Resource Utilization Potential of Red Mud: A Study on the Micro-Mechanism of the Synergistic Effect of Multiple Solid Waste Filling Materials
Abstract
:1. Introduction
2. Materials and Methods
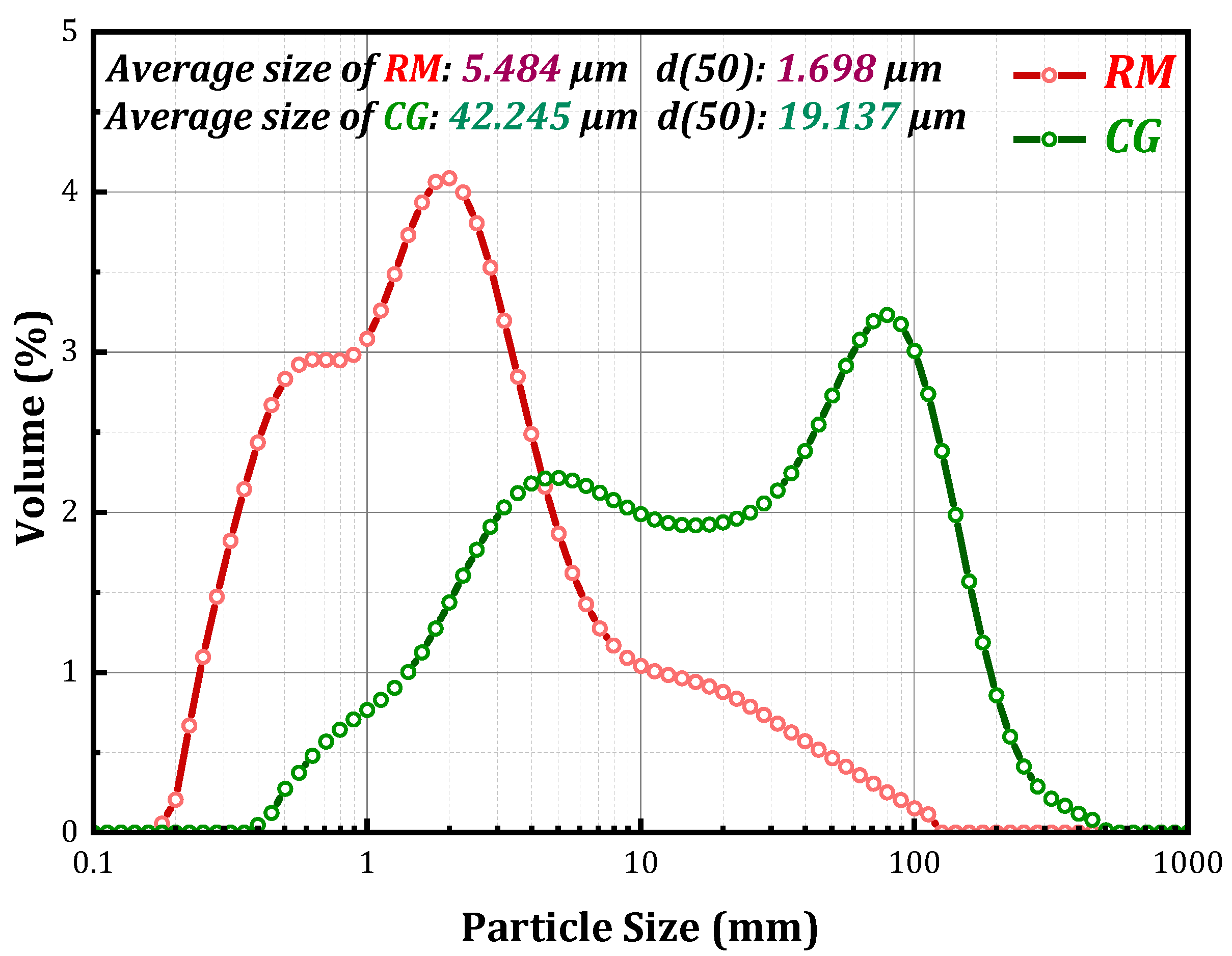
2.1. Materials
2.2. Orthogonal Experimental Design
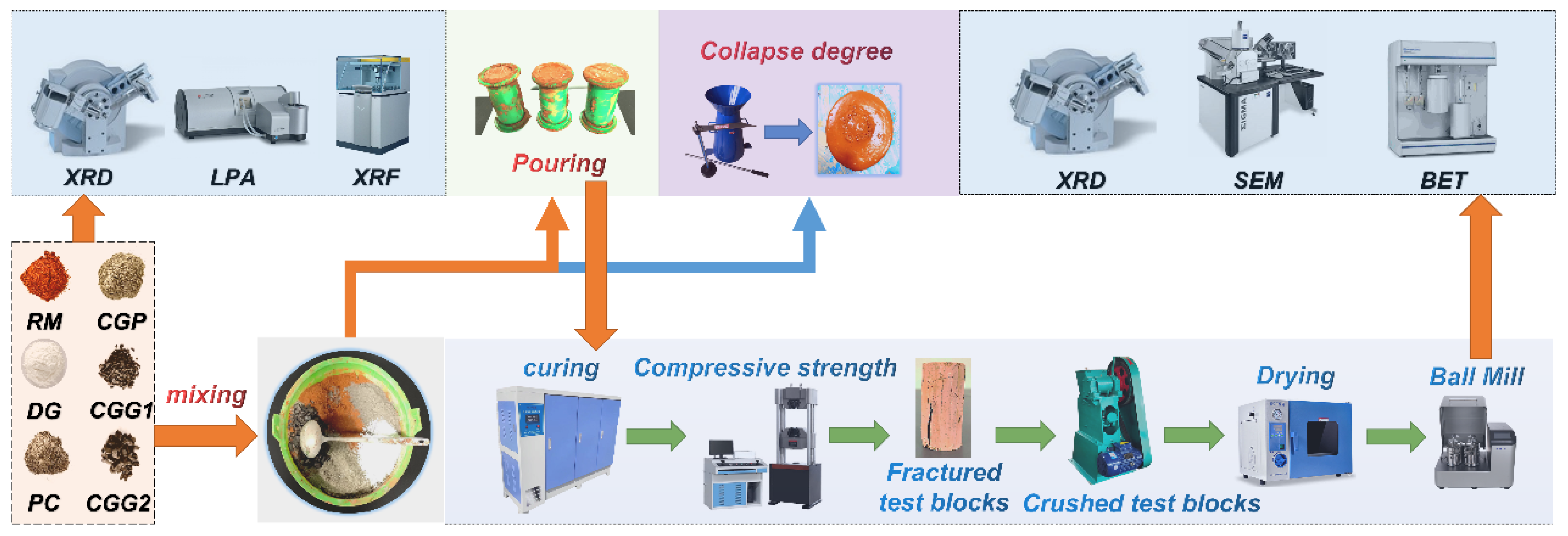
2.3. Experimental Procedure
2.4. Experimental Methods
2.4.1. Flow and Setting Time Tests
2.4.2. Uniaxial Compressive Strength Test
2.4.3. Microstructure Characterization
3. Results and Discussion
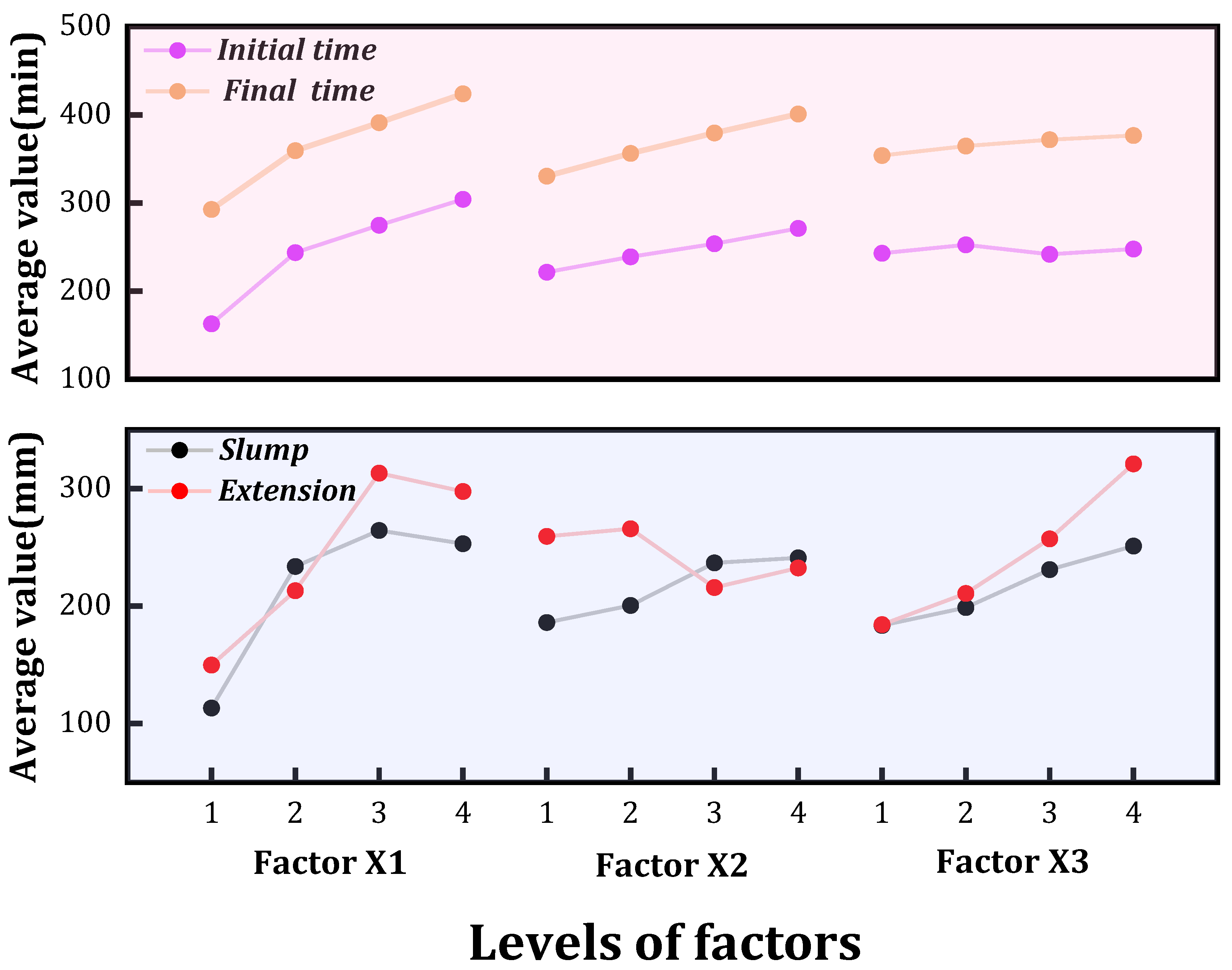
3.1. Flow and Setting Time Analysis
3.2. Analysis of Uniaxial Compression Results
