholar] [CrossRef]
- Chirdon, W.M.; O’Brien, W.J.; Robertson, R.E. Adsorption of Catechol and Comparative Solutes on Hydroxyapatite. J. Biomed. Mater. Res.-Part B Appl. Biomater. 2003, 66, 532–538. [Google Scholar] [CrossRef] [PubMed]
- Subair, R.; Tripathi, B.P.; Formanek, P.; Simon, F.; Uhlmann, P.; Stamm, M. Polydopamine Modified Membranes with in Situ Synthesized Gold Nanoparticles for Catalytic and Environmental Applications. Chem. Eng. J. 2016, 295, 358–369. [Google Scholar] [CrossRef]
- Zhang, C.; Xiang, L.; Zhang, J.; Gong, L.; Han, L.; Xu, Z.K.; Zeng, H. Tough and Alkaline-Resistant Mussel-Inspired Wet Adhesion with Surface Salt Displacement via Polydopamine/Amine Synergy. Langmuir 2019, 35, 5257–5263. [Google Scholar] [CrossRef] [PubMed]
- Pang, H.; Zhao, S.; Mo, L.; Wang, Z.; Zhang, W.; Huang, A.; Zhang, S.; Li, J. Mussel-Inspired Bio-Based Water-Resistant Soy Adhesives with Low-Cost Dopamine Analogue-Modified Silkworm Silk Fiber. J. Appl. Polym. Sci. 2020, 137, 48785. [Google Scholar] [CrossRef]
- Van der Steen, M.; Stevens, C.V. Undecylenic Acid: A Valuable and Physiologically Active Renewable Building Block from Castor Oil. ChemSusChem 2009, 2, 692–713. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Li, C.; Sun, X.S. Improved Water Resistance in Undecylenic Acid (UA)-Modified Soy Protein Isolate (SPI)-Based Adhesives. Ind. Crop. Prod. 2015, 74, 577–584. [Google Scholar] [CrossRef]
- Zhou, Y.; Zeng, G.; Zhang, F.; Luo, J.; Li, K.; Li, X.; Li, J.; Fang, Z. High Strength and Flame Retardant Soybean Polysaccharide-Based Wood Adhesive Produced by Borate Chemistry and Crosslinking Strategy. Eur. Polym. J. 2022, 164, 110973. [Google Scholar] [CrossRef]
- Xue, F.; Zhang, H.; Hu, J.; Liu, Y. Hyaluronic Acid Nanofibers Crosslinked with a Nontoxic Reagent. Carbohydr. Polym. 2021, 259, 117757. [Google Scholar] [CrossRef] [PubMed]
- Yang, B.; Song, J.; Jiang, Y.; Li, M.; Wei, J.; Qin, J.; Peng, W.; Lasaosa, F.L.; He, Y.; Mao, H.; et al. Injectable Adhesive Self-Healing Multicross-Linked Double-Network Hydrogel Facilitates Full-Thickness Skin Wound Healing. ACS Appl. Mater. Interfaces 2020, 12, 57782–57797. [Google Scholar] [CrossRef] [PubMed]
- Li, K.; Jin, S.; Zeng, G.; Zhou, Y.; Zhang, F.; Li, J.; Shi, S.Q.; Li, J. Biomimetic Development of a Strong, Mildew-Resistant Soy Protein Adhesive via Mineral–Organic System and Phenol-Amine Synergy. Ind. Crop. Prod. 2022, 187, 115412. [Google Scholar] [CrossRef]
- Yu, Y.; He, Y.; Mu, Z.; Zhao, Y.; Kong, K.; Liu, Z.; Tang, R. Biomimetic Mineralized Organic–Inorganic Hybrid Macrofiber with Spider Silk-Like Supertoughness. Adv. Funct. Mater. 2020, 30, 1908556. [Google Scholar] [CrossRef]
- Jiang, Y.; Pan, X.; Yao, M.; Han, L.; Zhang, X.; Jia, Z.; Weng, J.; Chen, W.; Fang, L.; Wang, X.; et al. Bioinspired Adhesive and Tumor Microenvironment Responsive NanoMOFs Assembled 3D-Printed Scaffold for Anti-Tumor Therapy and Bone Regeneration. Nano Today 2021, 39, 101182. [Google Scholar] [CrossRef]
- Liu, J.; Li, Y.; Mo, H.; Xie, E.; Fang, J.; Gan, W. Current Utilization of Waste Biomass as Filler for Wood Adhesives: A Review. J. Ind. Eng. Chem. 2022, 115, 48–61. [Google Scholar] [CrossRef]
- Hasan Faris, A. Fillers in Wood Adhesives. In Fillers; Intechopen: London, UK, 2021. [Google Scholar] [CrossRef]
- Qi, G.; Li, N.; Wang, D.; Sun, X.S. Development of High-Strength Soy Protein Adhesives Modified with Sodium Montmorillonite Clay. JAOCS J. Am. Oil Chem. Soc. 2016, 93, 1509–1517. [Google Scholar] [CrossRef]
- Yang, Z.; Peng, H.; Wang, W.; Liu, T. Crystallization Behavior of Poly(ε-Caprolactone)/Layered Double Hydroxide Nanocomposites. J. Appl. Polym. Sci. 2010, 116, 2658–2667. [Google Scholar] [CrossRef]
- Pojanavaraphan, T.; Magaraphan, R.; Chiou, B.S.; Schiraldi, D.A. Development of Biodegradable Foamlike Materials Based on Casein and Sodium Montmorillonite Clay. Biomacromolecules 2010, 11, 2640–2646. [Google Scholar] [CrossRef]
- Qi, G. Modified Soy Protein Based Adhesives and Their Physicochemical Properties. Ph.D. Thesis, Kansas State University, Manhattan, KS, USA, 2011. [Google Scholar]
- Zhang, L.; Sun, X.S. Effect of Sodium Bisulfite on Properties of Soybean Glycinin. J. Agric. Food Chem. 2008, 56, 11192–11197. [Google Scholar] [CrossRef]
- Ciannamea, E.M.; Stefani, P.M.; Ruseckaite, R.A. Medium-Density Particleboards from Modified Rice Husks and Soybean Protein Concentrate-Based Adhesives. Bioresour. Technol. 2010, 101, 818–825. [Google Scholar] [CrossRef]
- ANSI A208.-1; Particleboard. American National Standards Institute: Washington, DC, USA, 2016.
