Study on the Interaction Mechanism between Residual Coal and Mine Water in Goaf of Coal Mine Underground Reservoir
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sample Collection
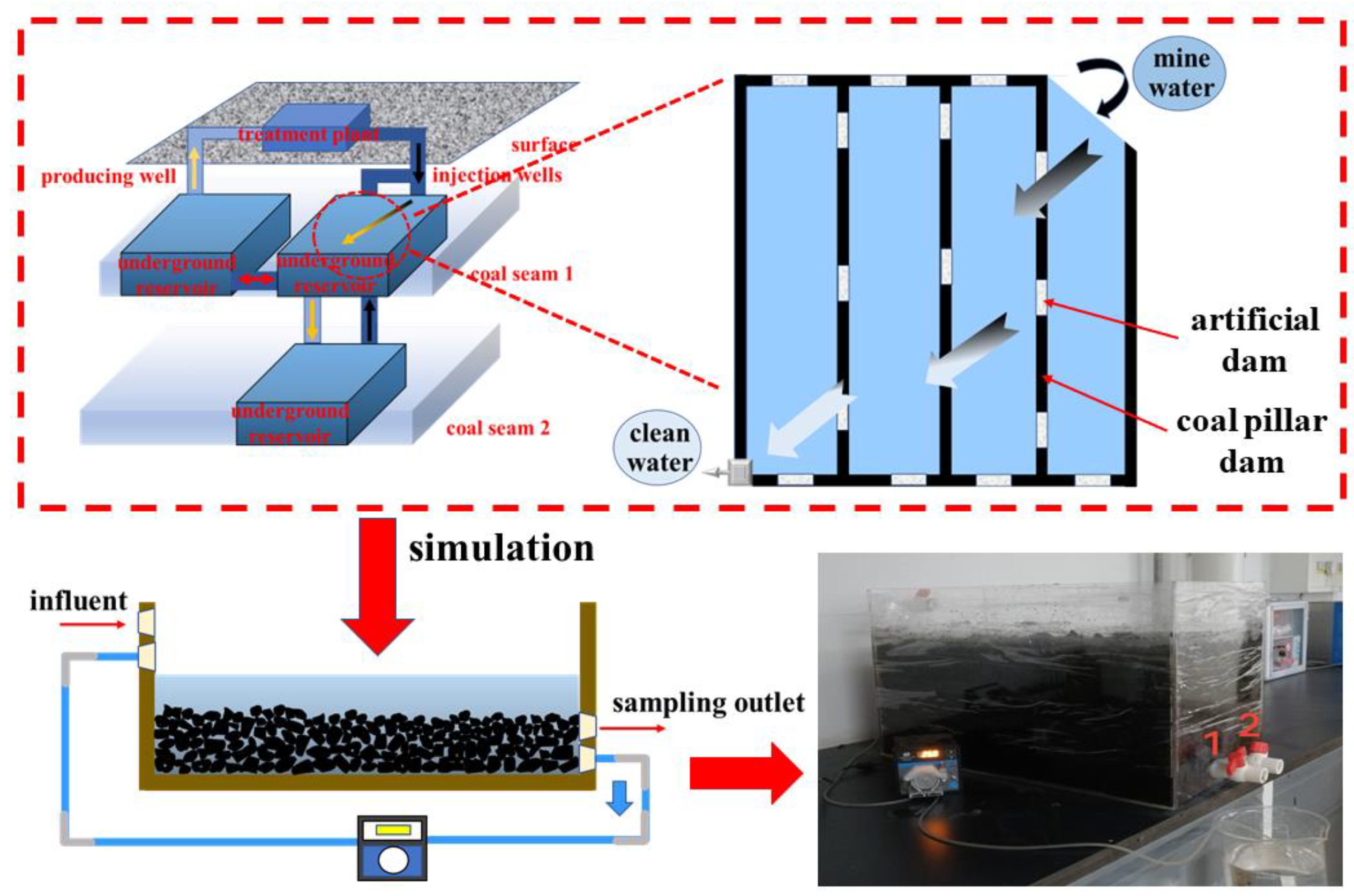
2.2. Dynamic Simulation
2.3. Methods to Character the Mine Water and Coal Samples
3. Results
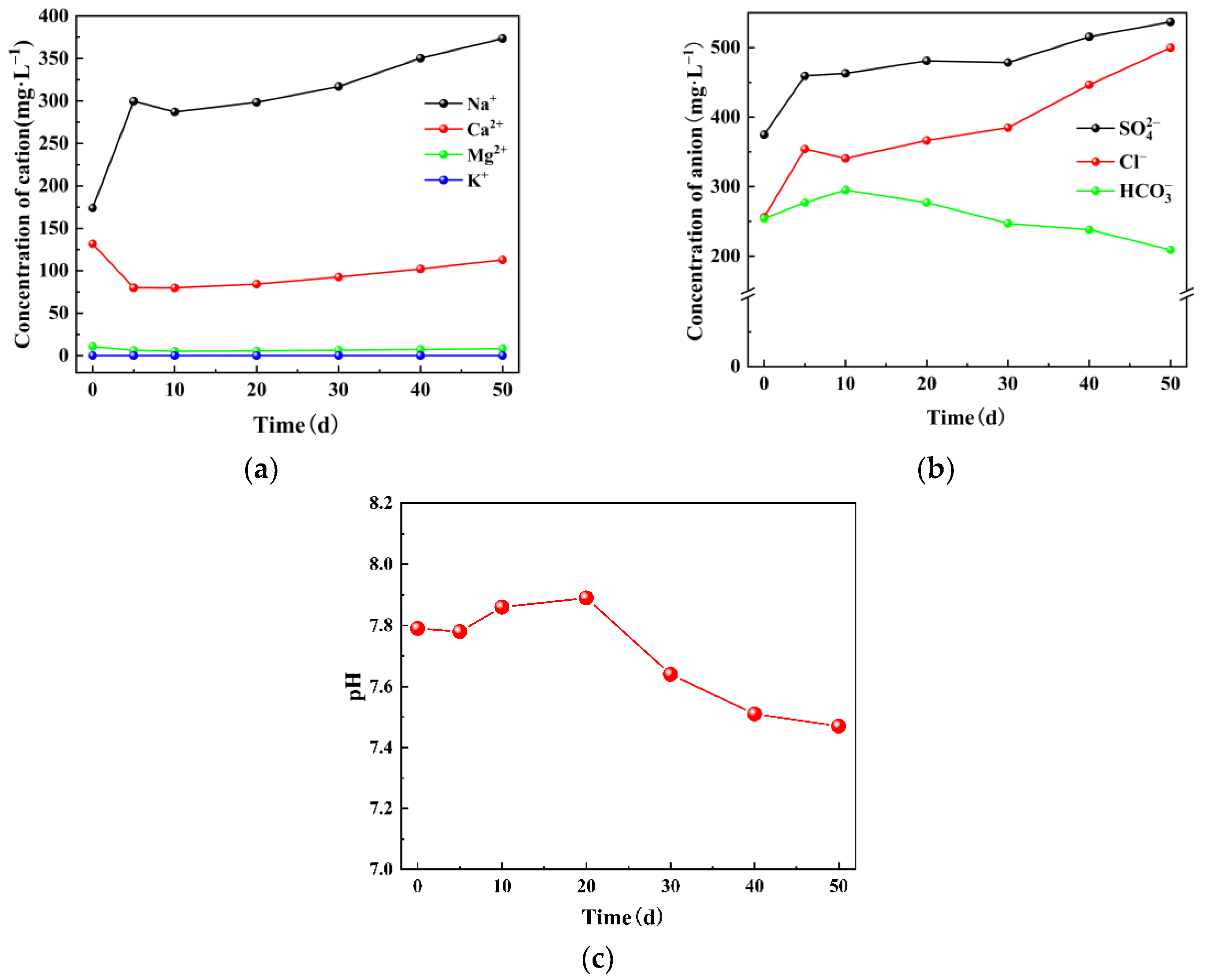
3.1. Changes of Inorganic Components in Coal under Water–Coal Interaction
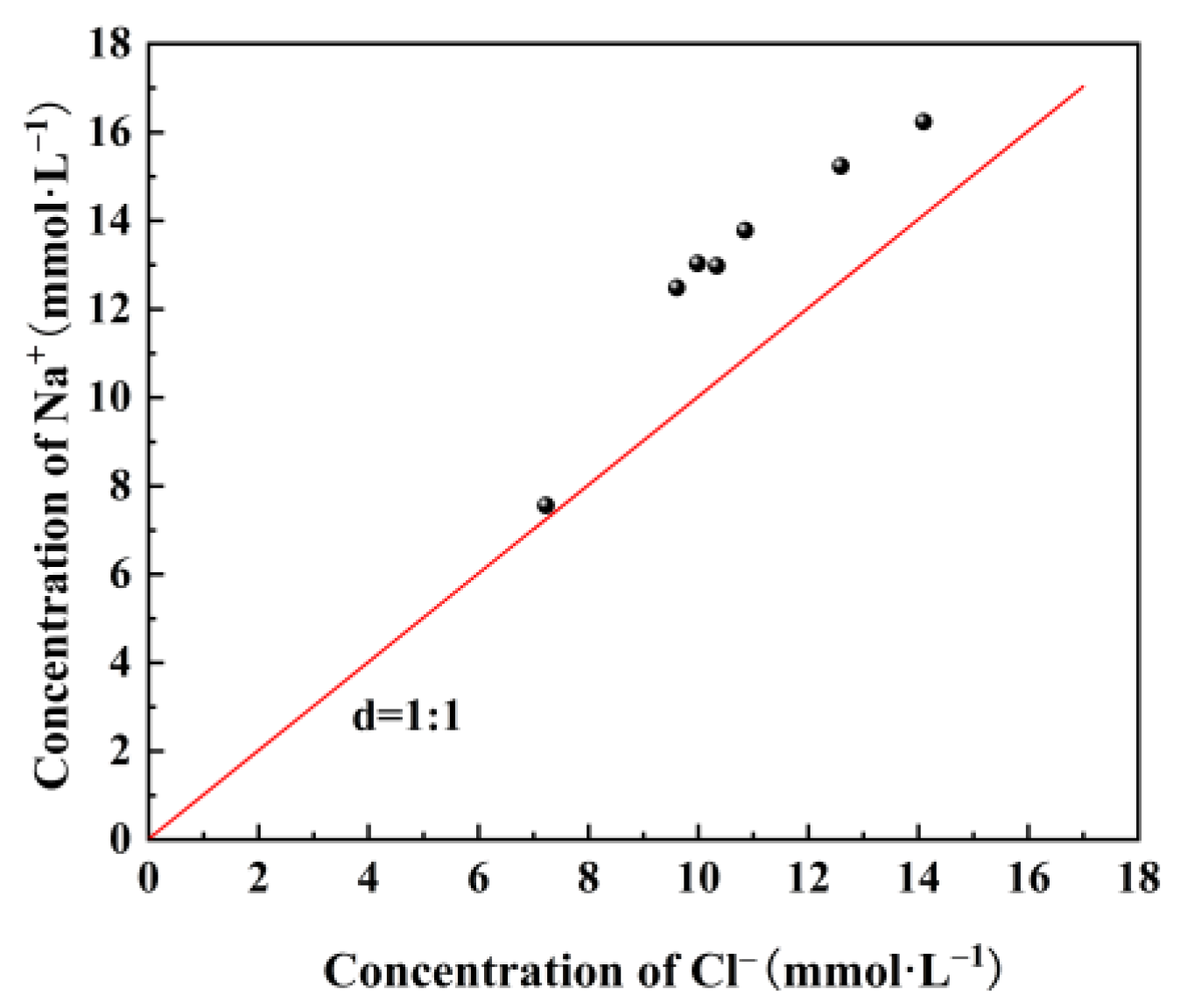
3.2. Ion Changes in Mine Water under Water–Coal Interaction
3.3. Mechanism Analysis of Water–Coal Interaction
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chi, M.B.; Li, Q.S.; Cao, Z.G.; Fang, J.; Wu, B.Y.; Zhang, Y.; Wei, S.R.; Liu, X.Q.; Yang, Y.M. Evaluation of water resources carrying capacity in ecologically fragile mining areas under the influence of underground reservoirs in coal mines. J. Clean. Prod. 2022, 379, 134449. [Google Scholar] [CrossRef]
- Zhang, S.Y.; Wang, H.; He, X.W.; Guo, S.Q.; Xia, Y.; Zhou, Y.X.; Liu, K.; Yang, S.P. Research progress, problems and prospects of mine water treatment technology and resource utilization in China. Crit. Rev. Environ. Sci. Technol. 2020, 50, 331–383. [Google Scholar] [CrossRef]
- Zhang, C.H.; Luo, B.; Xu, Z.M.; Sun, Y.J.; Feng, L. Research on the capacity of underground reservoirs in coal mines to protect the groundwater resources: A case of Zhangshuanglou coal mine in Xuzhou, China. Water 2023, 15, 1468. [Google Scholar] [CrossRef]
- Jiang, B.B.; Gao, J.; Du, K. Insight into the water–rock interaction process and purification mechanism of mine water in underground reservoir of Daliuta coal mine in China. Environ. Sci. Pollut. Res. 2022, 29, 28538–28551. [Google Scholar] [CrossRef]
