Aquatic Macrophytes Metal and Nutrient Concentration Variations, with Implication for Phytoremediation Potential in a Subtropical River System
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Area
2.2. Macrophytes Sampling and Processing
3. Data Analysis
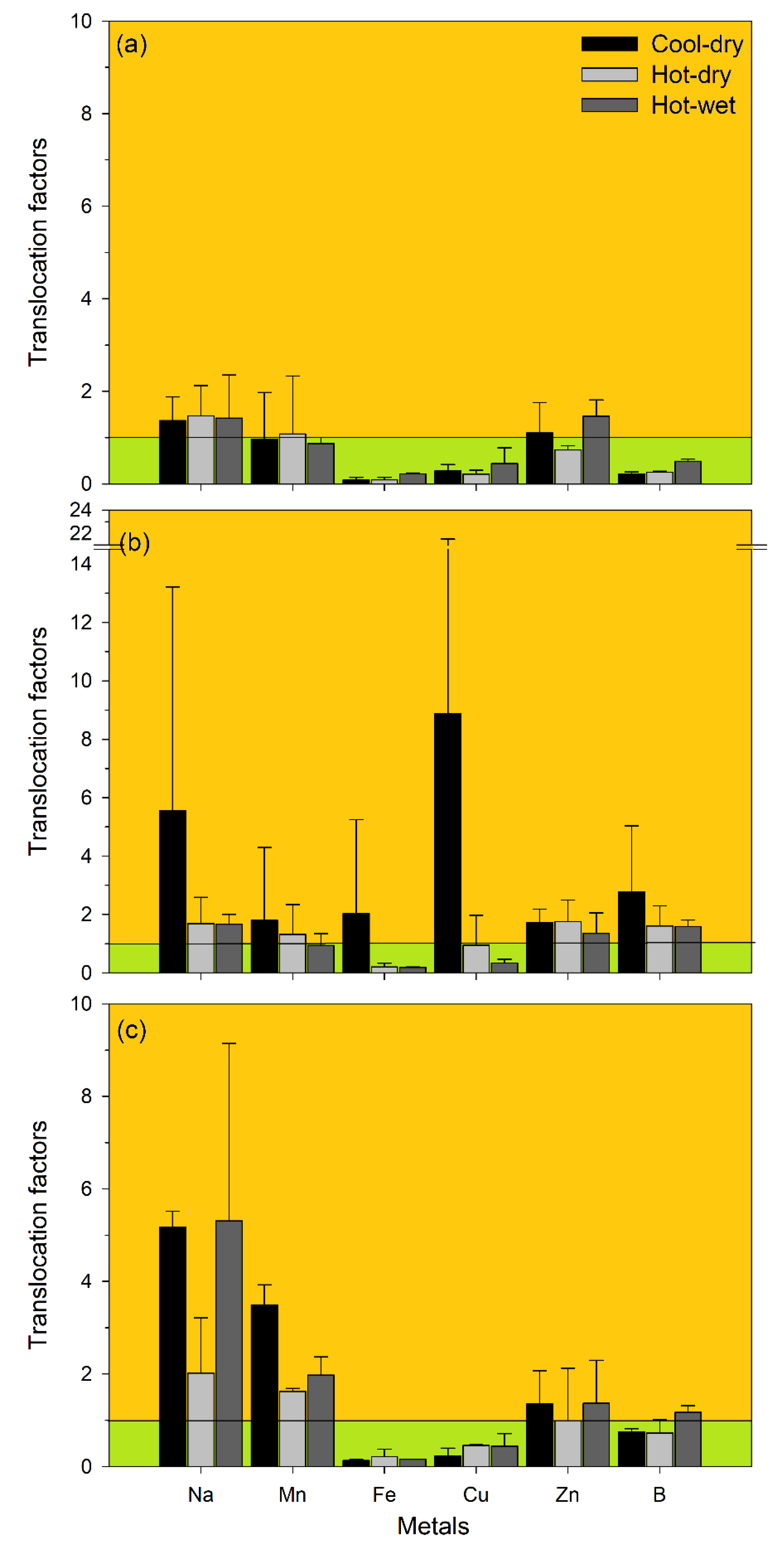
4. Results
5. Discussion
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dhir, B. Phytoremediation: Role of Aquatic Plants in Environmental Clean-Up; Springer: New Delhi, India, 2013. [Google Scholar]
- Altınsaçlı, S.; Altınsaçlı, S.; Paçal, F.P. Species composition and qualitative distribution of the macrophytes in three Turkish lakes (Kandira, Kocaeli, Turkey). Phytol. Balc. 2014, 20, 89–98. [Google Scholar]
- Demarco, C.F.; Afonso, T.F.; Pieniz, S.; Selau, F.C.; Machado, F.M.; Andreazza, R. Potential Phytoremediation of Aquatic Macrophyte Species for Heavy Metals in Urban Environments in the Southern Area of Brazil. Sustainability 2022, 15, 419. [Google Scholar] [CrossRef]
- Piedade, M.T.F.; Parolin, P.; Junk, W.; Schöngart, J.; Wittmann, F.; Demarchi, L.O.; Lopes, A. Vegetation. In Fundamentals of Tropical Freshwater Wetlands: From Ecology to Conservation Management; Dalu, T., Wasserman, R.J., Eds.; Elsevier: Cambridge, UK, 2022. [Google Scholar]
- Carniatto, N.; Fugi, R.; Quirino, B.A.; Cunha, E.R.; Thomaz, S.M. An invasive and a native macrophyte species provide similar feeding habitat for fish. Ecol. Freshw. Fish 2020, 29, 112–120. [Google Scholar] [CrossRef]
- Brito, J.S.; Michelan, T.S.; Juen, L. Aquatic macrophytes are important substrates for Libellulidae (Odonata) larvae and adults. Limnology 2021, 22, 139–149. [Google Scholar] [CrossRef]
- Xin, J.; Ma, S.; Li, Y.; Zhao, C.; Tian, R. Pontederia cordata, an ornamental aquatic macrophyte with great potential in phytoremediation of heavy–metal–contaminated wetlands. Ecotoxicol. Environ. Saf. 2020, 203, 111024. [Google Scholar] [CrossRef] [PubMed]
- Venkateswarlu, V.; Venkatrayulu, C.H.; Bai, T.J.L. Phytoremediation of heavy metal Copper (II) from aqueous environment by using aquatic macrophytes Hydrilla verticillata and Pistia stratiotes. Int. J. Fish. Aquat. Stud. 2019, 7, 390–393. [Google Scholar]
- Queiroz, R.D.C.S.D.; Lôbo, I.P.; Ribeiro, V.D.S.; Rodrigues, L.B.; Almeida Neto, J.A.D. Assessment of autochthonous aquatic macrophytes with phytoremediation potential for dairy wastewater treatment in floating constructed wetlands. Int. J. Phytoremediat. 2020, 22, 518–528. [Google Scholar] [CrossRef] [PubMed]
- Dean, S.; Akhtar, M.S.; Ditta, A.; Valipour, M.; Aslam, S. Microcosm Study on the Potential of Aquatic Macrophytes for Phytoremediation of Phosphorus–Induced Eutrophication. Sustainability 2022, 14, 16415. [Google Scholar] [CrossRef]