3.3. Microstructure Analysis
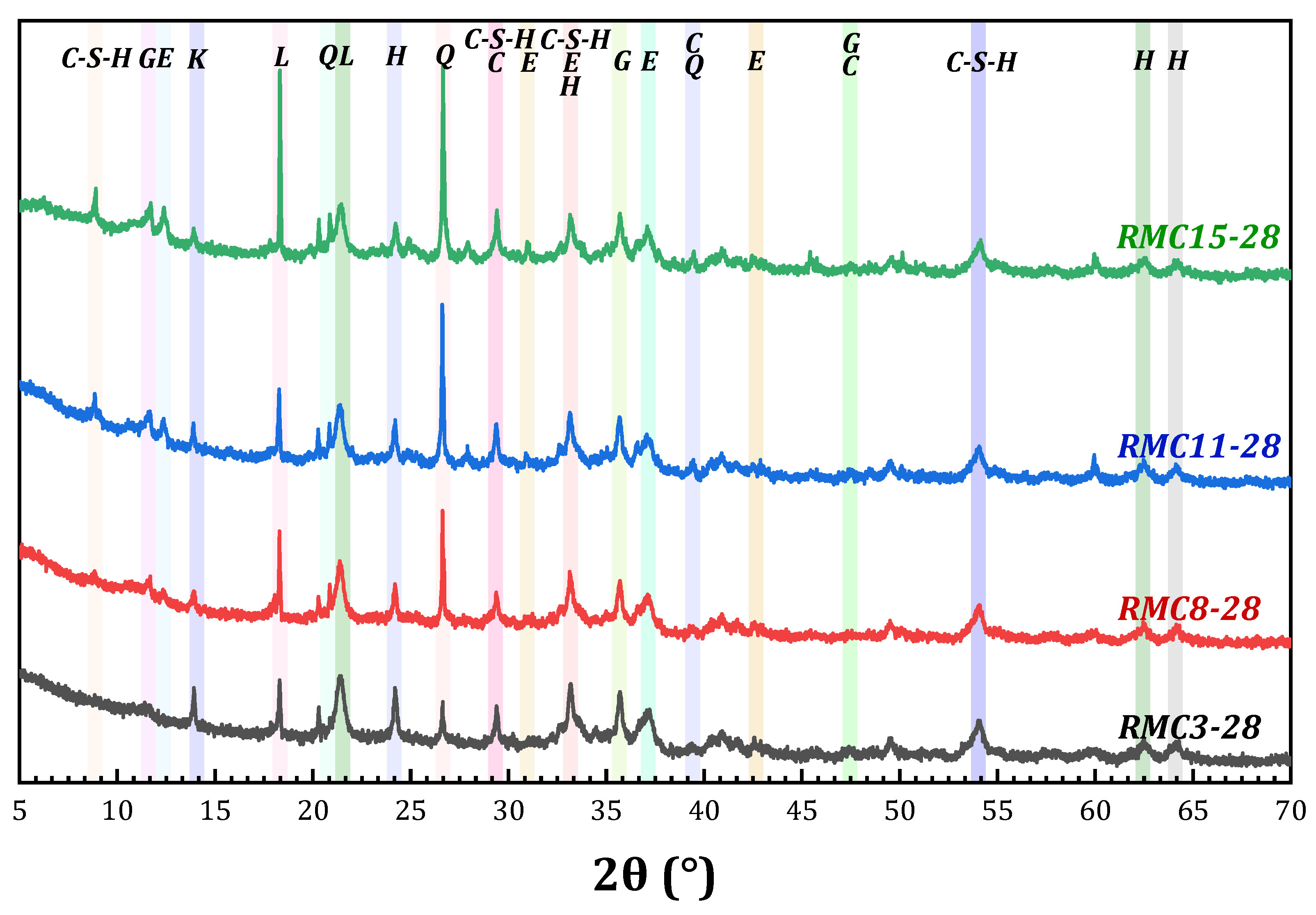
3.3.1. Analysis of Hydration Products
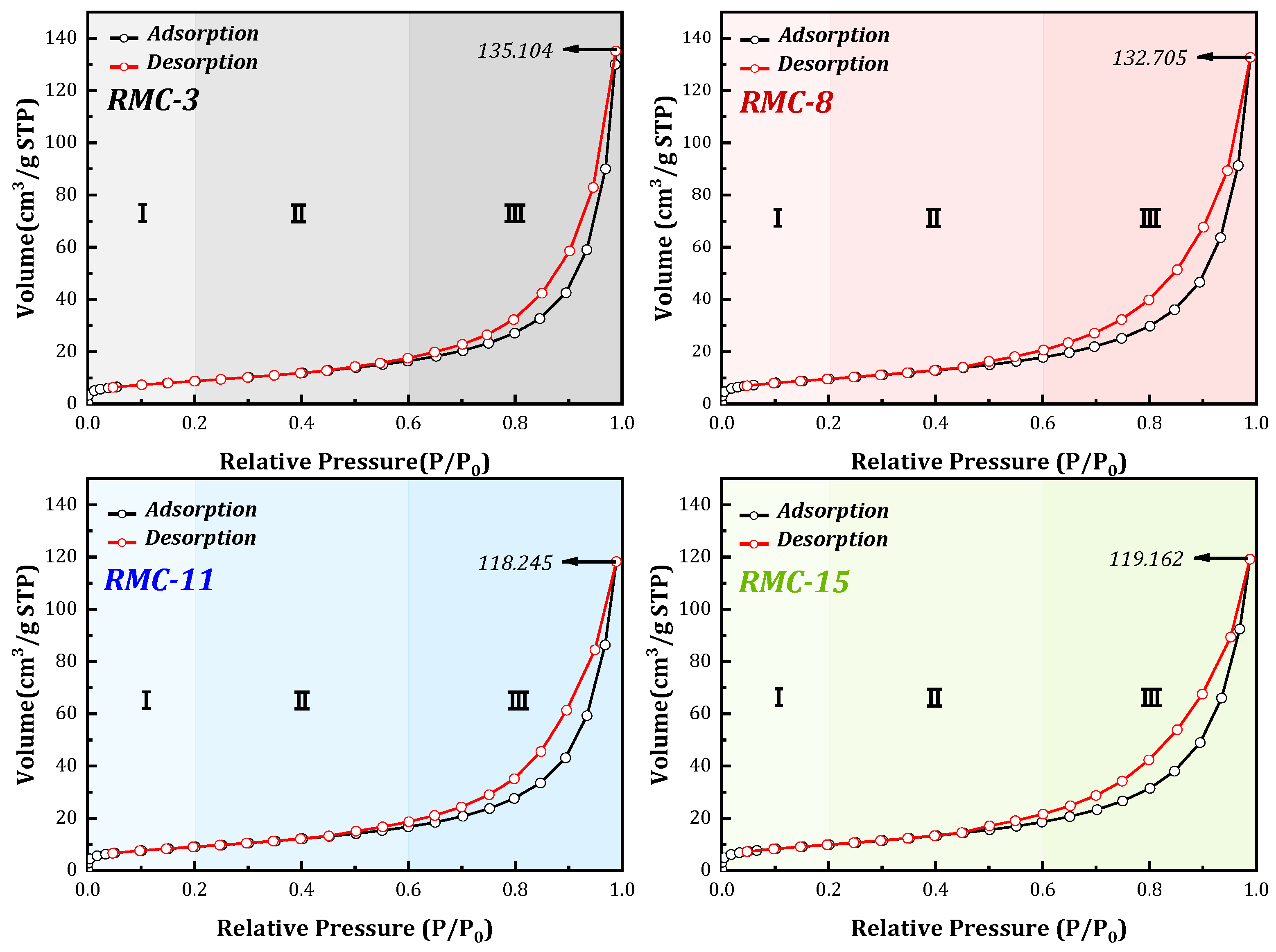
3.3.2. N2 Adsorption Curves and Pore Distribution Analysis
3.3.3. SEM Analysis
3.4. Cost Analysis
3.5. Leachate Testing
3.6. Strengthening Mechanism Analysis of RMC
3.6.1. Early Intensity
3.6.2. Final Intensity
4. Conclusions
- (1)
- The macroscopic experimental results show that the mass ratio of RM/CG has the greatest influence on the flow properties of RMC. In terms of setting time, both initial setting time and final setting time increase with the increase in the level values of each factor. The order of influence of each factor on the initial setting time and final setting time is X1 > X2 > X3. This is because coarse aggregate does not participate in the hydration reaction, so the mass ratio of RM/CG determines the mass of the material participating in the hydration reaction. The more fine the aggregate, the faster the setting rate. The hydration products of FGD in the composite system hinder the progress of the hydration reaction and play a role in slowing the setting process.
- (2)
- The uniaxial compressive strength test results indicate that the compressive strength of RMC generally follows the rule of increasing with the increase in curing time. Among them, RMC8 has the best compressive strength, with a compressive strength of 8.58 MPa after 28 days of curing. The crack propagation on the surface of the specimen shows that the failure mode of the specimen surface is single under early strength, but as the curing time of the specimen increases, the surface cracks first form in an approximately vertical direction and gradually appear, and then spread and intersect in a horizontal direction. The failure mode changes from cleavage failure to a combination of cleavage and shear failure, and the crack width decreases.
