Bioactive Compounds of Jambu (Acmella oleracea (L.) R. K. Jansen) as Potential Components of Biodegradable Food Packing: A Review
Abstract
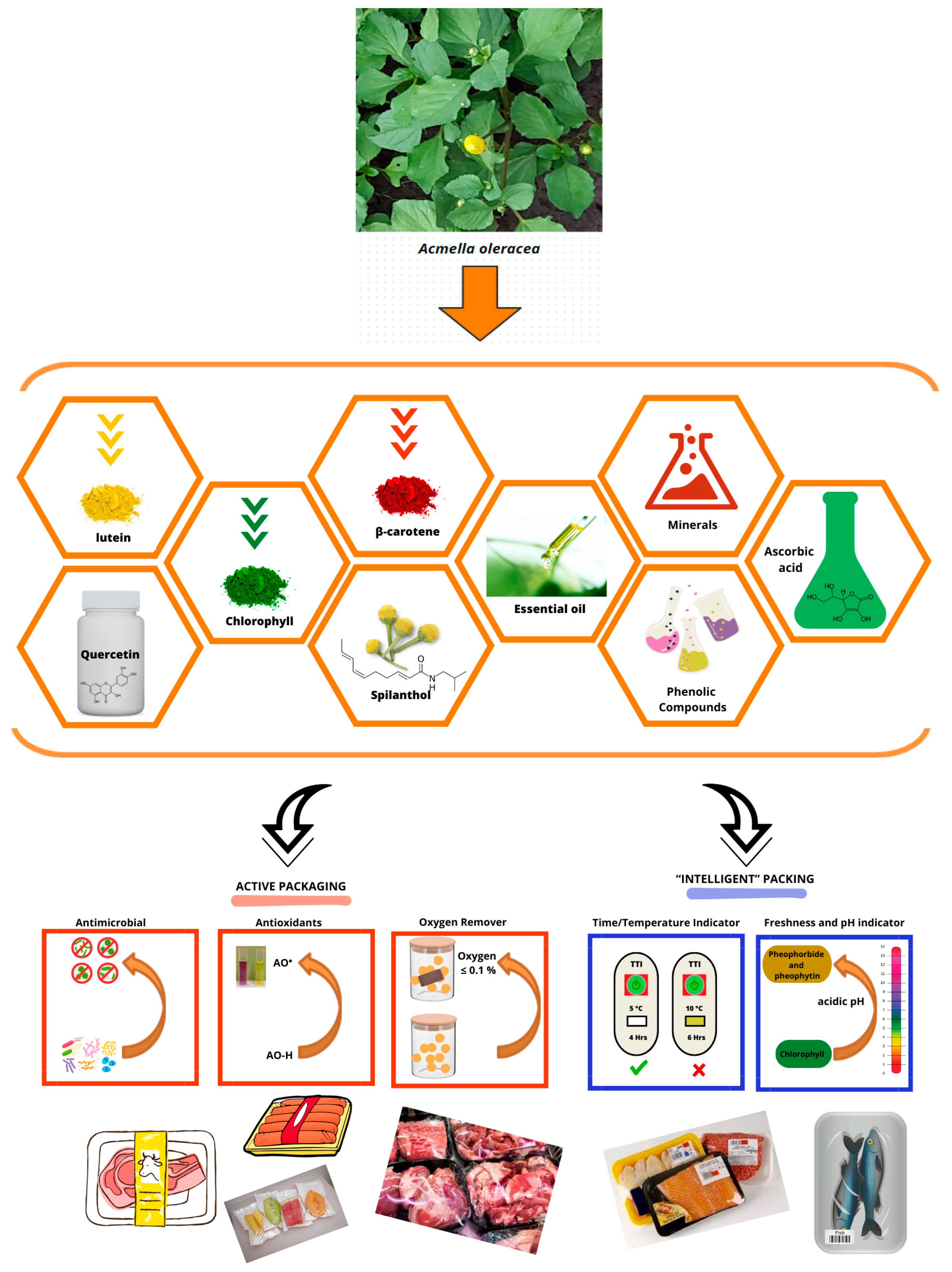
1. Introduction
2. Acmella oleracea (Jambu)
2.1. Primary and Secondary Compounds of Jambu

2.2. Biological Properties Attributed to Jambu
3. Application of Jambu Bioactive Compounds in Packaging
3.1. Active Packaging
3.1.1. Antioxidant and Antimicrobial Active Packaging
3.1.2. Active Oxygen Removal Packaging
3.2. “Intelligent” Packaging
3.2.1. “Intelligent” Packaging with Time/Temperature Indicators
3.2.2. “Intelligent” Packaging with Freshness and pH Indicators
4. Toxic Effects of Bioactive Compounds in Jambu
5. Conclusions and Future Prospects
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Priyadarshi, R.; Rhim, J.W. Chitosan-based biodegradable functional films for food packaging applications. Innov. Food Sci. Emerg. Technol. 2020, 62, 102346. [Google Scholar] [CrossRef]
- Ahmad, A.A.; Sarbon, N.M. A comparative study: Physical, mechanical and antibacterial properties of bio-composite gelatin films as influenced by chitosan and zinc oxide nanoparticles incorporation. Food Biosci. 2021, 43, 101250. [Google Scholar] [CrossRef]
- UNEP (United Nations Environment Programme). From Pollution to Solution: A Global Assessment of Marine Litter and Plastic Pollution; UNEP: Nairobi, Kenya, 2021. [Google Scholar]
- Zhao, Z.; Li, Y.; Du, Z. Seafood Waste-Based Materials for Sustainable Food Packing: From Waste to Wealth. Sustainability 2022, 14, 16579. [Google Scholar] [CrossRef]
- Alias, A.R.; Wan, M.K.; Sarbon, N.M. Emerging materials and technologies of multi-layer film for food packaging application: A review. Food Control 2022, 136, 108875. [Google Scholar] [CrossRef]
- Soltani Firouz, M.; Mohi-Alden, K.; Omid, M. A critical review on intelligent and active packaging in the food industry: Research and development. Food Res. Int. 2021, 141, 110113. [Google Scholar] [CrossRef] [PubMed]
- Candido, G.S.; Natarelli, C.V.L.; Carvalho, E.E.N.; Oliveira, J.E. Bionanocomposites of pectin and pracaxi oil nanoemulsion as active packaging for butter. Food Packag. Shelf Life 2022, 32, 100862. [Google Scholar] [CrossRef]
- Etxabide, A.; Arregi, M.; Cabezudo, S.; Guerrero, P.; Caba, K. Whey Protein Films for Sustainable Food Packaging: Effect of Incorporated Ascorbic Acid and Environmental Assessment. Polymers 2023, 15, 387. [Google Scholar] [CrossRef]
- Sadeghi, A.; Razavi, S.M.A.; Shahrampour, D. Fabrication and characterization of biodegradable active films with modified morphology based on polycaprolactone-polylactic acid-green tea extract. Int. J. Biol. Macromol. 2022, 205, 341–356. [Google Scholar] [CrossRef]
- Vieira, D.M.; Pereira, C.; Calhelha, R.C.; Barros, L.; Petrovic, J.; Sokovic, M.; Barreiro, M.F.; Ferreira, I.C.F.R.; Castro, M.C.R.; Rodrigues, P.V.; et al. Evaluation of plant extracts as an efficient source of additives for active food packaging. Food Front. 2022, 3, 480–488. [Google Scholar] [CrossRef]
- Yang, C.M.; Chathuranga, K.; Lee, J.S.; Park, W.H. Effects of polyphenols on the thermal decomposition, antioxidative, and antimicrobial properties of poly(vinyl alcohol) and poly(vinyl pyrrolidone). Polym. Test. 2022, 116, 107786. [Google Scholar] [CrossRef]
- Barbosa-Pereira, L.; Aurrekoetxea, G.P.; Ângulo, I.; Paseiro-Losada, P.; Cruz, J.M. Development of new active packaging films coated with natural phenolic compounds to improve the oxidative stability of beef. Meat Sci. 2014, 97, 249–254. [Google Scholar] [CrossRef] [PubMed]
- Pirsa, S.; Shamusi, T. Intelligent and active packaging of chicken thigh meat by conducting nano structure cellulose-polypyrrole-ZnO film. Mater. Sci. Eng. C 2019, 102, 798–809. [Google Scholar] [CrossRef]
- Marcillo-Parra, V.; Tupuna-Yerovi, D.S.; González, Z.; Ruales, J. Encapsulation of bioactive compounds from fruit and vegetable by-products for food application—A review. Trends Food Sci. Technol. 2021, 116, 11–23. [Google Scholar] [CrossRef]
- Sagar, N.A.; Pareek, S.; Sharma, S.; Yahia, E.M.; Lobo, M.G. Fruit and Vegetable Waste: Bioactive Compounds, Their Extraction, and Possible Utilization. Compr. Rev. Food Sci. Food Saf. 2018, 17, 512–531. [Google Scholar] [CrossRef] [PubMed]
- Villachica, H.; Carvalho, J.E.U.; Muller, C.H.; Diaz, S.C.; Almanza, M. Frutales y Hortalizas Promisorios de la Amazonia; Tratado de Cooperacion Amazonica, Secretaria-Pro-Tempore: Lima, Peru, 1996; 367p. [Google Scholar]
- Poltronieri, M.C.; Mulle, N.R.M.; Poltronieri, L.S. Recomendações Para Produção de Jambu: Cultivar Nazaré. EMBRAPA, 2000, 13, Technical Circular n. 11. Available online: https://www.embrapa.br/busca-de-publicacoes (accessed on 25 October 2022).
