Microplastics Residence Time in Marine Copepods: An Experimental Study
Abstract
:1. Introduction
2. Material and Methods
3. Results
3.1. Parvocalanus Crassirostris
3.2. Acartia Pacifica
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lusher, A.; McHugh, M.; Thompson, R. Occurrence of microplastics in the gastrointestinal tract of pelagic and demersal fish from the English Channel. Mar. Pollut. Bull. 2013, 67, 94–99. [Google Scholar] [CrossRef] [PubMed]
- Rochman, C.M. The complex mixture, fate and toxicity of chemicals associated with plastic debris in the marine environment. In Marine Anthropogenic Litter; Springer: Berlin/Heidelberg, Germany, 2015; pp. 117–140. [Google Scholar]
- Naji, A.; Nuri, M.; Vethaak, A.D. Microplastics contamination in molluscs from the northern part of the Persian Gulf. Environ. Pollut. 2018, 235, 113–120. [Google Scholar] [CrossRef] [PubMed]
- Devriese, L.I.; van der Meulen, M.D.; Maes, T.; Bekaert, K.; Paul-Pont, I.; Frère, L.; Robbens, J.; Vethaak, A.D. Microplastic contamination in brown shrimp (Crangon crangon, Linnaeus 1758) from coastal waters of the Southern North Sea and Channel area. Mar. Pollut. Bull. 2015, 98, 179–187. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Bao, Z.; Wan, Z.; Fu, Z.; Jin, Y. Polystyrene microplastic exposure disturbs hepatic glycolipid metabolism at the physiological, biochemical, and transcriptomic levels in adult zebrafish. Sci. Total Environ. 2020, 710, 136279. [Google Scholar] [CrossRef] [PubMed]
- De Felice, B.; Sabatini, V.; Antenucci, S.; Gattoni, G.; Santo, N.; Bacchetta, R.; Ortenzi, M.A.; Parolini, M. Polystyrene microplastics ingestion induced behavioral effects to the cladoceran Daphnia magna. Chemosphere 2019, 231, 423–431. [Google Scholar] [CrossRef] [PubMed]
- Khan, F.R.; Syberg, K.; Shashoua, Y.; Bury, N.R. Influence of polyethylene microplastic beads on the uptake and localization of silver in zebrafish (Danio rerio). Environ. Pollut. 2015, 206, 73–79. [Google Scholar] [CrossRef] [PubMed]
- Prinz, N.; Korez, Š. Understanding How Microplastics Affect Marine Biota on the Cellular Level Is Important for Assessing Ecosystem Function: A Review. In YOUMARES 9—The Oceans: Our Research, Our Future: Proceedings of the 2018 Conference for YOUng MArine RESearcher in Oldenburg, Germany; Jungblut, S., Liebich, V., Bode-Dalby, M., Eds.; Springer International Publishing: Cham, Switzerland, 2020; pp. 101–120. [Google Scholar] [CrossRef]
- Alimi, O.S.; Farner Budarz, J.; Hernandez, L.M.; Tufenkji, N. Microplastics and nanoplastics in aquatic environments: Aggregation, deposition, and enhanced contaminant transport. Environ. Sci. Technol. 2018, 52, 1704. [Google Scholar] [CrossRef]
- Eerkes-Medrano, D.; Thompson, R.C.; Aldridge, D.C. Microplastics in freshwater systems: A review of the emerging threats, identification of knowledge gaps and prioritisation of research needs. Water Res. 2015, 75, 63. [Google Scholar] [CrossRef]
- Habib, R.; Thiemann, T.; Kendi, R. Microplastics and Wastewater Treatment Plants—A Review. J. Water Resour. Prot. 2020, 12, 1–35. [Google Scholar] [CrossRef]
- Ivar do Sul, J.A.; Costa, M.F. The present and future of microplastic pollution in the marine environment. Environ. Pollut. 2014, 185, 352–364. [Google Scholar] [CrossRef]
- United Nations. The State of Plastics. World Environment Day Outlook; United Nations: New York, NY, USA, 2018.