- (3)
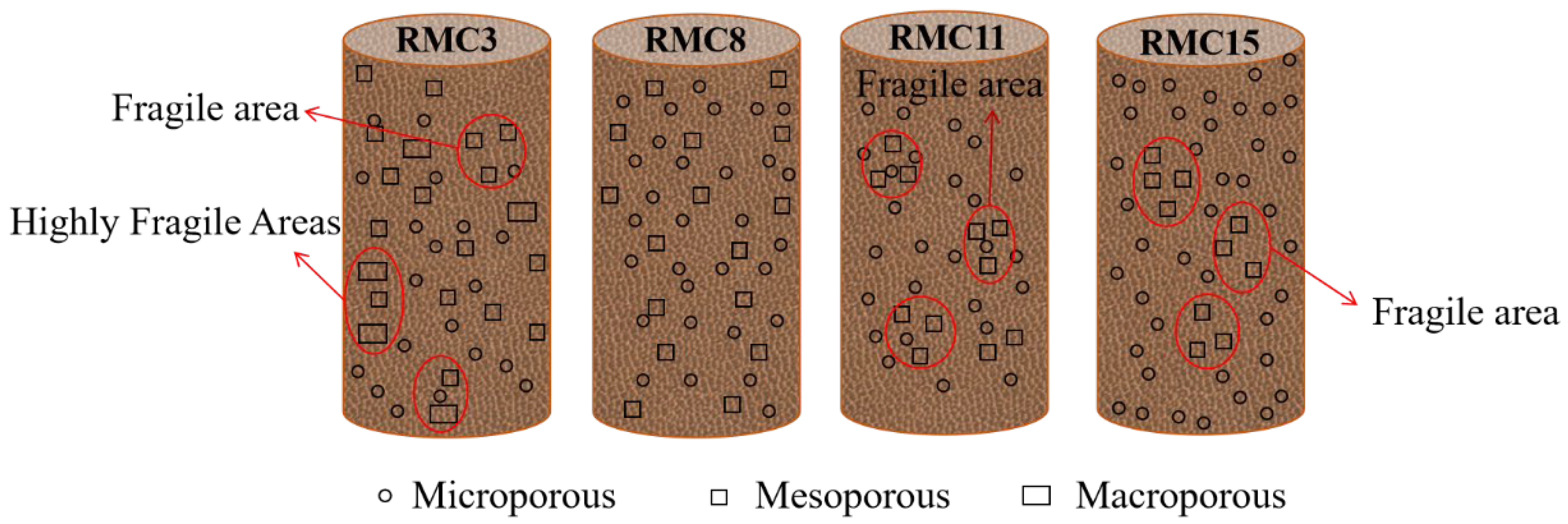
- The microscopic analysis shows that the XRD patterns of the samples of the preferred group (cured for 28 days) reveal that the hydration products are basically the same, and C-S-H gel and ettringite play key roles in the compressive strength of the composite system. The BET test revealed that coarse aggregate and hydration products (gel) increased the number and volume of pores in RMC. It was found that large pores and mesopores gathered to form highly fragile and fragile regions, respectively. The SEM observation of the pore analysis found that there is a critical value for the mass ratio of RM/CG, which is the optimal aggregate grading. A value higher or lower than this critical value in this system will have a detrimental effect on compressive strength.
- (4)
- The strengthening mechanism analysis showed that the early strength of the composite system was provided by the hydration products of PC and FGD (ettringite, lawsonite, and C-S-H gel). The alkaline environment provided by RM and PC stimulated the pozzolanic activity of RM and CGP, which led to the secondary hydration of silica and aluminum oxides in the system and promoted the late strength of RMC to increase. At the same time, the formation of hydration products (gmelinite) increased the solid alkali effect of the gelling system, which can reduce the pH value of RMC to a certain extent and reduce the adverse impact on the environment.
