Comprehensive Analysis of PM1 Composition in the Eastern Indo-Gangetic Basin: A Three-Year Urban Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Monitoring Sites, PM1 Sampler, and Monitoring Schedule
2.2. Surface Morphology and Elemental Composition of PM1
2.3. Source Apportionment of PM1
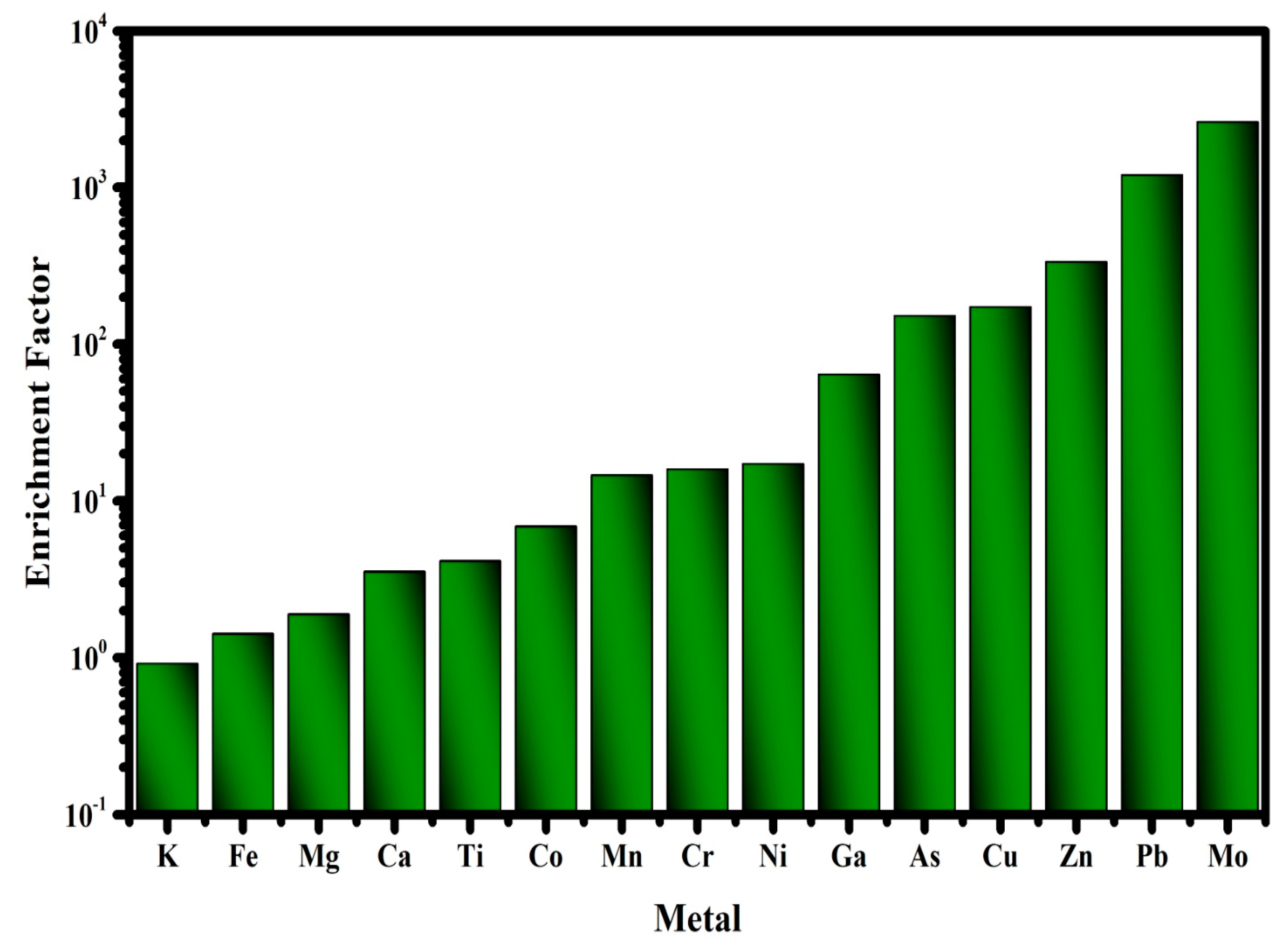
2.3.1. Enrichment Factor (EF) Analysis
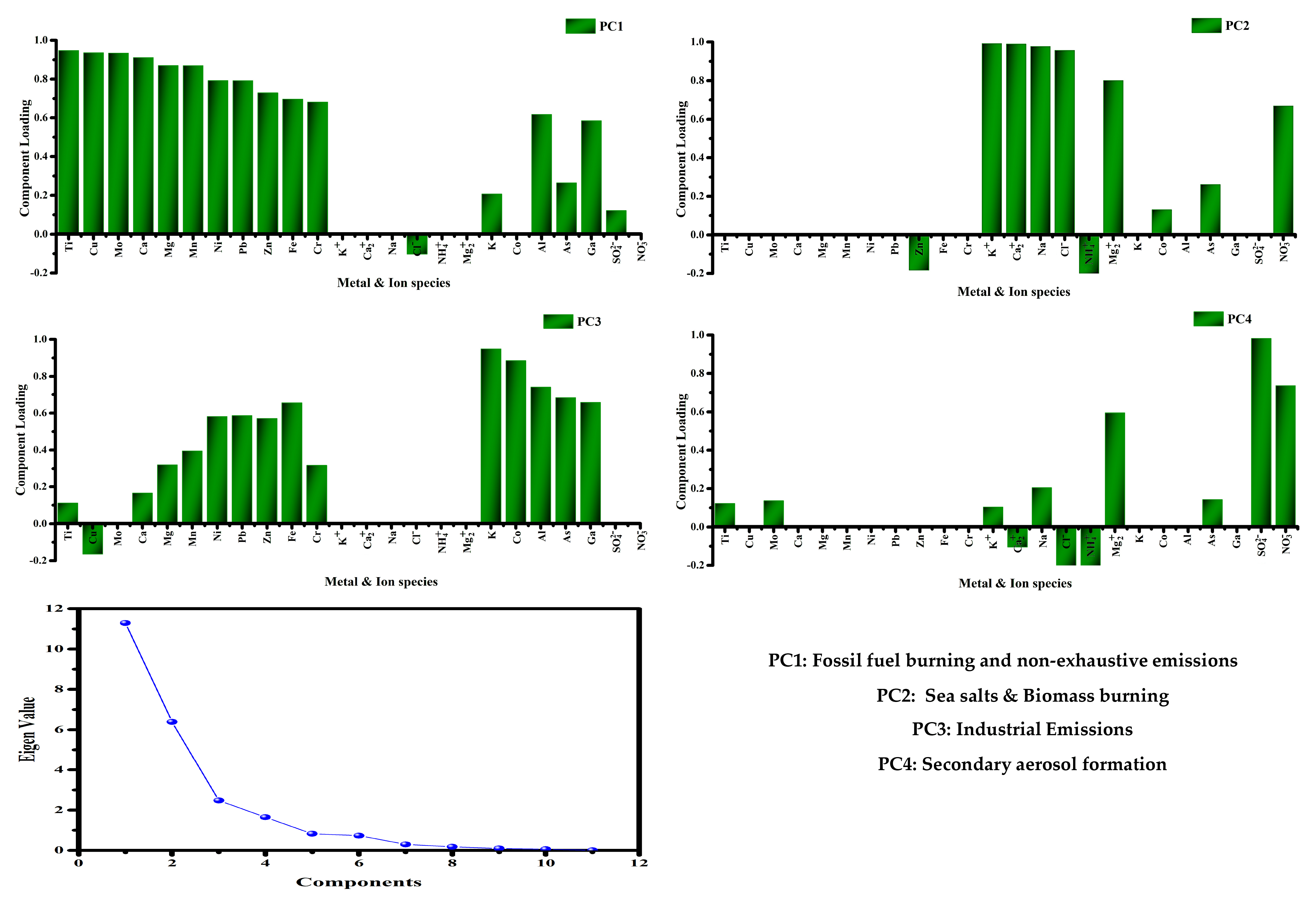
2.3.2. Principal Component Analysis (PCA)
2.4. Statistical Analysis
3. Results and Discussion
3.1. Meteorological Conditions at the Sites
3.2. Overview of PM1 Concentrations
3.3. Morphology and Relevant Elements of PM1
3.4. Ion Composition of PM1
3.5. Pollution Source Apportionment
3.5.1. Enrichment Factor Analysis
3.5.2. Principle Component Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kelly, F.J.; Fussell, J.C. Air pollution and public health: Emerging hazards and improved understanding of risk. Environ. Geochem. Health 2015, 37, 631–649. [Google Scholar] [CrossRef] [PubMed]
- Balakrishnan, K.; Dey, S.; Gupta, T.; Dhaliwal, R.S.; Brauer, M.; Cohen, A.J.; Stanaway, J.D.; Beig, G.; Joshi, T.K.; Aggarwal, A.N. The impact of air pollution on deaths, disease burden, and life expectancy across the states of India: The Global Burden of Disease Study 2017. Lancet Planet. Health 2019, 3, e26–e39. [Google Scholar] [CrossRef] [PubMed]
- Gupta, L.; Bansal, M.; Nandi, P.; Habib, G.; Raman, R.S. Source apportionment and potential source regions of size-resolved particulate matter at a heavily polluted industrial city in the Indo-Gangetic Plain. Atmos. Environ. 2023, 298, 119614. [Google Scholar] [CrossRef]
- Holland, N.A.; Fraiser, C.R.; Sloan, R.C.; Devlin, R.B.; Brown, D.A.; Wingard, C.J. Ultrafine particulate matter increases cardiac ischemia/reperfusion injury via mitochondrial permeability transition pore. Cardiovasc. Toxicol. 2017, 17, 441–450. [Google Scholar] [CrossRef] [PubMed]
- Talbi, A.; Kerchich, Y.; Kerbachi, R.; Boughedaoui, M. Assessment of annual air pollution levels with PM1, PM2.5, PM10 and associated heavy metals in Algiers, Algeria. Environ. Pollut. 2018, 232, 252–263. [Google Scholar] [CrossRef]
- Caggiano, R.; Sabia, S.; Speranza, A. Trace elements and human health risks assessment of finer aerosol atmospheric particles (PM1). Environ. Sci. Pollut. Res. 2019, 26, 36423–36433. [Google Scholar] [CrossRef]