- Hind, N.; Biggs, N. Plate 460. Acmella Oleracea Compositae. Curtis’s Bot. Mag. 2003, 20, 31–39. [Google Scholar] [CrossRef]
- Joly, A.B. Botânica: Introdução à Taxonomia Vegetal, 13th ed.; Companhia Editora Nacional: São Paulo, Brazil, 2002; 808p. [Google Scholar]
- Souza, V.C.; Lorenzi, H. Botânica Sistemática—Guia Ilustrado para Identificação das Famílias de Angiospermas da Flora Brasileira; Instituto Plantarum de Estudos da Flora: Nova Odessa, Brazil, 2005; 640p. [Google Scholar]
- Revilla, J. Plantas da Amazônia: Oportunidades Econômicas Sustentáveis; Sebrae: Rio de Janeiro, Brazil, 2001; 405p. [Google Scholar]
- Coutinho, L.N.; Aparecido, C.C.; Figueiredo, M.B. Galhas e deformações em jambu (Spilanthes oleraceae) causadas por Tecaphora spilanthes (Ustilaginales). Summa Phytopathol. 2006, 32, 283–285. [Google Scholar] [CrossRef]
- Favoreto, R.; Gilbert, B. Acmella oleracea (L.) R. K. Jansen (Asteraceae)—Jambu. Rev. Fitos 2010, 5, 83–91. [Google Scholar]
- Kliebenstein, D.J. Secondary metabolites and plant/environment interactions: A view through Arabidopsis thaliana tinged glasses. Plant Cell Environ. 2004, 27, 675–684. [Google Scholar] [CrossRef]
- Simões, C.M.O. Farmacognosia. Da Planta ao Medicamento; UFRGS: Florianópolis, Brazil, 2010; 1104p. [Google Scholar]
- Seal, T.; Chaudhuri, K.; Pillai, B. Evaluation of Proximate and Mineral Composition of Wild Edible Leaves, Traditionally used by the Local People of Meghalaya State In India. Asian J. Plant Sci. 2013, 12, 171–175. [Google Scholar] [CrossRef]
- Neves, D.A.; Schmiele, M.; Pallone, J.A.L.; Orlando, E.A.; Risso, E.M.; Cunha, E.C.E.; Godoy, H.T. Chemical and nutritional characterization of raw and hydrothermal processed jambu (Acmella oleracea (L.) R.K. Jansen). Food Res. Int. 2019, 116, 1144–1152. [Google Scholar] [CrossRef] [PubMed]
- Gomes, F.P.; Resende, O.; Sousa, E.P.; Damasceno, L.F. Comparison of powdered and fresh jambu (Acmella oleracea). Heliyon 2020, 6, e05349. [Google Scholar] [CrossRef] [PubMed]
- Nakatani, N.; Nagashima, M. Pungent Alkamides from Spilanthes acmella L. var. oleracea Clarke. Biosci. Biotechnol. Biochem. 1992, 56, 759–762. [Google Scholar] [CrossRef]
- Yasuda, I.; Koichi, T.; Hideji, I. The structure of spilanthol. Chem. Farm. Bull. 1980, 28, 2251–2253. [Google Scholar] [CrossRef]
- Velozo, L.S.M.; Vulpi, T.S.; Morais, C.P.M.; Trindade, A.P.F.; Lima, M.H.P.; Kaplan, M.A.C. Análise do Óleo Essencial dos Diferentes Órgãos de Acmella ciliata Kunth (Asteraceae). Rev. Bras. Biociências 2008, 5 (Suppl. S2), 1128–1130. [Google Scholar]
- Phrutivorapongkul, A.; Chaiwon, A.; Vejabhikul, S.; Netisingha, W.; Chansakaow, S. An Anesthetic Alkamide and Fixed Oil from Acmella oleracea. J. Health Res. 2008, 22, 97–99. [Google Scholar]
- Boonen, J.; Baert, B.; Burvenich, C.; Blondeel, P.; Saeger, S.; Spiegeleer, B. LC–MS profiling of N-alkylamides in Spilanthes acmella extract and the transmucosal behaviour of its main bio-active spilanthol. J. Pharm. Biomed. Anal. 2010, 53, 243–249. [Google Scholar] [CrossRef]
- Mbeunkui, F.; Grace, M.H.; Lategan, C.; Smith, P.J.; Raskin, I.; Lila, M.A. Isolation and identification of antiplasmodial N-alkylamides from Spilanthes acmella flowers using centrifugal partition chromatography and ESI-IT-TOF-MS. J. Chromatogr. B 2011, 879, 1886–1892. [Google Scholar] [CrossRef]
- Pandey, V.; Chopra, M.; Agrawal, V. In vitro isolation and characterization of biolarvicidal compounds from micropropagated plants of Spilanthes acmella. Parasitol. Res. 2011, 108, 297–305. [Google Scholar] [CrossRef]
- Ramsewak, R.S.; Erickson, A.J.; Nair, M.G. Bioactive N-isobutylamides from the flower buds of Spilanthes acmella. Phytochemistry 1999, 51, 729–732. [Google Scholar] [CrossRef]
- Sharma, V.; Boonen, J.; Chauhan, N.S.; Thakur, M.; Spiegeleer, B.; Dixit, V.K. Spilanthes acmella ethanolic flower extract: LC–MS alkylamide profiling and its effects on sexual behavior in male rats. Phytomedicine 2011, 18, 1161–1169. [Google Scholar] [CrossRef] [PubMed]
- Prachayasittikul, S.; Suphapong, S.; Worachartcheewan, A.; Lawung, R.; Ruchirawat, S.; Prachayasittikul, V. Bioactive Metabolites from Spilanthes acmella Murr. Molecules 2009, 14, 850–867. [Google Scholar] [CrossRef] [PubMed]
- Dubey, S.; Maity, S.; Singh, M.; Saraf, S.A.; Saha, S. Phytochemistry, pharmacology and toxicology of Spilanthes acmella: A review. Adv. Pharmacol. Sci. 2013, 2013, 423750. [Google Scholar] [PubMed]
- Nascimento, A.M.; Souza, L.M.; Baggio, C.H.; Werner, M.F.P.; Maria-Ferreira, D.; Silva, L.M.; Sassaki, G.L.; Gorin, P.A.J.; Iacomini, M.; Cipriani, T.R. Gastroprotective effect and structure of a rhamnogalacturonan from Acmella oleracea. Phytochemistry 2013, 85, 137–142. [Google Scholar] [CrossRef] [PubMed]
