Enrichment of Microbial Consortium with Hydrogenotrophic Methanogens for Biological Biogas Upgrade to Biomethane in a Bubble Reactor under Mesophilic Conditions
Abstract
:1. Introduction
2. Materials and Methods
2.1. Inoculum and Nutrient Media Preparation
2.2. Experimental Setup
2.3. Analytical Methods and Calculations
2.4. DNA Extraction and 16S rDNA Amplification
2.5. Library Preparation and Illumina MiSeq Sequencing
2.6. Bioinformatics Analysis
3. Results
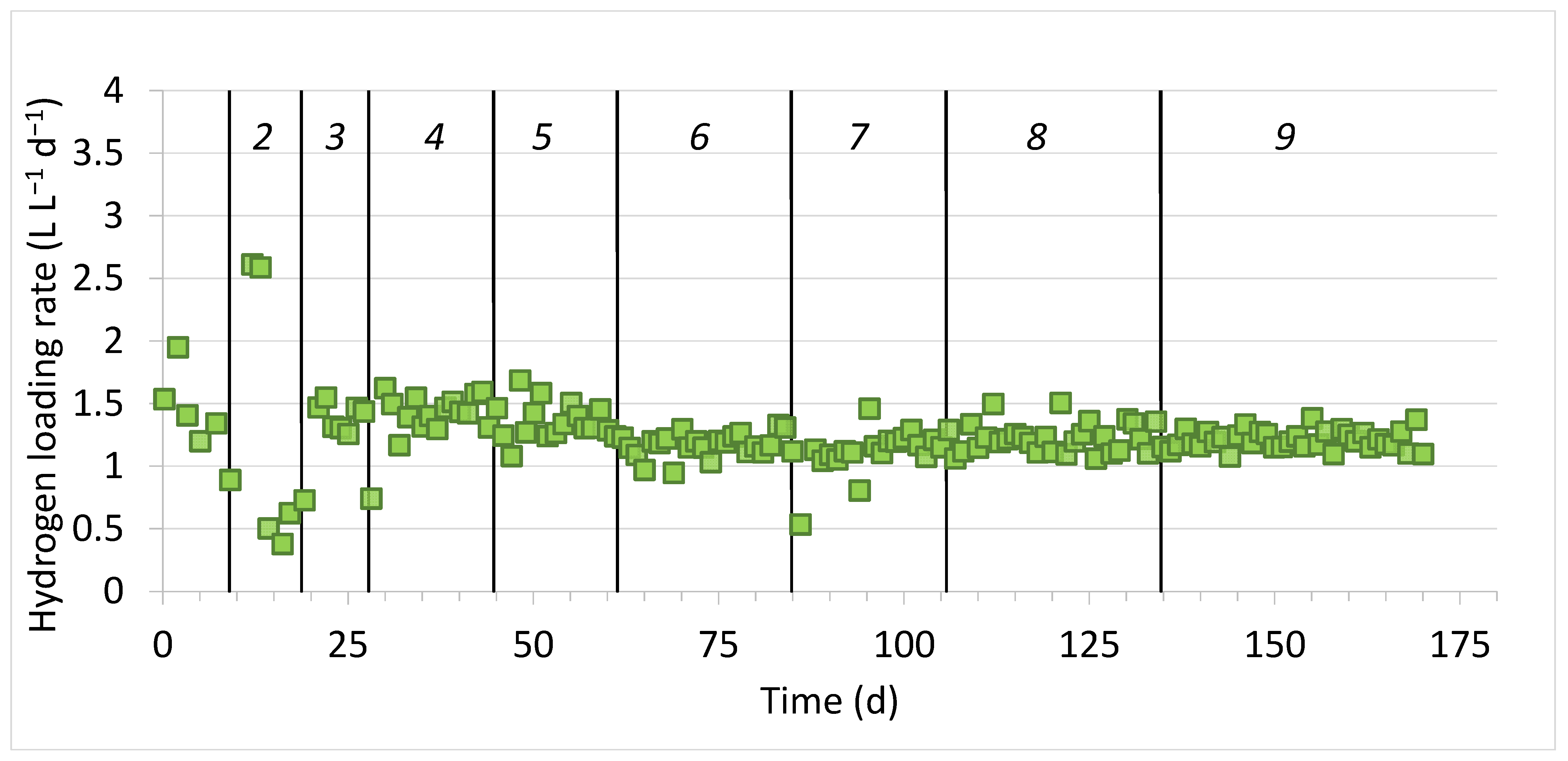
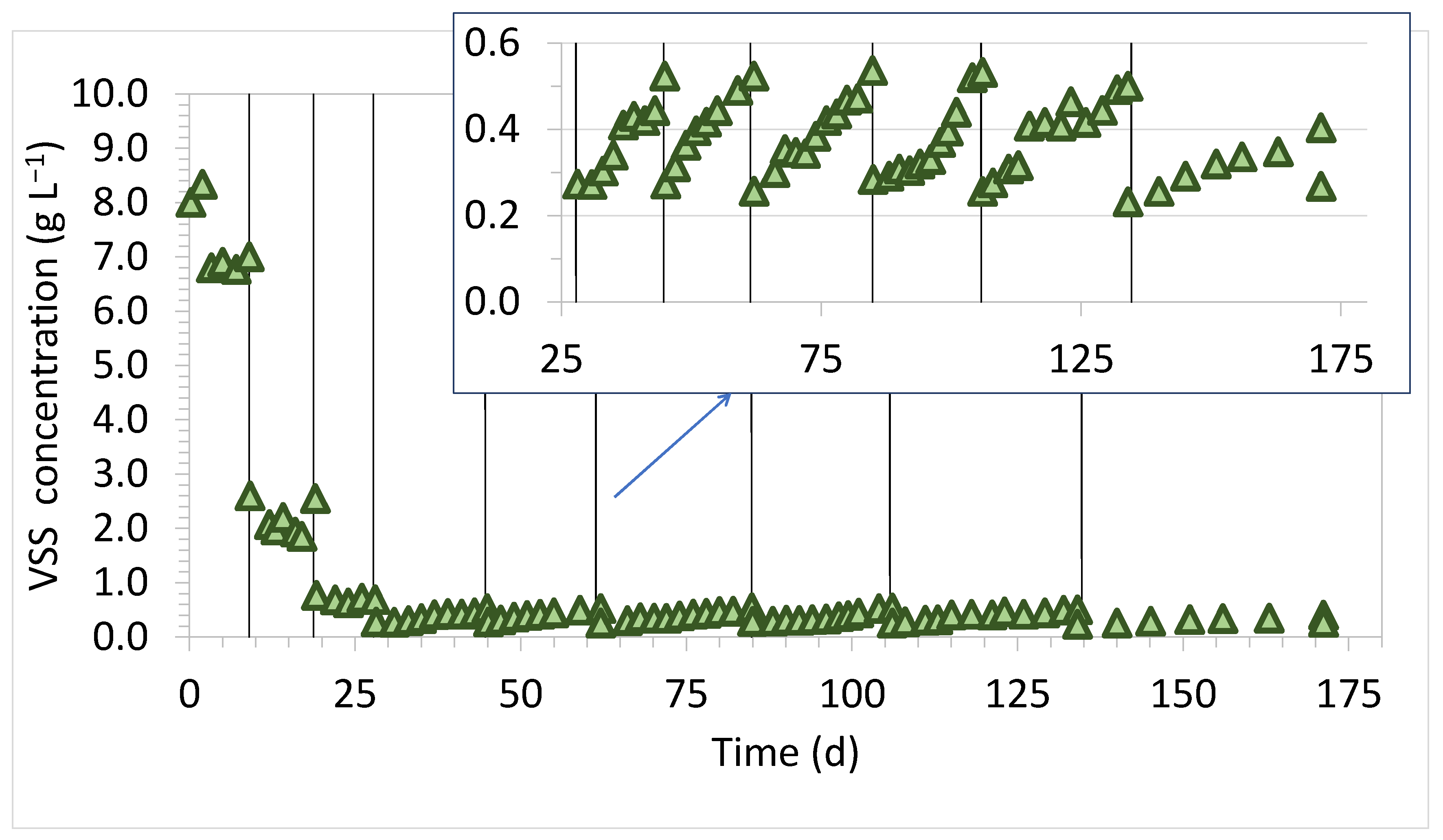
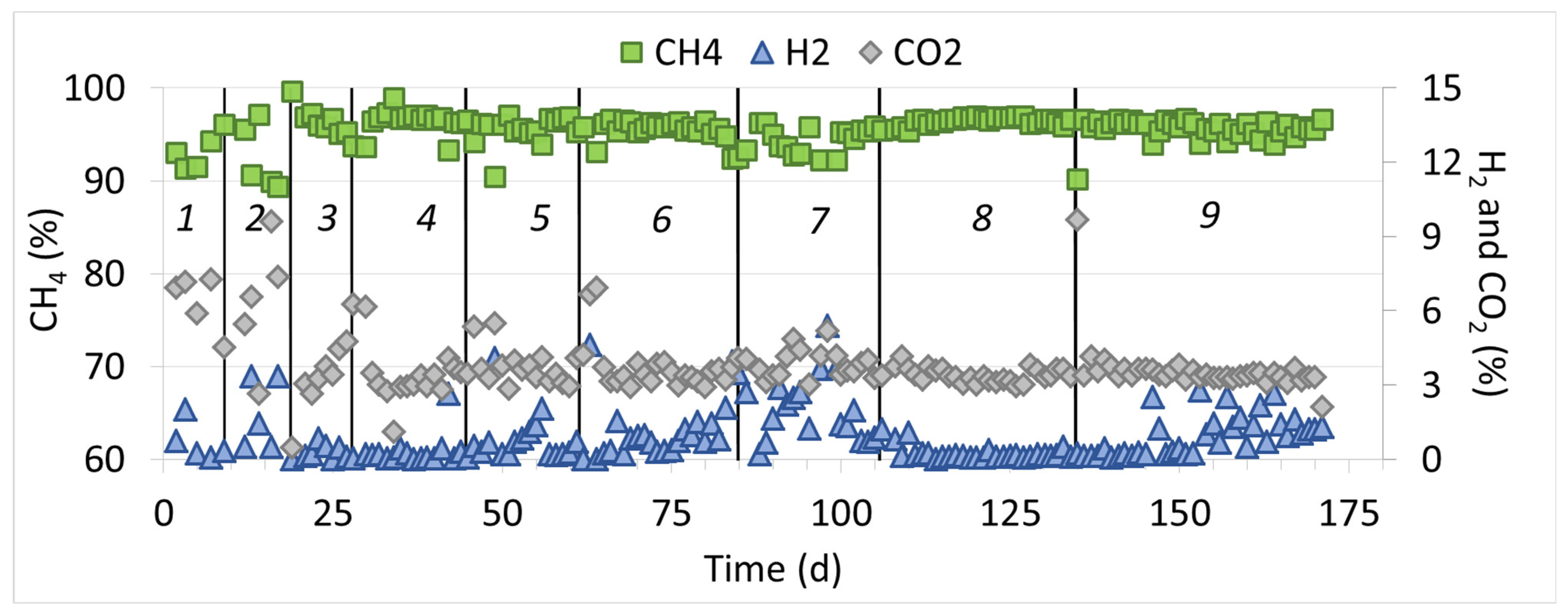
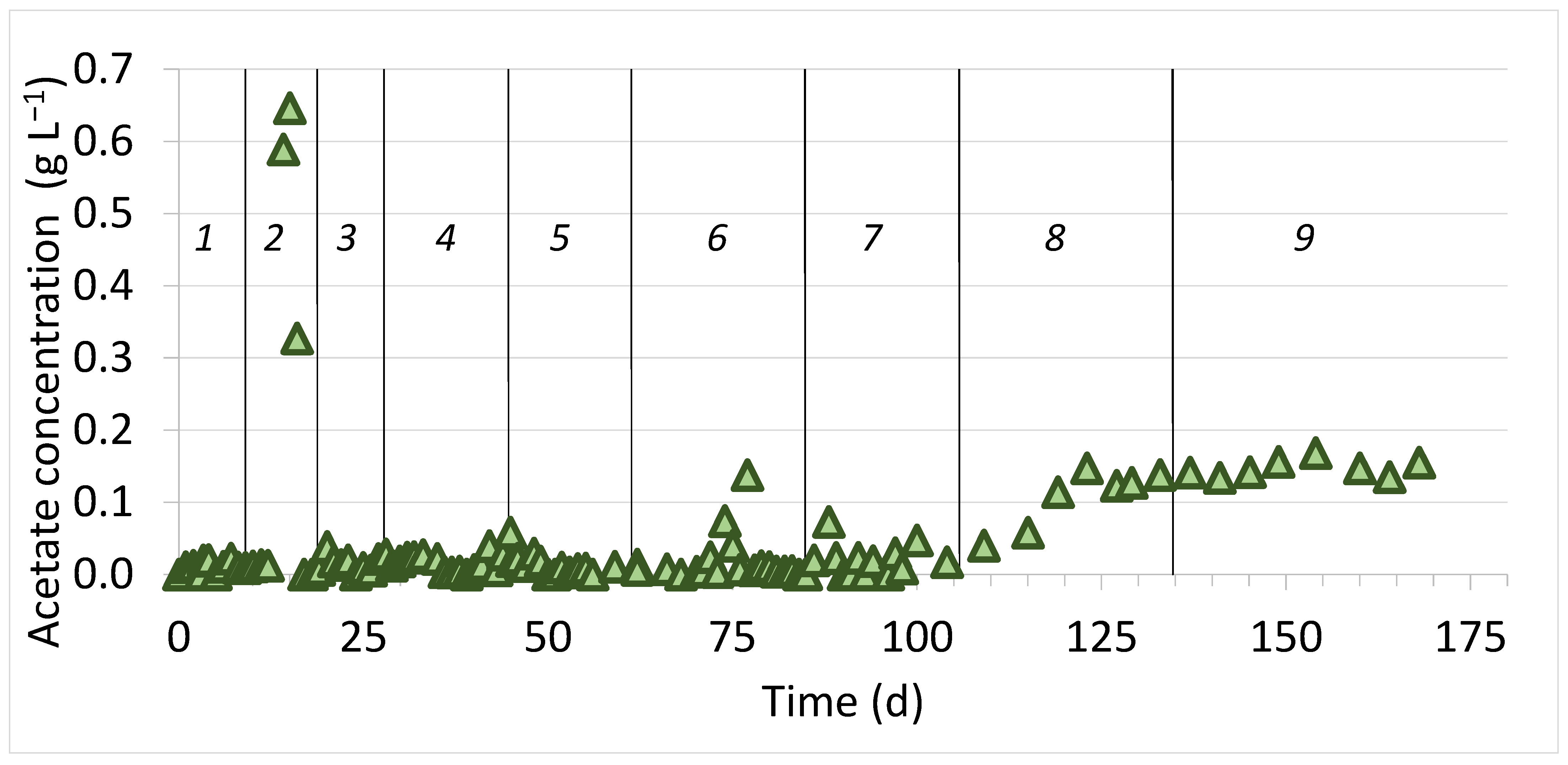
3.1. Bioreactor’s Performance
3.2. Prokaryotic Community Composition
3.2.1. Analysis of Bacterial 16S rRNA Sequences
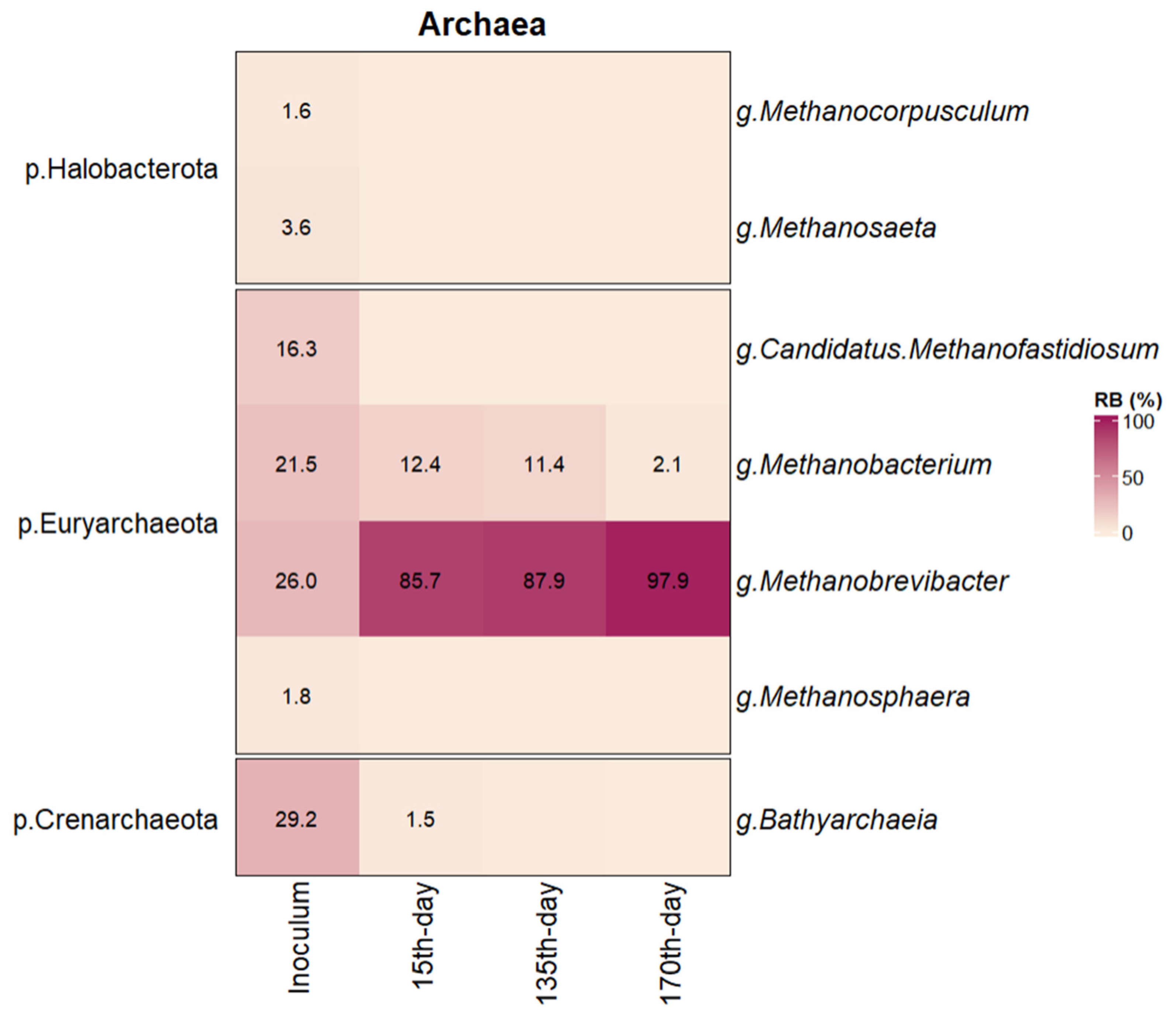
3.2.2. Analysis of Archaeal 16S rRNA Sequences
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Luo, G.; Angelidaki, I. Co-Digestion of Manure and Whey for in Situ Biogas Upgrading by the Addition of H2: Process Performance and Microbial Insights. Appl. Microbiol. Biotechnol. 2013, 97, 1373–1381. [Google Scholar] [CrossRef] [PubMed]
- EBA 2022; EBA Statistical Report 2022. EBA: Brussels, Belgium, 2022.
- EBA 2016; EBA Biomethane in Transport 2016. EBA: Brussels, Belgium, 2016.
- EBA 2023. Brussels, Belgium. Available online: https://www.Europeanbiogas.Eu/Benefits/#growth-Potential (accessed on 14 October 2023).
- D’Adamo, I.; Sassanelli, C. A Mini-Review of Biomethane Valorization: Managerial and Policy Implications for a Circular Resource. Waste Manag. Res. 2022, 40, 1745–1756. [Google Scholar] [CrossRef] [PubMed]
- Taifouris, M.; Martín, M. Towards Energy Security by Promoting Circular Economy: A Holistic Approach. Appl. Energy 2023, 333, 120544. [Google Scholar] [CrossRef]