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Panda, I.; Jain, S.; Das, S.K.; Jayabalan, R. Characterization of red mud as a structural fill and embankment material using bioremediation. Int. Biodeterior. Biodegrad. 2017, 119, 368–376. [Google Scholar] [CrossRef]
- Mustafa, J.; Mourad, A.; Al-Marzouqi, A.H.; El-Naas, M.H. Simultaneous treatment of reject brine and capture of carbon dioxide: A comprehensive review. Desalination 2020, 483, 114386. [Google Scholar] [CrossRef]
- Li, X.-F.; Zhang, T.-A.; Lv, G.-Z.; Wang, K.; Wang, S. Summary of Research Progress on Metallurgical Utilization Technology of Red Mud. Minerals 2023, 13, 737. [Google Scholar] [CrossRef]
- Pan, X.; Wu, H.; Lv, Z.; Yu, H.; Tu, G. Recovery of valuable metals from red mud: A comprehensive review. Sci. Total Environ. 2023, 904, 166686. [Google Scholar] [CrossRef]
- Liu, D.-Y.; Wu, C.-S. Stockpiling and Comprehensive Utilization of Red Mud Research Progress. Materials 2012, 5, 1232–1246. [Google Scholar] [CrossRef]
- Liu, H.B.; Liu, Z.L. Recycling utilization patterns of coal mining waste in China. Resour. Conserv. Recycl. 2010, 54, 1331–1340. [Google Scholar]
- Hu, Z.; Hu, F.; Li, J.-H.; Li, H. Impact of coal mining subsidence on farmland in eastern China. Int. J. Surf. Min. Reclam. Environ. 1997, 11, 91–94. [Google Scholar] [CrossRef]
- Wang, A.; Ma, L.; Wang, Z.; Zhang, D.; Li, K.K.; Zhang, Y.; Yi, X. Soil and water conservation in mining area based on ground surface subsidence control: Development of a high-water swelling material and its application in backfilling mining. Environ. Earth Sci. 2016, 75, 698. [Google Scholar] [CrossRef]
- Guo, W.; Guo, M.; Tan, Y.; Bai, E.; Zhao, G. Sustainable Development of Resources and the Environment: Mining-Induced Eco-Geological Environmental Damage and Mitigation Measures—A Case Study in the Henan Coal Mining Area, China. Sustainability 2019, 11, 4366. [Google Scholar] [CrossRef]
- Liu, J.-G.; Li, X.-W.; He, T. Current situation and development of coal mine filling mining application in China. J. China Coal Soc. 2020, 45, 141–150. [Google Scholar]
- Chen, S.-J.; Yin, D.; Cao, F.; Liu, Y.; Ren, K. An overview of integrated surface subsidence-reducing technology in mining areas of China. Nat. Hazards 2015, 81, 1129–1145. [Google Scholar] [CrossRef]
- Wang, S.; Jin, H.; Deng, Y.; Xiao, Y. Comprehensive utilization status of red mud in China: A critical review. J. Clean. Prod. 2021, 289, 125136. [Google Scholar] [CrossRef]
- Dodoo-Arhin, D.; Nuamah, R.A.; Agyei-Tuffour, B.; Obada, D.O.; Yaya, A. Awaso bauxite red mud-cement based composites: Characterisation for pavement applications. Case Stud. Constr. Mater. 2017, 7, 45–55. [Google Scholar] [CrossRef]
- Sushil, S.; Batra, V.S. Catalytic applications of red mud, an aluminium industry waste: A review. Appl. Catal. B Environ. 2008, 81, 64–77. [Google Scholar] [CrossRef]
- López, A.; de Marco, I.; Caballero, B.M.; Laresgoiti, M.F.; Adrados, A.; Aranzabal, A. Catalytic pyrolysis of plastic wastes with two different types of catalysts: ZSM-5 zeolite and Red Mud. Appl. Catal. B Environ. 2011, 104, 211–219. [Google Scholar] [CrossRef]
- Senff, L.; Hotza, D.; Labrincha, J.A. Effect of red mud addition on the rheological behaviour and on hardened state characteristics of cement mortars. Constr. Build. Mater. 2011, 25, 163–170. [Google Scholar] [CrossRef]
- He, J.; Jie, Y.; Zhang, J.; Yu, Y.; Zhang, G. Synthesis and characterization of red mud and rice husk ash-based geopolymer composites. Cem. Concr. Compos. 2013, 37, 108–118. [Google Scholar] [CrossRef]
- Singh, M.; Upadhayay, S.N.; Prasad, P.M. Preparation of iron rich cements using red mud. Cem. Concr. Res. 1997, 27, 1037–1046. [Google Scholar] [CrossRef]
- Aygörmez, Y. Evaluation of the red mud and quartz sand on reinforced metazeolite-based geopolymer composites. J. Build. Eng. 2021, 43, 102528. [Google Scholar] [CrossRef]
- Bonenfant, D.; Kharoune, L.; Sauvé, S.; Hausler, R.; Niquette, P.; Mimeault, M.; Kharoune, M. CO2 Sequestration by Aqueous Red Mud Carbonation at Ambient Pressure and Temperature. Ind. Eng. Chem. Res. 2008, 47, 7617–7622. [Google Scholar] [CrossRef]