- Prachayasittikul, V.; Prachayasittikul, S.; Ruchirawat, S.; Prachayasittikul, V. High therapeutic potential of Spilanthes acmella: A review. EXCLI J. 2012, 12, 291–312. [Google Scholar]
- Rodriguez-Amaya, D.B. A Guide to Carotenoid Analysis in Foods; International Life Sciences Institute: Washington, DC, USA, 2001; 71p. [Google Scholar]
- Bellumori, M.; Zonfrillo, B.; Maggini, V.; Bogani, P.; Gallo, E.; Firenzuoli, F.; Mulinacci, N.; Innocenti, M. Acmella oleracea (L.) R.K. Jansen: Alkylamides and phenolic compounds in aerial parts and roots of in vitro seedlings. J. Pharm. Biomed. Anal. 2022, 220, 114991. [Google Scholar] [CrossRef]
- Nascimento, L.E.S.; Arriola, N.D.A.; Silva, L.A.L.; Faqueti, L.G.; Sandjo, L.P.; Araújo, C.E.S.; Biavatti, M.W.; Barcelos-Oliveira, J.L.; Amboni, R.D.M.C. Phytochemical profile of different anatomical parts of jambu (Acmella oleracea (L.) R.K. Jansen): A comparison between hydroponic and conventional cultivation using PCA and cluster analysis. Food Chem. 2020, 332, 127393. [Google Scholar] [CrossRef]
- Barbosa, A.F.; Carvalho, M.G.; Smith, R.E.; Sabaa-Srur, A.U.O. Spilanthol: Occurrence, extraction, chemistry and biological activities. Rev. Bras. Farmacogn. 2016, 26, 128–133. [Google Scholar] [CrossRef]
- Stein, R.; Berger, M.; Cecco, B.S.; Mallmann, L.P.; Terraciano, P.B.; Driemeier, D.; Rodrigues, E.; Beys-da-Silva, W.O.; Konrath, E.L. Chymase inhibition: A key factor in the anti-inflammatory activity of ethanolic extracts and spilanthol isolated from Acmella oleracea. J. Ethnopharmacol. 2021, 270, 113610. [Google Scholar] [CrossRef]
- Wu, L.; Fan, N.; Lin, M.; Chu, I.; Huang, S.; Hu, C.; Ha, S. Anti-inflammatory Effect of Spilanthol from Spilanthes acmella on Murine Macrophage by Down-Regulating LPS-Induced Inflammatory Mediators. J. Agric. Food Chem. 2008, 56, 2341–2349. [Google Scholar] [CrossRef]
- Soares, C.P.; Lemos, V.R.; Silva, A.G.; Campoy, R.M.; Silva, C.A.P.; Menegon, R.F.; Rojahn, I.; Joaquim, W.M. Effect of Spilanthes acmella hydroethanolic extract activity on tumour cell actin cytoskeleton. Cell Biol. Int. 2014, 38, 131–135. [Google Scholar] [CrossRef]
- Abeysinghe, D.C.; Wijerathne, S.M.N.K.; Dharmadasa, R.M. Secondary Metabolites Contents and Antioxidant Capacities of Acmella Oleraceae Grown under Different Growing Systems. World J. Agric. Res. 2014, 2, 163–167. [Google Scholar] [CrossRef][Green Version]
- Ratnasooriya, W.D.; Pieris, K.P.P.; Samaratunga, U.; Jayakody, J.R.A.C. Diuretic activity of Spilanthes acmella flowers in rats. J. Ethnopharmacol. 2004, 91, 317–320. [Google Scholar] [CrossRef] [PubMed]
- Daisy, M.J.; Raju, A.; Subin, M. Qualitative Phytochemical Analysis and in vitro Antibacterial Activity of Acmella ciliata (H.B.K) Cassini and Ichnocarpus frutescens (Linn.) R.Br. Against Two Pathogenic Bacteria. Nat. Environ. Pollut. Technol. Int. Q. Sci. J. 2012, 12, 167–170. [Google Scholar]
- Rani, S.; Murty, S. Antifungal potential of flower head extract of Spilanthes acmella Linn. Afr. J. Biomed. Res. 2006, 9, 67–69. [Google Scholar] [CrossRef]
- Cheng, Y.B.; Liu, R.H.; Ho, M.C.; Wu, T.Y.; Chen, C.Y.; Lo, I.W.; Hou, M.; Yuan, S.; Wu, Y.; Chang, F. Alkylamides of Acmella oleracea. Molecules 2015, 20, 6970–6977. [Google Scholar] [CrossRef]
- Abeysiri, G.R.P.I.; Dharmadasa, R.M.; Abeysinghe, D.C.; Samarasinghe, K. Screening of phytochemical, physico-chemical and bioactivity of different parts of Acmella oleraceae Murr. (Asteraceae), a natural remedy for toothache. Ind. Crops Prod. 2013, 50, 852–856. [Google Scholar] [CrossRef]
- Ley, J.P.; Blings, M.; Krammer, G.; Reinders, G.; Schmidt, C.O.; Bertram, H.J. Isolation and synthesis of acmellonate, a new unsaturated long chain 2-ketol ester from Spilanthes acmella. Nat. Prod. Res. 2006, 20, 798–804. [Google Scholar] [CrossRef]
- Maria-Ferreira, D.; Silva, L.M.; Mendes, D.A.G.B.; Cabrini, D.A.; Nascimento, A.M.; Iacomini, M.; Cipriani, T.R.; Santos, A.R.S.; Werner, M.F.P.; Baggio, C.H. Rhamnogalacturonan from Acmella oleracea (L.) R.K. Jansen: Gastroprotective and Ulcer Healing Properties in Rats. PLoS ONE 2014, 9, e84762. [Google Scholar] [CrossRef]
- Huang, W.C.; Peng, H.L.; Hu, S.; Wu, S.J. Spilanthol from Traditionally Used Spilanthes acmella Enhances AMPK and Ameliorates Obesity in Mice Fed High-Fat Diet. Nutrients 2019, 11, 991. [Google Scholar] [CrossRef]
- Rahim, R.A.; Jayusman, P.A.; Lim, V.; Ahmad, N.H.; Hamid, Z.A.A.; Mohamed, S.; Muhammad, N.; Ahmad, F.; Mokhtar, N.; Mohamed, N.; et al. Phytochemical Analysis, Antioxidant and Bone Anabolic Effects of Blainvillea acmella (L.) Philipson. Front. Pharmacol. 2022, 12, 796509. [Google Scholar] [CrossRef] [PubMed]
