Biomonitoring of Atmospheric PAHs and PMs Using Xanthoria parietina and Cupressus sempervirens in Bouira (Algeria)
Abstract
:1. Introduction
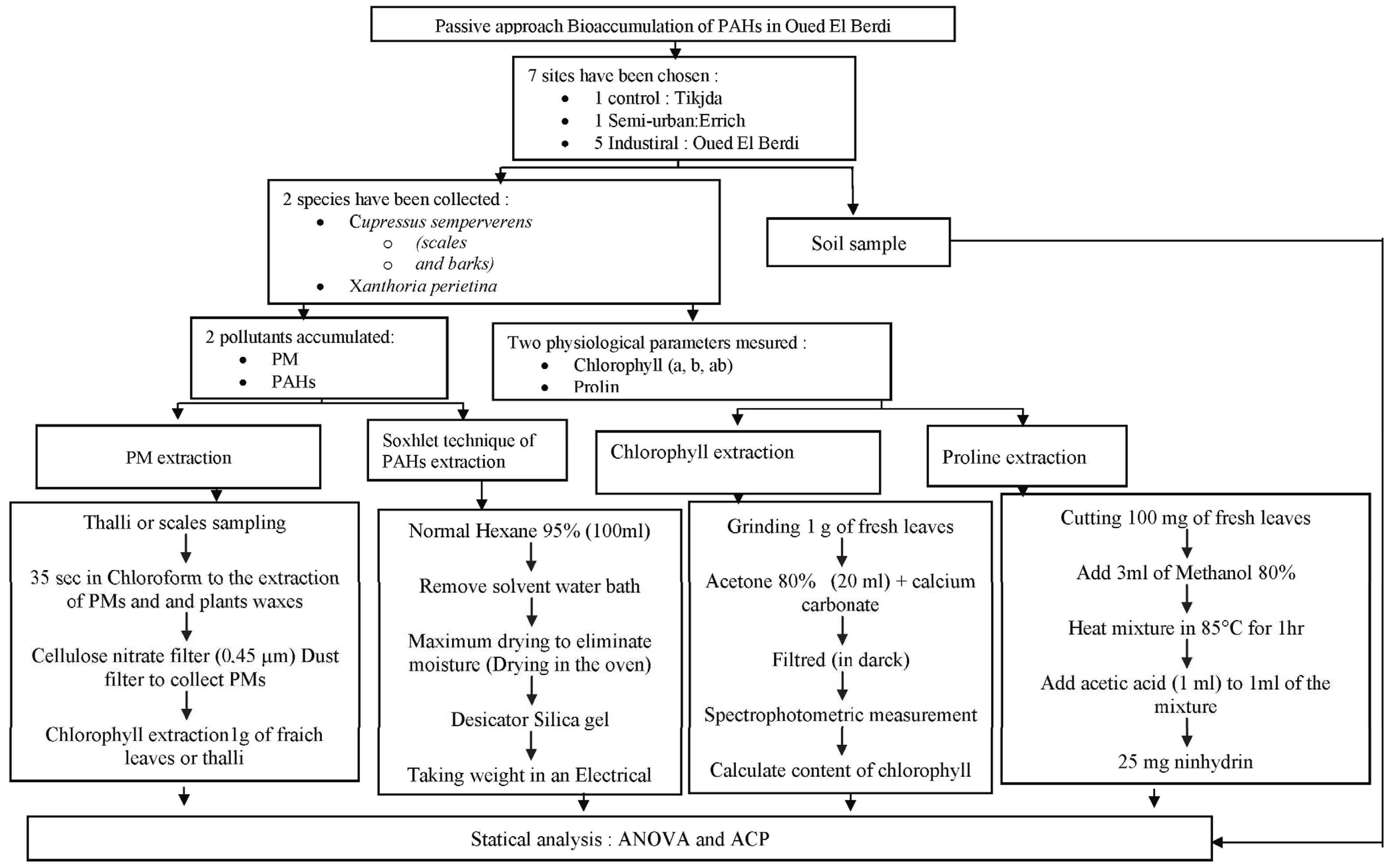
2. Materials and Methods
2.1. Description of the Area
2.2. Sampling Techniques
2.2.1. Xanthoria parietina Samples
2.2.2. Cupressus sempervirens Samples
2.2.3. Soil Samples
2.3. Air Pollutants Extraction
2.4. Chlorophyll and Proline Extraction
2.5. Statistical Analysis
3. Results
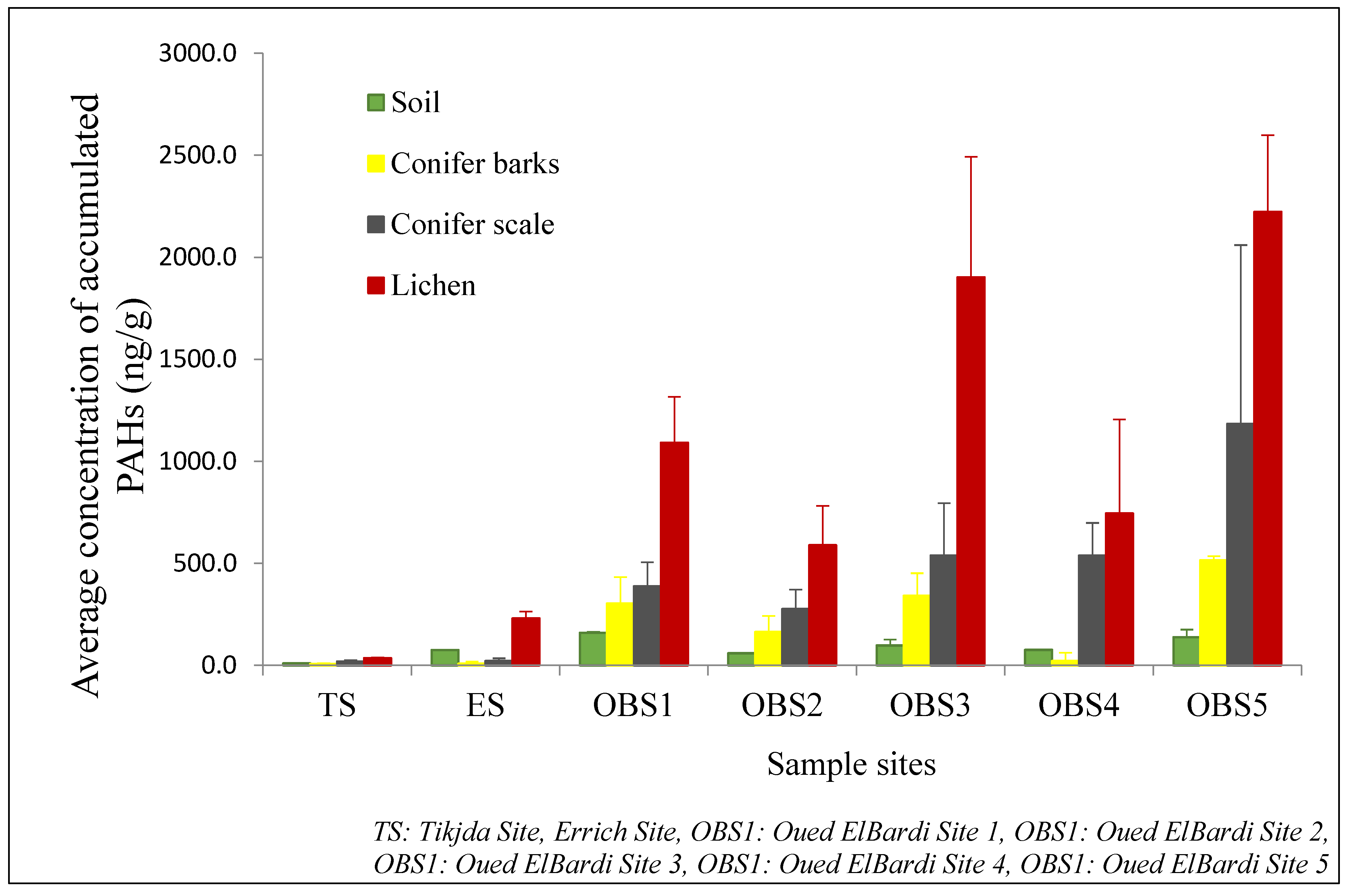
3.1. Estimation of Accumulated PAHs
3.1.1. PAHs in Xanthoria parietina
3.1.2. PAHs in Cupressus sempervirens
3.1.3. PAHs in the Soil
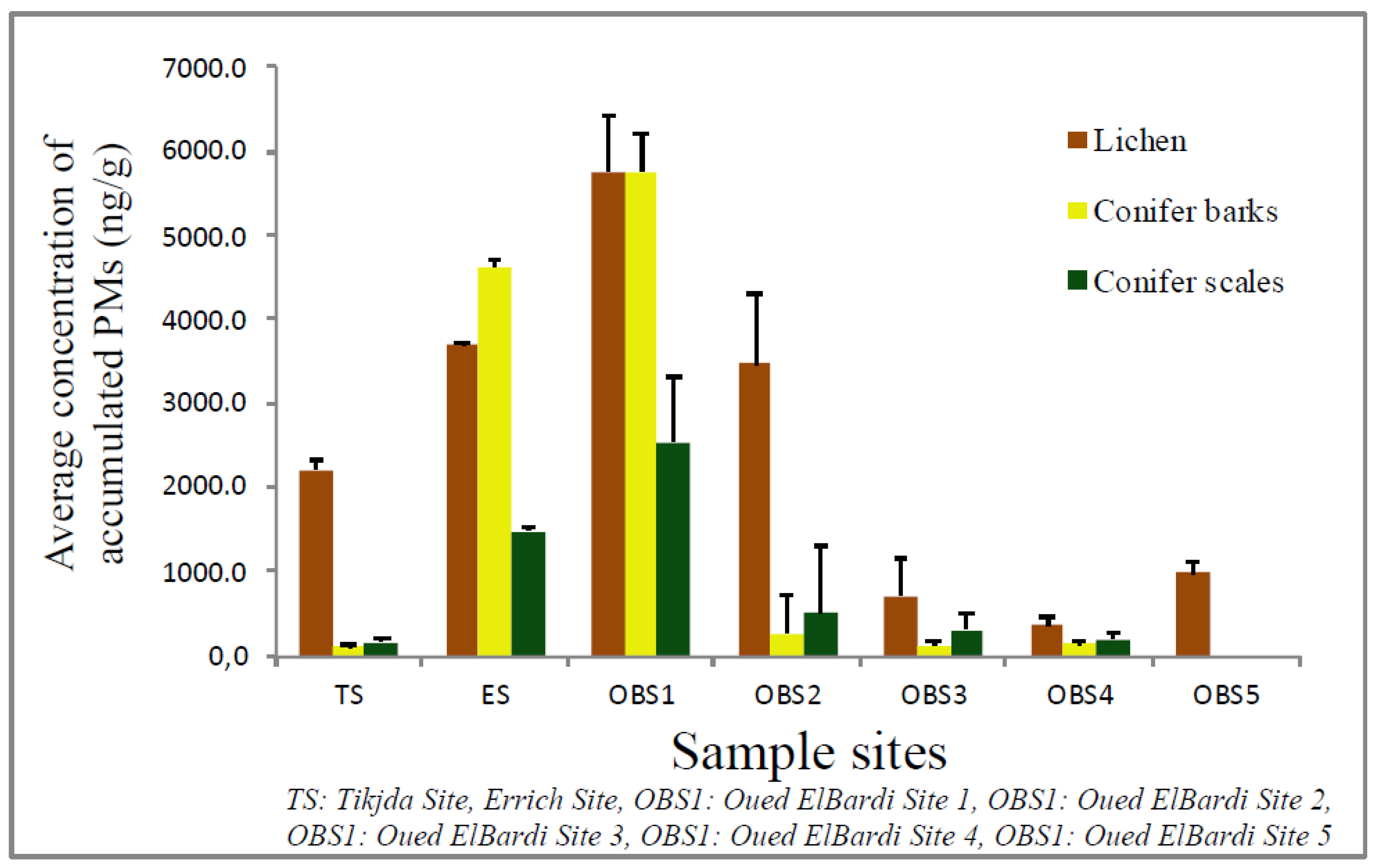
3.2. Estimation of Dust Deposited on Lichen and Conifer
3.2.1. PM Concentration in Xanthoria parietina Thalli
3.2.2. PM Concentration in Cupressus sempervirens
3.3. Physiological Parameters
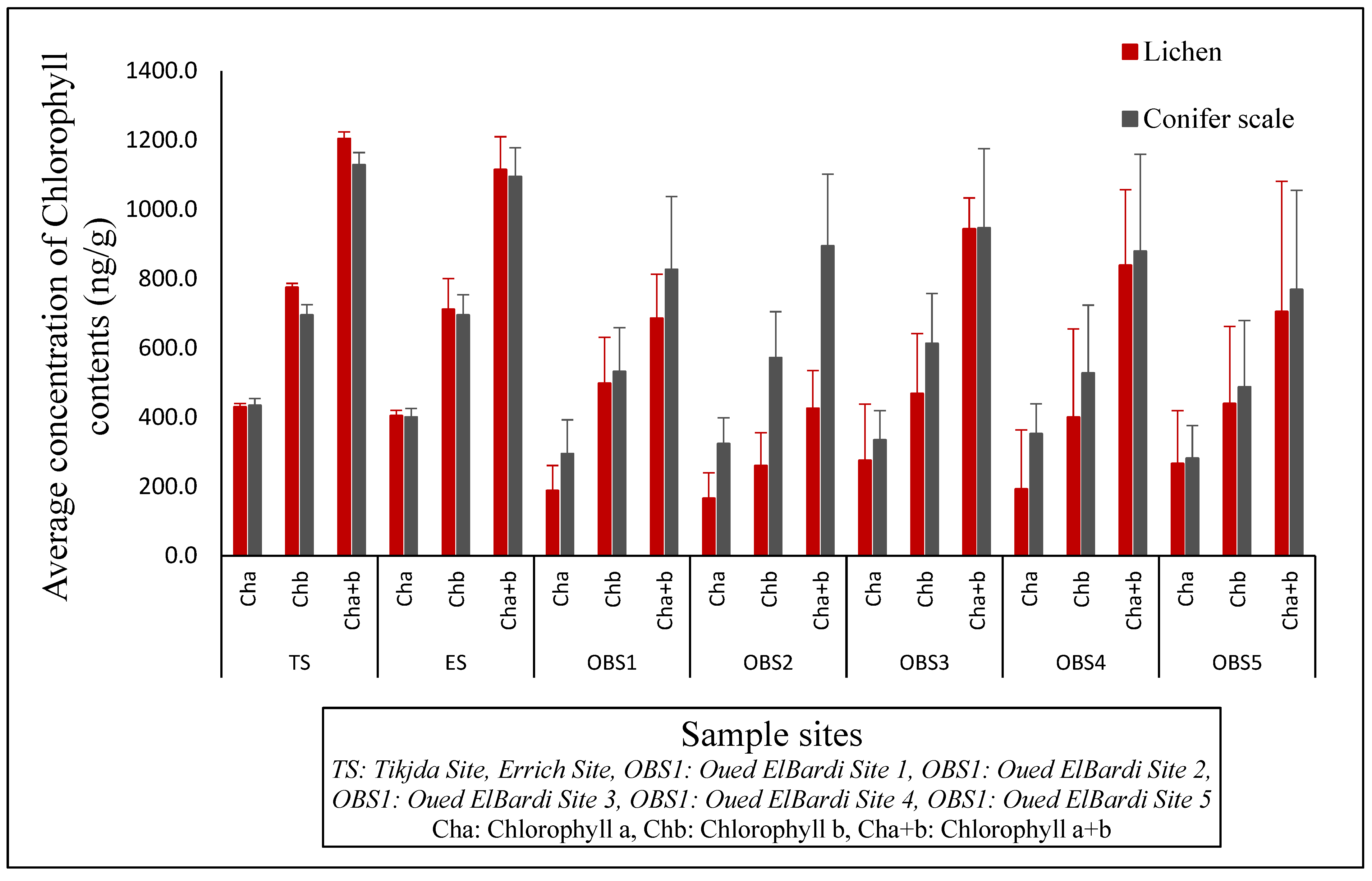
3.3.1. Chlorophyll Variations
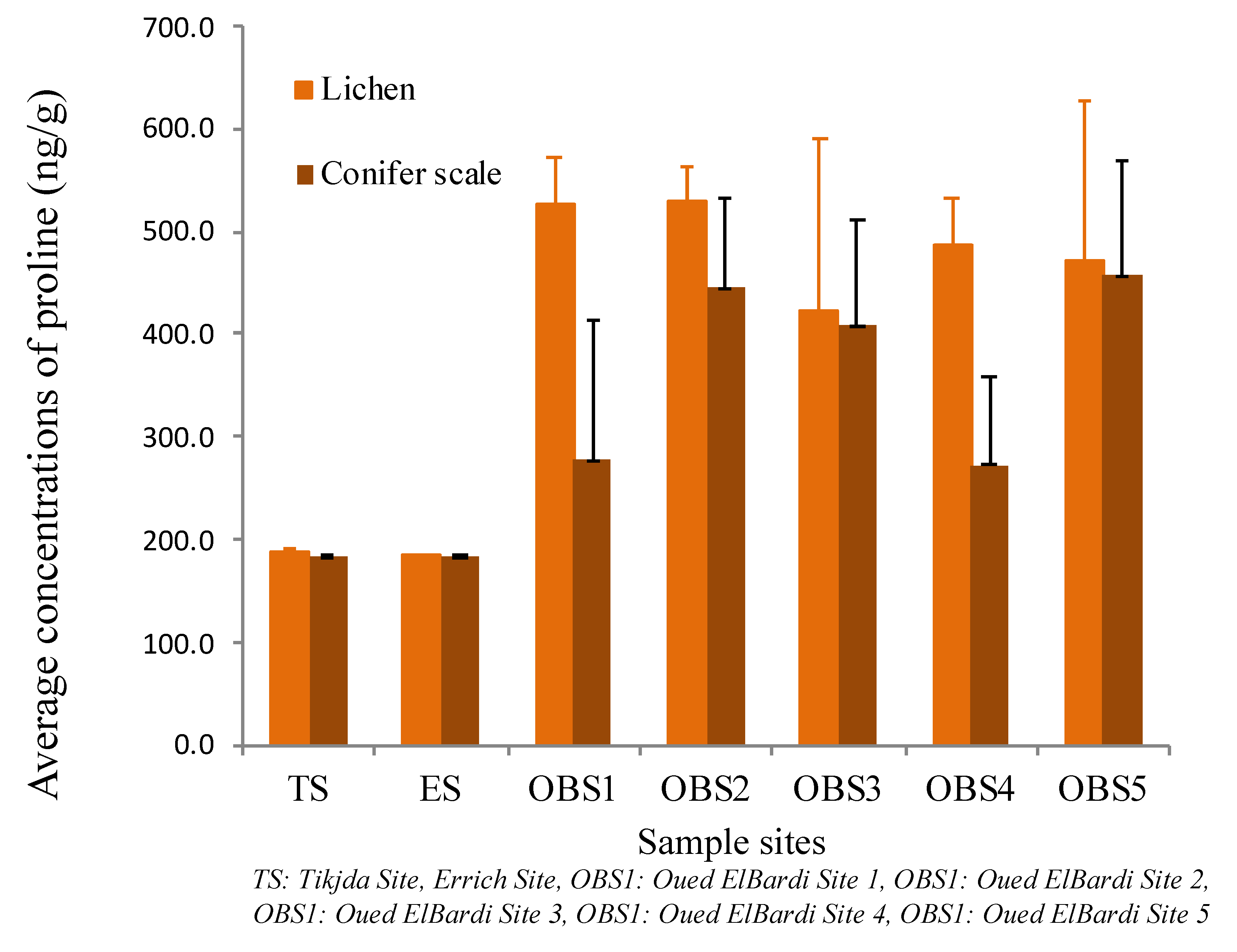
3.3.2. Proline
3.4. Comparison between Xanthoria parietina, Cupressus sempervirens, and Soil
4. Discussion and Conclusions
| Parameters | Current Study | Results from Literature | |
|---|---|---|---|
| Lichen | PAHs (ng/g dw) | (35.6–2222) | Spain (108 and 330) [40], Portugal (95.5–873.8) [41] |
| Chl (ng/g dw) Proline | (425.3–1204.4) (184–597) | Algeria (1870) [47], Algeria (50–380) [48] | |
| Conifer | PAH (g/kg dw) | Scales (18.8 et 1183.5) Barks (07–515.3) | Japan (83.0–466.8) [42], Portugal (185.4± 113.5) [41], Italy (33–1015) [43] |
| Chl rate (ng/g dw) | Scales (894.8–1128.7) | India (2250–3640) [10] | |
| Soil | PAHs (ng/g dw) | (9.9–138.2) | (0.8 and 30) [44]. |
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Nouchi, I. Plants as Bioindicators of Air Pollutants. In Air Pollution and Plant Biotechnology: Prospects for Phytomonitoring and Phytoremediation; Springer: Tokyo, Japan, 2002; pp. 41–60. [Google Scholar] [CrossRef]
- Agbaire, P.O. Impact of Air Pollution on Proline and Soluble Sugar Content of Selected Plant Species. Chem. Mater. Res. 2016, 8, 72–76. [Google Scholar]
- Giri, S.; Deepali, S.; Ketki, D.; Pallavi, D. Effect of air pollution on chlorophyll content of leaves. Curr. Agric. Res. 2013, 1, 93–98. [Google Scholar] [CrossRef]