- Trippetta, S.; Sabia, S.; Caggiano, R. Fine aerosol particles (PM1): Natural and anthropogenic contributions and health risk assessment. Air Qual. Atmos. Health 2016, 9, 621–629. [Google Scholar] [CrossRef]
- India Should Monitor PM1, the Least Visible Particle in Air. Available online: https://www.downtoearth.org.in/news/india-should-monitor-pm1-the-least-visible-particle-in-air-68293 (accessed on 5 August 2023).
- Ganguly, T.; Selvaraj, K.L.; Guttikunda, S.K. National Clean Air Programme (NCAP) for Indian cities: Review and outlook of clean air action plans. Atmos. Environ. X 2020, 8, 100096. [Google Scholar] [CrossRef]
- Moreno-Ríos, A.L.; Tejeda-Benítez, L.P.; Bustillo-Lecompte, C.F. Sources, characteristics, toxicity, and control of ultrafine particles: An overview. Geo. Front. 2022, 13, 101147. [Google Scholar] [CrossRef]
- Schraufnagel, D.E. The health effects of ultrafine particles. Exp. Mol. Med. 2020, 52, 311–317. [Google Scholar] [CrossRef]
- Kasimov, N.S.; Vlasov, D.V.; Kosheleva, N.E. Enrichment of road dust particles and adjacent environments with metals and metalloids in eastern Moscow. Urban Clim. 2020, 32, 100638. [Google Scholar] [CrossRef]
- Klimont, Z.; Kupiainen, K.; Heyes, C.; Purohit, P.; Cofala, J.; Rafaj, P.; Borken-Kleefeld, J.; Schöpp, W. Global anthropogenic emissions of particulate matter including black carbon. Atmos. Chem. Phys. 2017, 17, 8681–8723. [Google Scholar] [CrossRef]
- Mandal, M.; Das, S.; Roy, A.; Rakwal, R.; Jones, O.A.; Popek, R.; Agrawal, G.K.; Sarkar, A. Interactive relations between plants, phyllosphere microbial community, and particulate matter pollution. Sci. Total Environ. 2023, 890, 164352. [Google Scholar] [CrossRef] [PubMed]
- Xue, W.; Xue, J.; Mousavi, A.; Sioutas, C.; Kleeman, M.J. Positive matrix factorization of ultrafine particle mass (PM0.1) at three sites in California. Sci. Total Environ. 2020, 715, 136902. [Google Scholar] [CrossRef] [PubMed]
- Rai, P.; Chakraborty, A.; Mandariya, A.K.; Gupta, T. Composition and source apportionment of PM1 at urban site Kanpur in India using PMF coupled with CBPF. Atmos. Res. 2016, 178, 506–520. [Google Scholar] [CrossRef]
- Jaiprakash. Chemical characterization of PM1.0 aerosol in Delhi and source apportionment using positive matrix factorization. Environ. Sci. Pollut. Res. 2017, 24, 445–462. [Google Scholar] [CrossRef]