- Gupta, V.K.; Gupta, M.; Sharma, S. Process development for the removal of lead and chromium from aqueous solutions using red mud—An aluminium industry waste. Water Res. 2001, 35, 1125–1134. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Boyjoo, Y.; Choueib, A.; Zhu, Z.H. Removal of dyes from aqueous solution using fly ash and red mud. Water Res. 2005, 39, 129–138. [Google Scholar] [CrossRef] [PubMed]
- Yadav, V.S.; Prasad, M.; Khan, J.; Amritphale, S.S.; Singh, M.; Raju, C.B. Sequestration of carbon dioxide (CO2) using red mud. J. Hazard. Mater. 2010, 176, 1044–1050. [Google Scholar] [CrossRef] [PubMed]
- Sahu, R.C.; Patel, R.K.; Ray, B.C. Neutralization of red mud using CO2 sequestration cycle. J. Hazard. Mater. 2010, 179, 28–34. [Google Scholar] [CrossRef]
- Liu, W.; Yang, J.; Xiao, B. Application of Bayer red mud for iron recovery and building material production from alumosilicate residues. J. Hazard. Mater. 2009, 161, 474–478. [Google Scholar] [CrossRef]
- Liu, Y.; Naidu, R. Hidden values in bauxite residue (red mud): Recovery of metals. Waste Manag. 2014, 34, 2662–2673. [Google Scholar] [CrossRef]
- Agatzini-Leonardou, S.; Oustadakis, P.; Tsakiridis, P.E.; Markopoulos, C. Titanium leaching from red mud by diluted sulfuric acid at atmospheric pressure. J. Hazard. Mater. 2008, 157, 579–586. [Google Scholar] [CrossRef]
- Pasechnik, L.A.; Skachkov, V.M.; Bibanaeva, S.A.; Medyankina, I.S.; Bamburov, V.G. Composition and Properties of Iron Oxides in the Products of Hydrothermal Treatment of Red Mud and Bauxites. Russ. J. Inorg. Chem. 2022, 67, 1101–1107. [Google Scholar] [CrossRef]
- Zinoveev, D.; Pasechnik, L.; Fedotov, M.; Dyubanov, V.; Grudinsky, P.; Alpatov, A. Extraction of Valuable Elements from Red Mud with a Focus on Using Liquid Media—A Review. Recycling 2021, 6, 38. [Google Scholar] [CrossRef]
- Pasechnik, L.A.; Skachkov, V.M.; Chufarov, A.Y.; Suntsov, A.Y.; Yatsenko, S.P. High purity scandium extraction from red mud by novel simple technology. Hydrometallurgy 2021, 202, 105597. [Google Scholar] [CrossRef]
- Grudinsky, P.; Zinoveev, D.; Yurtaeva, A.; Kondratiev, A.; Dyubanov, V.; Petelin, A. Iron Recovery from Red Mud Using Carbothermic Roasting with Addition of Alkaline Salts. J. Sustain. Metall. 2021, 7, 858–873. [Google Scholar] [CrossRef]
- Pyagai, I.N.; Pasechnik, L.A.; Yatsenko, A.S.; Skachkov, V.M.; Yatsenko, S.P. Recovery of sludge from alumina production. Russ. J. Appl. Chem. 2012, 85, 1649–1653. [Google Scholar] [CrossRef]
- Zhang, J.; Liu, S.; Yao, Z.; Wu, S.; Jiang, H.; Liang, M.; Qiao, Y. Environmental aspects and pavement properties of red mud waste as the replacement of mineral filler in asphalt mixture. Constr. Build. Mater. 2018, 180, 605–613. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, X.; Tang, B.; Li, Y.; Zhang, W.; Xue, Y. Effect of Ca/(Si + Al) on red mud based eco-friendly revetment block: Microstructure, durability and environmental performance. Constr. Build. Mater. 2021, 304, 124618. [Google Scholar] [CrossRef]
- Zhang, G.; He, J.; Gambrell, R.P. Synthesis, Characterization, and Mechanical Properties of Red Mud–Based Geopolymers. Transp. Res. Rec. 2010, 2167, 1–9. [Google Scholar] [CrossRef]
- Cheng, W.; Yuan, S.-S.; Yuan, B. Design of red mud-excited fly ash filling material and excitation mechanism. J. Wuhan Univ. Technol. 2019, 41, 20–23+32. [Google Scholar]
- Ye, N.; Yang, J.; Liang, S.; Hu, Y.; Hu, J.; Xiao, B.; Huang, Q. Synthesis and strength optimization of one-part geopolymer based on red mud. Constr. Build. Mater. 2016, 111, 317–325. [Google Scholar] [CrossRef]
- Wang, Z.; Wang, Y.; Wu, L.; Wu, A.; Ruan, Z.; Zhang, M.; Zhao, R. Effective reuse of red mud as supplementary material in cemented paste backfill: Durability and environmental impact. Constr. Build. Mater. 2022, 328, 127002. [Google Scholar] [CrossRef]
- Zou, G.; Xu, J.; Wu, C. Evaluation of factors that affect rutting resistance of asphalt mixes by orthogonal experiment design. Int. J. Pavement Res. Technol. 2017, 10, 282–288. [Google Scholar] [CrossRef]
- Deng, L.; Feng, B.; Zhang, Y. An optimization method for multi-objective and multi-factor designing of a ceramic slurry: Combining orthogonal experimental design with artificial neural networks. Ceram. Int. 2018, 44, 15918–15923. [Google Scholar] [CrossRef]
- Li, X.; Hao, J. Orthogonal test design for optimization of synthesis of super early strength anchoring material. Constr. Build. Mater. 2018, 181, 42–48. [Google Scholar] [CrossRef]