- Uddin, S.; Fowler, S.W.; Behbehani, M. An assessment of microplastic inputs into the aquatic environment from wastewater streams. Mar. Pollut. Bull. 2020, 160, 111538. [Google Scholar] [CrossRef] [PubMed]
- Naji, A.; Azadkhah, S.; Farahani, H.; Uddin, S.; Khan, F.R. Microplastics in wastewater outlets of Bandar Abbas city (Iran): A potential point source of microplastics into the Persian Gulf. Chemosphere 2021, 262, 128039. [Google Scholar] [CrossRef] [PubMed]
- Uddin, S.; Fowler, S.W.; Uddin, M.F.; Behbehani, M.; Naji, A. A review of microplastic distribution in sediment profiles. Mar. Pollut. Bull. 2021, 163, 111973. [Google Scholar] [CrossRef] [PubMed]
- Jinadasa, B.K.K.K.; Uddin, S.; Fowler, S.W. Microplastics (MPs) in marine food chains: Is it a food safety issue? In Advances in Food and Nutrition Research; Ozogul, F., Ed.; Academic Press: Cambridge, MA, USA, 2023; Volume 103, p. 101140. [Google Scholar]
- Baztan, J.; Bergmann, M.; Booth, A.; Broglio, E.; Carrasco, A.; Chouinard, O.; Clüsener-Godt, M.; Cordier, M.; Cozar, A.; Devrieses, L.; et al. Breaking Down the Plastic Age. In Fate and Impact of Microplastics in Marine Ecosystems; Baztan, J., Jorgensen, B., Pahl, S., Thompson, R.C., Vanderlinden, J.-P., Eds.; Elsevier: Amsterdam, The Netherlands, 2017; pp. 177–181. [Google Scholar] [CrossRef]
- Bergmann, M.; Wirzberger, V.; Krumpen, T.; Lorenz, C.; Primpke, S.; Tekman, M.B.; Gerdts, G. High Quantities of Microplastic in Arctic Deep-Sea Sediments from the HAUSGARTEN Observatory. Environ. Sci. Technol. 2017, 51, 11000. [Google Scholar] [CrossRef] [PubMed]
- Eriksen, M.; Lebreton, L.C.M.; Carson, H.S.; Thiel, M.; Moore, C.J.; Borerro, J.C.; Galgani, F.; Ryan, P.G.; Reisser, J. Plastic Pollution in the World’s Oceans: More than 5 Trillion Plastic Pieces Weighing over 250,000 Tons Afloat at Sea. PLoS ONE 2014, 9, e111913. [Google Scholar] [CrossRef]
- van Sebille, E.; Wilcox, C.; Lebreton, L.; Maximenko, N.; Hardesty, B.D.; van Franeker, J.A.; Eriksen, M.; Siegel, D.; Galgani, F.; Law, K.L. A Global Inventory of Small Floating Plastic Debris. Environ. Res. Lett. 2015, 10, 124006. [Google Scholar] [CrossRef]
- Freeman, S.; Booth, A.; Sabbah, I.; Tiller, R.; Dierking, J.; Klun, K.; Rotter, A.; Ben David, E.; Javidpour, J.; Angel, D. Between source and sea: The role of wastewater treatment in reducing marine microplastics. J. Environ. Manag. 2020, 266, 110642. [Google Scholar] [CrossRef]
- Jambeck, J.R.; Geyer, R.; Wilcox, C.; Siegler, T.R.; Perryman, M.; Andrady, A.; Narayan, R.; Law, K.L. Plastic waste inputs from land into the ocean. Science 2015, 347, 768–771. [Google Scholar] [CrossRef]
- Mason, S.A.; Garneau, D.; Sutton, R.; Chu, Y.; Ehmann, K.; Barnes, J.; Fink, P.; Papazissimos, D.; Rogers, D.L. Microplastic pollution is widely detected in US municipal wastewater treatment plant effluent. Environ. Pollut. 2016, 218, 1045–1054. [Google Scholar] [CrossRef]
- Mintenig, S.; Int-Veen, I.; Löder, M.G.; Primpke, S.; Gerdts, G. Identification of microplastic in effluents of waste water treatment plants using focal plane array-based micro-Fourier-transform infrared imaging. Water Res. 2017, 108, 365–372. [Google Scholar] [CrossRef]
- Murphy, F.; Ewins, C.; Carbonnier, F.; Quinn, B. Wastewater treatment works (WwTW) as a source of microplastics in the aquatic environment. Environ. Sci. Technol. 2016, 50, 5800–5808. [Google Scholar] [CrossRef] [PubMed]