- Tao, M.; Liu, Q.; Schauer, J.J. Direct measurement of the deposition of submicron soot particles on leaves of Platanus acerifolia tree. Environ. Sci. Process. Impacts 2022, 24, 2336–2344. [Google Scholar] [CrossRef]
- Prakash, J.; Lohia, T.; Mandariya, A.K.; Habib, G.; Gupta, T.; Gupta, S.K. Chemical characterization and quantitative assessment of source-specific health risk of trace metals in PM1.0 at a road site of Delhi, India. Environ. Sci. Pollut. Res. 2018, 25, 8747–8764. [Google Scholar] [CrossRef]
- Levy, J.I.; Hanna, S.R. Spatial and temporal variability in urban fine particulate matter concentrations. Environ. Pollut. 2011, 159, 2009–2015. [Google Scholar] [CrossRef]
- National Highways Authority of India, Ministry of Road Transport and Highways. Memo No- 63013/1/2017-18/OTHS/Misc/103/1461; NHAI’s Office: Malda, India, 2017.
- Chakraborty, A.; Gupta, T. Chemical characterization and source apportionment of submicron (PM1) aerosol in Kanpur region, India. Aerosol Air Qual. Res. 2010, 10, 433–445. [Google Scholar] [CrossRef]
- Gupta, T.; Chakraborty, A.; Ujinwal, K.K. Development and performance evaluation of an indigenously developed air sampler designed to collect submicron aerosol. Ann. Natl. Acad. Eng. 2010, 7, 189–193. [Google Scholar]
- Lagouvardos, K.; Kotroni, V.; Bezes, A.; Koletsis, I.; Kopania, T.; Lykoudis, S.; Mazarakis, N.; Papagiannaki, K.; Vougioukas, S. The automatic weather stations NOANN network of the National Observatory of Athens: Operation and database. Geosci. Data J. 2017, 4, 4–16. [Google Scholar] [CrossRef]
- Usman, F.; Zeb, B.; Alam, K.; Huang, Z.; Shah, A.; Ahmad, I.; Ullah, S. In-depth analysis of physicochemical properties of particulate matter (PM10, PM2. 5 and PM1) and its characterization through FTIR, XRD and SEM–EDX Techniques in the Foothills of the Hindu Kush Region of Northern Pakistan. Atmosphere 2022, 13, 124. [Google Scholar] [CrossRef]
- Tao, M.; Xu, Y.; Gong, J.; Liu, Q. Estimation of aerosol acidity at a suburban site of Nanjing using the machine learning method. J. Atmos. Chem. 2022, 79, 141–151. [Google Scholar] [CrossRef]
- Sharma, S.K.; Kumar, M.; Gupta, N.C.; Saxena, M.; Mandal, T.K. Characteristics of ambient ammonia over Delhi, India. Meteorol. Atmos. Phys. 2014, 124, 67–82. [Google Scholar] [CrossRef]