- Ogagaoghene, A.J. pH level, Ascorbic Acid, Proline and Soluble Sugar as Bio-indicators for Pollution. Chem. Search J. 2017, 8, 41–49. [Google Scholar]
- Garrec, J.; Van Haluwyn, C. Biosurveillance Végétale de la Qualité de L’air; Lavoisier: Paris, France, 2002; 177p. [Google Scholar]
- Alkio, M.; Tabuchi, T.M.; Wang, X.; Ada Colon-Carmona, A. Stress responses to polycyclic aromatic hydrocarbons in Arabidopsis include growth inhibition and hypersensitive response-like symptoms. J. Exp. Bot. 2005, 56, 2983–2994. [Google Scholar] [CrossRef]
- Gostin, I.N. Air Pollution Effects on the Leaf Structure of some Fabaceae Species. Not. Bot. Hort. Agrobot. Cluj 2009, 37, 57–63. [Google Scholar]
- Seyyednejad, S.M.; Niknejad, M.; Koochak, H. A Review of Some Different Effects of Air Pollution on Plants. Res. J. Environ. Sci. 2011, 5, 302–309. [Google Scholar] [CrossRef]
- Talukdar, P.; Ghosh, N.; Malik, S.; Atikur Rahaman, C.H. Effect of air pollution on plant growth and pollen viability. WSN 2018, 109, 131–142. [Google Scholar]
- Allahnouri, M.; Ghasemi, A.F.; Pazhouhan, I. Traffic effects on leaf macro- and micro-morphological traits. Folia Oecologica 2018, 45, 92–101. [Google Scholar] [CrossRef]
- Mukhopadhyay, S.; Dutta, R.; Das, P. A critical review on plant biomonitors for determination of polycyclic aromatic hydrocarbons (PAHs) in air through solvent extraction techniques. Chemosphere 2020, 251, 126441. [Google Scholar] [CrossRef] [PubMed]
- Sharma, B.; Sharma, S.; Bhardwaj, S.K.; Alam, L.N. Effect of pollution on total chlorophyll content in temperate species growing along national in Himachal Pradesh. IJASEAT 2017, 5, 72–75. [Google Scholar]
- Güvenç, S.; Yıldız, G.; Dere, E. Physiological Responses of Epiphytic Lichens to the Urban and Rural Environment in the City of Bursa (Turkey). AKU J. Sci. Eng. 2018, 18, 33–43. [Google Scholar] [CrossRef]
- Thawale, P.R.; Satheesh Babu, S.; Wakode, R.R.; Kumar Singh, S.; Kumar, S.; Juwarkar, A.A. Biochemical changes in plant leaves as a biomarker of pollution due to anthropogenic activity. Environ. Monit. Assess. 2011, 177, 527–535. [Google Scholar] [CrossRef] [PubMed]
- Rydzyński, D.; Piotrowicz-Cieślak, A.I.; Grajek, H.; Michalczyk, D.J. Chlorophyll degradation by tetracycline and cadmium in spinach (Spinacia oleracea L.) leaves. Int. J. Environ. Sci. Technol. 2019, 16, 6301–6314. [Google Scholar] [CrossRef]