- D’Adamo, I.; Ribichini, M.; Tsagarakis, K.P. Biomethane as an Energy Resource for Achieving Sustainable Production: Economic Assessments and Policy Implications. Sustain. Prod. Consum. 2023, 35, 13–27. [Google Scholar] [CrossRef]
- Padi, R.K.; Douglas, S.; Murphy, F. Techno-Economic Potentials of Integrating Decentralised Biomethane Production Systems into Existing Natural Gas Grids. Energy 2023, 283, 128542. [Google Scholar] [CrossRef]
- Kupiec, B. Directive (EU) 2018/2001 of the European Parliament and of the Council of 11 December 2018 on the Promotion of the Use of Energy from Renewable Sources. Off. J. Eur. Union 2018. [Google Scholar]
- Angelidaki, I.; Treu, L.; Tsapekos, P.; Luo, G.; Campanaro, S.; Wenzel, H.; Kougias, P.G. Biogas Upgrading and Utilization: Current Status and Perspectives. Biotechnol. Adv. 2018, 36, 452–466. [Google Scholar] [CrossRef]
- Bassani, I.; Kougias, P.G.; Treu, L.; Porté, H.; Campanaro, S.; Angelidaki, I. Optimization of Hydrogen Dispersion in Thermophilic Up-Flow Reactors for Ex Situ Biogas Upgrading. Bioresour. Technol. 2017, 234, 310–319. [Google Scholar] [CrossRef]
- Tsapekos, P.; Treu, L.; Campanaro, S.; Centurion, V.B.; Zhu, X.; Peprah, M.; Zhang, Z.; Kougias, P.G.; Angelidaki, I. Pilot-Scale Biomethanation in a Trickle Bed Reactor: Process Performance and Microbiome Functional Reconstruction. Energy Convers. Manag. 2021, 244, 114491. [Google Scholar] [CrossRef]
- Ghofrani-Isfahani, P.; Tsapekos, P.; Peprah, M.; Kougias, P.; Zhu, X.; Kovalovszki, A.; Zervas, A.; Zha, X.; Jacobsen, C.S.; Angelidaki, I. Ex-Situ Biogas Upgrading in Thermophilic up-Flow Reactors: The Effect of Different Gas Diffusers and Gas Retention Times. Bioresour. Technol. 2021, 340, 125694. [Google Scholar] [CrossRef]