- Tasića, M.; Rajšića, S.; Tomaševića, M.; Mijića, Z.; Aničića, M.; Novakovića, V.; Markovic, D.M.; Markovic, D.A.; Lazic, L.; Radenkovic, M.; et al. Assessment of air quality in an urban area of Belgrade, Serbia. In Environmental Technologies: New Developments, 1st ed.; Burcu Özkaraova Güngör, E., Ed.; I-Tech Education and Publishing: Vienna, Austria, 2008; pp. 209–244. [Google Scholar]
- Wang, X.; Sato, T.; Xing, B.; Tamamura, S.; Tao, S. Source identification, size distribution and indicator screening of airborne trace metals in Kanazawa, Japan. J. Aerosol Sci. 2005, 36, 197–210. [Google Scholar] [CrossRef]
- Chaudhary, I.J.; Rathore, D. Dust pollution: Its removal and effect on foliage physiology of urban trees. Sustain. Cities Soc. 2019, 51, 101696. [Google Scholar] [CrossRef]
- Khan, J.Z.; Sun, L.; Tian, Y.; Shi, G.; Feng, Y. Chemical characterization and source apportionment of PM1 and PM2.5 in Tianjin, China: Impacts of biomass burning and primary biogenic sources. J. Environ. Sci. 2021, 99, 196–209. [Google Scholar] [CrossRef]
- Pérez, I.A.; García, M.Á.; Sánchez, M.L.; Pardo, N.; Fernández-Duque, B. Key Points in Air Pollution Meteorology. Int. J. Environ. Res. Public Health 2020, 17, 8349. [Google Scholar] [CrossRef]
- Rajput, P.; Mandaria, A.; Kachawa, L.; Singh, D.K.; Singh, A.K.; Gupta, T. Chemical characterisation and source apportionment of PM1 during massive loading at an urban location in Indo-Gangetic Plain: Impact of local sources and long-range transport. Tellus B Chem. Phys. Meteorol. 2016, 68, 30659. [Google Scholar] [CrossRef]
- Singh, D.K.; Gupta, T. Source apportionment and risk assessment of PM1 bound trace metals collected during foggy and non-foggy episodes at a representative site in the Indo-Gangetic plain. Sci. Total Environ. 2016, 550, 80–94. [Google Scholar] [CrossRef]
- Halek, F.; Kianpour-Rad, M.; Kavousirahim, A. Seasonal variation in ambient PM mass and number concentrations (case study: Tehran, Iran). Environ. Monit. Assess. 2010, 169, 501–507. [Google Scholar] [CrossRef]
- Reyes-Villegas, E.; Panda, U.; Darbyshire, E.; Cash, J.M.; Joshi, R.; Langford, B.; Di Marco, C.F.; Mullinger, N.; Acton, W.J.F.; Drysdale, W.; et al. PM1 composition and source apportionment at two sites in Delhi, India across multiple seasons. Atmos. Chem. Phys. 2021, 21, 11655–11667. [Google Scholar] [CrossRef]