- Hoang, H.G.; Lin, C.; Tran, H.T.; Chiang, C.F.; Bui, X.T.; Cheruiyot, N.K.; Shern, C.C.; Lee, C.W. Heavy metal contamination trends in surface water and sediments of a river in a highly–industrialized region. Environ. Technol. Innov. 2020, 20, 101043. [Google Scholar] [CrossRef]
- Dash, S.; Borah, S.S.; Kalamdhad, A.S. Heavy metal pollution and potential ecological risk assessment for surficial sediments of Deepor Beel, India. Ecol. Indic. 2021, 122, 107265. [Google Scholar] [CrossRef]
- Ali, H.; Khan, E.; Ilahi, I. Environmental chemistry and ecotoxicology of hazardous heavy metals: Environmental persistence, toxicity, and bioaccumulation. J. Chem. 2019, 2019, 6730305. [Google Scholar] [CrossRef]
- Dalu, T.; Tavengwa, N. (Eds.) Emerging Freshwater Pollutants: Analysis, Fate and Regulations; Elsevier: Cambridge, UK, 2022. [Google Scholar]
- Anand, S.; Bharti, S.K.; Kumar, S.; Barman, S.C.; Kumar, N. Phytoremediation of heavy metals and pesticides present in water using aquatic macrophytes. Phyto Rhizo Remediat. 2019, 9, 89–119. [Google Scholar]
- Topaldemir, H.; Taş, B.; Yüksel, B.; Ustaoğlu, F. Potentially hazardous elements in sediments and Ceratophyllum demersum: An ecotoxicological risk assessment in Miliç Wetland, Samsun, Türkiye. Environ. Sci. Pollut. Res. 2023, 30, 26397–26416. [Google Scholar] [CrossRef] [PubMed]
- Prajapati, M.; van Bruggen, J.J.A.; Dalu, T.; Malla, R. Assessing the effectiveness of pollutant removal by macrophytes in a floating wetland for wastewater treatment. Appl. Water Sci. 2017, 7, 4801–4809. [Google Scholar] [CrossRef]
- Kapahi, M.; Sachdeva, S. Bioremediation options for heavy metal pollution. J. Health Pollut. 2019, 9, 191203. [Google Scholar] [CrossRef] [PubMed]
- Netshiongolwe, N.R.; Cuthbert, R.N.; Maenetje, M.M.; Chari, L.D.; Motitsoe, S.N.; Wasserman, R.J.; Munyai, L.F.; Dalu, T. Quantifying metal contamination and potential uptake by Phragmites australis Adans. (Poaceae) along a subtropical river system. Plants 2020, 9, 846. [Google Scholar] [CrossRef]
- Haq, S.; Bhatti, A.A.; Dar, Z.A.; Bhat, S.A. Phytoremediation of heavy metals: An eco–friendly and sustainable approach. In Bioremediation and Biotechnology: Sustainable Approaches to Pollution Degradation; Springer: Cham, Switzerland, 2020; pp. 215–231. [Google Scholar]
- Khan, S.; Dilawar, S.; Hassan, S.; Ullah, A.; Yasmin, H.; Ayaz, T.; Akhtar, F.; Gaafar, A.R.Z.; Sekar, S.; Butt, S. Phytoremediation of Cu and Mn from industrially polluted soil: An eco–friendly and sustainable approach. Water 2023, 15, 3439. [Google Scholar] [CrossRef]