- Gui, C.; Zhu, J.; Liu, X.; Zhang, Z. Preparation of Water-Resistant Adhesives from Soy Flour with Less Water-Soluble Components for Wood Bonding. Pigment. Resin Technol. 2017, 46, 253–258. [Google Scholar] [CrossRef]
- Zhang, B.; Fan, B.; Li, M.; Zhang, Y. Effects of Thermal Treatment on the Properties of Defatted Soya Bean Flour and Its Adhesion to Plywood. R. Soc. Open Sci. 2018, 5, 180015. [Google Scholar] [CrossRef] [PubMed]
- Qi, G.; Li, N.; Wang, D.; Sun, X.S. Physicochemical Properties of Soy Protein Adhesives Modified by 2-Octen-1-Ylsuccinic Anhydride. Ind. Crop. Prod. 2013, 46, 165–172. [Google Scholar] [CrossRef]
- Zhang, W.; Sun, H.; Zhu, C.; Wan, K.; Zhang, Y.; Fang, Z.; Ai, Z. Mechanical and Water-Resistant Properties of Rice Straw Fiberboard Bonded with Chemically-Modified Soy Protein Adhesive. RSC Adv. 2018, 8, 15188–15195. [Google Scholar] [CrossRef] [PubMed]
- Pang, H.; Zhao, S.; Wang, Z.; Zhang, W.; Zhang, S.; Li, J. Development of Soy Protein-Based Adhesive with High Water Resistance and Bonding Strength by Waterborne Epoxy Crosslinking Strategy. Int. J. Adhes. Adhes. 2020, 100, 102600. [Google Scholar] [CrossRef]
- Arias, A.; González-Rodríguez, S.; Vetroni Barros, M.; Salvador, R.; de Francisco, A.C.; Moro Piekarski, C.; Moreira, M.T. Recent Developments in Bio-Based Adhesives from Renewable Natural Resources. J. Clean. Prod. 2021, 314, 127892. [Google Scholar] [CrossRef]

| Protein and Cross-Linking Agent | Wet Bonding Strength (MPa) | Reference |
|---|---|---|
| Wheat protein | 0.44 | [44] |
| GPPEI | 2.02 | |
| Soy protein | 0 | [43] |
| EPR + MF/SP | 0.85 | |
| Soy protein | 0.22 | [47] |
| EG/SP | 1.12 | |
| Soymeal | 0.22 | [49] |
| SM/TGIC/HD | 1.0 | |
| Wheat flour | 0.29 | [55] |
| T-SDS-WF | 1.22 | |
| Soy protein | 0.65 | [56] |
| WPU/SP | 1.1 | |
| Cottonmeal | 0.65 | [58] |
| CM/Isocyanate | 1.68 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Oni, O.V.; Lawrence, M.A.; Zappi, M.E.; Chirdon, W.M. A Review of Strategies to Enhance the Water Resistance of Green Wood Adhesives Produced from Sustainable Protein Sources. Sustainability 2023, 15, 14779. https://doi.org/10.3390/su152014779
Oni OV, Lawrence MA, Zappi ME, Chirdon WM. A Review of Strategies to Enhance the Water Resistance of Green Wood Adhesives Produced from Sustainable Protein Sources. Sustainability. 2023; 15(20):14779. https://doi.org/10.3390/su152014779
Chicago/Turabian StyleOni, Olatunji V., Michael A. Lawrence, Mark E. Zappi, and William M. Chirdon. 2023. "A Review of Strategies to Enhance the Water Resistance of Green Wood Adhesives Produced from Sustainable Protein Sources" Sustainability 15, no. 20: 14779. https://doi.org/10.3390/su152014779
APA StyleOni, O. V., Lawrence, M. A., Zappi, M. E., & Chirdon, W. M. (2023). A Review of Strategies to Enhance the Water Resistance of Green Wood Adhesives Produced from Sustainable Protein Sources. Sustainability, 15(20), 14779. https://doi.org/10.3390/su152014779







