Research on Water Stability and Moisture Damage Mechanism of a Steel Slag Porous Asphalt Mixture
Abstract
:1. Introduction
2. Materials and Test Methods
2.1. Raw Materials
2.2. Preparation of the Steel Slag Asphalt Mortar
2.3. Preparation of the SSPA Mixture
2.4. Test Methods
2.4.1. Time–Temperature H2O-Immersion Testing
2.4.2. Pull-Out Testing
2.4.3. H2O-Immersion Immersion Marshall Testing
2.4.4. Scanning Electron Microscopy (SEM) Testing
2.4.5. Fourier Transform Infrared (FTIR) Spectroscopy Testing
2.4.6. X-ray Diffraction (XRD) Testing
3. Experimental Test Results and Analysis
3.1. Macro Test
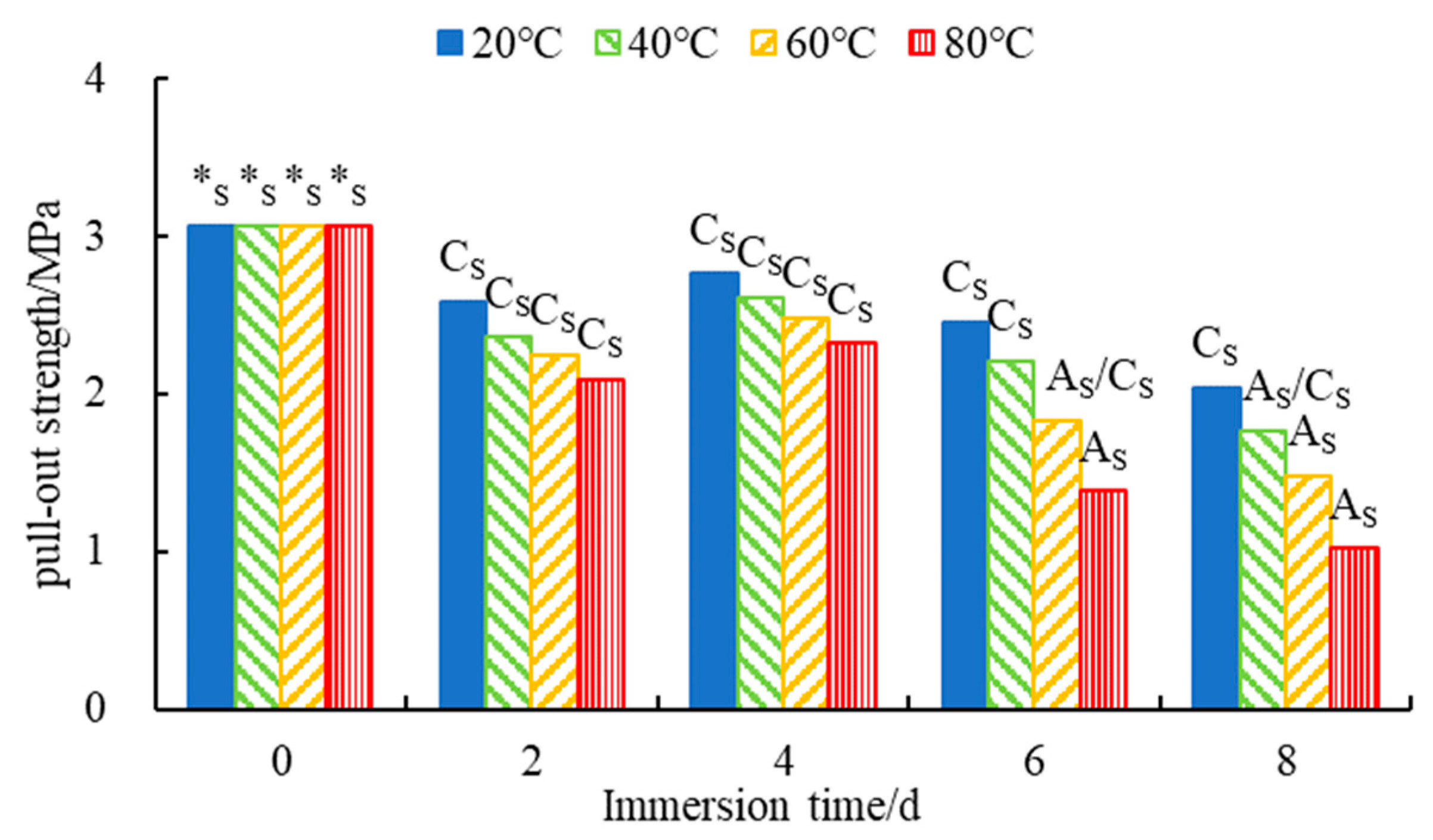
3.1.1. Analysis of the Results of the Pull-Out Test
- (1)
- Damage Evaluation Indicators
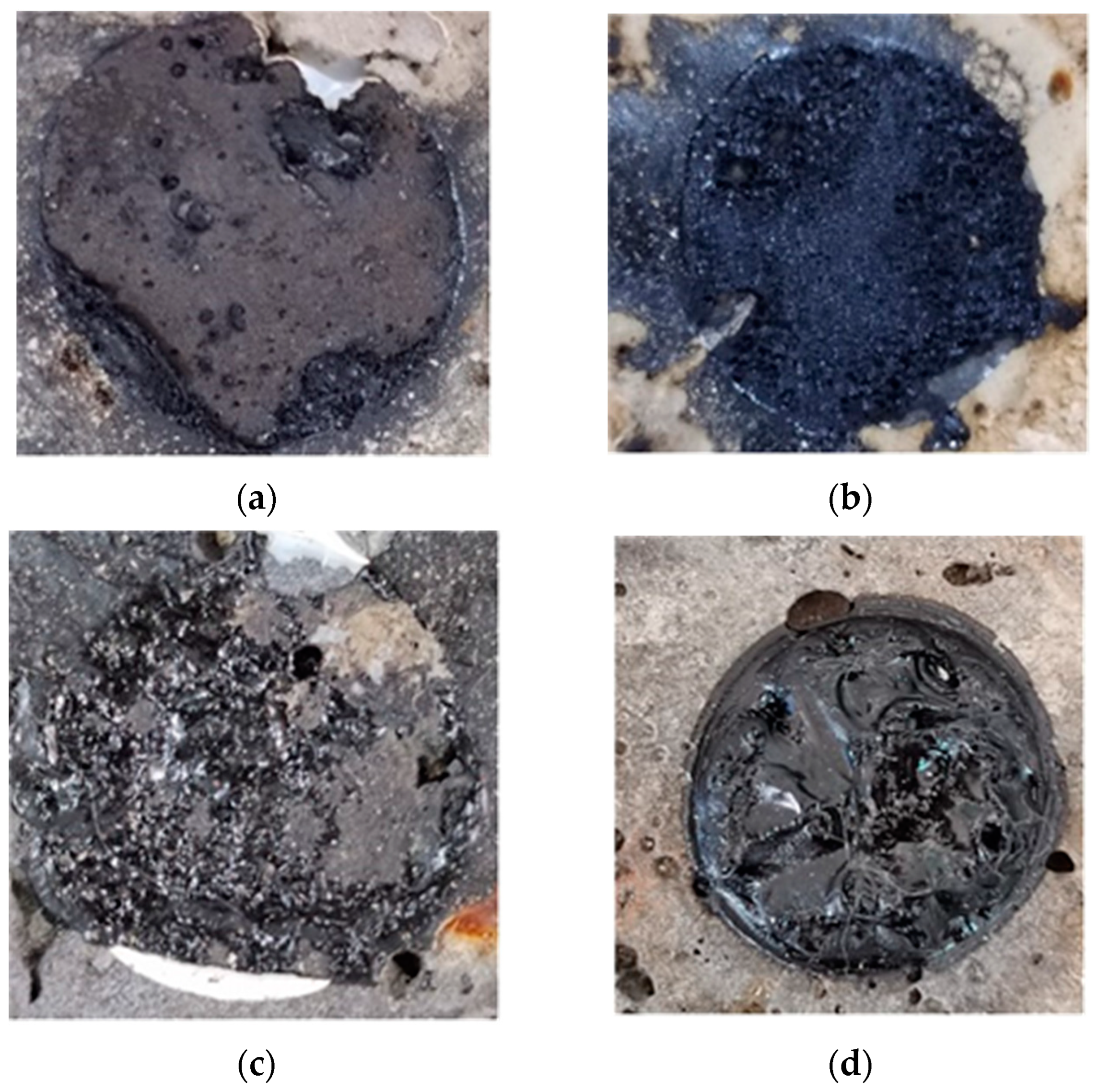
- As shown in Figure 3a, adhesion damage refers to the damage of the adhesive interface between the asphalt and aggregate [69]. When this occurs, there is either almost no residual asphalt film or only a small amount of asphalt film on the aggregate surface. However, there is typically no significant damage within the aggregate or within the asphalt.
- As shown in Figure 3c, mixed damage refers to the damage of the adhesive interface between the asphalt and aggregate, accompanied by internal damage within the asphalt [69]. With this damage mechanism, some asphalt film remains on the aggregate surface, but cannot be completely determined as adhesion or cohesive damage.
- As shown in Figure 3d, dislodgement damage refers to the form of damage that occurs between the asphalt and the pull-out head [69]; that is, there is dislodgement of the asphalt from the pull-out head. Under this condition, the surface of the asphalt film on the surface of the aggregate is flat, with all the asphalt film remaining on the surface of the aggregates. On the surface of the pull-out head, there would be none or only a very small amount of the asphalt film remaining.