- Conley, K.; Clum, A.; Deepe, J.; Lane, H.; Beckingham, B. Wastewater treatment plants as a source of microplastics to an urban estuary: Removal efficiencies and loading per capita over one year. Water Res. X 2019, 3, 100030. [Google Scholar] [CrossRef] [PubMed]
- Abbasi, S.; Keshavarzi, B.; Moore, F.; Turner, A.; Kelly, F.J.; Dominguez, A.O.; Jaafarzadeh, N. Distribution and potential health impacts of microplastics and microrubbers in air and street dusts from Asaluyeh County, Iran. Environ. Pollut. 2019, 244, 153–164. [Google Scholar] [CrossRef] [PubMed]
- Boucher, J.; Friot, D. Primary Microplastics in the Oceans: A Global Evaluation of Sources; IUCN: Gland, Switzerland, 2017. [Google Scholar]
- Senavirathna, M.; Zhaozhi, L.; Fujino, T. Short-duration exposure of 3-µm polystyrene microplastics affected morphology and physiology of watermilfoil (sp. roraima). Environ. Sci. Pollut. Res. Int. 2022, 29, 34475–34485. [Google Scholar] [CrossRef] [PubMed]
- Li, J.N.; Lusher, A.L.; Rotchell, J.M.; Deudero, S.; Turra, A.; Brate, I.L.N.; Sun, C.J.; Hossain, M.S.; Li, Q.P.; Kolandhasamy, P.; et al. Using mussel as a global bioindicator of coastal microplastic pollution. Environ. Pollut. 2019, 244, 522. [Google Scholar] [CrossRef]
- Lusher, A.L.; Welden, N.A.; Sobral, P.; Cole, M. Sampling, isolating and identifying microplastics ingested by fish and invertebrates. Anal. Methods 2017, 9, 1346. [Google Scholar] [CrossRef]
- Alimba, C.G.; Faggio, C. Microplastics in the marine environment: Current trends in environmental pollution and mechanisms of toxicological profile. Environ. Toxicol. Pharmacol. 2019, 68, 61–74. [Google Scholar] [CrossRef]
- Rodrigues, J.P.; Duarte, A.C.; Santos-Echeandía, J.; Rocha-Santos, T. Significance of interactions between microplastics and POPs in the marine environment: A critical overview. TrAC Trends Anal. Chem. 2019, 111, 252–260. [Google Scholar] [CrossRef]
- Al-Salem, S.M.; Uddin, S.; Al-Yamani, F. An assessment of microplastics threat to the marine environment: A short review in context of the Arabian/Persian Gulf. Mar. Environ. Res. 2020, 159, 104961. [Google Scholar] [CrossRef]
- Al-Salem, S.M.; Uddin, S.; Lyons, B. Evidence of microplastics (MP) in gut content of major consumed marine fish species in the State of Kuwait (of the Arabian/Persian Gulf). Mar. Pollut. Bull. 2020, 154, 111052. [Google Scholar] [CrossRef]
- Uddin, S.; Fowler, S.W.; Saeed, T. Microplastic particles in the Persian/Arabian Gulf—A review on sampling and identification. Mar. Pollut. Bull. 2020, 154, 111100. [Google Scholar] [CrossRef]
- Uddin, S.; Fowler, S.W.; Saeed, T.; Naji, A.; Al-Jandal, N. Standardized protocols for microplastics determinations in environmental samples from the Gulf and marginal seas. Mar. Pollut. Bull. 2020, 158, 111374. [Google Scholar] [CrossRef] [PubMed]
- Zakeri, M.; Naji, A.; Akbarzadeh, A.; Uddin, S. Microplastic ingestion in important commercial fish in the southern Caspian Sea. Mar. Pollut. Bull. 2020, 160, 111598. [Google Scholar] [CrossRef] [PubMed]
- Moller, L.; Torndal, U.B.; Eriksson, L.C.; Gustafsson, J.A. The air pollutant 2-nitrofluorene as initiator and promoter in a liver model for chemical carcinogenesis. Carcinogenesis 1989, 10, 435–440. [Google Scholar] [CrossRef]
- Uddin, A.S.; Uddin, S.; Fowler, S.W. Environmental Microplastics: A Significant Pollutant of the Anthropocene. In Microplastic Sources, Fate and Solution; Khan, A., Wang, C., Asiri, A.M., Eds.; Springer Nature Singapore: Singapore, 2023; pp. 89–105. [Google Scholar] [CrossRef]