- Wrona, M.; Silva, F.; Salafranca, J.; Nerín, C.; Alfonso, M.J.; Caballero, M.Á. Design of new natural antioxidant active packaging: Screening flowsheet from pure essential oils and vegetable oils to ex vivo testing in meat samples. Food Control 2021, 120, 107536. [Google Scholar] [CrossRef]
- Bertan, D.W.; Aparecida, G.L.; Bonilla, J.; Lourenço, R.V.; Bittante, A.M.Q.B.; Sobral, P.J.A. Boldo (Peumus boldus) leaf’s hydroethanolic extracts on gelatin-based active films. J. Food Process. Preserv. 2021, 45, e15936. [Google Scholar] [CrossRef]
- Galindo, M.V.; Paglione, I.S.; Balan, G.C.; Sakanaka, L.S.; Shirai, M.A. Atividade antimicrobiana e antioxidante de filmes comestíveis de gelatina e quitosana adicionados de óleos essenciais. Segurança Aliment. Nutricional. 2019, 26, e019008. [Google Scholar] [CrossRef]
- Damani, M.H.; Partovi, R.; Shahavi, M.H.; Azizkhani, M. Nanoemulsions of Trachyspermum copticum, Mentha pulegium and Satureja hortensis essential oils: Formulation, physicochemical properties, antimicrobial and antioxidant efficiency. Food Meas. 2022, 16, 1807–1819. [Google Scholar] [CrossRef]
- Sobhan, A.; Muthukumarappan, K.; Wei, L. Biosensors and biopolymer-based nanocomposites for smart food packaging: Challenges and opportunities. Food Packag. Shelf Life 2021, 30, 100745. [Google Scholar] [CrossRef]
- Estevez-Areco, S.; Guz, L.; Candal, R.; Goyanes, S. Release kinetics of rosemary (Rosmarinus officinalis) polyphenols from polyvinyl alcohol (PVA) electrospun nanofibers in several food simulants. Food Packag. Shelf Life 2018, 18, 42–50. [Google Scholar] [CrossRef]
- Kaya, M.; Khadem, S.; Cakmak, Y.S.; Mujtaba, M.; Ilk, S.; Akyuz, L.; Salaberria, A.M.; Labidi, J.; Abdulqadir, A.H.; Deligöz, E. Antioxidative and antimicrobial edible chitosan films blended with stem, leaf and seed extracts of Pistacia terebinthus for active food packaging. RSC Adv. 2018, 8, 3941–3950. [Google Scholar] [CrossRef]
- Priyadarshi, R.; Sauraj; Kumar, B.; Deeba, F.; Kulshreshtha, A.; Negi, Y.S. Chitosan films incorporated with Apricot (Prunus armeniaca) kernel essential oil as active food packaging material. Food Hydrocoll. 2018, 85, 158–166. [Google Scholar] [CrossRef]
- Bolumar, T.; LaPeña, D.; Skibsted, L.H.; Orlien, V. Rosemary and oxygen scavenger in active packaging for prevention of high-pressure induced lipid oxidation in pork patties. Food Packag. Shelf Life 2016, 7, 26–33. [Google Scholar] [CrossRef]
- Eltabakh, M.; Kassab, H.; Badawy, W.; Abdin, M.; Abdelhady, S. Active Bio-composite Sodium Alginate/Maltodextrin Packaging Films for Food Containing Azolla pinnata Leaves Extract as Natural Antioxidant. J. Polym. Environ. 2022, 30, 1355–1365. [Google Scholar] [CrossRef]
- Kanatt, S.R. Development of active/intelligent food packaging film containing Amaranthus leaf extract for shelf life extension of chicken/fish during chilled storage. Food Packag. Shelf Life 2020, 24, 100506. [Google Scholar] [CrossRef]
- Guo, Q.; Yuan, Y.; He, M.; Zhang, X.; Li, L.; Zhang, Y.; Li, B. Development of a multifunctional food packaging for meat products by incorporating carboxylated cellulose nanocrystal and beetroot extract into sodium alginate films. Food Chem. 2023, 415, 135799. [Google Scholar] [CrossRef] [PubMed]
- Silveira, N.; Sandjo, L.P.; Biavatti, M.W. Spilanthol-containing products: A patent review (1996–2016). Trends Food Sci. Technol. 2018, 74, 107–111. [Google Scholar] [CrossRef]
- González-Peña, M.A.; Ortega-Regules, A.E.; Parrodi, C.A.; Lozada-Ramírez, J.D. Chemistry, Occurrence, Properties, Applications, and Encapsulation of Carotenoids—A Review. Plants 2023, 12, 313. [Google Scholar] [CrossRef]
- Sharma, S.; Barkauskaite, S.; Jaiswal, A.K.; Jaiswal, S. Essential oils as additives in active food packaging. Food Chem. 2021, 343, 128403. [Google Scholar] [CrossRef]
- Sankaran, S.; Panigrahi, S.; Mallik, S. Odorant binding protein based biomimetic sensors for detection of alcohols associated with Salmonella contamination in packaged beef. Biosens. Bioelectron. 2011, 26, 3103–3109. [Google Scholar] [CrossRef]
- Bajer, D.; Burkowska-But, A. Innovative and environmentally safe composites based on starch modified with dialdehyde starch, caffeine, or ascorbic acid for applications in the food packaging industry. Food Chem. 2022, 374, 131639. [Google Scholar] [CrossRef]
- Gao, J.; Zhu, Y.; Luo, F. Effects of ethanol combined with ascorbic acid and packaging on the inhibition of browning and microbial growth in fresh-cut Chinese yam. Food Sci. Nutr. 2018, 6, 998–1005. [Google Scholar] [CrossRef]