- Zhang, C.; Wang, F.T.; Bai, Q.S. Underground space utilization of coalmines in China; a review of underground water reservoir construction. Tunn. Undergr. Space Technol. 2021, 107, 103657. [Google Scholar] [CrossRef]
- Han, J.M.; Gao, J.; Du, K.; Chen, M.Y.; Jiang, B.B.; Zhang, K. Analysis of hydrochemical characteristics and formation mechanism in coal mine underground reservoir. Coal Sci. Technol. 2020, 48, 223–231. (In Chinese) [Google Scholar]
- Zhang, K.; Gao, J.; Jiang, B.B.; Han, J.M.; Chen, M.Y. Experimental study on the mechanism of water-rock interaction in the coal mine underground reservoir. Chin. J. Coal 2019, 44, 3760–3772. (In Chinese) [Google Scholar]
- Fang, M.Y.; Li, X.Y.; Zhang, G.; Liu, Q.; Tuo, K.Y.; Liu, S.Q. Research on water–rock interaction mechanism in coal mine underground reservoir–taking Daliuta Coal Mine as an example. Coal Sci. Technol. 2022, 50, 236–242. (In Chinese) [Google Scholar]
- Zhang, K.; Gao, J.; Men, D.P.; Zhao, X.Y.; Wu, S.S. Insight into the heavy metal binding properties of dissolved organic matter in mine water affected by water-rock interaction of coal seam goaf. Chemosphere 2021, 265, 129134. [Google Scholar] [CrossRef]
- Zhang, Q.; Luo, S.H.; Zhao, L.; Zhang, P.Q.; Wang, S.D.; Sun, C.; Zhang, L. Effect of Darcy flux on the release of dissolved organic matter and nitrogen from coal gangue in a coal mine underground reservoir: Column experiments. Chem. Geol. 2020, 545, 119652. [Google Scholar] [CrossRef]
- Zhao, L.; Sun, C.; Yan, P.X.; Zhang, Q.; Wang, S.D.; Luo, S.H.; Mao, Y.X. Dynamic changes of nitrogen and dissolved organic matter during the transport of mine water in a coal mine underground reservoir: Column experiments. J. Contam. Hydrol. 2019, 223, 103473. [Google Scholar] [CrossRef]