- Smith, M.; Love, D.C.; Rochman, C.M.; Neff, R.A. Microplastics in Seafood and the Implications for Human Health. Curr. Environ. Health Rep. 2018, 5, 375–386. [Google Scholar] [CrossRef]
- Barboza, L.G.A.; Lopes, C.; Oliveira, P.; Bessa, F.; Otero, V.; Henriques, B.; Raimundo, J.; Caetano, M.; Vale, C.; Guilhermino, L. Microplastic in wild fish from North East Atlantic Ocean and its potential for causing neurotoxic effects, lipid oxidative damage, and human health risks associated with ingestion exposure. Sci. Total Environ. 2020, 717, 134625. [Google Scholar] [CrossRef] [PubMed]
- Besseling, E.; Redondo-Hasselerharm, P.; Foekema, E.M.; Koelmans, A.A. Quantifying ecological risks of aquatic micro- and nanoplastic. Crit. Rev. Environ. Sci. Technol. 2019, 49, 32–80. [Google Scholar] [CrossRef]
- Browne, M.A.; Niven, S.J.; Galloway, T.S.; Rowland, S.J.; Thompson, R.C. Microplastic moves pollutants and additives to worms, reducing functions linked to health and biodiversity. Curr. Biol. 2013, 23, 2388–2392. [Google Scholar] [CrossRef]
- Browne, M.A. Sources and Pathways of Microplastics to Habitats. In Marine Anthropogenic Litter; Bergmann, M., Gutow, L., Klages, M., Eds.; Springer International Publishing: Cham, Switzerland, 2015; pp. 229–244. [Google Scholar] [CrossRef]
- Mohsen, M.; Wang, Q.; Zhang, L.; Sun, L.; Lin, C.; Yang, H. Microplastic ingestion by the farmed sea cucumber Apostichopus japonicus in China. Environ. Pollut. 2019, 245, 1071–1078. [Google Scholar] [CrossRef]
- Vroom, R.J.E.; Koelmans, A.A.; Besseling, E.; Halsband, C. Aging of microplastics promotes their ingestion by marine zooplankton. Environ. Pollut. 2017, 231, 987–996. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, L.; Kannan, K. Microplastics in house dust from 12 countries and associated human exposure. Environ. Int. 2020, 134, 105314. [Google Scholar] [CrossRef] [PubMed]
- Sussarellu, R.; Suquet, M.; Thomas, Y.; Lambert, C.; Fabioux, C.; Pernet, M.E.J.; Le Goïc, N.; Quillien, V.; Mingant, C.; Epelboin, Y.; et al. Oyster reproduction is affected by exposure to polystyrene microplastics. Proc. Natl. Acad. Sci. USA 2016, 113, 2430–2435. [Google Scholar] [CrossRef] [PubMed]
- Tang, Q.; Yang, J.; Sun, D. Functional diversity of copepod assemblages along a basin-scale latitudinal gradient in the North Pacific Ocean. Ecol. Indic. 2022, 141, 109112. [Google Scholar] [CrossRef]
- Carlotti, F.; Pagano, M.; Guilloux, L.; Donoso, K.; Valdés, V.; Grosso, O.; Hunt, B.P.V. Meso-zooplankton structure and functioning in the western tropical South Pacific along the 20th parallel south during the OUTPACE survey (February–April 2015). Biogeosciences 2018, 15, 7273–7297. [Google Scholar] [CrossRef]
- Behbehani, M.; Uddin, S.; Habibi, N.; Al-Sarawi, H.A.; Al-Enezi, Y. The Reproductive Capacities of the Calanoid Copepods Parvocalanus crassirostis and Acartia pacifica under Different pH and Temperature Conditions. Animals 2023, 13, 2160. [Google Scholar] [CrossRef]
- Zeldis, J.R.; Décima, M. Mesozooplankton connect the microbial food web to higher trophic levels and vertical export in the New Zealand Subtropical Convergence Zone. Deep Sea Res. Part I Oceanogr. Res. Pap. 2020, 155, 103146. [Google Scholar] [CrossRef]