- (2)
- Test Results
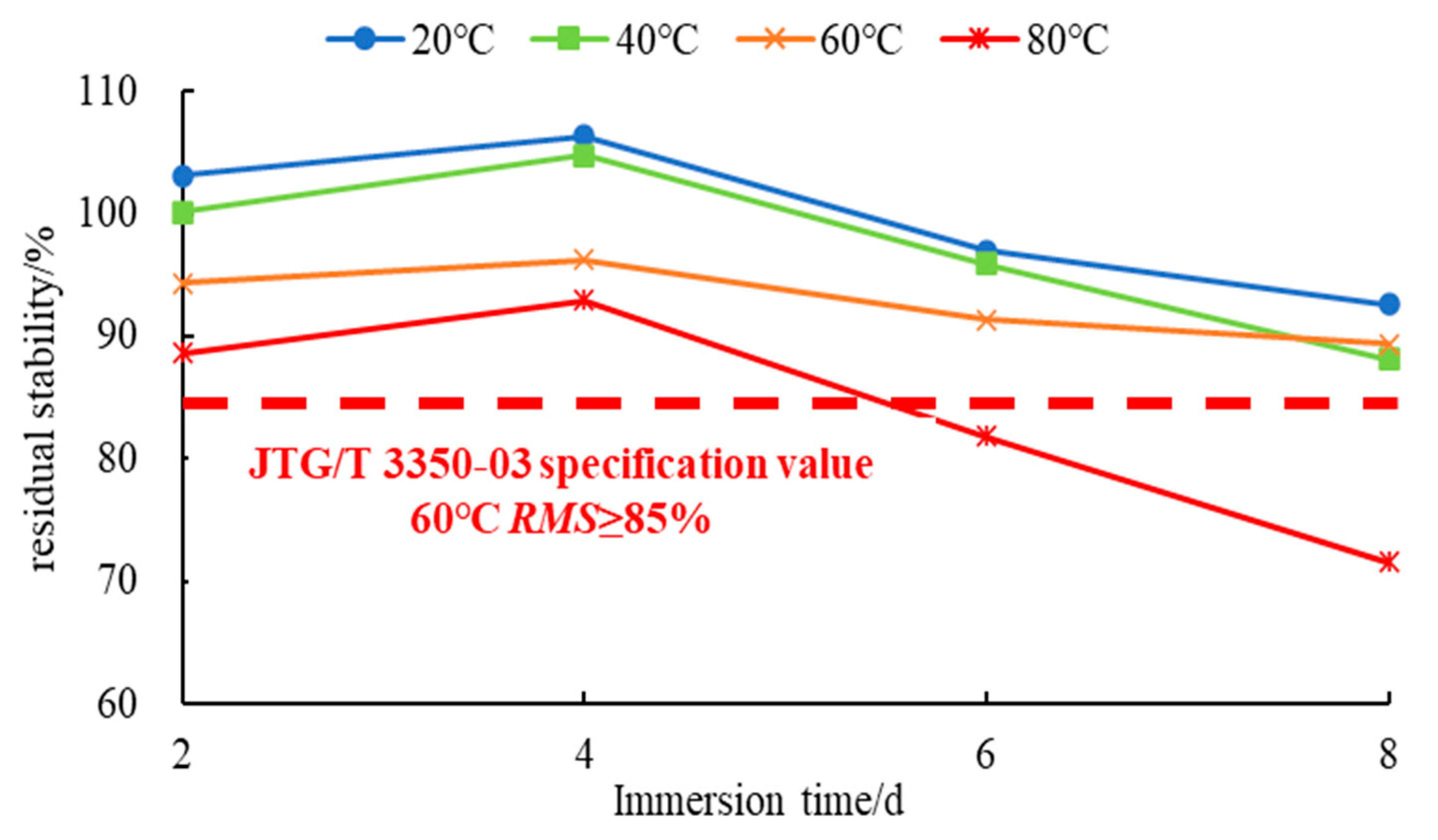
3.1.2. Analysis of the Results of the H2O-Immersion Marshall Test
3.2. Microscopic Test
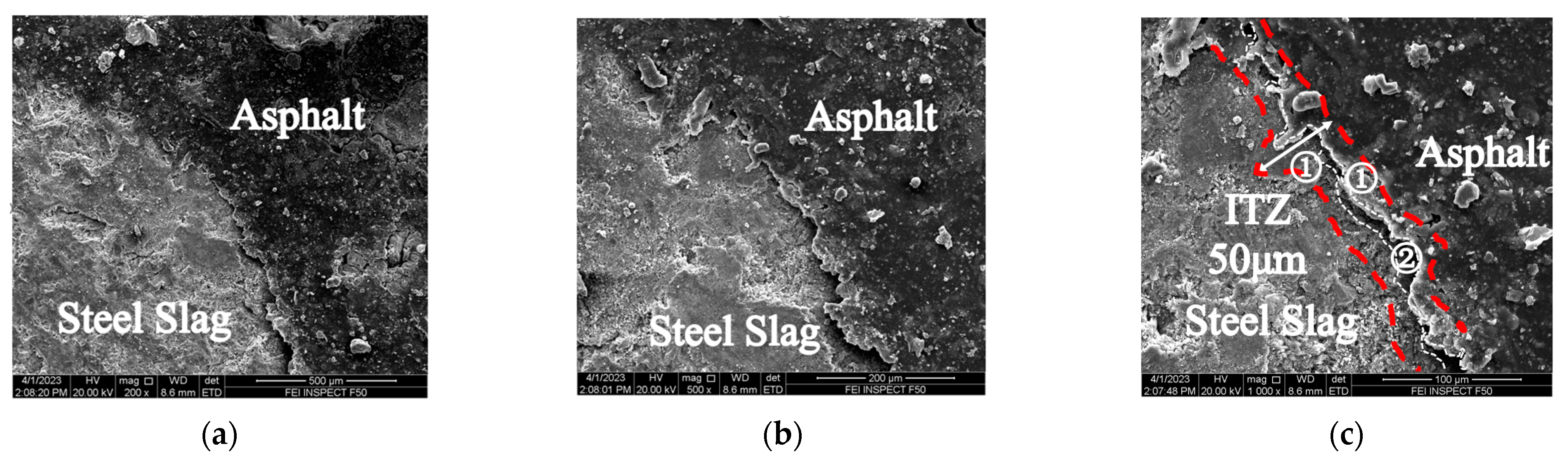
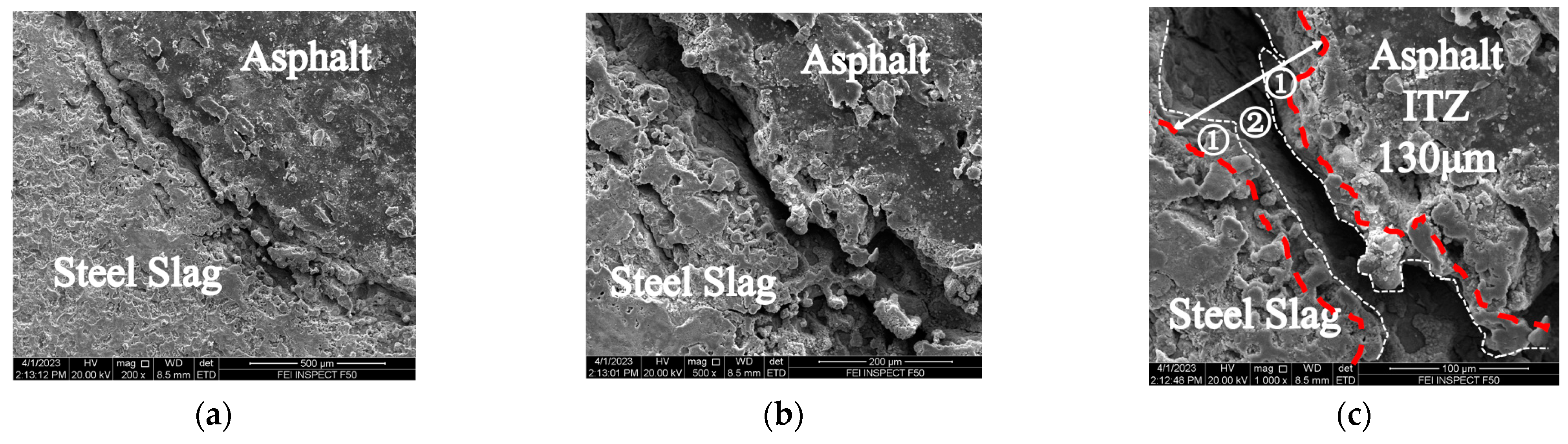
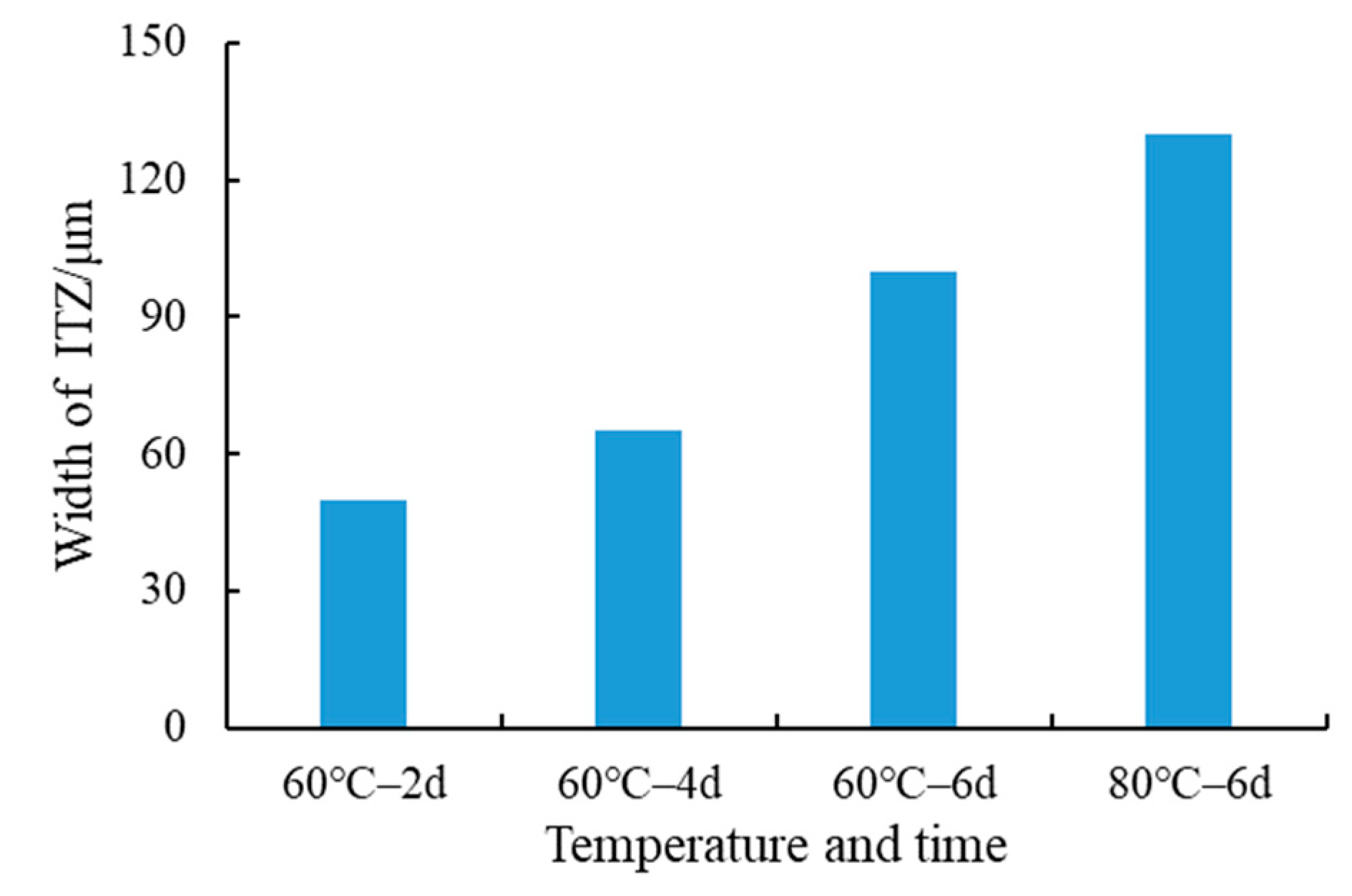
3.2.1. Changes in the SSPA’s Micro-Interface after Time–Temperature H2O-Immersion
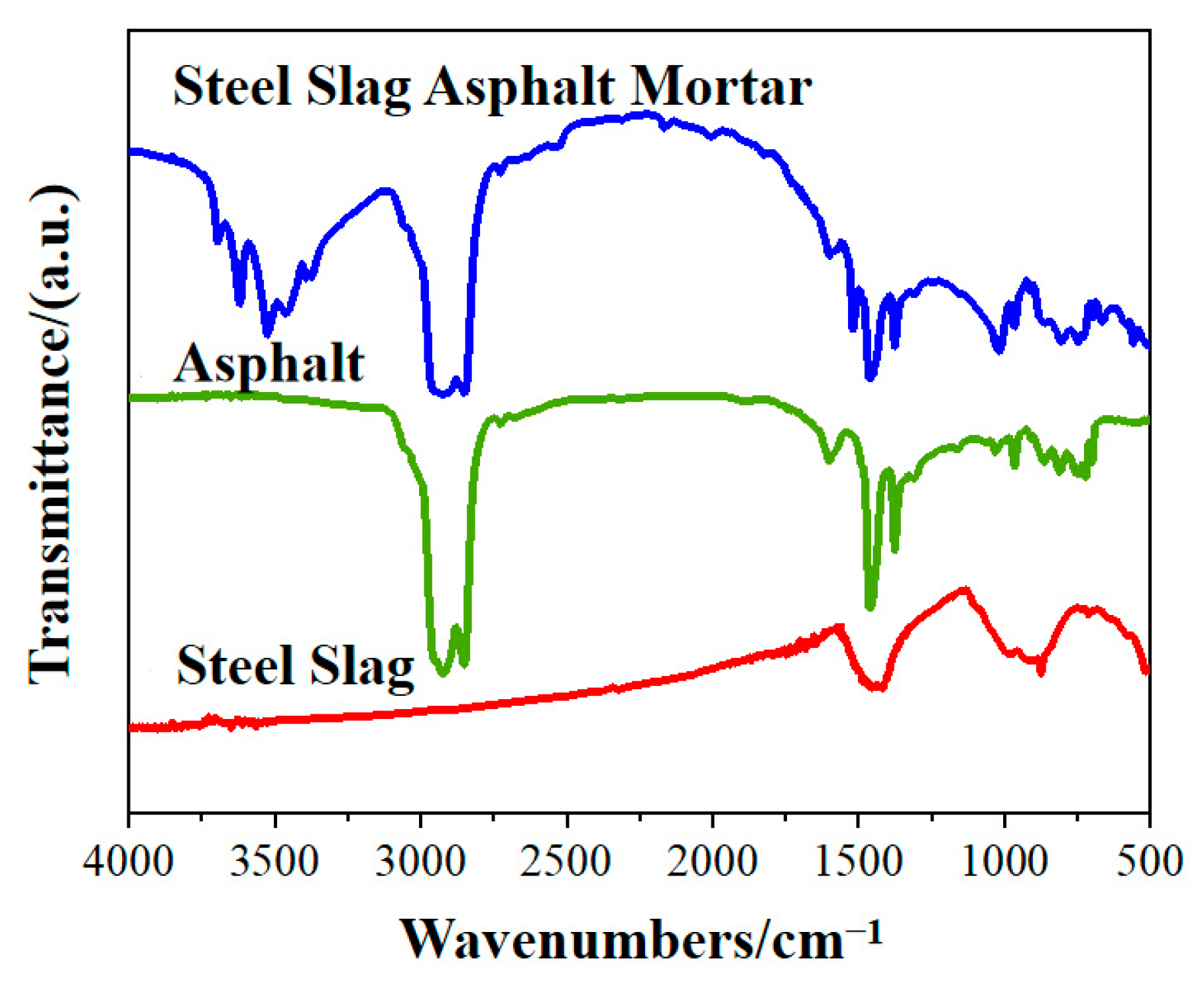
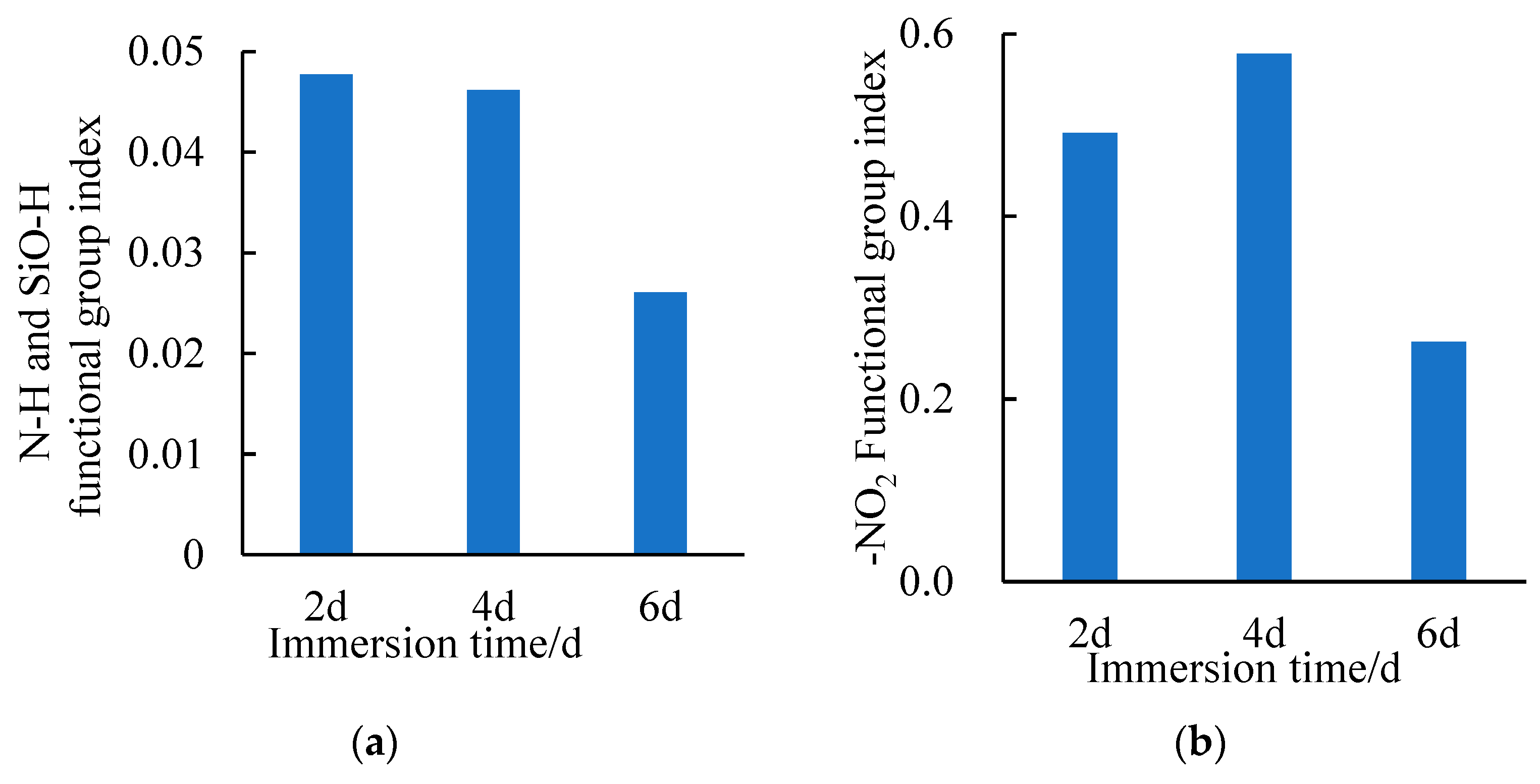
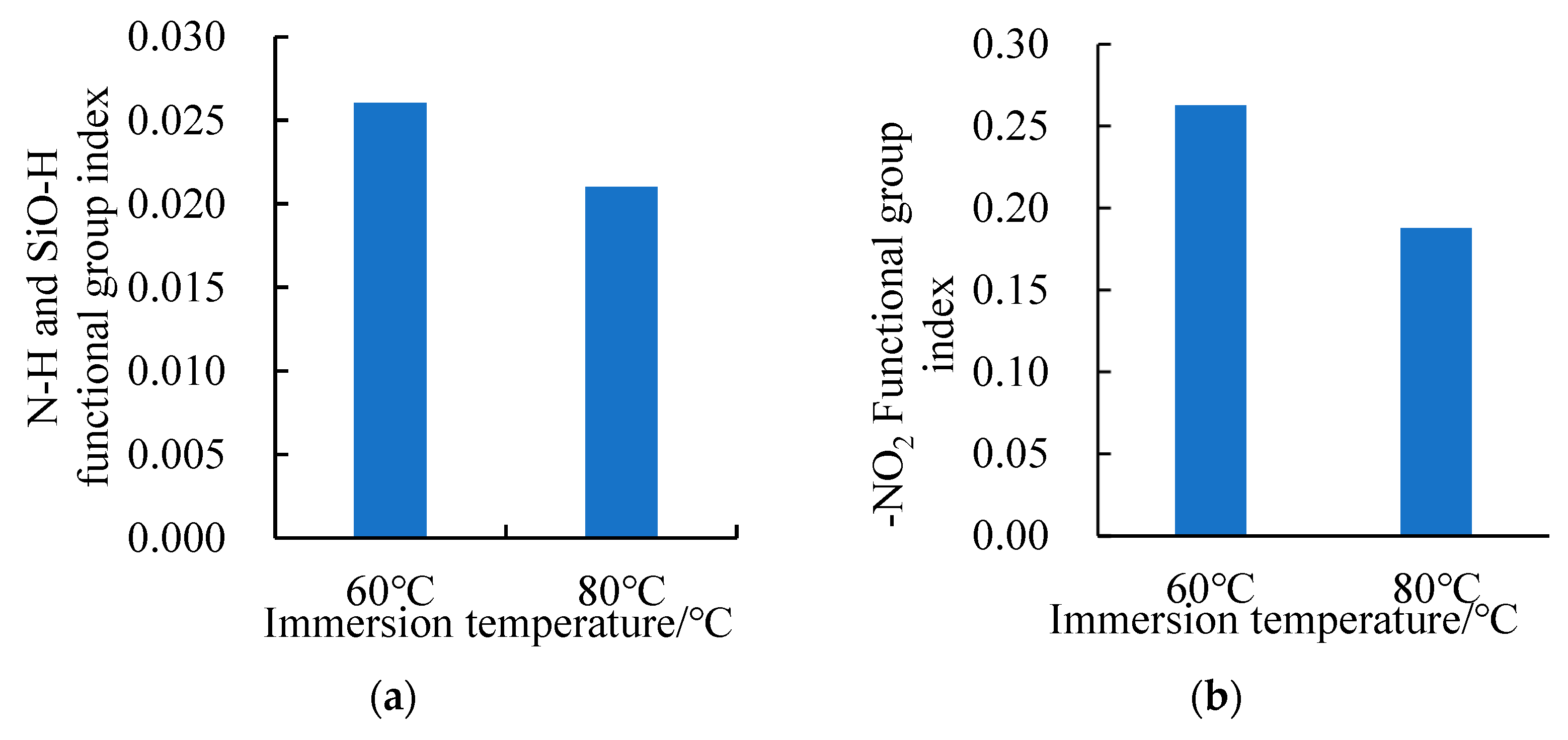
3.2.2. Changes in the SSAM’s Functional Groups after Time–Temperature H2O-Immersion