- Ekperusi, A.O.; Sikoki, F.D.; Nwachukwu, E.O. Application of common duckweed (Lemna minor) in phytoremediation of chemicals in the environment: State and future perspective. Chemosphere 2019, 223, 285–309. [Google Scholar] [CrossRef]
- Liu, Y.; Xu, H.; Yu, C.; Zhou, G. Multifaceted roles of duckweed in aquatic phytoremediation and bioproducts synthesis. Glob. Change Biol.—Bioenergy 2021, 13, 70–82. [Google Scholar] [CrossRef]
- Rezania, S.; Ponraj, M.; Talaiekhozani, A.; Mohamad, S.E.; Din, M.F.M.; Taib, S.M.; Sabbagh, F.; Sairan, F.M. Perspectives of phytoremediation using water hyacinth for removal of heavy metals, organic and inorganic pollutants in wastewater. J. Environ. Manag. 2015, 163, 125–133. [Google Scholar] [CrossRef]
- Ting, W.H.T.; Tan, I.A.W.; Salleh, S.F.; Wahab, N.A. Application of water hyacinth (Eichhornia crassipes) for phytoremediation of ammoniacal nitrogen: A review. J. Water Process Eng. 2018, 22, 239–249. [Google Scholar] [CrossRef]
- Yan, A.; Wang, Y.; Tan, S.N.; Mohd Yusof, M.L.; Ghosh, S.; Chen, Z. Phytoremediation: A promising approach for revegetation of heavy metal–polluted land. Front. Plant Sci. 2020, 11, 3. [Google Scholar] [CrossRef] [PubMed]
- Shen, X.; Dai, M.; Yang, J.; Sun, L.; Tan, X.; Peng, C.; Ali, I.; Naz, I. A critical review on the phytoremediation of heavy metals from environment: Performance and challenges. Chemosphere 2022, 291, 132979. [Google Scholar] [CrossRef] [PubMed]
- Demarco, C.F.; Quadro, M.S.; Selau Carlos, F.; Pieniz, S.; Morselli, L.B.G.A.; Andreazza, R. Bioremediation of aquatic environments contaminated with heavy metals: A review of mechanisms, solutions and perspectives. Sustainability 2023, 15, 1411. [Google Scholar] [CrossRef]
- Rai, P.K. Heavy metal phytoremediation from aquatic ecosystems with special reference to macrophytes. Crit. Rev. Environ. Sci. Technol. 2009, 39, 697–753. [Google Scholar] [CrossRef]
- Sinclair, J.S.; Mademann, J.A.; Haubrock, P.J.; Haase, P. Primarily neutral effects of river restoration on macroinvertebrates, macrophytes, and fishes after a decade of monitoring. Restor. Ecol. 2023, 31, e13840. [Google Scholar] [CrossRef]
- Dalu, T.; Banda, T.; Mutshekwa, T.; Munyai, L.F.; Cuthbert, R.N. Effects of urbanization and a wastewater treatment plant on microplastic densities along a subtropical river system. Environ. Sci. Pollut. Res. 2021, 28, 36102–36111. [Google Scholar] [CrossRef] [PubMed]
- Yan, M.; Sun, C.; Xu, J.; Dong, J.; Ke, W. Role of Fe oxides in corrosion of pipeline steel in a red clay soil. Corros. Sci. 2014, 80, 309–317. [Google Scholar] [CrossRef]
- Edokpayi, J.N.; Odiyo, J.O.; Popoola, O.E.; Msagati, T.A. Assessment of trace metals contamination of surface water and sediment: A case study of Mvudi River, South Africa. Sustainability 2016, 8, 135. [Google Scholar] [CrossRef]