- Uddin, S.; Behbehani, M.; Al-Ghadban, A.; Sajid, S.; Al-Zekri, W.; Ali, M.; Al-Jutaili, S.; Al-Musallam, L.; Vinod, V.; Al-Murad, M.; et al. 210Po concentration in selected calanoid copepods in the northern Arabian Gulf. Mar. Pollut. Bull. 2018, 133, 861–864. [Google Scholar] [CrossRef]
- Cherry, R.D.; Fowler, S.W.; Beasley, T.M.; Heyraud, M. Polonium-210: Its vertical oceanic transport by zooplankton metabolic activity. Mar. Chem. 1975, 3, 105–110. [Google Scholar] [CrossRef]
- Cole, M.; Lindeque, P.K.; Fileman, E.; Clark, J.; Lewis, C.; Halsband, C.; Galloway, T.S. Microplastics Alter the Properties and Sinking Rates of Zooplankton Faecal Pellets. Environ. Sci Technol. 2016, 50, 3239–3246. [Google Scholar] [CrossRef]
- Sun, J.; Dai, X.; Wang, Q.; van Loosdrecht, M.C.M.; Ni, B.-J. Microplastics in wastewater treatment plants: Detection, occurrence and removal. Water Res. 2019, 152, 21–37. [Google Scholar] [CrossRef]
- Sundt, P.; Schulze, P.-E.; Syversen, F. Sources of Microplastics-Pollution to the Marine Environment; Report No. M-321|2015; Norwegian Environment Agency: Oslo, Norway, 2014; p. 86. [Google Scholar]
- Gies, E.A.; LeNoble, J.L.; Noël, M.; Etemadifar, A.; Bishay, F.; Hall, E.R.; Ross, P.S. Retention of microplastics in a major secondary wastewater treatment plant in Vancouver, Canada. Mar. Pollut. Bull. 2018, 133, 553–561. [Google Scholar] [CrossRef]
- Al-Yamani, F.Y.; Prusova, I. Common Copepods of the Northwestern Arabian Gulf: Identification Guide; Kuwait Institute for Scientific Research: Kuwait City, Kuwait, 2003; ISBN 99906-41-18-8. [Google Scholar]
- Behbehani, M.; Uddin, S.; Dupont, S.; Fowler, S.W.; Gorgun, A.U.; Al-Enezi, Y.; Al-Musallam, L.; Kumar, V.V.; Faizuddin, M. Ocean Acidification-Mediated Food Chain Transfer of Polonium between Primary Producers and Consumers. Toxics 2023, 11, 14. [Google Scholar] [CrossRef] [PubMed]
- Naji, A.; Esmaili, Z.; Khan, F.R. Plastic debris and microplastics along the beaches of the Strait of Hormuz, Persian Gulf. Mar. Pollut. Bull. 2017, 114, 1057–1062. [Google Scholar] [CrossRef] [PubMed]
- Naji, A.; Esmaili, Z.; Mason, S.; Vethaak, D. The occurrence of microplastics contamination in littoral sediments of the Persian Gulf, Iran. Environ. Sci. Pollut. Res. 2017, 24, 20459–20468. [Google Scholar] [CrossRef] [PubMed]
- Naji, A.; Nuri, M.; Amiri, P.; Niyogi, S. Small microplastic particles (S-MPPs) in sediments of mangrove ecosystem on the northern coast of the Persian Gulf. Mar. Pollut. Bull. 2019, 146, 305–311. [Google Scholar] [CrossRef]
- Gaston, E.; Woo, M.; Steele, C.; Sukumaran, S.; Anderson, S. Microplastics Differ Between Indoor and Outdoor Air Masses: Insights from Multiple Microscopy Methodologies. Appl. Spectrosc. 2020, 74, 1079–1098. [Google Scholar] [CrossRef]
- Maes, T.; Jessop, R.; Wellner, N.; Haupt, K.; Mayes, A.G. A rapid-screening approach to detect and quantify microplastics based on fluorescent tagging with Nile Red. Sci. Rep. 2017, 7, 44501. [Google Scholar] [CrossRef]
- Van Cauwenberghe, L.; Devriese, L.; Galgani, F.; Robbens, J.; Janssen, C.R. Microplastics in sediments: A review of techniques, occurrence and effects. Mar. Environ. Res. 2015, 111, 5–17. [Google Scholar] [CrossRef]
- Rosner, B.; Glynn, R.J.; Lee, M.L. The Wilcoxon signed rank test for paired comparisons of clustered data. Biometrics 2006, 62, 185–192. [Google Scholar] [CrossRef]
- Lee, S.; Lee, D.K. What is the proper way to apply the multiple comparison test? Korean J. Anesthesiol. 2018, 71, 353–360. [Google Scholar] [CrossRef]