- Swain, S.; Narayan Mallick, S.; Prasad, P. Effect of industrial dust deposition on photosynthetic pigment chlorophyll and growth of selected plant species in Kalunga Industrial areas, Sundargarh, Odisha. Int. J. Bot. Stud. 2016, 1, 1–5. [Google Scholar]
- Tzvetkova, N.; Kolarov, D. Effect of air pollution on carbohydrate and nutrients concentrations in some deciduous tree species. Bulg. J. Plant. Physilo. 1996, 22, 53–63. [Google Scholar]
- Hu, R.; Liu, G.; Zhang, H.; Xue, H.; Wang, X.; Wang, R. Particle-Associated Polycyclic Aromatic Hydrocarbons (PAHs) in the Atmosphere of Hefei, China: Levels, Characterizations and Health Risks. Arch. Environ. Contam. Toxicol. 2018, 74, 442–451. [Google Scholar] [CrossRef]
- Geeta, C.; Namrata, C. Effect of Air Pollution on the Photosynthetic Pigments of Selected Plant Species along Roadsides in Jamshedpur, Jharkhand. Res. Plant Biol. 2014, 4, 65–68. [Google Scholar]
- Desalme, D.; Binet, P.; Chiapusio, G. Challenges in Tracing the Fate and Effects of Atmospheric Polycyclic Aromatic Hydrocarbon Deposition in Vascular Plants. Environ. Sci. Technol. 2013, 47, 3967–3981. [Google Scholar] [CrossRef]
- Shen, Y.; Li, J.; Gu, R.; Yue, L.; Wang, H.; Zhan, X.; Xing, B. Carotenoid and Superoxide Dismutase Are the Most Effective Antioxidants Participating in ROS Scavenging in Phenanthrene Accumulated Wheat Leaf. Chemosphere 2018, 197, 513–525. [Google Scholar] [CrossRef]
- Molina, L.; Segura, A. Biochemical and Metabolic Plant Responses toward Polycyclic Aromatic Hydrocarbons and Heavy Metals Present in Atmospheric Pollution. Plants 2021, 10, 2305. [Google Scholar] [CrossRef]
- Sethy, S.; Ghosh, S. Effect of Heavy Metals on Germination of Seeds. J. Nat. Sci. Biol. Med. 2013, 4, 272. [Google Scholar]
- Abdel-Shafy, H.I.; Mansour, M.S.M. A review on polycyclic aromatic hydrocarbons: Source, environmental impact, effect on human health and remediation. Egypt. J. Petrol. 2016, 25, 107–123. [Google Scholar] [CrossRef]
- Kaur, N.; Erickson, T.E.; Ball, A.S.; Ryan, M.H. A Review of Germination and Early Growth as a Proxy for Plant Fitness under Petrogenic Contamination—Knowledge Gaps and Recommendations. Sci. Total Environ. 2017, 603, 728–744. [Google Scholar] [CrossRef] [PubMed]