- Mbedzi, R.; Cuthbert, R.N.; Wasserman, R.J.; Murungweni, F.M.; Dalu, T. Spatiotemporal variation in microplastic contamination along a subtropical reservoir shoreline. Environ. Sci. Pollut. Res. 2020, 27, 23880–23887. [Google Scholar] [CrossRef]
- Świerk, D.; Szpakowska, B. Occurrence of heavy metals in aquatic macrophytes colonising small aquatic ecosystems. Ecol. Chem. Eng. 2011, 18, 369–378. [Google Scholar]
- Campbell, C.R.; Plank, C.O. Preparation of plant tissue for laboratory analysis. In Handbook of Reference Methods for Plant Analysis; Kalra, Y.P., Ed.; CRC Press: Boca Raton, FL, USA, 1998. [Google Scholar]
- Barron, M.G. Bioconcentration. Will water–borne organic chemicals accumulate in aquatic animals? Environ. Sci. Technol. 1990, 24, 1612–1618. [Google Scholar]
- Ghosh, M.; Singh, S.P. A comparative study of cadmium phytoextraction by accumulator and weed species. Environ. Pollut. 2005, 133, 365–371. [Google Scholar] [CrossRef] [PubMed]
- Xiao, W.; Sun, R.; Hu, S.; Meng, C.; Xie, B.; Yi, M.; Wu, Y. Recent advances and future perspective on lignocellulose-based materials as adsorbents in diverse water treatment applications. Int. J. Biol. Macromol. 2023, 253, 126984. [Google Scholar] [CrossRef] [PubMed]
- Kassaye, Y.A.; Skipperud, L.; Einset, J.; Salbu, B. Aquatic macrophytes in Ethiopian Rift Valley lakes; their trace elements concentration and use as pollution indicators. Aquat. Bot. 2016, 134, 18–25. [Google Scholar] [CrossRef]
- Mahar, A.; Wang, P.; Ali, A.; Awasthi, M.K.; Lahori, A.H.; Wang, Q.; Li, R.; Zhang, Z. Challenges and opportunities in the phytoremediation of heavy metals contaminated soils: A review. Ecotoxicol. Environ. Saf. 2016, 126, 111–121. [Google Scholar] [CrossRef] [PubMed]
- Mokarram, M.; Saber, A.; Sheykhi, V. Effects of heavy metal contamination on river water quality due to release of industrial effluents. J. Clean. Prod. 2020, 277, 123380. [Google Scholar] [CrossRef]
- Dalu, T.; Cuthbert, R.N.; Taylor, J.C.; Magoro, M.L.; Weyl, O.L.F.; Froneman, P.W.; Wasserman, R.J. Benthic diatom–based indices and isotopic biomonitoring of nitrogen pollution in a warm temperate Austral river system. Sci. Total Environ. 2020, 748, 142452. [Google Scholar] [CrossRef]
- Rani, R.; Sharma, P.; Kumar, R.; Hajam, Y.A. Effects of heavy metals and pesticides on fish. In Bacterial Fish Diseases; Dar, G.H., Bhat, R.A., Qadri, H., Al–Ghamdy, K.M., Hakeem, K.H., Eds.; Academic Press: London, UK, 2022. [Google Scholar]