- Wołosiak-Hnat, A.; Zych, K.; Mężyńska, M.; Kifonidis, A.; Dajworski, M.; Lisiecki, S.; Bartkowiak, A. LDPE/PET laminated films modified with FeO(OH) × H2O, Fe2O3, and ascorbic acid to develop oxygen scavenging system for food packaging. Packag. Technol. Sci. 2019, 32, 457–469. [Google Scholar] [CrossRef]
- Salleh, W.M.N.H.W.; Kammil, M.F.; Ahmad, F.; Sirat, H.M. Antioxidant and Anti-inflammatory Activities of Essential Oil and Extracts of Piper miniatum. Nat. Product. Commun. 2015, 10, 2005–2008. [Google Scholar]
- Costa, E.V.; Dutra, L.M.; Jesus, H.C.R.; Nogueira, P.C.L.; Moraes, V.R.S.; Salvador, M.J.; Cavalcanti, S.C.H.; Santos, R.L.C.; Prata, A.P.N. Chemical Composition and Antioxidant, Antimicrobial, and Larvicidal Activities of the Essential Oils of Annona salzmannii and A. pickelii (Annonaceae). Nat. Product. Commun. 2011, 6, 907–912. [Google Scholar] [CrossRef]
- Sarikurkcu, C.; Ozer, M.S.; Cakir, A.; Eskici, M.; Mete, E. GC/MS Evaluation and In Vitro Antioxidant Activity of Essential Oil and Solvent Extracts of an Endemic Plant Used as Folk Remedy in Turkey: Phlomis bourgaei Boiss. Evid. -Based Complement. Altern. Med. 2013, 2013, 293080. [Google Scholar] [CrossRef]
- Xiao, J.; Zhang, M.; Wang, W.; Teng, A.; Liu, A.; Ye, R.; Liu, Y.; Wang, K.; Ding, J.; Wu, X. An Attempt of Using β-Sitosterol-Corn Oil Oleogels to Improve Water Barrier Properties of Gelatin Film. J. Food Sci. 2019, 84, 1447–1455. [Google Scholar] [CrossRef] [PubMed]
- Hayes, J.E.; Stepanyan, V.; Allen, P.; O’Grady, M.N.; Kerry, J.P. Effect of lutein, sesamol, ellagic acid and olive leaf extract on the quality and shelf-life stability of packaged raw minced beef patties. Meat Sci. 2010, 84, 613–620. [Google Scholar] [CrossRef] [PubMed]
- Latos-Brozio, M.; Masek, A. The application of natural food colorants as indicator substances in intelligent biodegradable packaging materials. Food Chem. Toxicol. 2020, 135, 110975. [Google Scholar] [CrossRef]
- LakshmiBalasubramaniam, S.; Howell, C.; Tajvidi, M.; Skonberg, D. Characterization of novel cellulose nanofibril and phenolic acid-based active and hydrophobic packaging films. Food Chem. 2022, 374, 131773. [Google Scholar] [CrossRef]
- Masek, A.; Latos, M.; Piotrowska, M.; Zaborski, M. The potential of quercetin as an effective natural antioxidant and indicator for packaging materials. Food Packag. Shelf Life 2018, 16, 51–58. [Google Scholar] [CrossRef]
- Mohebi, E.; Marquez, L. Intelligent packaging in meat industry: An overview of existing solutions. J. Food Sci. Technol. 2015, 52, 3947–3964. [Google Scholar] [CrossRef]
- Yao, Y.; Ding, D.; Shao, H.; Peng, Q.; Huang, Y. Antibacterial Activity and Physical Properties of Fish Gelatin-Chitosan Edible Films Supplemented with D-Limonene. Int. J. Polym. Sci. 2017, 2017, 1837171. [Google Scholar] [CrossRef]
- Braga, L.R.; Pérez, L.M.; Soazo, M.D.V.; Machado, F. Evaluation of the antimicrobial, antioxidant and physicochemical properties of Poly(Vinyl chloride) films containing quercetin and silver nanoparticles. LWT 2019, 101, 491–498. [Google Scholar] [CrossRef]
- Huang, T.; Lin, J.; Fang, Z.; Yu, W.; Li, Z.; Xu, D.; Yang, W.; Zhang, J. Preparation and characterization of irradiated kafirin-quercetin film for packaging cod (Gadus morhua) during cold storage at 4 °C. Food Bioprocess Technol. 2020, 13, 522–532. [Google Scholar] [CrossRef]
- Vinhal, G.L.R.R.B.; Silva-Pereira, M.C.; Teixeira, J.A.; Barcia, M.T.; Pertuzatti, P.B.; Stefani, R. Gelatine/PVA copolymer film incorporated with quercetin as a prototype to active antioxidant packaging. J. Food Sci. Technol. 2021, 58, 3924–3932. [Google Scholar] [CrossRef]
- Andrés-Bello, A.; Barreto-Palacios, V.; García-Segovia, P.; Mir-Bel, J.; Martínez-Monzó, J. Effect of pH on Color and Texture of Food Products. Food Eng. Rev. 2013, 5, 158–170. [Google Scholar] [CrossRef]
- Demirhan, B.; Candoĝan, K. Active packaging of chicken meats with modified atmosphere including oxygen scavengers. Poult. Sci. 2017, 96, 1394–1401. [Google Scholar] [CrossRef] [PubMed]
- Naqash, S.; Naqash, F.; Fayaz, S.; Khan, S.; Dar, B.N.; Makroo, H.A. Application of Natural Antimicrobial Agents in Different Food Packaging Systems and Their Role in Shelf-life Extension of Food: A Review. J. Package Technol. Res. 2022, 6, 73–89. [Google Scholar] [CrossRef]
- Uthpala, T.G.G.; Navaratne, S.B. Acmella oleracea Plant; Identification, Applications and Use as an Emerging Food Source—Review. Food Rev. Int. 2021, 37, 399–414. [Google Scholar] [CrossRef]
- Bhargava, N.; Sharanagat, V.S.; Mor, R.S.; Kumar, K. Active and intelligent biodegradable packaging films using food and food waste-derived bioactive compounds: A review. Trends Food Sci. Technol. 2020, 105, 385–401. [Google Scholar] [CrossRef]