- Pratt, G.C.; Herbrandson, C.; Krause, M.J.; Schmitt, C.; Lippert, C.J.; McMahon, C.R.; Ellickson, K.M. Measurements of gas and particle polycyclic aromatic hydrocarbons (PAHs) in air at urban, rural and near-roadway sites. Atmos. Environ. 2018, 179, 268–278. [Google Scholar] [CrossRef]
- Martinez-Swatson, K.; Mihály, E.; Lange, C.; Ernst, M.; Dela Cruz, M.; Price, M.J.; Mikkelsen, T.N.; Christensen, J.H.; Lundholm, N.; Rønsted, N. Biomonitoring of Polycyclic Aromatic Hydrocarbon Deposition in Greenland Using Historical Moss Herbarium Specimens Shows a Decrease in Pollution During the 20th Century. Front. Plant Sci. 2020, 11, 1085. [Google Scholar] [CrossRef]
- Ristić, S.S.; Kosanić, M.M.; Ranković, B.R.; Stamenković, S.S. Lichens as biological indicators of air quality in the urban area of Kursumlija (Southern Serbia). Kragujev. J. Sci. 2017, 39, 165–175. [Google Scholar] [CrossRef]
- Terzaghi, E.; Wild, E.; Zacchello, G.; Cerabolini, B.E.L.; Jones, K.C.; Di Guardo, A. Forest Filter Effect: Role of leaves in capturing/releasing air particulate matter and its associated PAHs. Atmos. Environ. 2013, 74, 378–384. [Google Scholar] [CrossRef]
- Guéguen, F.; Stille, P.; Millet, M. Air quality assessment by tree bark biomonitoring in urban, industrial and rural environments of the Rhine Valley: PCDD/Fs, PCBs and trace metal evidence. Chemosphere 2011, 85, 195–202. [Google Scholar] [CrossRef]
- Brown, A.S.; Brown, R.J.C.; Coleman, P.J.; Conolly, C.; Sweetman, A.J.; Jones, K.C.; Butterfield, D.M.; Sarantaridis, D.; Donovan, B.J.; Roberts, I. Twenty years of measurement of polycyclic aromatic hydrocarbons (PAHs) in UK ambient air by nationwide air quality networks. Environ. Sci. Process. Impacts. 2013, 15, 1199–1215. [Google Scholar] [CrossRef]
- Niu, L.; Xu, C.; Zhou, Y.; Liu, W. Tree bark as a biomonitor for assessing the atmospheric pollution and associated human inhalation exposure risks of polycyclic aromatic hydrocarbons in rural China. Environ. Pollut. 2019, 246, 398–407. [Google Scholar] [CrossRef]
- Gonzalez, C.M.; Casanovas, S.S.; Pignata, M.L. Biomonitoring of air pollutants from traffic and industries employing Ramalina ecklonii (Spreng.) Mey. and Flot. in Cordoba, Argentina. Environ. Pollut. 1996, 91, 269–277. [Google Scholar] [CrossRef] [PubMed]