- Browne, M.A.; Galloway, T.; Thompson, R. Microplastic—an emerging contaminant of potential concern? Integr. Environ. Assess. Manag. 2007, 3, 559. [Google Scholar] [CrossRef] [PubMed]
- Browne, M.A. Environmental Consequences of Microplastic in Marine Habitats; Springer: Berlin/Heidelberg, Germany, 2008. [Google Scholar]
- Goldstein, M.C.; Rosenberg, M.; Cheng, L. Increased oceanic microplastic debris enhances oviposition in an endemic pelagic insect. Biol. Lett. 2012, 8, 817–820. [Google Scholar] [CrossRef] [PubMed]
- Cole, M.; Lindeque, P.; Fileman, E.; Halsband, C.; Goodhead, R.; Moger, J.; Galloway, T.S. Microplastic Ingestion by Zooplankton. Env. Sci Technol. 2013, 47, 6646–6655. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, M.; Ribeiro, A.; Hylland, K.; Guilhermino, L. Single and combined effects of microplastics and pyrene on juveniles (0+ group) of the common goby Pomatoschistus microps (Teleostei, Gobiidae). Ecol. Indic. 2013, 34, 641. [Google Scholar] [CrossRef]
- Wright, S.L.; Rowe, D.; Thompson, R.C.; Galloway, T.S. Microplastic ingestion decreases energy reserves in marine worms. Curr. Biol. 2013, 23, R1031–R1033. [Google Scholar] [CrossRef]
- Bakir, A.; Rowland, S.J.; Thompson, R.C. Enhanced desorption of persistent organic pollutants from microplastics under simulated physiological conditions. Environ. Pollut. 2014, 185, 16. [Google Scholar] [CrossRef] [PubMed]
- Chua, E.M.; Shimeta, J.; Nugegoda, D.; Morrison, P.D.; Clarke, B.O. Assimilation of Polybrominated Diphenyl Ethers from Microplastics by the Marine Amphipod, Allorchestes Compressa. Environ. Sci. Technol. 2014, 48, 8127–8134. [Google Scholar] [CrossRef]
- Hämer, J.; Gutow, L.; Köhler, A.; Saborowski, R. Fate of microplastics in the marine isopod idotea emarginata. Environ. Sci. Technol. 2014, 48, 13451–13458. [Google Scholar] [CrossRef]
- Van Cauwenberghe, L.; Janssen, C.R. Microplastics in bivalves cultured for human consumption. Environ. Pollut. 2014, 193, 65. [Google Scholar] [CrossRef]
- Au, S.Y.; Bruce, T.F.; Bridges, W.C.; Klaine, S.J. Responses of Hyalella azteca to acute and chronic microplastic exposures. Environ. Toxicol. Chem. 2015, 34, 2564–2572. [Google Scholar] [CrossRef]
- Avio, C.G.; Gorbi, S.; Milan, M.; Benedetti, M.; Fattorini, D.; d’Errico, G.; Pauletto, M.; Bargelloni, L.; Regoli, F. Pollutants bioavailability and toxicological risk from microplastics to marine mussels. Environ. Pollut. 2015, 198, 211. [Google Scholar] [CrossRef] [PubMed]
- Brennecke, D.; Ferreira, E.C.; Costa, T.M.M.; Appel, D.; da Gama, B.A.P.; Lenz, M. Ingested microplastics (>100 μm) are translocated to organs of the tropical fiddler crab Uca Rapax. Mar. Pollut. Bull. 2015, 96, 491–495. [Google Scholar] [CrossRef] [PubMed]
- de Sa, L.C.; Luís, L.G.; Guilhermino, L. Effects of microplastics on juveniles of the common goby (Pomatoschistus microps): Confusion with prey, reduction of the predatory performance and efficiency, and possible influence of developmental conditions. Environ. Pollut. 2015, 196, 359–361. [Google Scholar] [CrossRef] [PubMed]
- Corinaldesi, C.; Canensi, S.; Dell’Anno, A.; Tangherlini, M.; Di Capua, I.; Varrella, S.; Willis, T.J.; Cerrano, C.; Danovaro, R. Multiple impacts of microplastics can threaten marine habitat-forming species. Commun. Biol. 2021, 4, 431. [Google Scholar] [CrossRef] [PubMed]
- Neves, D.; Sobral, P.; Ferreira, J.L.; Pereira, T. Ingestion of microplastics by commercial fish off the Portuguese coast. Mar. Pollut. Bull. 2015, 101, 119–126. [Google Scholar] [CrossRef]