- EFSA (European Food Safety Authority). Scientific Opinion on Flavouring Group Evaluation 303, Revision 1 (FGE.303Rev1): Spilanthol from chemical group 30. EFSA J. 2015, 13, 3995. [Google Scholar] [CrossRef]

| Classification | Name |
|---|---|
| Phylum | Plantae |
| Division | Magnoliophyta |
| Class | Magnoliopsida ou dicotiledônea |
| Order | Asterales |
| Family | Asteraceae |
| Gender | Acmella |
| Species | Acmella oleracea (L.) R. K. Jansen |
| Compounds | Seal et al. [26] | Neves et al. [27] | Gomes et al. [28] |
|---|---|---|---|
| Moisture (%) | 87.6 ± 0.02 (w. b.) | 89.9 ± 0.44 (w. b.) | 92.9 ± 0.8 (w. b.) |
| Lipids (%) | 0.6 ± 0.03 (d. b.) | 1.5 ± 0.08 (d. b.) | 1.1 ± 0.1 (w. b.) |
| Protein (%) | 27.0 ± 0.03 (d. b.) | 24.1 ± 0.99 (d. b.) | 3.4 ± 0.2 (w. b.) |
| Carbohydrates (%) | 53.8 ± 0.06 (d. b.) | 63.4 ± 1.20 (d. b.) | |
| Ashes (%) | 12.5 ± 0.02 (d. b.) | 10.9 ± 0.10 (d. b.) | 1.4 ± 0.07 (w. b.) |
| Total fiber (%) | 62.6 ± 0.45 (d. b.) | ||
| Minerals (mg/100 g) | |||
| Ca | 2551.6 ± 73.6 (d. b.) | ||
| Mg | 734.6 ± 4.7 (d. b.) | ||
| Fe | 19.1 ± 0.4 (d. b.) | ||
| Zn | 9.3 ± 0.2 (d. b.) | ||
| K | 5833.5 ± 392.7 (d. b.) | ||
| Cu | 2.1 ± 0.0 (d. b.) | ||
| Mn | 10.4 ± 0.5 (d. b.) | ||
| Na | 15.9 ± 1.5 (d. b.) |
| Property | Main Information | Reference |
|---|---|---|
| Antioxidant | The therapeutic effect is mainly due to secondary metabolites with antioxidant activity present in different parts of the plant. | [54] |
| Anti-inflammatory | Several mechanisms, such as inhibition of chymase activity, suppression of the pro-inflammatory cytokine nitric oxide and antioxidant activities. | [46,47] |
| Sialagogue | When consumed, jambu’s flowers and leaves have a spicy flavor and cause a slight tingling and numbness of the tongue, followed by an increase in salivation called the sialagogue effect. The sensory effect is related to the structure of alkylamides. | [55] |
| Antiulcerogenic | Ramnogalacturonan (RGal) present in jambu has antiulcerogenic activity against acute lesions induced by ethanol. In the chronic ulcer model, oral administration of RGal accelerated gastric ulcer healing and increased cell proliferation and gastric mucus content, reducing inflammatory parameters and oxidative stress. | [40,56] |
| Antimutagenic | Jambu extract has proven efficacy in the cell adhesion process and the metabolism of tumor cells. | [48] |
| Bactericidal | The methanolic extract of leaves is a potential antibacterial agent against Bacillus subtilis and Escherichia coli. | [51] |
| Antifungal | Among the different fungal species, zones of inhibition were high in Fusarium oxysporium (2.3 cm), Fusarum moniliformis (2.1 cm), Aspergillus niger (2.0 cm) and Aspergillus paraciticus (1.8 cm). | [52] |
| Diuretic | It may cause a marked increase in urinary Na+ and K+ levels and a decrease in urine osmolarity, acting primarily as a diuretic in the Henle loop. It may also inhibit antidiuretic hormone (ADH) release and action. | [50] |
| Anti-obesity | The bioactive compounds can significantly inhibit the intracellular accumulation of lipids and significantly reduce lipogenesis-related proteins’ expression, including acetyl-CoA carboxylase and fatty acid synthase, and attenuating lipogenic and adipogenic transcription factors providing antiobesity effects. | [57] |
| Anti-osteoporosis | Compounds present in the extract such as α-cubebene terpenoids, caryophyllene, caryophyllene oxide, phytol and flavonoids of pinostrobin and apigenin were the compounds that contributed to the bone antioxidant and anabolic effects. | [58] |
| Extract Vegetable | Applications | Reference |
|---|---|---|
| Extracts of green tea leaves, rosemary leaves, cinnamon bark, fennel seeds, clove flowers, lemon balm leaves and curcumin rhizomes | Various aromatic natural extracts from different parts of plants (leaf, flower, seed, bark and rhizome) were evaluated for thermal stability, antioxidant, antimicrobial and antifungal activities. Leaves, seeds and rhizomes were the best candidates to incorporation into the polymeric matrix of active food packaging. | [10] |
| Green tea extract (Camellia sinensis) | The films showed antioxidant behavior and also better barrier properties (decrease of up to 6.25% of water vapor and 55.78% of the oxygen transition rate) and better mechanical properties (increase of 14.96%, 38.89% and 8.75% in elastic modulus, tensile strength and elongation at break, respectively). | [9] |
| Boldo leaf (Peumus boldus) | Films based on gelatin showed antioxidant activity provided by addition of hydroethanolic extracts of boldo leaf. | [60] |