- Jürgensen, L.; Ehimen, E.A.; Born, J.; Holm-Nielsen, J.B. Utilization of Surplus Electricity from Wind Power for Dynamic Biogas Upgrading: Northern Germany Case Study. Biomass Bioenergy 2014, 66, 126–132. [Google Scholar] [CrossRef]
- Alitalo, A.; Niskanen, M.; Aura, E. Biocatalytic Methanation of Hydrogen and Carbon Dioxide in a Fixed Bed Bioreactor. Bioresour. Technol. 2015, 196, 600–605. [Google Scholar] [CrossRef] [PubMed]
- Ashraf, M.T.; Yde, L.; Triolo, J.M.; Wenzel, H. Optimizing the Dosing and Trickling of Nutrient Media for Thermophilic Biomethanation in a Biotrickling Filter. Biochem. Eng. J. 2021, 176, 108220. [Google Scholar] [CrossRef]
- Thapa, A.; Park, J.-G.; Yang, H.-M.; Jun, H.-B. In-Situ Biogas Upgrading in an Anaerobic Trickling Filter Bed Reactor Treating a Thermal Post-Treated Digestate. J. Environ. Chem. Eng. 2021, 9, 106780. [Google Scholar] [CrossRef]
- Ashraf, M.T.; Sieborg, M.U.; Yde, L.; Rhee, C.; Shin, S.G.; Triolo, J.M. Biomethanation in a Thermophilic Biotrickling Filter—pH Control and Lessons from Long-Term Operation. Bioresour. Technol. Rep. 2020, 11, 100525. [Google Scholar] [CrossRef]
- Porté, H.; Kougias, P.G.; Alfaro, N.; Treu, L.; Campanaro, S.; Angelidaki, I. Process Performance and Microbial Community Structure in Thermophilic Trickling Biofilter Reactors for Biogas Upgrading. Sci. Total Environ. 2019, 655, 529–538. [Google Scholar] [CrossRef]
- Jensen, M.B.; Strübing, D.; De Jonge, N.; Nielsen, J.L.; Ottosen, L.D.M.; Koch, K.; Kofoed, M.V.W. Stick or Leave—Pushing Methanogens to Biofilm Formation for Ex Situ Biomethanation. Bioresour. Technol. 2019, 291, 121784. [Google Scholar] [CrossRef]
- Tang, Q.; Xu, J.; Liu, Z.; Huang, Z.; Zhao, M.; Shi, W.; Ruan, W. Optimal the Ex-Situ Biogas Biological Upgrading to Biomethane and Its Combined Application with the Anaerobic Digestion Stage. Energy Sources Part Recovery Util. Environ. Eff. 2021, 43, 2147–2159. [Google Scholar] [CrossRef]