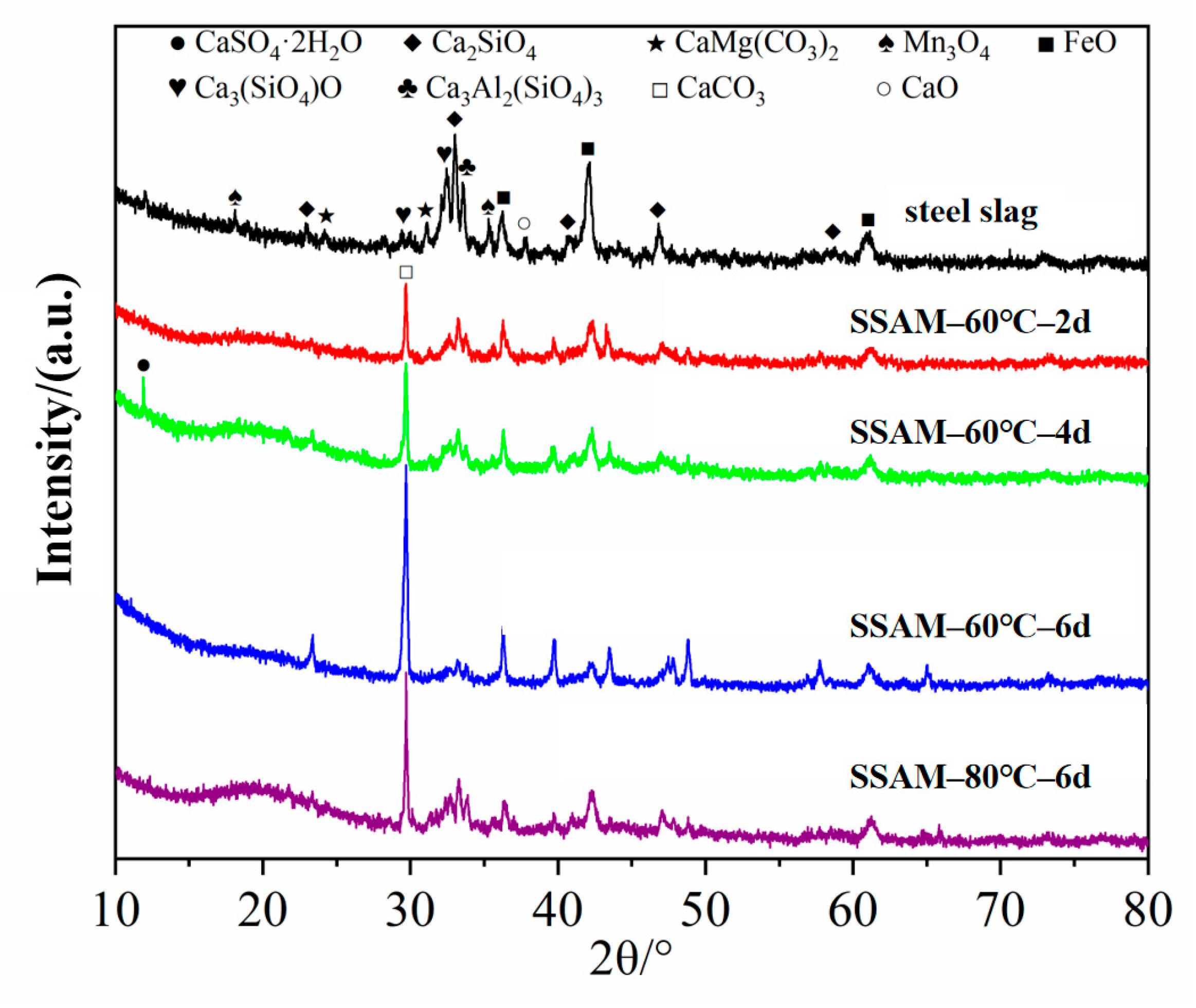
3.2.3. Changes in the SSAM’s Chemical Fraction after Time–Temperature H2O-Immersion
4. Conclusions
- Both the macroscopic and microscopic tests showed that at a constant H2O immersion temperature, the water stability of SSPA first increased and then decreased with an increase in the H2O immersion time. The optimum water stability for SSPA was attained at 4 days. The water stability of SSPA was generally excellent under short-term high-temperature or medium-temperature H2O immersion, but significantly deceased after long periods of high-temperature H2O-immersion schemes.
- The SEM results showed that under short-term, high-temperature H2O-immersion conditions, the interfacial phase of asphalt and steel slag was generally continuous and uniform; that is, the steel slag aggregates and asphalt contact had a firm mechanical bonding force that enhanced the interfacial strength between the asphalt and steel slag aggregates. The change in the spacing size of the ITZ reflected the chemical adhesion mechanism between the asphalt and steel slag aggregates.