- Mohammadi-Moghadam, F.; Heidari, M.; Farhadkhani, M.; Sadeghi, M.; Forouzandeh, S.; Ahmadi, A.; Khabaz-Ghasemi, E. TSP, PM10, PM2.5, and PM1 in ambient air of Shahr-e Kord, Iran’s rooftop; levels, characterisation and health risk assessment of particles-bound heavy metals. Int. J. Environ. Anal. Chem. 2022, 102, 5356–5372. [Google Scholar] [CrossRef]
- Popek, R.; Fornal-Pieniak, B.; Chyliński, F.; Pawełkowicz, M.; Bobrowicz, J.; Chrzanowska, D.; Piechota, N.; Przybysz, A. Not only trees matter—Traffic-related PM accumulation by vegetation of urban forests. Sustainability 2022, 14, 2973. [Google Scholar] [CrossRef]
- Onat, B.; Sahin, U.A.; Akyuz, T. Elemental characterization of PM2.5 and PM1 in dense traffic area in Istanbul, Turkey. Atmos. Pollut. Res. 2013, 4, 101–105. [Google Scholar] [CrossRef]
- Zhang, Y.; Lang, J.; Cheng, S.; Li, S.; Zhou, Y.; Chen, D.; Zhang, H.; Wang, H. Chemical composition and sources of PM1 and PM2.5 in Beijing in autumn. Sci. Total Environ. 2018, 630, 72–82. [Google Scholar] [CrossRef]
- Popek, R.; Łukowski, A.; Bates, C.; Oleksyn, J. Accumulation of particulate matter, heavy metals, and polycyclic aromatic hydrocarbons on the leaves of Tilia cordata Mill. in five Polish cities with different levels of air pollution. Int. J. Phytoremediat. 2017, 19, 1134–1141. [Google Scholar]
- Slezakova, K.; Pereira, M.C.; Alvim-Ferraz, M.C. Influence of tobacco smoke on the elemental composition of indoor particles of different sizes. Atmos. Environ. 2009, 43, 486–493. [Google Scholar] [CrossRef]
- Deshmukh, D.K.; Deb, M.K.; Tsai, Y.I.; Mkoma, S.L. Water soluble ions in PM2.5 and PM1 aerosols in Durg city, Chhattisgarh, India. Aerosol Air. Qual. Res. 2011, 11, 696–708. [Google Scholar] [CrossRef]
- Deshmukh, D.K.; Tsai, Y.I.; Deb, M.K.; Mkoma, S.L. Characterization of dicarboxylates and inorganic ions in urban PM10 aerosols in the eastern central India. Aerosol Air. Qual. Res. 2012, 12, 592–607. [Google Scholar] [CrossRef]
- Seinfeld, J.H.; Pandis, S.N. From air pollution to climate change. In Atmospheric Chemistry and Physics, 2nd ed.; John Wiley & Sons, Inc.: New York, NY, USA, 1998; p. 1326. [Google Scholar]