- Maatoug, M.; Medkour, K.; Ait Hammou, M.; Hellal, B. Cartographie de la pollution atmosphérique par le plomb d’origine routière à l’aide de transplantation d’un lichen bio-accumulateur Xanthoria parietina dans la ville de Tiaret (Algérie). Pollut. Atmosphérique 2010, 205, 93–101. [Google Scholar]
- Vitali, M.; Antonucci, A.; Owczarek, M.; Guidotti, M.; Astolfi, M.L.; Manigrasso, M.; Avino, P.; Bhattacharya, B.; Protano, C. Air quality assessment in different environmental scenarios by the determination of typical heavy metals and Persistent Organic Pollutants in native lichen Xanthoria parietina. Environ. Pollut. 2019, 254, 113013. [Google Scholar] [CrossRef] [PubMed]
- Protano, C.; Guidotti, M.; Owczarek, M.; Fantozzi, L.; Blasi, G.; Vitali, M. Polycyclic Aromatic Hydrocarbons and Metals in Transplanted Lichen (Pseudovernia furfuracea) at Sites Adjacent to a Solid-waste Landfill in Central Italy. Arch. Environ. Contam. Toxicol. 2014, 66, 471–481. [Google Scholar] [CrossRef] [PubMed]
- Protano, C.; Owczarek, M.; Fantozzi, L.; Guidotti, M.; Vitali, M. Transplanted Lichen Pseudovernia furfuracea as a Multi-Tracer Monitoring Tool Near a Solid Waste Incinerator in Italy: Assessment of Airborne Incinerator-Related Pollutants. Bull. Environ. Contam. Toxicol. 2015, 95, 644–653. [Google Scholar] [CrossRef] [PubMed]
- Conti, M.E.; Cecchetti, G. Biological monitoring: Lichens as bioindicators of air pollution assessment—A review. Environ. Pollut. 2001, 114, 471–492. [Google Scholar] [CrossRef] [PubMed]
- Monneveux, P.; Nemmar, M. Contribution à l’étude de la résistance à la sécheresse chez le blé tendre (Triticum aestivum L.) et chez le blé dur (Triticum durum Desf.): étude de l’accumulation de la proline au cours du cycle de développement. Agronomie 1986, 6, 583–590. [Google Scholar] [CrossRef]
- Domínguez-Morueco, N.; Augusto, S.; Trabalón, L.; Pocurull, E.; Borrull, F.; Schuhmacher, M.; Domingo, J.L.; Nadal, M. Monitoring PAHs in the petrochemical area of Tarragona County, Spain: Comparing passive air samplers with lichen transplants. Environ. Sci. Pollut. Res. Int. 2017, 24, 11890–11900. [Google Scholar] [CrossRef] [PubMed]