- The FTIR results showed that there was a chemical bonding reaction between the asphalt and steel slag aggregates. This inherently enhanced the adhesion properties between the asphalt and steel slag aggregates; however, the combination of long-time and high-temperature H2O-immersion severely weakened the adhesive bonding between the asphalt and steel slag aggregates.
- The XRD results showed that with an increase in the H2O immersion time, the crystallization peak of CaCO3 produced via the hydration reaction of steel slag appeared to be significantly enhanced. Furthermore, the CaSO4·2H2O formed via the chemical reaction of various acidic groups contained in asphalt and the CaCO3 can enhance the water stability of SSPA.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kutuk, S.; Kutuk-Sert, T. An examination of nanoparticle colemanite mineral added warm mix asphalt. Constr. Build. Mater. 2020, 243, 118252. [Google Scholar] [CrossRef]
- Kütük-Sert, T.K.-S.; Günbey, R.E. Investigation of nano ulexite mineral effects on mechanical behaviour of warm mix asphalt pavements. J. Croat. Assoc. Civ. Eng. 2020, 72, 503–513. [Google Scholar]
- Walubita, L.F.; Martinez-Arguelles, G.; Polo-Mendoza, R.; Ick-Lee, S.; Fuentes, L. Comparative Environmental Assessment of Rigid, Flexible, and Perpetual Pavements: A Case Study of Texas. Sustainability 2022, 14, 9983. [Google Scholar] [CrossRef]
- Zhang, J.; Simate, G.S.; Hu, X.D.; Souliman, M.; Walubita, L.F. Impact of recycled asphalt materials on asphalt binder properties and rutting and cracking performance of plant-produced mixtures. Constr. Build. Mater. 2017, 155, 654–663. [Google Scholar] [CrossRef]
- Sanchez-Cotte, E.H.; Fuentes, L.; Martinez-Arguelles, G.; Quintana, H.A.R.; Walubita, L.F.; Cantero-Durango, J.M. Influence of recycled concrete aggregates from different sources in hot mix asphalt design. Constr. Build. Mater. 2020, 259, 120427. [Google Scholar] [CrossRef]
- Walubita, L.F.; Djebou, D.C.S.; Faruk, A.M.; Lee, S.I.; Dessouky, S.; Hu, X.D. Prospective of Societal and Environmental Benefits of Piezoelectric Technology in Road Energy Harvesting. Sustainability 2018, 10, 383. [Google Scholar] [CrossRef]
- Li, J.; Zhang, J.; Ni, S.N.; Liu, L.B.; Walubita, L.F. Mechanical performance and environmental impacts of self-compacting concrete with recycled demolished concrete blocks. J. Clean. Prod. 2021, 293, 126129. [Google Scholar] [CrossRef]
- Yang, X.X.; Zhou, X.T.; Kan, T.; Strezov, V.; Nelson, P.; Evans, T.; Jiang, Y.J. Characterization of size resolved atmospheric particles in the vicinity of iron and steelmaking industries in China. Sci. Total Environ. 2019, 694, 133534. [Google Scholar] [CrossRef]
- Kılıçarslan, Z. Comparative analysis of the competitiveness in the steel sector: The case of top 10 steel-producing countries. Erciyes Üniversitesi İktisadi Ve İdari Bilim. Fakültesi Derg. 2021, 60, 755–773. [Google Scholar] [CrossRef]
- Liu, H.M.; Li, Q.Q.; Li, G.G.; Ding, R. Life Cycle Assessment of Environmental Impact of Steelmaking Process. Complexity 2020, 2020, 8863941. [Google Scholar] [CrossRef]
- Hainin, M.R.; Aziz, M.M.A.; Ali, Z.; Putra Jaya, R.; Elsergany, M.; Yaacob, H. Steel Slag as A Road Construction Material. J. Teknol. 2015, 73, 33–38. [Google Scholar] [CrossRef]
- Motz, H.; Geiseler, J. Products of steel slags an opportunity to save natural resources. Waste Manag. 2001, 21, 285–293. [Google Scholar] [CrossRef]
- Guo, J.L.; Bao, Y.P.; Wang, M. Steel slag in China: Treatment, recycling, and management. Waste Manag. 2018, 78, 318–330. [Google Scholar] [CrossRef] [PubMed]
- Yi, H.; Xu, G.P.; Cheng, H.G.; Wang, J.S.; Wan, Y.F.; Chen, H. An overview of utilization of steel slag. In Proceedings of the 7th International Conference on Waste Management and Technology (ICWMT), Beijing, China, 5–7 September 2012; pp. 791–801. [Google Scholar]
- Liu, W.H.; Li, H.; Zhu, H.M.; Xu, P.J. The Interfacial Adhesion Performance and Mechanism of a Modified Asphalt-Steel Slag Aggregate. Materials 2020, 13, 1180. [Google Scholar] [CrossRef] [PubMed]
- de Moura, B.L.R.; Teixeira, J.; Simao, R.A.; Khedmati, M.; Kim, Y.R.; Pires, P.J.M. Adhesion between steel slag aggregates and bituminous binder based on surface characteristics and mixture moisture resistance. Constr. Build. Mater. 2020, 264, 120685. [Google Scholar] [CrossRef]
- Wang, W.Z.; Shen, A.Q.; He, Z.M.; Liu, H.C. Evaluation of the adhesion property and moisture stability of rubber modified asphalt mixture incorporating waste steel slag. J. Adhes. Sci. Technol. 2023, 37, 296–318. [Google Scholar] [CrossRef]
- Zalnezhad, M.; Hesami, E. Effect of steel slag aggregate and bitumen emulsion types on the performance of microsurfacing mixture. J. Traffic Transp. Eng. Engl. Ed. 2020, 7, 215–226. [Google Scholar] [CrossRef]
- Walubita, L.F.; Martin, A.E.; Jung, S.H.; Glover, C.J.; Park, E.S.; Chowdhury, A.R.; Lytton, R.L. Comparison of Fatigue Analysis Approaches for Two Hot Mix Asphalt Concrete (HMAC) Mixtures; Texas A&M Transportation Institute: Bryan, TX, USA, 2005. [Google Scholar]
- Gan, Y.W.; Li, C.M.; Ke, W.; Deng, Q.H.; Yu, T. Study on pavement performance of steel slag asphalt mixture based on surface treatment. Case Stud. Constr. Mater. 2022, 16, e01131. [Google Scholar] [CrossRef]
- Yang, C.; Wu, S.; Cui, P.; Amirkhanian, S.; Zhao, Z.; Wang, F.; Zhang, L.; Wei, M.; Zhou, X.; Xie, J. Performance characterization and enhancement mechanism of recycled asphalt mixtures involving high RAP content and steel slag. J. Clean. Prod. 2022, 336, 130484. [Google Scholar] [CrossRef]
- Cui, P.; Wu, S.; Xiao, Y.; Hu, R.; Yang, T. Environmental performance and functional analysis of chip seals with recycled basic oxygen furnace slag as aggregate. J. Hazard. Mater. 2021, 405, 124441. [Google Scholar] [CrossRef]
- Liu, J.Z.; Xu, J.; Liu, Q.; Wang, S.Y.; Yu, B. Steel Slag for Roadway Construction: A Review of Material Characteristics and Application Mechanisms. J. Mater. Civ. Eng. 2022, 34, 03122001. [Google Scholar] [CrossRef]
- Alvarez, A.E.; Fernandez, E.M.; Martin, A.E.; Reyes, O.J.; Simate, G.S.; Walubita, L.F. Comparison of permeable friction course mixtures fabricated using asphalt rubber and performance-grade asphalt binders. Constr. Build. Mater. 2012, 28, 427–436. [Google Scholar] [CrossRef]
- Walubita, L.F.; Lee, S.I.; Faruk, A.N.M.; Scullion, T.; Nazarian, S.; Abdallah, I. Texas Flexible Pavements and Overlays: Year 5 Report—Complete Data Documentation; Texas A&M Transportation Institute: Bryan, TX, USA, 2017. [Google Scholar]
- Walubita, L.F.; Scullion, T.; Leidy, J.; Liu, W.T. Non-Destructive Testing Technologies Application of the Ground Penetrating Radar (GPR) to Perpetual Pavements. Road Mater. Pavement Des. 2009, 10, 259–286. [Google Scholar] [CrossRef]
- Zevenbergen, C.; Fu, D.F.; Pathirana, A. Transitioning to Sponge Cities: Challenges and Opportunities to Address Urban Water Problems in China. Water 2018, 10, 1230. [Google Scholar] [CrossRef]
- Sha, A.; Liu, Z.; Jiang, W.; Qi, L.; Hu, L.; Jiao, W.; Barbieri, D.M. Advances and development trends in eco-friendly pavements. J. Road Eng. 2021, 1, 1–42. [Google Scholar] [CrossRef]