- Zaranyika, M.F.; Nyati, W. Uptake of heavy metals by Typha capensis from wetland sites polluted by effluent from mineral processing plants: Implications of metal–metal interactions. 3 Biotech 2017, 7, 286. [Google Scholar] [CrossRef]
- Emenike, P.C.; Neris, J.B.; Tenebe, I.T.; Nnaji, C.C.; Jarvis, P. Estimation of some trace metal pollutants in River Atuwara southwestern Nigeria and spatio–temporal human health risks assessment. Chemosphere 2020, 239, 124770. [Google Scholar] [CrossRef]
- Westerlund, C.; Viklander, M. Particles and associated metals in road runoff during snowmelt and rainfall. Sci. Total Environ. 2006, 362, 143–156. [Google Scholar] [CrossRef] [PubMed]
- Lambers, H.; Hayes, P.E.; Laliberte, E.; Oliveira, R.S.; Turner, B.L. Leaf manganese accumulation and phosphorus–acquisition efficiency. Trends Plant Sci. 2015, 20, 83–90. [Google Scholar] [CrossRef] [PubMed]
- Yabanli, M.; Yozukmaz, A.; Sel, F. Heavy metal accumulation in the leaves, stem and root of the invasive submerged macrophyte Myriophyllum spicatum L. (Haloragaceae): An example of Kadin Creek (Mugla, Turkey). Braz. Arch. Biol. Technol. 2014, 57, 434–440. [Google Scholar] [CrossRef]
- Kushwaha, A.; Rani, R.; Kumar, S.; Gautam, A. Heavy metal detoxification and tolerance mechanisms in plants: Implications for phytoremediation. Environ. Rev. 2015, 24, 39–51. [Google Scholar] [CrossRef]
- Muthusaravanan, S.; Sivarajasekar, N.; Vivek, J.S.; Paramasivan, T.; Naushad, M.; Prakashmaran, J.; Gayathri, V.; Al–Duaij, O.K. Phytoremediation of heavy metals: Mechanisms, methods and enhancements. Environ. Chem. Lett. 2018, 16, 1339–1359. [Google Scholar] [CrossRef]
- Schröder, P.; Harvey, P.J.; Schwitzguébel, J.P. Prospects for the phytoremediation of organic pollutants in Europe. Environ. Sci. Pollut. Res. 2002, 9, 1–3. [Google Scholar] [CrossRef] [PubMed]
- Prasad, M.N.V.; Hagemeyer, J.; Saxena, P.K.; KrishnaRaj, S.; Dan, T.; Perras, M.R.; Vettakkorumakankav, N.N. Phytoremediation of heavy metal contaminated and polluted soils. In Heavy Metal Stress in Plants: From Molecules to Ecosystems; Prasad, M.N.V., Hagemeyer, J., Eds.; Springer: Berlin/Heidelberg, Germany, 1999. [Google Scholar]
- Zhu, D.; Schwab, A.P.; Banks, M.K. Heavy metal leaching from mine tailings as affected by plants. J. Environ. Qual. 1999, 28, 1727–1732. [Google Scholar] [CrossRef]
- Bhatia, M.; Goyal, D. Analyzing remediation potential of wastewater through wetland plants: A review. Environ. Prog. Sustain. Energy 2014, 33, 9–27. [Google Scholar] [CrossRef]


| Variables | Df | F | p | Variables | Df | F | p |
|---|---|---|---|---|---|---|---|
| Metal Concentrations | |||||||
| Species | Season | ||||||
| N | 2 | 9.206 | <0.001 | Cu | 2 | 7.707 | 0.001 |
| Ca | 2 | 65.126 | <0.001 | B | 2 | 10.53 | <0.001 |