- Van Cauwenberghe, L.; Claessens, M.; Vandegehuchte, M.B.; Janssen, C.R. Microplastics are taken up by mussels (Mytilus edulis) and lugworms (Arenicola marina) living in natural habitats. Environ. Pollut. 2015, 199, 10. [Google Scholar] [CrossRef]
- Terepocki, A.; Brush, A.; Kleine, L.; Shugart, G.; Hodum, P. Size and dynamics of microplastic in gastrointestinal tracts of Northern Fulmars (Fulmarus glacialis) and Sooty Shearwaters (Ardenna grisea). Mar. Pollut. Bull. 2017, 116, 143–150. [Google Scholar] [CrossRef]
- Abbasi, S.; Soltani, N.; Keshavarzi, B.; Moore, F.; Turner, A.; Hassanaghaei, M. Microplastics in different tissues of fish and prawn from the Musa Estuary, Persian Gulf. Chemosphere 2018, 205, 80–87. [Google Scholar] [CrossRef]
- Anbumani, S.; Kakkar, P. Ecotoxicological effects of microplastics on biota: A review. Environ. Sci. Pollut. Res. 2018, 25, 14373–14396. [Google Scholar] [CrossRef]
- Ma, Y.; Wang, L.; Wang, T.; Chen, Q.; Ji, R. Microplastics as Vectors of Chemicals and Microorganisms in the Environment; Springer: Berlin/Heidelberg, Germany, 2020; pp. 209–230. [Google Scholar] [CrossRef]
- Kvale, K.; Prowe, A.E.F.; Chien, C.T.; Landolfi, A.; Oschlies, A. Zooplankton grazing of microplastic can accelerate global loss of ocean oxygen. Nat. Commun. 2021, 12, 2358. [Google Scholar] [CrossRef]
- Qiu, X.; Qi, Z.; Ouyang, Z.; Liu, P.; Guo, X. Interactions between microplastics and microorganisms in the environment: Modes of action and influencing factors. Gondwana Res. 2022, 108, 102–119. [Google Scholar] [CrossRef]
- Setälä, O.; Lehtiniemi, M.; Coppock, R.; Cole, M. Chapter 11—Microplastics in Marine Food Webs. In Microplastic Contamination in Aquatic Environments; Zeng, E.Y., Ed.; Elsevier: Amsterdam, The Netherlands, 2018; pp. 339–363. [Google Scholar] [CrossRef]
- Botterell, Z.L.R.; Beaumont, N.; Cole, M.; Hopkins, F.E.; Steinke, M.; Thompson, R.C.; Lindeque, P.K. Bioavailability of Microplastics to Marine Zooplankton: Effect of Shape and Infochemicals. Environ. Sci. Technol. 2020, 54, 12024–12033. [Google Scholar] [CrossRef]
- Lee, K.-W.; Shim, W.J.; Kwon, O.Y.; Kang, J.-H. Size-Dependent Effects of Micro Polystyrene Particles in the Marine Copepod Tigriopus japonicus. Environ. Sci. Technol. 2013, 47, 11278–11283. [Google Scholar] [CrossRef] [PubMed]
- Bai, Z.; Wang, N.; Wang, M. Effects of microplastics on marine copepods. Ecotoxicol. Environ. Saf. 2021, 217, 112243. [Google Scholar] [CrossRef] [PubMed]
- Cole, M.; Lindeque, P.; Fileman, E.; Halsband, C.; Galloway, T.S. The impact of polystyrene microplastics on feeding, function and fecundity in the marine copepod Calanus helgolandicus. Environ. Sci. Technol. 2015, 49, 1130–1137. [Google Scholar] [CrossRef] [PubMed]
- Coppock, R.L.; Galloway, T.S.; Cole, M.; Fileman, E.S.; Queirós, A.M.; Lindeque, P.K. Microplastics alter feeding selectivity and faecal density in the copepod, Calanus helgolandicus. Sci. Total Environ. 2019, 687, 780–789. [Google Scholar] [CrossRef]
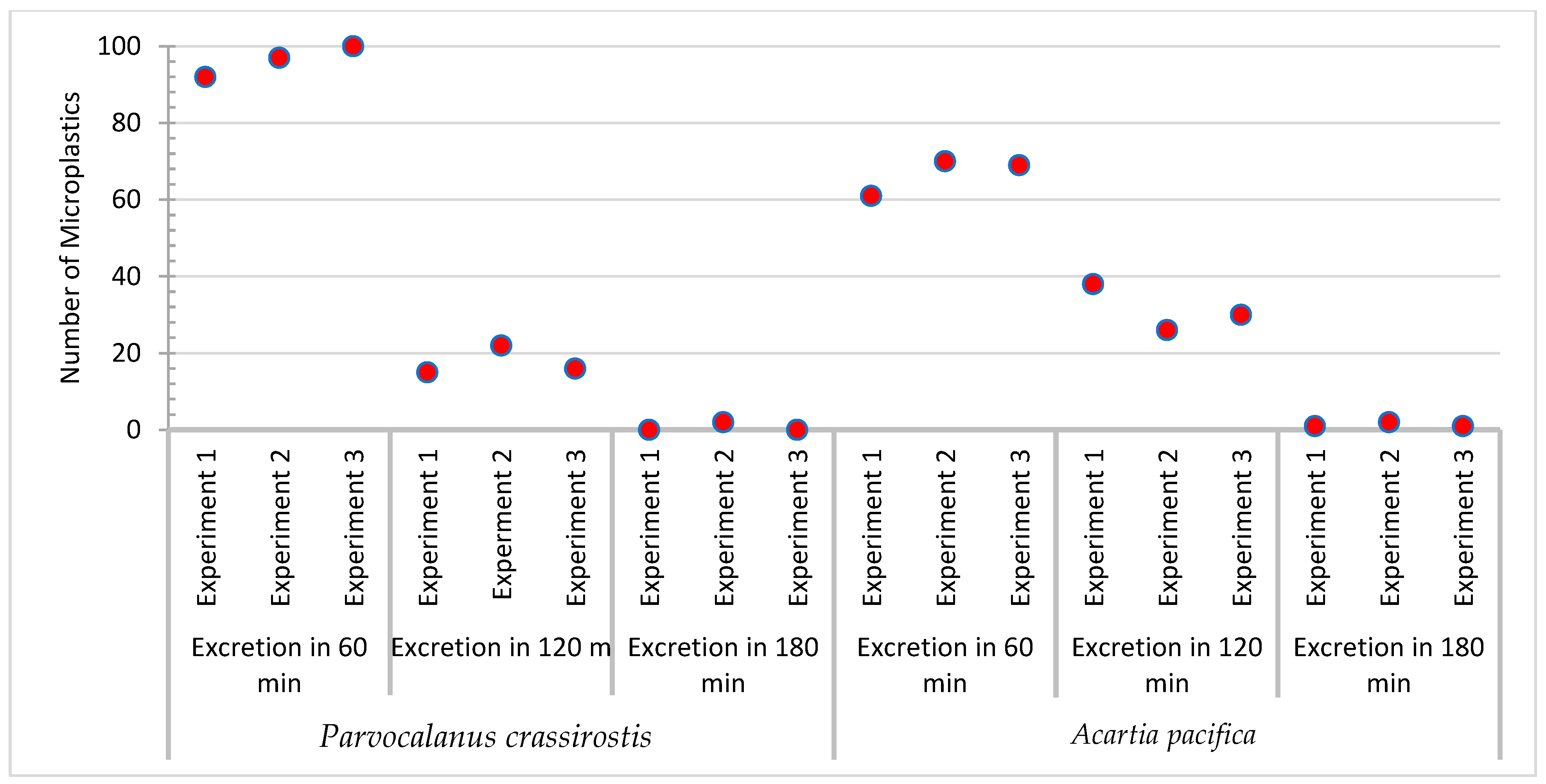
| Sample | No. of MPs Added | No. of MPs Ingested | No. of MPs Excreted | ||||
|---|---|---|---|---|---|---|---|
| 60 min | 120 min | 180 min | 1440 min | ||||
| Experiment 1 | Parvocalanus crassirostis | 100 | 92 | 77 | 15 | - | - |
| Acartia pacifica | 100 | 100 | 61 | 38 | 1 | - | |
| Experiment 2 | Parvocalanus crassirostis | 100 | 97 | 73 | 22 | 2 | - |