- Augusto, S.; Maguas, C.; Matos, J.; Pereira, M.J.; Branquinho, C. Lichens as an integrating tool for monitoring PAH atmospheric deposition: A comparison with soil, air and pine needles. Environ. Pollut. 2009, 2, 483–489. [Google Scholar] [CrossRef]
- Oishi, Y. Comparison of moss and pine needles as bioindicators of transboundary polycyclic aromatic hydrocarbon pollution in central Japan. Environ. Pollut. 2018, 234, 330–338. [Google Scholar] [CrossRef]
- Orecchio, S.; Gianguzza, A.; Culotta, L. Absorption of polycyclic aromatic hydrocarbons by Pinus bark: Analytical method and use for environmental pollution monitoring in the Palermo area (Sicily, Italy). Environ. Res. 2008, 107, 371. [Google Scholar] [CrossRef] [PubMed]
- Maliszewska-Kordybach, B. Sources, concentrations, fate and effects of polycyclic aromatic hydrocarbons (PAHs) in the environment. Part A: PAHs in the air. Pol. J. Environ. Stud. 1996, 8, 131–136. [Google Scholar]
- Ortega-Rosas, C.I.; Meza-Figueroa, D.; Vidal-Solano, J.R.; González-Grijalva, B.; Schiavo, B. Association of airborne particulate matter with pollen, fungal spores, and allergic symptoms in an arid urbanized area. Environ. Geochem. Health 2021, 43, 1761–1782. [Google Scholar] [CrossRef]
- Liu, X.; Yu, X.; Zhang, Z. PM2.5 Concentration Differences between Various Forest Types and Its Correlation with Forest Structure. Atmosphere 2015, 6, 1801–1815. [Google Scholar] [CrossRef]
- Khelil, R.; Ould El Hadj-Khelil, A.; Dadamoussa, B.; Cabello-Hurtado, F.; Esnault, M.A. Effet de la pollution atmosphérique par les hydrocarbures sur le lichen Xanthoria parietina (L.) Th (Teloschistaceae) dans la zone de Hassi Messaoud (Sahara septentrional Est algérien). Alger. J. Arid. Environ. 2014, 4, 98–106. [Google Scholar]
- Maizi, N.; Alioua, A.; Tahar, A. Jumelage des bio-indicateurs et d’un réseau de surveillance de la qualité de l’air pour la détection de la pollution par le SO2 dans la region de Annaba (Algérie). Biotechnol. Agron. Soc. Environ. 2012, 16, 149–158. [Google Scholar]
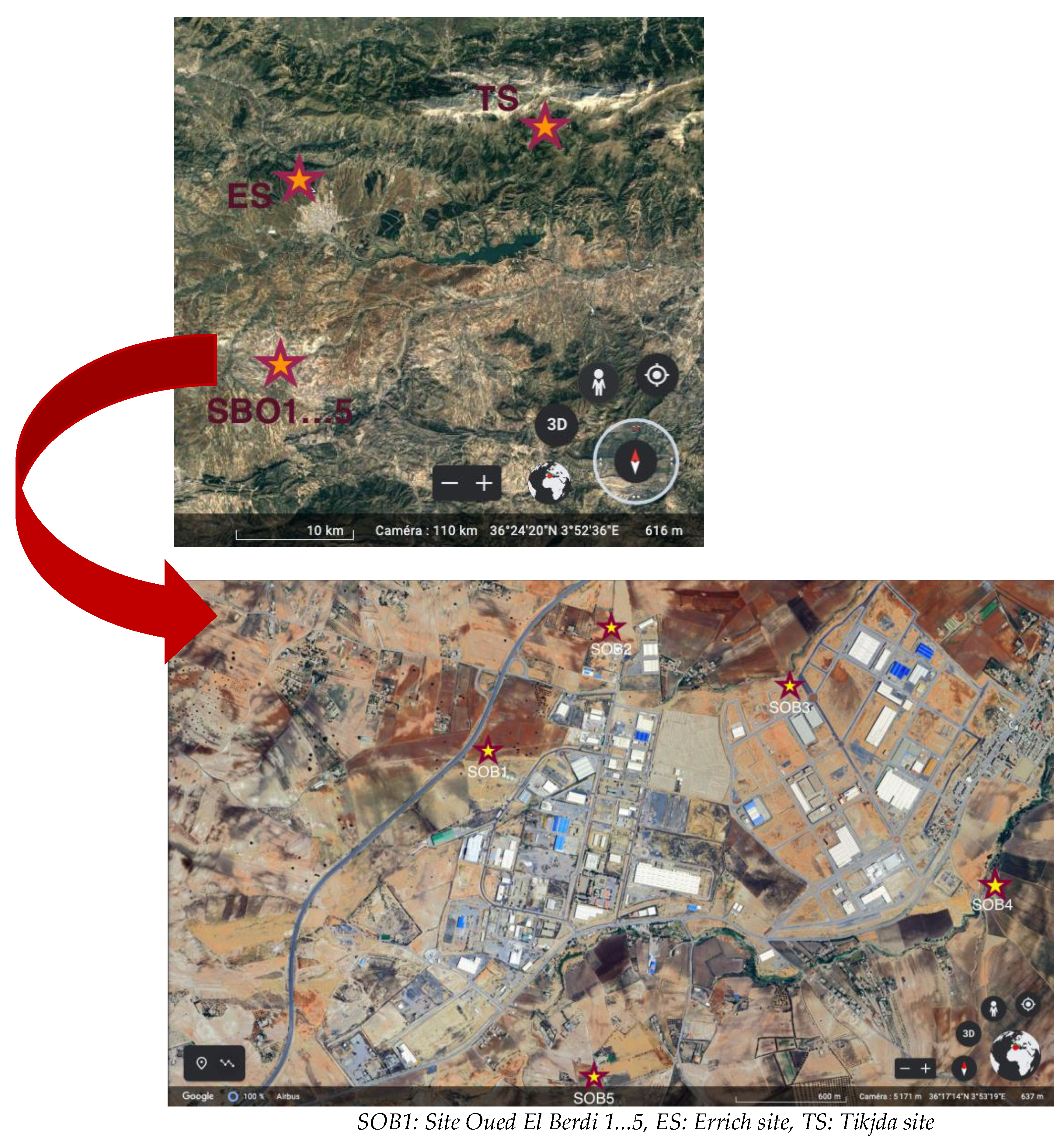
| Sites | Latitude | Longitude | Altitude (m) | Type | Vegetation Cover |
|---|---|---|---|---|---|
| (SOB1) | 361,718.23 | 35,310.23 | 650 | Industrial | Very rare |
| (SOB2) | 361,738.91 | 35,337.09 | 625 | Industrial | Rare |