- Lee, J.C.; Kim, J.H.; Chang, W.S.; Pak, D. Biological Conversion of CO2 to CH4 Using Hydrogenotrophic Methanogen in a Fixed Bed Reactor. J. Chem. Technol. Biotechnol. 2012, 87, 844–847. [Google Scholar] [CrossRef]
- Rachbauer, L.; Voitl, G.; Bochmann, G.; Fuchs, W. Biological Biogas Upgrading Capacity of a Hydrogenotrophic Community in a Trickle-Bed Reactor. Appl. Energy 2016, 180, 483–490. [Google Scholar] [CrossRef]
- Wahid, R.; Horn, S.J. The Effect of Mixing Rate and Gas Recirculation on Biological CO2 Methanation in Two-Stage CSTR Systems. Biomass Bioenergy 2021, 144, 105918. [Google Scholar] [CrossRef]
- Ghofrani-Isfahani, P.; Tsapekos, P.; Peprah, M.; Kougias, P.; Zervas, A.; Zhu, X.; Yang, Z.; Jacobsen, C.S.; Angelidaki, I. Ex-Situ Biogas Upgrading in Thermophilic Trickle Bed Reactors Packed with Micro-Porous Packing Materials. Chemosphere 2022, 296, 133987. [Google Scholar] [CrossRef] [PubMed]
- Sieborg, M.U.; Jønson, B.D.; Ashraf, M.T.; Yde, L.; Triolo, J.M. Biomethanation in a Thermophilic Biotrickling Filter Using Cattle Manure as Nutrient Media. Bioresour. Technol. Rep. 2020, 9, 100391. [Google Scholar] [CrossRef]
- Thapa, A.; Park, J.-G.; Jun, H.-B. Enhanced Ex-Situ Biomethanation of Hydrogen and Carbon Dioxide in a Trickling Filter Bed Reactor. Biochem. Eng. J. 2022, 179, 108311. [Google Scholar] [CrossRef]
- Tsapekos, P.; Alvarado-Morales, M.; Angelidaki, I. H2 Competition between Homoacetogenic Bacteria and Methanogenic Archaea during Biomethanation from a Combined Experimental-Modelling Approach. J. Environ. Chem. Eng. 2022, 10, 107281. [Google Scholar] [CrossRef]