| Mg | 2 | 70.452 | <0.001 | Species × Location | |||
| Na | 2 | 58.734 | <0.001 | N | 4 | 7.123 | <0.001 |
| B | 2 | 11.452 | <0.001 | K | 4 | 4.872 | 0.002 |
| Location | Ca | 4 | 9.615 | <0.001 | |||
| N | 2 | 53.067 | <0.001 | Mg | 4 | 4.98 | 0.002 |
| P | 2 | 26.549 | <0.001 | Na | 4 | 4.666 | 0.003 |
| K | 2 | 30.387 | <0.001 | Cu | 4 | 5.102 | 0.001 |
| Ca | 2 | 21.065 | <0.001 | Zn | 4 | 2.281 | 0.072 |
| Mg | 2 | 5.251 | 0.008 | B | 4 | 10.788 | <0.001 |
| Na | 2 | 10.645 | <0.001 | Species × Location × Season | |||
| Fe | 2 | 14.197 | <0.001 | Cu | 8 | 4.216 | 0.001 |
| Cu | 2 | 23.159 | <0.001 | ||||
| Zn | 2 | 5.232 | 0.008 | ||||
| B | 2 | 33.227 | <0.001 | ||||
| Bioconcentration factors | |||||||
| Species | Species × Location | ||||||
| Na | 2 | 24.721 | <0.001 | B | 4 | 10.327 | <0.001 |
| Mn | 2 | 6.089 | 0.004 | Species × Season | |||
| Zn | 2 | 2.979 | 0.059 | Mn | 4 | 3.222 | 0.019 |
| B | 2 | 17.342 | <0.001 | Zn | 4 | 3.049 | 0.024 |
| Location | B | 4 | 7.268 | <0.001 | |||
| Na | 2 | 5.342 | 0.008 | Location × Season | |||
| Fe | 2 | 36.821 | <0.001 | Fe | 4 | 10.575 | <0.001 |
| Cu | 2 | 37.569 | <0.001 | Cu | 4 | 6.539 | <0.001 |
| Zn | 2 | 3.762 | 0.030 | Zn | 4 | 2.866 | 0.032 |
| B | 2 | 25.134 | <0.001 | B | 4 | 9.604 | <0.001 |
| Season | Species × Location × Season | ||||||
| Na | 2 | 8.461 | 0.001 | B | 8 | 4.378 | <0.001 |
| Mn | 2 | 7.082 | 0.002 | ||||
| Fe | 2 | 17.335 | <0.001 | ||||
| Cu | 2 | 13.889 | <0.001 | ||||
| Zn | 2 | 7.563 | 0.001 | ||||
| B | 2 | 79.260 | <0.001 | ||||
| Variables | Cool–Dry | Hot–Dry | Hot–Wet | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Roots | Stem | Leaves | Roots | Stem | Leaves | Roots | Stem | Leaves | |
| Phragmites australis | |||||||||
| Na | 1.39 ± 0.66 | 4.89 ± 1.57 | 5.35 ± 2.66 | 4.21 ± 0.46 | 22.88 ± 7.15 | 23.28 ± 12.37 | 4.51 ± 2.11 | 19.61 ± 9.61 | 19.71 ± 8.63 |
| Mn | 0.96 ± 1.26 | 5.10 ± 8.02 | 0.58 ±0.61 | 5.60 ± 1.26 | 7.17 ± 7.03 | 1.39 ± 2.44 | 0.47 ± 1.26 | 1.06 ± 0.34 | 0.44 ± 0.20 |
| Fe | 0.04 ± 0.03 | 1.11 ± 0.57 | 0.04 ± 0.03 | 7.38 ± 0.03 | 170.7 ± 136.6 | 4.60 ± 5.63 | 4.57 ± 0.03 | 84.48 ± 8.86 | 13.46 ± 2.87 |
| Cu | 0.11 ± 0.05 | 0.86 ± 0.33 | 0.12 ± 0.06 | 0.74 ± 0.05 | 6.40 ± 3.12 | 0.61 ± 0.49 | 0.28 ± 0.05 | 3.19 ± 2.40 | 0.66 ± 0.56 |
| Zn | 0.73 ± 0.43 | 1.22 ± 0.46 | 0.57 ± 0.25 | 6.24 ± 0.43 | 15.46 ± 6.57 | 4.62 ± 4.07 | 6.35 ± 0.43 | 10.78 ± 10.77 | 10.03 ± 11.96 |
| B | 0.21 ± 0.13 | 1.54 ± 0.80 | 0.12 ± 0.07 | 16.54 ± 0.13 | 92.60 ± 19.90 | 5.54 ± 5.42 | 10.76 ± 0.13 | 43.42 ± 15.72 | 10.09 ± 2.92 |
| Schoenoplectus corymbosus | |||||||||