| (SOB3) | 361,729.58 | 35,415.12 | 600 | Urban | More or less dense |
| (SOB4) | 361,655.57 | 3546.59 | 630 | Industrial | Low |
| (SOB5) | 361,618.74 | 35,332.84 | 645 | Industrial | Rare |
| Errich (ES) | 362,437.25 | 35,240.95 | 610 | Semi-urban | Very dense |
| Tikjda (TS) | 362,650.95 | 40,733.64 | 1400 | Forest | Very dense |
| PAHs (ng/g dw) | Xanthoria parietina | Conifer Scales | Conifer Barks | Soil | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Min | Me | M | SD | Max | Min | Me | M | SD | Max | Min | Me | M | SD | Max | Min | Me | M | SD | Max | |
| TS | 21.5 | 32.1 | 35.6 | 3 | 58.5 | 9.9 | 20.2 | 18.8 | 7.0 | 30.3 | 3 | 9 | 7 | 3 | 13 | 8.7 | 9.6 | 9.9 | 1.1 | 10.6 |
| ES | 102 | 200 | 229.9 | 33.2 | 267 | 12.2 | 17.3 | 21.6 | 13.0 | 44.2 | 0 | 7 | 8 | 11 | 28.4 | 70 | 73.9 | 75.3 | 0.2 | 76.5 |
| OBS1 | 429.6 | 993.1 | 1091.1 | 224 | 1988 | 183.2 | 416.4 | 387.2 | 118.0 | 690.8 | 88.9 | 290.3 | 302.6 | 130 | 530 | 148 | 150.6 | 159.7 | 4.8 | 162 |
| OBS2 | 443.1 | 545 | 588.8 | 192 | 765.4 | 99.6 | 296.3 | 276.1 | 95.0 | 290.5 | 50.4 | 157.3 | 163.3 | 79 | 186.1 | 59.5 | 60.1 | 59.4 | 2.4 | 64.4 |
| OBS3 | 1400.1 | 2154.1 | 1901.6 | 590 | 2668 | 331.3 | 544.5 | 538.2 | 257.0 | 766.4 | 256.1 | 320.1 | 340.3 | 111 | 608.4 | 87.1 | 100.3 | 97.6 | 29 | 108 |
| OBS4 | 280 | 703.3 | 744.4 | 461 | 921.3 | 497.2 | 577.1 | 537.9 | 160.0 | 642.1 | 14.4 | 19.5 | 21.4 | 41 | 50.6 | 73.6 | 75.5 | 76.2 | 2.7 | 80.1 |
| OBS5 | 1967.1 | 2099.9 | 2222.2 | 376 | 2695.4 | 2922 | 2176.4 | 1183.5 | 876 | 9012.5 | 498.9 | 500.2 | 515.3 | 19 | 574 | 119 | 129.6 | 138.2 | 37 | 140 |
| PAHs | PMs | Cha | Chb | Cha + b | Proline | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| F | p | F | p | F | p | F | p | F | p | F | p | |
| D | 10.24 | *** | 4.91 | 0.002 | 17.17 | *** | 33.78 | *** | 25.84 | *** | 18.68 | *** |
| Sites | 4.1 | *** | 3.61 | *** | 22.28 | *** | 35.34 | *** | 23.81 | *** | 50.94 | *** |
| Inter | 3.02 | *** | 1.83 | 0.005 | 7.60 | *** | 11.90 | *** | 8.35 | *** | 5.58 | *** |
| PMs (µg/g/dw) | Xanthoria parietina | Conifer Scales | Conifer Barks | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Min | Me | M | SD | Max | Min | Me | M | SD | Max | Min | Me | M | SD | Max | |