- Zhao, B.H.; Fan, S.; Bian, W.; Jiang, B.B.; Su, C.; Li, J.; Zhu, Y.H.; Li, Y.Q. Study of Dissolved organic matter adsorption removal by coal gangue in high salinity mine water. J. Beijing Univ. Technol. 2022, 48, 989–997. (In Chinese) [Google Scholar]
- Fan, L.M.; Ma, X.D. A review on investigation of water-preserved coal mining in western China. Int. J. Coal Sci. Technol. 2018, 5, 411–416. [Google Scholar] [CrossRef]
- Liu, S.Y.; Zhang, K.; Gao, J.; Yang, Y.M.; Bai, L.; Yan, J.Y. Research on temporal patterns of water–Rock interaction in the coal mine underground reservoir based on the dynamic simulation test. ACS Omega 2023, 8, 13819–13832. [Google Scholar] [CrossRef]
- Chen, S.S. Research on the Key Technology of Water Resources Recycling Utilization in the Underground Goaf Reservoir in Shendong Mining Area; Xi’an University of Science and Technology: Xi’an, China, 2016. (In Chinese) [Google Scholar]
- Qin, W. Study on hydraulic connection and seepage law of goaf groups in coal mine underground reservoir. Geofluids 2022, 2022, 4316878. [Google Scholar] [CrossRef]
- Shi, X.C. Research progress and prospect of underground reservoirs in coal mines. Coal Sci. Technol. 2022, 50, 216–225. (In Chinese) [Google Scholar]
- Finkelman, R.B.; Dai, S.F.; French, D. The importance of minerals in coal as the hosts of chemical elements A review. Int. J. Coal Geol. 2019, 212, 103251. [Google Scholar] [CrossRef]
- Couch, G. Understanding slagging and fouling in PF combustion. IEA Rep. 1994, 74, 1541–1542. [Google Scholar]
- Yang, J.; Wang, H.; Wang, Q.M.; Zhang, X.Y.; Wang, T.T. Characteristics and sources of typical pollution components in mine water in the border area of Inner Mongolia and Shaanxi. Chin. J. Coal 2023, 48, 1687–1696. (In Chinese) [Google Scholar]