- Kougias, P.G.; Treu, L.; Benavente, D.P.; Boe, K.; Campanaro, S.; Angelidaki, I. Ex-Situ Biogas Upgrading and Enhancement in Different Reactor Systems. Bioresour. Technol. 2017, 225, 429–437. [Google Scholar] [CrossRef]
- Figeac, N.; Trably, E.; Bernet, N.; Delgenès, J.-P.; Escudié, R. Temperature and Inoculum Origin Influence the Performance of Ex-Situ Biological Hydrogen Methanation. Molecules 2020, 25, 5665. [Google Scholar] [CrossRef]
- Dupnock, T.L.; Deshusses, M.A. High-Performance Biogas Upgrading Using a Biotrickling Filter and Hydrogenotrophic Methanogens. Appl. Biochem. Biotechnol. 2017, 183, 488–502. [Google Scholar] [CrossRef]
- Fenske, C.F.; Kirzeder, F.; Strübing, D.; Koch, K. Biogas Upgrading in a Pilot-Scale Trickle Bed Reactor—Long-Term Biological Methanation under Real Application Conditions. Bioresour. Technol. 2023, 376, 128868. [Google Scholar] [CrossRef]
- Ebrahimian, F.; Bernardini, N.D.; Tsapekos, P.; Treu, L.; Zhu, X.; Campanaro, S.; Karimi, K.; Angelidaki, I. Effect of Pressure on Biomethanation Process and Spatial Stratification of Microbial Communities in Trickle Bed Reactors under Decreasing Gas Retention Time. Bioresour. Technol. 2022, 361, 127701. [Google Scholar] [CrossRef]
- Logroño, W.; Kluge, P.; Kleinsteuber, S.; Harms, H.; Nikolausz, M. Effect of Inoculum Microbial Diversity in Ex Situ Biomethanation of Hydrogen. Bioengineering 2022, 9, 678. [Google Scholar] [CrossRef] [PubMed]
- Latif, M.A.; Mehta, C.M.; Batstone, D.J. Influence of Low pH on Continuous Anaerobic Digestion of Waste Activated Sludge. Water Res. 2017, 113, 42–49. [Google Scholar] [CrossRef] [PubMed]
- Standard Methods for the Examination of Water and Wastewater, 20th ed.; American Public Health Association/American Water Works Association/Water Environment Federation: Washington DC, USA, 1999.