| Linseed oil (Linum usitassimum); ginger root essential oil (Zingiber officinalis); grape seed essential oil and rose oil (Rosa eglanteria) | The addition of these agents is possible in active packaging films for fresh meat and application on an industrial scale is also possible. The optimal packaging corresponded to a 50 μm low-density polyethylene (LDPE) film with linseed oil that could prolong the shelf life of fresh meat by 22%. | [59] |
| Rosemary extract (Rosmarinus officinalis L.) | Polyalcohol films vinyl (PVA) combined with plant extract of rosemary for application in food packaging providing antioxidant action. The PVA/Rosemary films showed a phenolic compounds content of 15.4 ± 0.5 mgEAG/g and achieved an antioxidant activity of 120 ± 8 μmol ET/g, showing promising values as an antioxidant agent. | [64] |
| Methanolic extracts of stem, leaf and seed of Pistachio terebinthus | The addition of plant extracts in chitosan films effectively improved the antioxidant and antimicrobial activity. This plant extract has flavonoids with anti-inflammatory and antioxidant action, as well as omega-3 fatty acids, tannins and other valuable compounds. | [65] |
| Apricot kernel essential oil (Prunus Armenian) | The addition of essential oil caused an increase in the water vapor barrier. The mechanical test revealed an increase in elongation. The antibacterial and antioxidant characteristics yielded superior results to pure chitosan film. | [66] |
| Rosemary extract (Rosmarinus officinalis L.) | Active packaging containing films with rosemary extract was effective in protecting pork burgers from lipid oxidation. | [67] |
| Natural extract from brewery waste and commercial rosemary extract (Rosmarinus officinalis L.) | Applying films containing natural extracts in active packaging can improve the oxidative stability of meat products. | [12] |
| Phenolic extract of fern leaves (Azolla pinnata) | The films exhibited satisfactory antioxidant and antibacterial activity. The most substantial effect of eliminating free radicals was attributed to the presence of phenolic compounds. The antimicrobial properties were attributed to the presence of ferulic acid, rutin, thiamine, tamarixetin, astragalin, quercetin, chlorogenic acid and epicatechin present in the extracts. | [68] |
| Amaranth leaf extract | The film with active and “intelligent” property developed with polyvinyl alcohol (PVA), gelatin and amaranth leaf extract showed a high concentration of phenolic compounds with potential as an antioxidant. The films showed antioxidant and antimicrobial behavior, indicating the freshness of fish and chicken meat. More protection against ultraviolet light, reduced water solubility, water vapor permeability and better mechanical properties were observed. The color change occurred with the decrease in the quality of the food due to the change in the pH of the medium. In addition, active films had a lifespan four times longer than pure films. | [69] |
| Carboxylated cellulose and beetroot extract | The literature reports the development of sodium alginate films with carboxylated cellulose nanocrystal (C-CNC) and beet extract to obtain active and “intelligent” films. The incorporation of C-CNC can improve the mechanical properties of the films. Beet extract can add antioxidant properties and pH responsiveness without significantly altering the thermal stability of the film. | [70] |
| Properties | Active Materials | |||
|---|---|---|---|---|
| Biopolymers A,C | Nanomaterials | Essential Oils D | Jambu Bioactive Compounds B | |
| Antioxidants | Yes/No | No | Yes | Yes |
| Antimicrobial | Yes/No | Yes | Yes | Yes |
| Sustainable | Yes | No | Yes | Yes |
| Bioactive Compounds from Jambu | Application Potential | Reference |
|---|---|---|
| Alkylamides: (2E,6Z,8E)-N-Isobutyl-2,6,8-decatrienamide (spilanthol); Undeca-2E,7Z,9E-trienoic acid isobutylamide Undeca-2E-en-8,10-diyonic acid isobutylamide; 2E-N-(2-Methylbutyl)-2-undecene-8,10-diynamide; 2E,7Z-N-Isobutyl-2,7-tridecadiene-10,12-diynamide; 7Z-N-Isobutyl-7-tridecene-10,12-diynamide | - They have antioxidant and antimicrobial action for application in active and “intelligent” packaging films; - They detect pathogenic bacteria such as E. coli and Salmonella. Applicable in packaging for fish, meat and dairy products. | [29,36,46,63,74] |
| Acid ascorbic | - Stimulates the formation of the crystalline structure and improves the hydrophilicity and antioxidant activity of films made of starch; - Can be applied in oxygen scavenging system for active food packaging. In this system, the oxygen scavenging reaction occurs through the oxidation of ascorbate to dehydroascorbic acid, which is catalyzed by the reduction of Fe3+ ions to Fe2+. - Can improve the UV-Vis light absorption capacity of whey protein films, a relevant improvement to protect foods susceptible to UV-Vis light-induced lipid oxidation. | [8,75,76,77] |