- Chen, S.D.; Tao, S.; Tian, W.G.; Tang, D.Z.; Zhang, B.; Liu, P.C. Hydrogeological control on the accumulation and production of coalbed methane in the Anze Block, southern Qinshui Basin, China. J. Petrol. Sci. Eng. 2021, 198, 108138. [Google Scholar] [CrossRef]
- Magaritz, M.; Nadler, A.; Koyumdjisky, H. The use of Na/Cl ratios to trace solute sources in a semiarid zone. Water Resour. Res. 1981, 17, 602–608. [Google Scholar] [CrossRef]
- Sami, K. Recharge mechanisms and geochemical processes in a semi-arid sedimentary basin, Eastern Cape, South Africa. J. Hydrol. 1992, 139, 27–48. [Google Scholar] [CrossRef]
- Liang, D.C.; Xie, Q.; Zhou, H.B.; Yang, M.S.; Cao, J.Y.; Zhang, J. Catalytic effect of alkali and alkaline earth metals in different occurrence modes in Zhundong coals. Asia–Pac. J. Chem. Eng. 2018, 13, e2190. [Google Scholar] [CrossRef]
- Sun, C.; Wei, X.L.; Kang, R.N.; Bin, F.; Li, S. Intrinsic sodium occurrence in Zhundong coal: Experimental observations and molecular modeling. Fuel 2021, 305, 121491. [Google Scholar] [CrossRef]
- Liang, D.C.; Xie, Q.; Liu, J.C.; Liu, D.Q.; Wan, C.R.; Yang, S. Transformation of alkali and alkaline earth metals during the preparation of activated carbon from Zhundong high-alkali coal. RSC Adv. 2021, 11, 3870–3878. [Google Scholar] [CrossRef]
- Zhao, J.; Wei, X.L.; Li, T.; Li, S. Effect of alkali metals on nitrogen oxide emission: Role of Na and its occurrence in coal. Proc. Combust. Inst. 2021, 38, 5299–5309. [Google Scholar] [CrossRef]
- Susmita, S.G.; Krishna, G.B. Adsorption of metal ions by clays and inorganic solids. RSC Adv. 2014, 4, 28537–28586. [Google Scholar]
- Fang, Z.Y. Research on Mechanism of Purifying Underground Reservoir Water Storage by Collapsed Coal Rock in Goaf of Wanli No.1 Coal Mine; China University of Mining and Technology: Beijing, China, 2020. (In Chinese) [Google Scholar]