| Na | 1.21 ± 0.39 | 4.09 ± 2.91 | 5.53 ± 5.98 | 8.35 ± 5.38 | 35.50 ± 19.41 | 51.70 ± 32.00 | 6.65 ± 4.89 | 25.25 ± 12.83 | 35.75 ± 19.4 |
| Mn | 0.55 ± 0.11 | 1.96 ± 1.32 | 0.59 ± 0.48 | 3.00 ± 3.87 | 4.26 ± 3.04 | 4.45 ± 6.01 | 1.70 ± 1.53 | 6.23 ± 5.58 | 4.57 ± 5.93 |
| Fe | 0.07 ± 0.06 | 0.59 ± 0.52 | 0.06 ± 0.05 | 25.13 ± 23.28 | 181.9 ± 111.5 | 20.68 ± 28.66 | 10.75 ± 6.63 | 103.59 ± 23.22 | 9.03 ± 4.01 |
| Cu | 1.77 ± 1.51 | 0.51 ± 0.60 | 0.12 ± 0.08 | 1.49 ± 0.68 | 4.54 ± 2.87 | 0.90 ± 0.38 | 0.84 ± 0.49 | 3.73 ± 0.93 | 0.48 ± 0.27 |
| Zn | 0.70 ± 0.13 | 0.68 ± 0.12 | 0.53 ± 0.28 | 11.40 ± 2.10 | 11.58 ± 3.72 | 7.18 ± 1.69 | 7.74 ± 2.97 | 9.68 ± 1.37 | 4.78 ± 2.22 |
| B | 1.14 ± 1.05 | 0.79 ± 0.65 | 0.22 ± 0.19 | 145.0 ± 70.2 | 105.76 ± 7.98 | 23.14 ± 2.73 | 58.94 ± 16.40 | 50.79 ± 18.93 | 19.95 ± 8.07 |
| Typha capensis | |||||||||
| Na | 38.96 ± 21.19 | 18.48 ± 10.23 | 54.88 ± 25.41 | 81.92 ± 96.63 | 83.43 ± 73.99 | 130.7 ± 152.5 | 66.04 ± 24.11 | 40.23 ± 14.99 | 118.8 ± 50.5 |
| Mn | 2.99 ± 1.34 | 1.28 ± 0.51 | 1.58 ± 1.00 | 34.49 ± 45.55 | 28.14 ± 36.14 | 10.14 ± 11.45 | 9.59 ± 3.82 | 7.59 ± 4.70 | 4.48 ± 2.47 |
| Fe | 0.02 ± 0.02 | 0.64 ± 0.35 | 0.06 ± 0.01 | 5.80 ± 4.82 | 167.7 ± 66.0 | 24.80 ± 18.39 | 4.44 ± 1.93 | 135.9 ± 2.45 | 16.89 ± 2.71 |
| Cu | 0.09 ± 0.01 | 1.68 ± 1.52 | 0.15 ± 0.03 | 0.66 ± 0.04 | 4.74 ± 0.66 | 1.50 ± 0.33 | 0.45 ± 0.19 | 3.31 ± 0.75 | 0.90 ± 0.38 |
| Zn | 0.56 ± 0.34 | 1.18 ± 0.12 | 1.08 ± 0.68 | 7.80 ± 3.88 | 114.7 ± 143.5 | 24.35 ± 8.02 | 4.77 ± 2.26 | 10.02 ± 0.03 | 8.93 ± 7.01 |
| B | 0.36 ± 0.10 | 0.89 ± 0.23 | 0.30 ± 0.02 | 23.58 ± 5.67 | 86.3 ± 14.5 | 40.82 ± 30.05 | 29.28 ± 10.72 | 52.77 ± 10.23 | 33.21 ± 9.05 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Munyai, L.F.; Dalu, T. Aquatic Macrophytes Metal and Nutrient Concentration Variations, with Implication for Phytoremediation Potential in a Subtropical River System. Sustainability 2023, 15, 14933. https://doi.org/10.3390/su152014933
Munyai LF, Dalu T. Aquatic Macrophytes Metal and Nutrient Concentration Variations, with Implication for Phytoremediation Potential in a Subtropical River System. Sustainability. 2023; 15(20):14933. https://doi.org/10.3390/su152014933
Chicago/Turabian StyleMunyai, Linton F., and Tatenda Dalu. 2023. "Aquatic Macrophytes Metal and Nutrient Concentration Variations, with Implication for Phytoremediation Potential in a Subtropical River System" Sustainability 15, no. 20: 14933. https://doi.org/10.3390/su152014933
APA StyleMunyai, L. F., & Dalu, T. (2023). Aquatic Macrophytes Metal and Nutrient Concentration Variations, with Implication for Phytoremediation Potential in a Subtropical River System. Sustainability, 15(20), 14933. https://doi.org/10.3390/su152014933