- Li, Q.; Yang, Z.; Li, X.; Ding, S.; Du, F. Seasonal characteristics of sulfate and nitrate in size-segregated particles in ammonia-poor and-rich atmospheres in Chengdu, Southwest China. Aerosol Air Qual. Res. 2019, 19, 2697–2706. [Google Scholar] [CrossRef]
- Luo, L.; Zhang, Y.Y.; Xiao, H.Y.; Xiao, H.W.; Zheng, N.J.; Zhang, Z.Y.; Xie, Y.J.; Liu, C. Spatial distributions and sources of inorganic chlorine in PM2.5 across China in winter. Atmosphere 2019, 10, 505. [Google Scholar] [CrossRef]
- Kayee, J.; Sompongchaiyakul, P.; Sanwlani, N.; Bureekul, S.; Wang, X.; Das, R. Metal concentrations and source apportionment of PM2.5 in Chiang Rai and Bangkok, Thailand during a biomass burning season. ACS Earth Space Chem. 2020, 4, 1213–1226. [Google Scholar] [CrossRef]
- Kong, S.; Ji, Y.; Lu, B.; Chen, L.; Han, B.; Li, Z.; Bai, Z. Characterization of PM10 source profiles for fugitive dust in Fushun-a city famous for coal. Atmos. Environ. 2011, 45, 5351–5365. [Google Scholar] [CrossRef]
- Matawle, J.L.; Pervez, S.; Dewangan, S.; Shrivastava, A.; Tiwari, S.; Pant, P.; Deb, M.K.; Pervez, Y. Characterization of PM2.5 source profiles for traffic and dust sources in Raipur, India. Aerosol Air Qual. Res. 2015, 15, 2537–2548. [Google Scholar] [CrossRef]
- Adamiec, E.; Jarosz-Krzemińska, E.; Wieszała, R. Heavy metals from non-exhaust vehicle emissions in urban and motorway road dusts. Environ. Monit. Assess. 2016, 188, 369. [Google Scholar] [CrossRef]
- Murphy, L.U.; Cochrane, T.A.; O’Sullivan, A. The influence of different pavement surfaces on atmospheric copper, lead, zinc, and suspended solids attenuation and wash-off. Water Air Soil Pollut. 2015, 226, 232. [Google Scholar] [CrossRef]




| Years | Seasons | Mean Temperature (°C) | Mean Relative Humidity (%) | Total Rainfall (mm) | ||
|---|---|---|---|---|---|---|
| Max | Min | Max | Min | |||
| 2017 | Pre-monsoon | 31.4 ± 0.3 b | 20.2 ± 0.4 b | 85.8 ± 0.7 b | 52.8 ± 1.5 c | 174.9 b |
| Monsoon | 33.7 ± 0.1 a | 27 ± 0.08 a | 89.7 ± 0.3 a | 71.1 ± 0.8 a | 942.2 a | |
| Post-Monsoon | 26.5 ± 0.4 c | 17.6 ± 0.5 c | 91.2 ± 0.4 a | 60.5 ± 1.0 a, b | 180.7 b | |
| 2018 | Pre-monsoon | 31.3 ± 0.2 b | 20.1 ± 0.3 b | 87.5 ± 0.6 b | 53.3 ± 1.3 b | 411.8 b |
| Monsoon | 33.8 ± 0.1 a | 27.0 ± 0.1 a | 89.9 ± 0.3 a | 71.1 ± 0.8 a | 740.1 a | |