- Li, H.L.; Chen, J.; Peng, C.L.; Min, F.F.; Song, S.X. Salt coagulation or flocculation? In situ zeta potential study on ion correlation and slime coating with the presence of clay: A case of coal slurry aggregation. Environ. Res. 2020, 189, 109875. [Google Scholar] [CrossRef]
- Zou, Y.; Zheng, C.; Sheikhi, S. Role of ion exchange in the brine-rock interaction systems; a detailed geochemical modeling study. Chem. Geol. 2021, 559, 119992. [Google Scholar] [CrossRef]
- Shi, W.G.; Wang, Q.R.; Danlami, M.S. A novel analytical model of solute transport in a layered aquifer system with mixing processes in the reservoirs. Environ. Sci. Pollut. Res. 2022, 29, 67953–67968. [Google Scholar] [CrossRef]
- Han, J.M. Study on Water-Rock Interaction and Water Purification Mechanism of Coal Mine Underground Reservoir; China University of Mining and Technology: Beijing, China, 2020. (In Chinese) [Google Scholar]
- Liu, X.M.; Zhu, Y.C.; Bennett, J.M.; Wu, L.S.; Li, H. Effects of sodium adsorption ratio and electrolyte concentration on soil saturated hydraulic conductivity. Geoderma 2022, 414, 115772. [Google Scholar] [CrossRef]
- Zhu, J.X.; Xian, H.Y.; Lin, X.J.; Tang, H.M.; Du, R.X.; Yang, Y.P.; Zhu, R.L.; Liang, X.L.; Wei, J.M.; Teng, H.H.; et al. Surface structure-dependent pyrite oxidation in relatively dry and moist air: Implications for the reaction mechanism and sulfur evolution. Geochim. Et Cosmochim. Acta 2018, 228, 259–274. [Google Scholar] [CrossRef]
- Feng, J.L.; Tian, H.; Huang, Y.L.; Ding, Z.Y.; Yin, Z.L. Pyrite oxidation mechanism in aqueous medium. J. Chin. Chem. Soc. 2019, 66, 345–354. [Google Scholar] [CrossRef]