- Spyridonidis, A.; Skamagkis, T.; Lambropoulos, L.; Stamatelatou, K. Modeling of Anaerobic Digestion of Slaughterhouse Wastes after Thermal Treatment Using ADM1. J. Environ. Manag. 2018, 224, 49–57. [Google Scholar] [CrossRef] [PubMed]
- Klindworth, A.; Pruesse, E.; Schweer, T.; Peplies, J.; Quast, C.; Horn, M.; Glöckner, F.O. Evaluation of General 16S Ribosomal RNA Gene PCR Primers for Classical and Next-Generation Sequencing-Based Diversity Studies. Nucleic Acids Res. 2013, 41, e1. [Google Scholar] [CrossRef] [PubMed]
- Ntougias, S.; Polkowska, Ż.; Nikolaki, S.; Dionyssopoulou, E.; Stathopoulou, P.; Doudoumis, V.; Ruman, M.; Kozak, K.; Namieśnik, J.; Tsiamis, G. Bacterial Community Structures in Freshwater Polar Environments of Svalbard. Microbes Environ. 2016, 31, 401–409. [Google Scholar] [CrossRef]
- Edgar, R.C. Search and Clustering Orders of Magnitude Faster than BLAST. Bioinformatics 2010, 26, 2460–2461. [Google Scholar] [CrossRef]
- Bolyen, E.; Rideout, J.R.; Dillon, M.R.; Bokulich, N.A.; Abnet, C.C.; Al-Ghalith, G.A.; Alexander, H.; Alm, E.J.; Arumugam, M.; Asnicar, F.; et al. Reproducible, Interactive, Scalable and Extensible Microbiome Data Science Using QIIME 2. Nat. Biotechnol. 2019, 37, 852–857. [Google Scholar] [CrossRef]
- Edgar, R.C. UPARSE: Highly Accurate OTU Sequences from Microbial Amplicon Reads. Nat. Methods 2013, 10, 996–998. [Google Scholar] [CrossRef]
- Edgar, R.C. UNCROSS2: Identification of Cross-Talk in 16S rRNA OTU Tables. BioRxiv 2018, 400762. [Google Scholar]
- Camacho, C.; Coulouris, G.; Avagyan, V.; Ma, N.; Papadopoulos, J.; Bealer, K.; Madden, T.L. BLAST+: Architecture and Applications. BMC Bioinform. 2009, 10, 421. [Google Scholar] [CrossRef]
- Quast, C.; Pruesse, E.; Yilmaz, P.; Gerken, J.; Schweer, T.; Yarza, P.; Peplies, J.; Glöckner, F.O. The SILVA Ribosomal RNA Gene Database Project: Improved Data Processing and Web-Based Tools. Nucleic Acids Res. 2012, 41, D590–D596. [Google Scholar] [CrossRef] [PubMed]
- Oksanen, J.; Blanchet, F.G.; Kindt, R.; Legendre, P.; Minchin, P.; O’Hara, B.; Simpson, G.; Solymos, P.; Stevens, H.; Wagner, H. Vegan: Community Ecology Package, R Package Version 2.1-2; 2015. Available online: https://CRAN.R-project.org/package=vegan (accessed on 14 October 2023).
- Wickham, H. Ggplot2: Elegant Graphics for Data Analysis; Use R! Springer International Publishing: Berlin/Heidelberg, Germany, 2016; ISBN 978-3-319-24275-0. [Google Scholar]
- Chen, J.; Bittinger, K.; Charlson, E.; Hoffmann, C.; Lewis, J.; Wu, G.; Collman, R.; Bushman, F.; Li, H. Associating Microbiome Composition with Environmental Covariates Using Generalized UniFrac Distances. Bioinforma. Oxf. Engl. 2012, 28, 2106–2113. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Zhang, X.; Yang, L. GUniFrac: Generalized UniFrac Distances, Distance-Based Multivariate Methods and Feature-Based Univariate Methods for Microbiome Data Analysis. R Package Version 1.3. (Internet). Available online: https://CRAN.R-project.org/package=GUniFrac (accessed on 14 October 2023).