- Qin, Z.; Yao, Y.J.; Zhao, J.W.; Fu, H.L.; Zhang, S.; Qiu, L.Y. Investigation of migration rule of rainwater for sponge city roads under different rainfall intensities. Environ. Geochem. Health 2022, 44, 3395–3407. [Google Scholar] [CrossRef]
- Zhang, L.; Lu, Q.; Ding, Y.F.; Peng, P.; Yao, Y. Design and Performance Simulation of Road Bioretention Media for Sponge Cities. J. Perform. Constr. Facil. 2018, 32, 04018061. [Google Scholar] [CrossRef]
- Guan, X.; Wang, J.Y.; Xiao, F.P. Sponge city strategy and application of pavement materials in sponge city. J. Clean. Prod. 2021, 303, 127022. [Google Scholar] [CrossRef]
- Liu, W.H.; Li, H.; Zhu, H.M.; Xu, P.J. Properties of a Steel Slag-Permeable Asphalt Mixture and the Reaction of the Steel Slag-Asphalt Interface. Materials 2019, 12, 3603. [Google Scholar] [CrossRef]
- Walubita, L.F.; Faruk, A.N.M.; Zhang, J.; Hu, X.D.; Lee, S.I. The Hamburg rutting test—Effects of HMA sample sitting time and test temperature variation. Constr. Build. Mater. 2016, 108, 22–28. [Google Scholar] [CrossRef]
- Chen, Z.W.; Gong, Z.L.; Jiao, Y.Y.; Wang, Y.; Shi, K.; Wu, J.C. Moisture stability improvement of asphalt mixture considering the surface characteristics of steel slag coarse aggregate. Constr. Build. Mater. 2020, 251. [Google Scholar] [CrossRef]
- Liang, Y.C.; Bai, T.; Zhou, X.L.; Wu, F.; Chenxin, C.; Peng, C.; Fuentes, L.; Walubita, L.F.; Li, W.; Wang, X.C. Assessing the Effects of Different Fillers and Moisture on Asphalt Mixtures’ Mechanical Properties and Performance. Coatings 2023, 13, 288. [Google Scholar] [CrossRef]
- Walubita, L.F.; Hugo, F.; Martin, A.E. Indirect tensile fatigue performance of asphalt after MMLS3 trafficking under different environmental conditions. J. Van Die Suid-Afr. Inst. Van Siviele Ingenieurswese 2002, 44, 2–11. [Google Scholar]
- Liu, H.B.; Zhu, B.; Wei, H.B.; Chai, C.; Chen, Y. Laboratory Evaluation on the Performance of Porous Asphalt Mixture with Steel Slag for Seasonal Frozen Regions. Sustainability 2019, 11, 6924. [Google Scholar] [CrossRef]
- Liu, Y.W.; Apeagyei, A.; Ahmad, N.; Grenfell, J.; Airey, G. Examination of moisture sensitivity of aggregate-bitumen bonding strength using loose asphalt mixture and physico-chemical surface energy property tests. Int. J. Pavement Eng. 2014, 15, 657–670. [Google Scholar] [CrossRef]
- Walubita, L.F. Comparison of Fatigue Analysis Approaches for Predicting Fatigue Lives of Hot-Mix Asphalt Concrete (HMAC) Mixtures. Doctoral Dissertation, Texas A&M University, Bryan, TX, USA, 2006. [Google Scholar]
- Xu, F.; Nie, X.; Gan, W.X.; Hongzhi, E.; Xu, P.Y.; Cao, H.Q.; Gong, R.F.; Zhang, Y.X. Moisture Sensitivity Evaluation of the Asphalt Mortar-Aggregate Filler Interface Using Pull-Out Testing and 3-D Structural Imaging. Coatings 2023, 13, 868. [Google Scholar] [CrossRef]
- Walubita, L.F.; Martin, A.E.; Cleveland, G.S.; Lytton, R.L. Computation of pseudo strain energy and Paris law fracture coefficients from surface energy and uniaxial strain-controlled tension test data. Int. J. Pavement Eng. 2006, 7, 167–178. [Google Scholar] [CrossRef]
- Hu, X.D.; Jiang, M.; Bai, T.; Pan, P.; Walubita, L.F. Development and validation of an enhanced test setup for assessing HMA stripping potential under hydrodynamic pressure. Road Mater. Pavement Des. 2020, 21, 2024–2039. [Google Scholar] [CrossRef]
- Mabui, D.S.; Tjaronge, M.W.; Adisasmita, S.A.; Pasra, M. Resistance to cohesion loss in cantabro test on specimens of porous asphalt containing modificated asbuton. In Proceedings of the 3rd International Conference on Civil and Environmental Engineering (ICCEE), Bali, Indonesia, 29–30 August 2019. [Google Scholar]
- Cox, B.C.; Smith, B.T.; Howard, I.L.; James, R.S. State of Knowledge for Cantabro Testing of Dense Graded Asphalt. J. Mater. Civ. Eng. 2017, 29, 04017174. [Google Scholar] [CrossRef]
- Mirhosseini, A.F.; Tahami, A.; Hoff, I.; Dessouky, S.; Kavussi, A.; Fuentes, L.; Walubita, L.F. Performance Characterization of Warm-Mix Asphalt Containing High Reclaimed-Asphalt Pavement with Bio-Oil Rejuvenator. J. Mater. Civ. Eng. 2020, 32, 04020382. [Google Scholar] [CrossRef]
- Do, T.C.; Tran, V.P.; Le, V.P.; Lee, H.J.; Kim, W.J. Mechanical characteristics of tensile strength ratio method compared to other parameters used for moisture susceptibility evaluation of asphalt mixtures. J. Traffic Transp. Eng. -Engl. Ed. 2019, 6, 621–630. [Google Scholar] [CrossRef]
- Bai, T.; Hu, Z.A.; Hu, X.D.; Liu, Y.; Fuentes, L.; Walubita, L.F. Rejuvenation of short-term aged asphalt-binder using waste engine oil. Can. J. Civ. Eng. 2020, 47, 822–832. [Google Scholar] [CrossRef]
- Polo-Mendoza, R.; Martinez-Arguelles, G.; Walubita, L.F.; Moreno-Navarro, F.; Giustozzi, F.; Fuentes, L.; Navarro-Donado, T. Ultraviolet ageing of bituminous materials: A comprehensive literature review from 2011 to 2022. Constr. Build. Mater. 2022, 350, 128889. [Google Scholar] [CrossRef]
- Mubaraki, M. The Effect of Modified Asphalt Binders by Fourier Transform Infrared Spectroscopy, X-ray Diffraction, and Scanning Electron Microscopy. J. Mater. Eng. Struct. 2019, 6, 5–14. [Google Scholar]
- Zhang, Y.; Gu, Q.L.; Kang, A.H.; Ding, X.H.; Ma, T. Characterization of mesoscale fracture damage of asphalt mixtures with basalt fiber by environmental scanning electron microscopy. Constr. Build. Mater. 2022, 344, 128889. [Google Scholar] [CrossRef]
- Application of Nano-Technology in Pavement Engineering: A Literature Review. Application of Nanotechnology in Pavements, Geological Disasters, and Foundation Settlement Control Technology. In Proceedings of the Geo-Hubei 2014 International Conference on Sustainable Civil Infrastructure, Yichang, China, 20–22 July 2014; pp. 9–16.
- Wang, P.; Dong, Z.J.; Tan, Y.Q.; Liu, Z.Y. Identifying the rheological properties of polymer-modified bitumen based on its morphology. Road Mater. Pavement Des. 2017, 18, 249–258. [Google Scholar] [CrossRef]
- Zhang, X.R.; Wang, J.T.; Zhou, X.X.; Zhang, Z.Q.; Chen, X.B. Mechanical Properties of the Interfacial Bond between Asphalt-Binder and Aggregates under Different Aging Conditions. Materials 2021, 14, 1221. [Google Scholar] [CrossRef]
- JTG E20-2011; Standard Test Methods of Bitumen and Bituminous Mixtures for Highway Engineering. China Communications Press: Beijing, China, 2011.
- JTG F40-2004; Technical Specification for Construction of Highway Asphalt Pavements. China Communications Press: Beijing, China, 2004.
- GB/T 24766-2009; Steel Slag for Pervious Asphalt Pave. China Communications Press: Beijing, China, 2009.
- JTG/T 3350-03—2020; Technical Specifications for Design and Construction of Porous Asphalt Pavement. China Communications Press: Beijing, China, 2020.
- JT/T 860.2-2013; Modifier for Asphalt Mixture (Part 2: High Viscosity Additive). China Communications Press: Beijing, China, 2013.
- JT/T 533-2020; Fiber for Asphalt Pavements. China Communications Press: Beijing, China, 2020.
- Zhang, J.P.; Liu, G.Q.; Zhu, C.Z.; Pei, J.Z. Evaluation indices of asphalt-filler interaction ability and the filler critical volume fraction based on the complex modulus. Road Mater. Pavement Des. 2017, 18, 1338–1352. [Google Scholar] [CrossRef]
- CJJ/T 190-2012; Technical Specification for Permeable Asphalt Pavement. China Communications Press: Beijing, China, 2012.