| Post-Monsoon | 27.7 ± 0.3 c | 16.2 ± 0.4 c | 88.2 ± 0.5 b | 51 ± 0.8 b | 79.8 c | |
| 2019 | Pre-monsoon | 32 ± 0.3 b | 20.2 ± 0.4 a,b | 86.8 ± 0.6 b | 51.1 ± 1.3 c | 261.7 b |
| Monsoon | 33.9 ± 0.2 a | 26.9 ± 0.1 a | 90.8 ± 0.3 a | 72.1 ± 0.9 a | 918 a | |
| Post-Monsoon | 26.9 ± 0.4 c | 16.9 ± 0.4 c | 91.3 ± 0.3 a | 65.4 ± 1.1 b | 157.5 b | |
| Total Ions | Pre-Monsoon | Monsoon | Post Monsoon |
|---|---|---|---|
| Concentration (µg m−3) | Concentration (µg m−3) | Concentration (µg m−3) | |
| Na+ | 0.9 ± 0.9 a | 0.36 ± 0.2 b | 1.3 ± 1.0 a |
| NH4+ | 2.4 ± 0.8 a, b | 3.6 ± 0.4 a | 2.1±1.1 b |
| K+ | 0.8 ± 0.4 a | 0.33 ± 0.2 a | 1.3 ± 0.8 a |
| Mg2+ | 0.1 ± 1.0 a | 0.03 ± 0.0 b | 0.15 ± 0.04 a |
| Ca2+ | 0.9 ± 0.5 b | 0.18 ± 0.1 b | 2.6 ± 0.5 a |
| Cl− | 0.8 ± 1.0 b | 0.69 ± 0.6 b | 1.5 ± 0.8 a |
| NO3− | 2.8 ± 1.2 a | 0.86 ± 0.4 b | 2.4 ± 0.9 a |
| SO42− | 6.3 ± 1.7 a | 2.6 ± 1.3 b | 3.4 ± 1.8 b |
| Total Anions (R) | 9.9 | 4.1 | 9.6 |
| Total Cations (r) | 5.1 | 4.5 | 10.4 |
| Ionic ratio (r/R) | 0.5 | 1.1 | 1.1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Das, S.; Roy, A.; Masiwal, R.; Mandal, M.; Popek, R.; Chakraborty, M.; Prasad, D.; Chyliński, F.; Awasthi, A.; Sarkar, A. Comprehensive Analysis of PM1 Composition in the Eastern Indo-Gangetic Basin: A Three-Year Urban Study. Sustainability 2023, 15, 14894. https://doi.org/10.3390/su152014894
Das S, Roy A, Masiwal R, Mandal M, Popek R, Chakraborty M, Prasad D, Chyliński F, Awasthi A, Sarkar A. Comprehensive Analysis of PM1 Composition in the Eastern Indo-Gangetic Basin: A Three-Year Urban Study. Sustainability. 2023; 15(20):14894. https://doi.org/10.3390/su152014894
Chicago/Turabian StyleDas, Sujit, Anamika Roy, Renu Masiwal, Mamun Mandal, Robert Popek, Monojit Chakraborty, Dinesh Prasad, Filip Chyliński, Amit Awasthi, and Abhijit Sarkar. 2023. "Comprehensive Analysis of PM1 Composition in the Eastern Indo-Gangetic Basin: A Three-Year Urban Study" Sustainability 15, no. 20: 14894. https://doi.org/10.3390/su152014894
APA StyleDas, S., Roy, A., Masiwal, R., Mandal, M., Popek, R., Chakraborty, M., Prasad, D., Chyliński, F., Awasthi, A., & Sarkar, A. (2023). Comprehensive Analysis of PM1 Composition in the Eastern Indo-Gangetic Basin: A Three-Year Urban Study. Sustainability, 15(20), 14894. https://doi.org/10.3390/su152014894