- Anderson, M. A New Method for Non-Parametric Multivariate Analysis of Variance. Austral Ecol. 2001, 26, 32–46. [Google Scholar] [CrossRef]
- Clarke, K.; Gorley, R.N. PRIMER v6: User Manual/Tutorial; PRIMER-E: Plymouth, UK, 2006; Volume 29, pp. 1060–1065. [Google Scholar]
- Anderson, M.; Gorley, R.N.; Clarke, K. PERMANOVA+ for Primer: Guide to Software and Statistical Methods; PRIMER-E: Plymouth, UK, 2008. [Google Scholar]
- Rittmann, B.E.; McCarty, P.L. Environmental Biotechnology: Principles and Applications; McGraw-Hill Education: New York, NY, USA, 2018; ISBN 978-1-260-44059-1. [Google Scholar]
- Chen, Y.; Cheng, J.J.; Creamer, K.S. Inhibition of Anaerobic Digestion Process: A Review. Bioresour. Technol. 2008, 99, 4044–4064. [Google Scholar] [CrossRef]
- Asimakopoulos, K.; Łężyk, M.; Grimalt-Alemany, A.; Melas, A.; Wen, Z.; Gavala, H.N.; Skiadas, I.V. Temperature Effects on Syngas Biomethanation Performed in a Trickle Bed Reactor. Chem. Eng. J. 2020, 393, 124739. [Google Scholar] [CrossRef]
- Giuliano, A.; Cellamare, C.M.; Chiarini, L.; Tabacchioni, S.; Petta, L. Evaluation of the Controlled Hydrodynamic Cavitation as Gas Mass Transfer System for Ex-Situ Biological Hydrogen Methanation. Chem. Eng. J. 2023, 471, 144475. [Google Scholar] [CrossRef]
- Laura, M.; Jo, P. No Acetogen Is Equal: Strongly Different H2 Thresholds Reflect Diverse Bioenergetics in Acetogenic Bacteria. Environ. Microbiol. 2023, 25, 2032–2040. [Google Scholar] [CrossRef]
- Agneessens, L.M.; Ottosen, L.D.M.; Andersen, M.; Berg Olesen, C.; Feilberg, A.; Kofoed, M.V.W. Parameters Affecting Acetate Concentrations during In-Situ Biological Hydrogen Methanation. Bioresour. Technol. 2018, 258, 33–40. [Google Scholar] [CrossRef]
- Hahnke, S.; Langer, T.; Koeck, D.E.; Klocke, M. Description of Proteiniphilum saccharofermentans sp. nov., Petrimonas mucosa sp. nov. and Fermentimonas caenicola gen. nov., sp. nov., Isolated from Mesophilic Laboratory-Scale Biogas Reactors, and Emended Description of the Genus Proteiniphilum. Int. J. Syst. Evol. Microbiol. 2016, 66, 1466–1475. [Google Scholar] [CrossRef]
- Feng, G.; Zeng, Y.; Wang, H.-Z.; Chen, Y.-T.; Tang, Y.-Q. Proteiniphilum and Methanothrix Harundinacea Became Dominant Acetate Utilizers in a Methanogenic Reactor Operated under Strong Ammonia Stress. Front. Microbiol. 2023, 13, 1098814. [Google Scholar] [CrossRef]
- Li, M.-T.; Rao, L.; Wang, L.; Gou, M.; Sun, Z.-Y.; Xia, Z.-Y.; Song, W.-F.; Tang, Y.-Q. Bioaugmentation with Syntrophic Volatile Fatty Acids-Oxidizing Consortia to Alleviate the Ammonia Inhibition in Continuously Anaerobic Digestion of Municipal Sludge. Chemosphere 2022, 288, 132389. [Google Scholar] [CrossRef] [PubMed]
- Nikitina, A.A.; Kallistova, A.Y.; Grouzdev, D.S.; Kolganova, T.V.; Kovalev, A.A.; Kovalev, D.A.; Panchenko, V.; Zekker, I.; Nozhevnikova, A.N.; Litti, Y.V. Syntrophic Butyrate-Oxidizing Consortium Mitigates Acetate Inhibition through a Shift from Acetoclastic to Hydrogenotrophic Methanogenesis and Alleviates VFA Stress in Thermophilic Anaerobic Digestion. Appl. Sci. 2023, 13, 173. [Google Scholar] [CrossRef]
- Sun, Z.; He, J.; Yu, N.; Chen, Y.; Chen, Y.; Tang, Y.; Kida, K. Biomethane Production and Microbial Strategies Corresponding to High Organic Loading Treatment for Molasses Wastewater in an Upflow Anaerobic Filter Reactor. Bioprocess Biosyst. Eng. 2023, 46, 1033–1043. [Google Scholar] [CrossRef] [PubMed]
- Cheng, G.; Gabler, F.; Pizzul, L.; Olsson, H.; Nordberg, Å.; Schnürer, A. Microbial Community Development during Syngas Methanation in a Trickle Bed Reactor with Various Nutrient Sources. Appl. Microbiol. Biotechnol. 2022, 106, 5317–5333. [Google Scholar] [CrossRef]
- Kampmann, K.; Ratering, S.; Kramer, I.; Schmidt, M.; Zerr, W.; Schnell, S. Unexpected Stability of Bacteroidetes and Firmicutes Communities in Laboratory Biogas Reactors Fed with Different Defined Substrates. Appl. Environ. Microbiol. 2012, 78, 2106–2119. [Google Scholar] [CrossRef]