- Walubita, L.F.; Fuentes, L.; Lee, S.I.; Dawd, I.; Mahmoud, E. Comparative evaluation of five HMA rutting-related laboratory test methods relative to field performance data: DM, FN, RLPD, SPST, and HWTT. Constr. Build. Mater. 2019, 215, 737–753. [Google Scholar] [CrossRef]
- Holleran, I.; Wilson, D.J.; Holleran, G.; Walubita, L.; James, B. Porous Asphalt—More Than Just Safety. In Proceedings of the 2016 IPENZ Transportation Group Conference: Design, Innovation, Technology, Auckland, New Zealand, 22–24 March 2016. [Google Scholar]
- Gao, Y.; Huang, X.M.; Yu, W.B. The Compaction Characteristics of Hot Mixed Asphalt Mixtures. J. Wuhan Univ. Technol. Mater. Sci. Ed. 2014, 29, 956–959. [Google Scholar] [CrossRef]
- Jiang, W.; Sha, A.; Pei, J.Z.; Chen, S.; Zhou, H. Study on the fatigue characteristic of porous asphalt concrete. Jianzhu Cailiao Xuebao/J. Build. Mater. 2012, 15, 513–517+543. [Google Scholar] [CrossRef]
- Xu, F.; Chan, T.; Luo, M. Different changes in dry and humid heat waves over China. Int. J. Climatol. 2020, 41, 1369–1382. [Google Scholar] [CrossRef]
- Tschegg Elmar, K.; Kroyer, G.; Tan, D.-M.; Stanzl-Tschegg Stefanie, E.; Litzka, J. Investigation of Bonding between Asphalt Layers on Road Construction. J. Transp. Eng. 1995, 121, 309–316. [Google Scholar] [CrossRef]
- Lu, Z.; Feng, Z.-g.; Yao, D.; Li, X.; Jiao, X.; Zheng, K. Bonding performance between ultra-high performance concrete and asphalt pavement layer. Constr. Build. Mater. 2021, 312, 125375. [Google Scholar] [CrossRef]
- Mohammed, A.; Abdullah, A. Scanning Electron Microscopy (SEM): A Review. In Proceedings of the 2018 International Conference on Hydraulics and Pneumatics–HERVEX, Baile Govora, Romania, 13–15 November 2019. [Google Scholar]
- Lamontagne, J.; Dumas, P.; Mouillet, V.; Kister, J. Comparison by Fourier transform infrared (FTIR) spectroscopy of different ageing techniques: Application to road bitumens. Fuel 2001, 80, 483–488. [Google Scholar] [CrossRef]
- Souliman, M.I.; Gc, H.; Isied, M.; Walubita, L.F.; Sousa, J.; Bastola, N.R. Mechanistic analysis and cost-effectiveness evaluation of asphalt rubber mixtures. Road Mater. Pavement Des. 2020, 21, S76–S90. [Google Scholar] [CrossRef]
- Li, Z.Y.; Li, R.L.; Li, C.L.; Wang, K.R.; Fan, J.Y.; Gu, R. Identification of Tibetan Medicine Zhaxun by Infrared Spectroscopy Combined With Chemometrics. Spectrosc. Spectr. Anal. 2023, 43, 526–532. [Google Scholar] [CrossRef]
- Epp, J. X-ray diffraction (XRD) Techniques for Materials Characterization. In Materials Characterization Using Nondestructive Evaluation (NDE) Methods; Hübschen, G., Altpeter, I., Tschuncky, R., Herrmann, H.-G., Eds.; Woodhead Publishing: Cambridge, UK, 2016; pp. 81–124. [Google Scholar]
- Li, Z.T.; Wang, C.B. Multi-scale Ag/CuO Photothermal Materials: Preparation and Application in Seawater Desalination. Chin. J. Inorg. Chem. 2020, 36, 1457–1464. [Google Scholar] [CrossRef]
- Zhou, L.; Huang, W.; Lu, Q.; Zheng, M. Effects of Various Modifiers on the Bond Property and Moisture Damage Resistance of Asphalt. J. Build. Mater. 2021, 24, 377–384. [Google Scholar] [CrossRef]
- Bagampadde, U.; Isacsson, U.; Kiggundu, B.M. Classical and Contemporary Aspects of Stripping in Bituminous Mixes. Road Mater. Pavement Des. 2004, 5, 7–43. [Google Scholar] [CrossRef]
- Alnadish, A.M.; Aman, M.Y.; Katman, H.Y.B.; Ibrahim, M.R. Laboratory assessment of the performance and elastic behavior of asphalt mixtures containing steel slag aggregate and synthetic fibers. Int. J. Pavement Res. Technol. 2021, 14, 473–481. [Google Scholar] [CrossRef]
- Zhou, X.X.; Zhao, G.Y.; Tighe, S.; Chen, M.Z.; Wu, S.P.; Adhikari, S.; Gao, Y.M. Quantitative comparison of surface and interface adhesive properties of fine aggregate asphalt mixtures composed of basalt, steel slag, and andesite. Constr. Build. Mater. 2020, 246, 473–481. [Google Scholar] [CrossRef]
- Mi, G.; Qiang, W.; Wang, W. Destructive effect of steel slag coarse aggregate on the concrete under autoclaved condition. J. Tsinghua Univ. 2015, 55, 940–944. [Google Scholar]
- Liu, J.; Yu, B.; Hong, Q. Molecular dynamics simulation of distribution and adhesion of asphalt components on steel slag. Constr. Build. Mater. 2020, 255, 119332. [Google Scholar] [CrossRef]
- Shen, A.; Zhai, C.; Guo, Y.; Yang, X. Mechanism of adhesion property between steel slag aggregate and rubber asphalt. J. Adhes. Sci. Technol. 2018, 32, 2727–2740. [Google Scholar] [CrossRef]
- Chen, X.; Wen, W.; Zhou, J.; Zhou, X.; Ning, Y.; Liang, Z.; Ma, Z. Research on the Interaction Capability and Microscopic Interfacial Mechanism between Asphalt-Binder and Steel Slag Aggregate-Filler. Coatings 2022, 12, 1871. [Google Scholar] [CrossRef]
- Kutuk-Sert, T.; Ozturk, M.; Kutuk, S. Digital image processing of warm mix asphalt enriched with nanocolemanite and nanoulexite minerals. Constr. Build. Mater. 2023, 399, 132542. [Google Scholar] [CrossRef]
- Kara, C.; Kutuk, S.; Kutuk-Sert, T. Improvement of the durability of concrete by substitution of raw ground colemanite. Case Stud. Constr. Mater. 2023, 19, e02393. [Google Scholar] [CrossRef]
- Bai, T.; Cheng, Z.; Hu, X.D.; Fuentes, L.; Walubita, L.F. Viscoelastic modelling of an asphalt pavement based on actual tire-pavement contact pressure. Road Mater. Pavement Des. 2021, 22, 2458–2477. [Google Scholar] [CrossRef]
- Walubita, L.F.; Zhang, J.; Das, G.; Hu, X.D.; Mushota, C.; Alvarez, A.E.; Scullion, T. Hot-Mix Asphalt Permanent Deformation Evaluated by Hamburg Wheel Tracking, Dynamic Modulus, and Repeated Load Tests. Transp. Res. Rec. 2012, 22, 2458–2477. [Google Scholar] [CrossRef]
- Ling, M.; Lee, S.I.; Ji, J.; Fuentes, L.; Walubita, L.F. Assessing permanent deformation potential of asphalt mixtures based on viscoelastic characteristics. Int. J. Pavement Eng. 2023, 24, 2240472. [Google Scholar] [CrossRef]
- Chen, F.H.; Zhang, D.F.; Liu, G.; Huang, J.F.; Wu, S. Experimental investigation of improving anti-stripping performance of asphalt mixture. J. Wuhan Univ. Technol. 2007, 29, 9–11. [Google Scholar]
- Jonczy, I.; Grzesik, B. Polymorphic transformations of dicalcium silicates in steel slags used in the production of road aggregates. Gospod. Surowcami Miner. Miner. Resour. Manag. 2021, 37, 97–116. [Google Scholar] [CrossRef]