| α-Cubebene | Application in active antioxidant packaging. | [78] |
| β-Caryophyllene; caryophyllene oxide | Application in active antioxidant packaging. | [33,79,80] |
| β-sitosterol | It decreases gelatin films’ water vapor permeability and has little negative influence on film strength. Furthermore, sitosterol can increase the number of ordered crystals in the film, which contributes to compacting and smoothing the material’s surface. | [81] |
| β- carotene; Lutein; Chlorophyll | - They can be used as indicators, changing color under the influence of external factors and giving materials the characteristics of “intelligent” packaging, where color changes indicate the lifetime of materials. - The lutein present in leaf extracts can reduce the oxidation of oxymyoglobin in raw meat; - They can be applied as gas sensors due to their chemosensitive characteristic. Packaging for fruits, vegetables and foods susceptible to rancidity is the most promising option. | [6,82,83] |
| Phenolic Compounds | - They feature attractive and bright colors and are susceptible to temperature changes. Therefore, its application is Time/Temperature (ITT) indicators in “intelligent” packaging. It is applied to frozen and chilled meats. - They can react with non-volatile metabolites produced during the deterioration of food, functioning as a pH indicator. The application is mainly to fish, meat and poultry packaging. - Can improve hydrophobicity and provide additional active functionalities such as antioxidant properties to active and hydrophobic packaging films. | [11,84,85,86] |
| Germacrene D; Limonene | These compounds, present in plant extracts, can be incorporated into food packaging materials to increase the shelf life of foods due to their antimicrobial and antioxidant properties. Limonene in films can provide resistance to light and water penetration due to the molecule’s hydrophobicity. Elongation at break also increases with limonene, indicating that this compound utilization is a strong plasticizer. Film with limonene also exhibits strong antibacterial activity against Escherichia coli. | [58,62,87] |
| Quercetin | - Oxidation causes a change in the color of quercetin and, therefore, can serve as a colorimetric indicator compound during food storage. Cycloolefin copolymer films containing quercetin exhibited color changes with variations in climatic factors such as weathering and thermo-oxidation. | [88,89,90] |
| Essential oils | Essential oils could eliminate free radicals and improve the barrier capacity of the packaging film. It is important to emphasize that different plants present, in their essential oils, different bioactive compounds that provide different responses to the functional properties of the package. | [73] |
| Spilantol | Present in the jambu extract, it is presented as an antimicrobial, antifungal and antioxidant agent, being a promising biomolecule for incorporation into films or coatings of active packaging of foods conducive to deterioration by fungi and bacteria and also oxidative deterioration. | [10,51,52,54] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moura, J.d.S.; Gemaque, E.d.M.; Bahule, C.E.; Martins, L.H.d.S.; Chisté, R.C.; Lopes, A.S. Bioactive Compounds of Jambu (Acmella oleracea (L.) R. K. Jansen) as Potential Components of Biodegradable Food Packing: A Review. Sustainability 2023, 15, 15231. https://doi.org/10.3390/su152115231
Moura JdS, Gemaque EdM, Bahule CE, Martins LHdS, Chisté RC, Lopes AS. Bioactive Compounds of Jambu (Acmella oleracea (L.) R. K. Jansen) as Potential Components of Biodegradable Food Packing: A Review. Sustainability. 2023; 15(21):15231. https://doi.org/10.3390/su152115231
Chicago/Turabian StyleMoura, Jardilene da Silva, Eveline de Matos Gemaque, Celina Eugenio Bahule, Luiza Helena da Silva Martins, Renan Campos Chisté, and Alessandra Santos Lopes. 2023. "Bioactive Compounds of Jambu (Acmella oleracea (L.) R. K. Jansen) as Potential Components of Biodegradable Food Packing: A Review" Sustainability 15, no. 21: 15231. https://doi.org/10.3390/su152115231
APA StyleMoura, J. d. S., Gemaque, E. d. M., Bahule, C. E., Martins, L. H. d. S., Chisté, R. C., & Lopes, A. S. (2023). Bioactive Compounds of Jambu (Acmella oleracea (L.) R. K. Jansen) as Potential Components of Biodegradable Food Packing: A Review. Sustainability, 15(21), 15231. https://doi.org/10.3390/su152115231