- Murillo-Roos, M.; Uribe-Lorío, L.; Fuentes-Schweizer, P.; Vidaurre-Barahona, D.; Brenes-Guillén, L.; Jiménez, I.; Arguedas, T.; Liao, W.; Uribe, L. Biogas Production and Microbial Communities of Mesophilic and Thermophilic Anaerobic Co-Digestion of Animal Manures and Food Wastes in Costa Rica. Energies 2022, 15, 3252. [Google Scholar] [CrossRef]
- Vanwonterghem, I.; Evans, P.N.; Parks, D.H.; Jensen, P.D.; Woodcroft, B.J.; Hugenholtz, P.; Tyson, G.W. Methylotrophic Methanogenesis Discovered in the Archaeal Phylum Verstraetearchaeota. Nat. Microbiol. 2016, 1, 16170. [Google Scholar] [CrossRef]
- Agrimonti, C.; Visoli, G.; Ferrari, G.; Sanangelantoni, A.M. Comparison of Bacterial And Archaeal Microbiome In Two Bioreactors Fed With Cattle Sewage And Corn Biomass. Waste Biomass Valor. 2021, 13, 4533–4547. [Google Scholar] [CrossRef]
- Maus, I.; Rumming, M.; Bergmann, I.; Heeg, K.; Pohl, M.; Nettmann, E.; Jaenicke, S.; Blom, J.; Pühler, A.; Schlüter, A.; et al. Characterization of Bathyarchaeota Genomes Assembled from Metagenomes of Biofilms Residing in Mesophilic and Thermophilic Biogas Reactors. Biotechnol. Biofuels 2018, 11, 167. [Google Scholar] [CrossRef]
- Bassani, I.; Kougias, P.G.; Treu, L.; Angelidaki, I. Biogas Upgrading via Hydrogenotrophic Methanogenesis in Two-Stage Continuous Stirred Tank Reactors at Mesophilic and Thermophilic Conditions. Environ. Sci. Technol. 2015, 49, 12585–12593. [Google Scholar] [CrossRef] [PubMed]




| Parameters | Value |
|---|---|
| pH | 8.11 |
| Conductivity (mS/cm @25 °C) | 11.8 |
| Total Suspended Solids, TSS (g L−1) | 11.23 |
| Volatile Suspended Solids, VSS (g L−1) | 8.33 |
| Dilution Cycle | VSS Increase Rate (mg VSS d−1) |
|---|---|
| 1 | - |
| 2 | - |
| 3 | - |
| 4 | 14.92 |
| 5 | 14.73 |
| 6 | 12.25 |
| 7 | 12.35 |
| 8 | 8.73 |
| 9 | 4.63 |
| Dilution Cycle | H2 Loading Rate L L−1 d−1 | Output Gas Composition * (%) | CH4 Production Rate (L L−1 d−1) | nH2 (%) | nCO2 (%) | ||
|---|---|---|---|---|---|---|---|
| CH4 | CO2 | H2 | |||||
| 1 | 1.5 ± 0.3 | 93.2 ± 2.0 | 6.3 ± 1.2 | 0.7 ± 0.8 | 0.32 ± 0.07 | 99.5 ± 0.6 | 83.9 ± 3.2 |
| 2 | 2.6 ± 0.1 | 93.7 ± 4.3 | 5.3 ± 3.3 | 1.5 ± 1.5 | 0.29 ± 0.18 | 99.0 ± 1.0 | 85.9 ± 9.4 |
| 3 | 1.4 ± 0.1 | 95.8 ± 1.1 | 3.9 ± 1.2 | 0.3 ± 0.3 | 0.27 ± 0.06 | 99.8 ± 0.2 | 88.7 ± 3.8 |
| 4 | 1.4 ± 0.1 | 96.4 ± 1.3 | 3.3 ± 1.0 | 0.3 ± 0.6 | 0.28 ± 0.06 | 99.7 ± 0.6 | 90.3 ± 2.5 |
| 5 | 1.4 ± 0.2 | 95.4 ± 1.6 | 3.7 ± 0.8 | 0.8 ± 1.0 | 0.30 ± 0.04 | 99.3 ± 0.9 | 89.4 ± 3.4 |
| 6 | 1.2 ± 0.1 | 95.4 ± 1.2 | 3.7 ± 1.0 | 1.2 ± 1.2 | 0.27 ± 0.04 | 99.0 ± 1.0 | 89.3 ± 3.1 |
| 7 | 1.1 ± 0.2 | 94.4 ± 1.4 | 3.8 ± 0.6 | 2.0 ± 1.3 | 0.27 ± 0.04 | 98.4 ± 1.1 | 88.8 ± 2.2 |
| 8 | 1.2 ± 0.1 | 96.4 ± 0.5 | 3.3 ± 0.3 | 0.2 ± 0.3 | 0.29 ± 0.04 | 99.8 ± 0.2 | 90.1 ± 0.8 |
| 9 | 1.2 ± 0.1 | 95.5 ± 1.2 | 3.5 ± 1.1 | 0.9 ± 0.8 | 0.28 ± 0.02 | 99.3 ± 0.7 | 89.5 ± 2.4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Spyridonidis, A.; Vasiliadou, I.A.; Stathopoulou, P.; Tsiamis, A.; Tsiamis, G.; Stamatelatou, K. Enrichment of Microbial Consortium with Hydrogenotrophic Methanogens for Biological Biogas Upgrade to Biomethane in a Bubble Reactor under Mesophilic Conditions. Sustainability 2023, 15, 15247. https://doi.org/10.3390/su152115247
Spyridonidis A, Vasiliadou IA, Stathopoulou P, Tsiamis A, Tsiamis G, Stamatelatou K. Enrichment of Microbial Consortium with Hydrogenotrophic Methanogens for Biological Biogas Upgrade to Biomethane in a Bubble Reactor under Mesophilic Conditions. Sustainability. 2023; 15(21):15247. https://doi.org/10.3390/su152115247
Chicago/Turabian StyleSpyridonidis, Apostolos, Ioanna A. Vasiliadou, Panagiota Stathopoulou, Athanasios Tsiamis, George Tsiamis, and Katerina Stamatelatou. 2023. "Enrichment of Microbial Consortium with Hydrogenotrophic Methanogens for Biological Biogas Upgrade to Biomethane in a Bubble Reactor under Mesophilic Conditions" Sustainability 15, no. 21: 15247. https://doi.org/10.3390/su152115247
APA StyleSpyridonidis, A., Vasiliadou, I. A., Stathopoulou, P., Tsiamis, A., Tsiamis, G., & Stamatelatou, K. (2023). Enrichment of Microbial Consortium with Hydrogenotrophic Methanogens for Biological Biogas Upgrade to Biomethane in a Bubble Reactor under Mesophilic Conditions. Sustainability, 15(21), 15247. https://doi.org/10.3390/su152115247