| TS | 1879.3 | 2115.2 | 2213.3 | 113.1 | 2680.2 | 36.6 | 50.1 | 54.1 | 45.6 | 87.6 | 55.8 | 68.3 | 72.9 | 13.4 | 103.3 |
| ES | 3110.5 | 3765.1 | 3713.3 | 21.9 | 3801.3 | 95.4 | 104.3 | 162.6 | 54.9 | 210.6 | 73.5 | 128.4 | 125.2 | 63.6 | 201.9 |
| OBS1 | 3090.4 | 5709.6 | 5763.3 | 678.3 | 6984.5 | 1019 | 1506.4 | 1474.2 | 795 | 1659.4 | 3020.1 | 4321.7 | 4631.6 | 428.9 | 4861.1 |
| OBS2 | 1983.9 | 3354.3 | 3468.9 | 850.0 | 5769.6 | 1879.3 | 2343.6 | 2529.1 | 780 | 3665.5 | 5331.7 | 5692.1 | 5766.6 | 434.7 | 6210.1 |
| OBS3 | 577.4 | 654.4 | 700.0 | 466.6 | 899.6 | 398.4 | 488.5 | 511.2 | 188.1 | 843.1 | 222.8 | 267.5 | 273.7 | 63 | 376.2 |
| OBS4 | 102.5 | 348.7 | 366.7 | 109.8 | 521.2 | 264.5 | 255.1 | 295.5 | 87.6 | 411.4 | 98.9 | 120.1 | 121 | 18.1 | 157.7 |
| OBS5 | 820.4 | 1032.3 | 983.3 | 123.1 | 1178.6 | 118.4 | 181 | 201.6 | 92.8 | 465 | 132.2 | 147 | 143.3 | 23.7 | 183.3 |
| Variable | Ch.a | Ch.a + b | Ch.a | PAH | PM | Proline |
|---|---|---|---|---|---|---|
| Ch.a | 1 | |||||
| Ch.a + b | 0.92 | 1 | ||||
| Ch. B | 0.84 | 0.95 | 1 | |||
| PAH | −0.60 | −0.64 | −0.63 | 1 | ||
| PM | −0.47 | −0.47 | −0.43 | 0.3 | 1 | |
| Proline | −0.79 | −0.80 * | −0.79 | 0.65 * | 0.4 | 1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Benaissa, F.; Bourfis, N.; Ferhoum, F.; Annesi-Measano, I. Biomonitoring of Atmospheric PAHs and PMs Using Xanthoria parietina and Cupressus sempervirens in Bouira (Algeria). Sustainability 2023, 15, 15174. https://doi.org/10.3390/su152015174
Benaissa F, Bourfis N, Ferhoum F, Annesi-Measano I. Biomonitoring of Atmospheric PAHs and PMs Using Xanthoria parietina and Cupressus sempervirens in Bouira (Algeria). Sustainability. 2023; 15(20):15174. https://doi.org/10.3390/su152015174
Chicago/Turabian StyleBenaissa, Fatima, Nassima Bourfis, Fatiha Ferhoum, and Isabella Annesi-Measano. 2023. "Biomonitoring of Atmospheric PAHs and PMs Using Xanthoria parietina and Cupressus sempervirens in Bouira (Algeria)" Sustainability 15, no. 20: 15174. https://doi.org/10.3390/su152015174
APA StyleBenaissa, F., Bourfis, N., Ferhoum, F., & Annesi-Measano, I. (2023). Biomonitoring of Atmospheric PAHs and PMs Using Xanthoria parietina and Cupressus sempervirens in Bouira (Algeria). Sustainability, 15(20), 15174. https://doi.org/10.3390/su152015174