- Gao, J. Study on the Law of Ion Migration and Transformation during Water-Rock Action of Coal Mine Underground Reservoir; China University of Mining and Technology: Beijing, China, 2021. (In Chinese) [Google Scholar]






| Elements | Occurrence Form |
|---|---|
| Si | quartz (SiO2), kaolinite (Al2Si2O5(OH)4)), calcium montmorillonite (Ca0.145Mg0.26Al1.77Si3.97O10(OH)2), illite ((K1.5Al4(Si6.5Al1.5)O20(OH)2), chlorite (Mg2.5Fe2.5Al2Si3O10(OH)8), albite (NaAlSi3O8), potash feldspar (KAlSi3O8) |
| Al | kaolinite, calcium montmorillonite, illite, chlorite and other clay minerals, albite (NaAlSi3O8), potash feldspar (KAlSi3O8) |
| Ca | calcite (CaCO3), gypsum (CaSO4·2H2O), dolomite (CaMg(CO3)2), a small part of silicate minerals |
| Mg | chlorite |
| S | pyrite (FeS2) |
| Na | rock salt (NaCl), albite (NaAlSi3O8) |
| K | potash feldspar (KAlSi3O8), illite (K1.5Al4(Si6.5Al1.5)O20(OH)2) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jiang, B.; Zhao, Z.; Liu, D.; Cao, Z.; Tang, J.; Wu, M.; Zhang, H.; Li, P.; Liang, D. Study on the Interaction Mechanism between Residual Coal and Mine Water in Goaf of Coal Mine Underground Reservoir. Sustainability 2023, 15, 15106. https://doi.org/10.3390/su152015106
Jiang B, Zhao Z, Liu D, Cao Z, Tang J, Wu M, Zhang H, Li P, Liang D. Study on the Interaction Mechanism between Residual Coal and Mine Water in Goaf of Coal Mine Underground Reservoir. Sustainability. 2023; 15(20):15106. https://doi.org/10.3390/su152015106
Chicago/Turabian StyleJiang, Binbin, Ze Zhao, Deqian Liu, Zhiguo Cao, Jiawei Tang, Min Wu, Haiqin Zhang, Peng Li, and Dingcheng Liang. 2023. "Study on the Interaction Mechanism between Residual Coal and Mine Water in Goaf of Coal Mine Underground Reservoir" Sustainability 15, no. 20: 15106. https://doi.org/10.3390/su152015106