- Feng, X.; Zhao, M.; Chen, W.; Xie, M. Research of Road Performances and Microcosmic Mechanisms of Coal Waste Powder Asphalt Mortar. J. Build. Mater. 2019, 22, 113–119. [Google Scholar] [CrossRef]
- Yao, H.; Dai, Q.L.; You, Z.P. Fourier Transform Infrared Spectroscopy characterization of aging-related properties of original and nano-modified asphalt binders. Constr. Build. Mater. 2015, 101, 1078–1087. [Google Scholar] [CrossRef]
- Fernández-Gómez, W.D.; Rondón Quintana, H.A.; Daza, C.E.; Reyes Lizcano, F.A. The effects of environmental aging on Colombian asphalts. Fuel 2014, 115, 321–328. [Google Scholar] [CrossRef]
- Zhang, L.; Long, N.Q.; Liu, Y.; Wang, L. Cross-scale study on the influence of moisture-temperature coupling conditions on adhesive properties of rubberized asphalt and steel slag. Constr. Build. Mater. 2022, 332, 127401. [Google Scholar] [CrossRef]
- Liu, W.H.; Li, H.; Zhu, H.M.; Xu, P.J. Effects of Steel-Slag Components on Interfacial-Reaction Characteristics of Permeable Steel-Slag-Bitumen Mixture. Materials 2020, 13, 3885. [Google Scholar] [CrossRef]
- Jing, W.; Jiang, J.P.; Ding, S.; Duan, P. Hydration and Microstructure of Steel Slag as Cementitious Material and Fine Aggregate in Mortar. Molecules 2020, 25, 4456. [Google Scholar] [CrossRef]
- Li, H.; Liu, W.; Lu, J.; Lu, Y.; Shi, S.; Wang, Z.; Ye, Q.; Jia, Z. Effect of microwave-assisted acidification on the microstructure of coal: XRD, 1H-NMR, and SEM studies. Int. J. Min. Sci. Technol. 2023, 33, 919–926. [Google Scholar] [CrossRef]
- Wang, Q.; Yan, P.Y. Hydration properties of basic oxygen furnace steel slag. Constr. Build. Mater. 2010, 24, 1134–1140. [Google Scholar] [CrossRef]
- Wang, Q.; Yan, P.Y.; Han, S. Activity index for steel slag. Mag. Concr. Res. 2011, 63, 737–742. [Google Scholar] [CrossRef]
- Wang, Q.A.; Yan, P.Y.; Feng, J.W. A discussion on improving hydration activity of steel slag by altering its mineral compositions. J. Hazard. Mater. 2011, 186, 1070–1075. [Google Scholar] [CrossRef] [PubMed]
- Xin-li, L.Y.W.B.-h.N.W.M. Synergies in Early Hydration Reaction of Slag-Steel Slag-Gypsum System. J. Northeast. Univ. 2020, 41, 581–586. [Google Scholar] [CrossRef]




| Test | Unit | Test Result | Spec Requirement [55] | |
|---|---|---|---|---|
| Penetration (25 °C, 100 g, 5 s) | 0.1 mm | 55.9 | 40~60 | |
| Softening point | °C | 82.5 | ≥60 | |
| 5 °C ductility | cm | 34.6 | ≥20 | |
| Elastic recovery (25 °C) | % | 94 | ≥70 | |
| Kinematic viscosity (135 °C) | Pa∙s | 2.36 | ≤3 | |
| After RTFOT | Mass loss ratio | % | 0.14 | ≤0.6 |
| Penetration ratio (25 °C) | % | 80 | ≥65 | |
| Ductility (5 °C) | cm | 22.6 | ≥15 | |
| Test | Unit | Test Result | Spec Requirement [56] |
|---|---|---|---|
| Apparent relative density | - | 3.549 | ≥2.90 |
| Water absorption rate | % | 1.59 | ≤3.0 |
| Crushing value | % | 13.4 | ≤26 |
| Los Angeles abrasion loss | % | 10.7 | ≤26 |
| Flat elongated particles content | % | 10.1 | ≤12 |
| Water washing method <0.075 mm particle content | % | 0.47 | ≤1 |
| Adhesive performance | - | 5 | ≥4 |
| Polished stone value (PSV) | - | 52 | ≥42 |
| Test | Unit | Test Result | Spec Requirement [56] |
|---|---|---|---|
| Apparent relative density | - | 3.408 | ≥2.90 |
| Water absorption rate | % | 4.75 | - |
| Sand equivalent | % | 95 | ≥60 |
| Water washing method <0.075 mm particle content | s | 2.0 | ≤3 |
| Angularity (flow time) | s | 45 | ≥40 |
| Test | Test Result | Spec Requirement [57] | |
|---|---|---|---|
| Apparent relative density | 2.684 | ≥2.60 | |
| Water content/% | 0.6 | ≤1 | |
| Particle size range | <0.6 mm | 100 | 100 |
| <0.15 mm | 100 | 90~100 | |
| <0.075 mm | 99.3 | 75~100 | |
| Appearance | Uniform, no agglomerate | No agglomerate | |
| Hydrophilicity coefficient | 0.52 | ≤0.8 | |
| Heating stability | No change in color | No change in color | |
| Test | Unit | Test Result | Spec Requirement [58] |
|---|---|---|---|
| Appearance | - | Uniform, yellow, granular, and full | Granular, uniform, and full-bodied |
| Density | g/cm3 | 0.58 | ≤1.0 |
| Melt index | g/10 min | 8 | ≥2.0 |
| Single particle mass | g | 0.023 | ≤0.03 |
| Test | Unit | Test Result | Spec Requirement [59] |
|---|---|---|---|
| Oil absorption rate | times | 5.5 | - |
| Average length | mm | 6 | 4.5~7.5 |
| Average diameter | μm | 15.2 | 15~25 |
| Fracture strength | MPa | 527 | ≥450 |
| Fracture elongation | % | 25 | ≥20 |
| Density | g/cm3 | 1.34 | - |
| Melting point | °C | 255~265 | ≥240 |
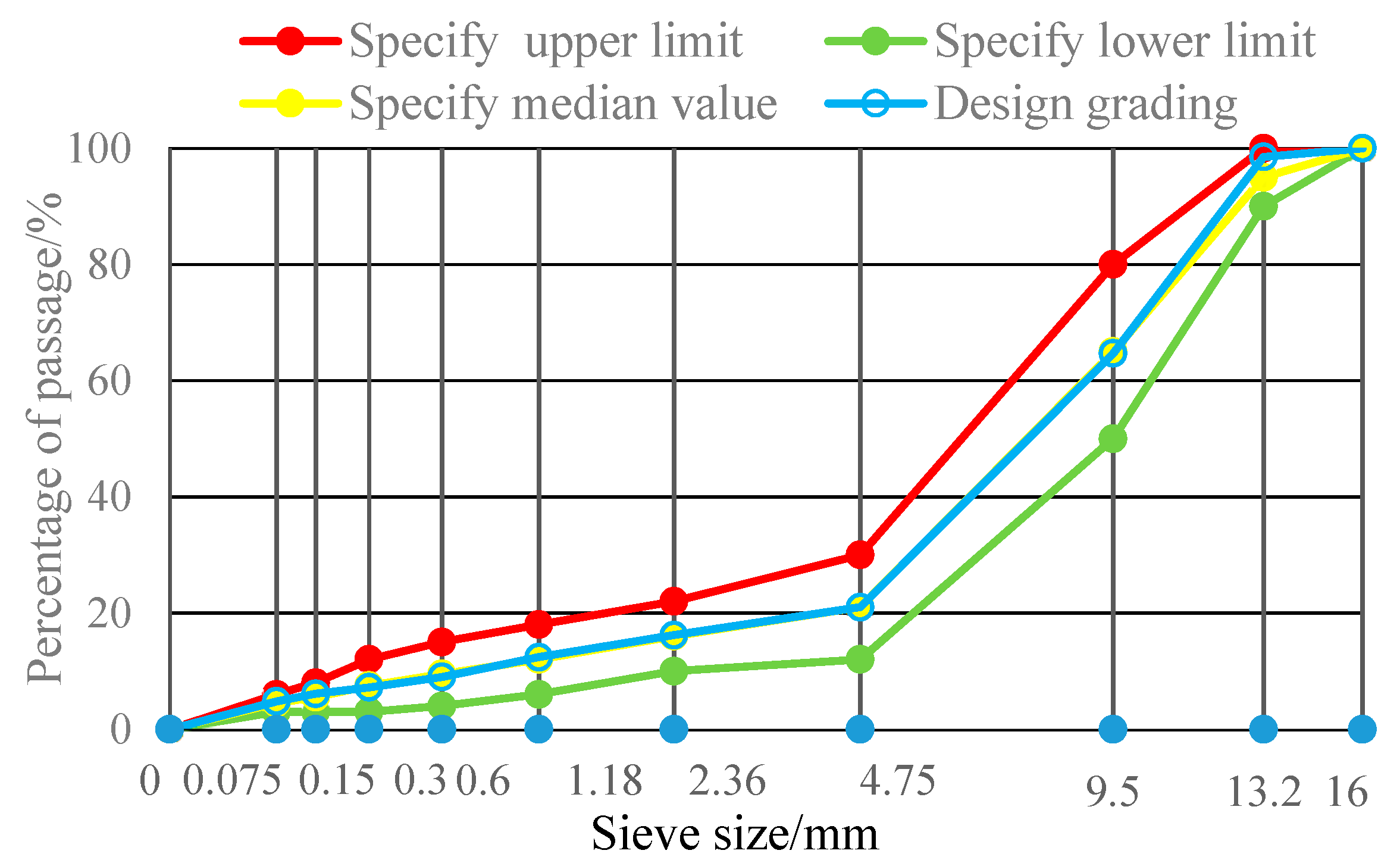
| Gradation | Mass Percentage (%) Passing in Each Sieve Size (mm) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 16 | 13.2 | 9.5 | 4.75 | 2.36 | 1.18 | 0.6 | 0.3 | 0.15 | 0.075 | |
| Design grading | 100 | 98.5 | 64.7 | 21.0 | 16.2 | 12.4 | 9.0 | 7.2 | 6.1 | 4.8 |
| Upper limit | 100 | 100 | 80 | 30 | 22 | 18 | 15 | 12 | 8 | 6 |
| Lower limit | 100 | 90 | 50 | 12 | 10 | 6 | 4 | 3 | 3 | 3 |
| Median | 100 | 95 | 65 | 21 | 16 | 12 | 9.5 | 7.5 | 5.5 | 4.5 |
| Immersion Time/d | Damage Mechanism at Different Temperatures | |||
|---|---|---|---|---|
| 20 °C | 40 °C | 60 °C | 80 °C | |
| 0 | Dislodgement | Dislodgement | Dislodgement | Dislodgement |
| 2 | Cohesive | Cohesive | Cohesive | Cohesive |
| 4 | Cohesive | Cohesive | Cohesive | Cohesive |
| 6 | Cohesive | Cohesive | Mixed | Adhesion |
| 8 | Cohesive | Mixed | Adhesion | Adhesion |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, X.; Zhang, M.; Yao, J.; Zhang, X.; Wen, W.; Yin, J.; Liang, Z. Research on Water Stability and Moisture Damage Mechanism of a Steel Slag Porous Asphalt Mixture. Sustainability 2023, 15, 14958. https://doi.org/10.3390/su152014958
Chen X, Zhang M, Yao J, Zhang X, Wen W, Yin J, Liang Z. Research on Water Stability and Moisture Damage Mechanism of a Steel Slag Porous Asphalt Mixture. Sustainability. 2023; 15(20):14958. https://doi.org/10.3390/su152014958
Chicago/Turabian StyleChen, Xiaobing, Miao Zhang, Jianming Yao, Xiaofei Zhang, Wei Wen, Jinhai Yin, and Zhongshan Liang. 2023. "Research on Water Stability and Moisture Damage Mechanism of a Steel Slag Porous Asphalt Mixture" Sustainability 15, no. 20: 14958. https://doi.org/10.3390/su152014958
APA StyleChen, X., Zhang, M., Yao, J., Zhang, X., Wen, W., Yin, J., & Liang, Z. (2023). Research on Water Stability and Moisture Damage Mechanism of a Steel Slag Porous Asphalt Mixture. Sustainability, 15(20), 14958. https://doi.org/10.3390/su152014958