| Acartia pacifica | 100 | 98 | 70 | 26 | 2 | - | |
| Experiment 3 | Parvocalanus crassirostis | 100 | 100 | 84 | 16 | - | - |
| Acartia pacifica | 100 | 100 | 69 | 30 | 1 | - | |
| Groups: | 60 min | 120 min | 180 min |
|---|---|---|---|
| Skewness: | 0.7822 | 1.5971 | 1.7321 |
| Skewness Shape: |  Potentially Symmetrical (pval = 0.177) Potentially Symmetrical (pval = 0.177) |  Potentially Symmetrical (pval = 0.445) Potentially Symmetrical (pval = 0.445) |  Potentially Symmetrical (pval = 0.157) Potentially Symmetrical (pval = 0.157) |
| Excess kurtosis: | NaN | NaN | NaN |
| Tails Shape: |  Potentially Mesokurtic, normal like tails (pval = NaN) Potentially Mesokurtic, normal like tails (pval = NaN) |  Potentially Mesokurtic, normal like tails (pval = NaN) Potentially Mesokurtic, normal like tails (pval = NaN) |  Potentially Mesokurtic, normal like tails (pval = NaN) Potentially Mesokurtic, normal like tails (pval = NaN) |
| Normality | 0.9986 | 0.4335 | 0.1305 |
| Outliers: | |||
| Median: | 77 | 16 | 0 |
| Sample size (n): | 3 | 3 | 3 |
| Rank sum (R): | 24 | 15 | 6 |
| R2/n: | 192 | 75 | 12 |
| Pair | Mean Rank Difference | Z | SE | Critical Value | p-Value | p-Value/2 |
|---|---|---|---|---|---|---|
| x1–x2 | 3 | 1.3473 | 2.2267 | 5.3306 | 0.1779 | 0.08895 |
| x1–x3 | 6 | 2.6945 | 2.2267 | 5.3306 | 0.007049 | 0.003524 |
| x2–x3 | 3 | 1.3473 | 2.2267 | 5.3306 | 0.1779 | 0.08895 |
| Groups: | 60 min | 120 min | 180 min |
|---|---|---|---|
| Skewness: | −1.6523 | 0.9352 | 1.7321 |
| Skewness Shape: |  Potentially Symmetrical (pval = 0.177) Potentially Symmetrical (pval = 0.177) |  Potentially Symmetrical (pval = 0.445) Potentially Symmetrical (pval = 0.445) |  Potentially Symmetrical (pval = 0.157) Potentially Symmetrical (pval = 0.157) |
| Excess kurtosis: | NaN | NaN | NaN |
| Tails Shape: |  Potentially Mesokurtic, normal like tails (pval = NaN) Potentially Mesokurtic, normal like tails (pval = NaN) |  Potentially Mesokurtic, normal like tails (pval = NaN) Potentially Mesokurtic, normal like tails (pval = NaN) |  Potentially Mesokurtic, normal like tails (pval = NaN) Potentially Mesokurtic, normal like tails (pval = NaN) |
| Normality | 0.3369 | 0.9895 | 0.1305 |
| Outliers: | |||
| Median: | 69 | 30 | 1 |
| Sample size (n): | 3 | 3 | 3 |
| Rank sum (R): | 24 | 15 | 6 |
| R2/n: | 192 | 75 | 12 |
| Pair | Mean Rank Difference | Z | SE | Critical Value | p-Value | p-Value/2 |
|---|---|---|---|---|---|---|
| x1–x2 | 3 | 1.3473 | 2.2267 | 5.3306 | 0.1779 | 0.08895 |
| x1–x3 | 6 | 2.6945 | 2.2267 | 5.3306 | 0.007049 | 0.003524 |
| x2–x3 | 3 | 1.3473 | 2.2267 | 5.3306 | 0.1779 | 0.08895 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Uddin, S.; Behbehani, M.; Habibi, N.; Fowler, S.W.; Al-Sarawi, H.A.; Alonso-Hernandez, C. Microplastics Residence Time in Marine Copepods: An Experimental Study. Sustainability 2023, 15, 14970. https://doi.org/10.3390/su152014970
Uddin S, Behbehani M, Habibi N, Fowler SW, Al-Sarawi HA, Alonso-Hernandez C. Microplastics Residence Time in Marine Copepods: An Experimental Study. Sustainability. 2023; 15(20):14970. https://doi.org/10.3390/su152014970
Chicago/Turabian StyleUddin, Saif, Montaha Behbehani, Nazima Habibi, Scott W. Fowler, Hanan A. Al-Sarawi, and Carlos Alonso-Hernandez. 2023. "Microplastics Residence Time in Marine Copepods: An Experimental Study" Sustainability 15, no. 20: 14970. https://doi.org/10.3390/su152014970