- Mu, J.; Li, Y.; Hao, J.; Liu, Y.; Shen, J. Research on discrete element simulation of slump test for fresh self-compacting concrete. J. Build. Eng. 2023, 70, 106464. [Google Scholar] [CrossRef]
- Zhou, L.-B.; Sun, X.-H.; Liu, Z.; Wang, D.-M.; Zhu, L. Preparation, workability and microstructure of heavily doped fly ash-based mine filling materials. J. China Coal Soc. 2022, 359, 132043. [Google Scholar]
- Creber, K.J.; McGuinness, M.; Kermani, M.F.; Hassani, F.P. Investigation into changes in pastefill properties during pipeline transport. Int. J. Miner. Process. 2017, 163, 35–44. [Google Scholar] [CrossRef]
- Qu, H.-S.; Suo, Y.-L.; Liu, L.; Zhou, W.-W.; Yang, P.; Zhang, C.-X. Preparation and properties of modified coal gasification slag-based mining filling materials. J. China Coal Soc. 2022, 47, 1958–1973. [Google Scholar]
- Zhou, N.; Zhang, J.; Ouyang, S.; Deng, X.; Dong, C.; Du, E. Feasibility study and performance optimization of sand-based cemented paste backfill materials. J. Clean. Prod. 2020, 259, 120798. [Google Scholar] [CrossRef]
- Wang, P.; Liu, D.-Y. Physical and Chemical Properties of Sintering Red Mud and Bayer Red Mud and the Implications for Beneficial Utilization. Materials 2012, 5, 1800–1810. [Google Scholar] [CrossRef]
- Liu, H.; Sun, X.; Wang, Y.; Lu, X.; Du, H.; Tian, Z. Study on the Influence of Silica Fume (SF) on the Rheology, Fluidity, Stability, Time-Varying Characteristics, and Mechanism of Cement Paste. Materials 2022, 15, 90. [Google Scholar] [CrossRef]
- Wang, J.; Ma, T.; Luan, Y.; Wang, S.; Zhang, Y. Investigation on the effects of desulfurization gypsum on the engineering properties of ternary geopolymers: Improving the utilization of industrial wastes. J. Clean. Prod. 2023, 414, 137638. [Google Scholar] [CrossRef]
- Caillahua, M.C.; Moura, F.J. Technical feasibility for use of FGD gypsum as an additive setting time retarder for Portland cement. J. Mater. Res. Technol. 2018, 7, 190–197. [Google Scholar] [CrossRef]
- Wang, Z.; Zhao, N. Influence of coal gangue aggregate grading on strength properties of concrete. Wuhan Univ. J. Nat. Sci. 2015, 20, 66–72. [Google Scholar] [CrossRef]
- Tabsh, S.W.; Abdelfatah, A.S. Influence of recycled concrete aggregates on strength properties of concrete. Constr. Build. Mater. 2009, 23, 1163–1167. [Google Scholar] [CrossRef]
- Zhao, Y.-H.; Ran, H.-Y.; Feng, G.-R.; Guo, Y.-X.; Fan, Y.-J. Study on damage evolution and damage characteristics of gangue cemented filler with different aspect ratios under uniaxial compression. J. Min. Saf. Eng. 2022, 39, 674–682. [Google Scholar]
- Feng, G.; Du, X.; Zhang, Y. “Optical-acoustic-stress” responses in failure progress of cemented gangue-fly ash backfill material under uniaxial compression. Nondestruct. Test. Eval. 2019, 34, 135–146. [Google Scholar] [CrossRef]
- Feng, G.-R.; Xie, W.-S.; Guo, Y.-X.; Guo, J.; Ran, H.-Y.; Zhao, Y.-H. Influence of early loading on gangue cemented filling physical and mechanical properties and damage disruption. Chin. J. Rock Mech. Eng. 2022, 41, 775–784. [Google Scholar]
- Zhang, N.; Li, H.-X.; Liu, X.-M. Hydration kinetics of cementitious materials composed of red mud and coal gangue. Int. J. Miner. Metall. Mater. 2016, 23, 1215–1224. [Google Scholar] [CrossRef]
- Liu, S.-L.; Wang, Y.-M.; Wu, A.-X.; Zhang, M.-Z.; Wang, Z.-K.; Wu, L.-B. Leaching behavior and curing mechanism of red mud composite filling materials. Acta Mater. Compos. Sin. 2023, 40, 6736–6746. [Google Scholar]
- Zhang, N.; Sun, H.; Liu, X.; Zhang, J. Early-age characteristics of red mud–coal gangue cementitious material. J. Hazard. Mater. 2009, 167, 927–932. [Google Scholar] [CrossRef]
- Thommes, M.; Kaneko, K.; Neimark, A.V.; Olivier, J.P.; Rodriguez-Reinoso, F.; Rouquerol, J.; Sing, K.S.W. Physisorption of gases, with special reference to the evaluation of surface area and pore size distribution (IUPAC Technical Report). Pure Appl. Chem. 2015, 87, 1051–1069. [Google Scholar] [CrossRef]
- Koshy, N.; Dondrob, K.; Hu, L.; Wen, Q.; Meegoda, J.N. Synthesis and characterization of geopolymers derived from coal gangue, fly ash and red mud. Constr. Build. Mater. 2019, 206, 287–296. [Google Scholar] [CrossRef]
- GB 8978 1996; Comprehensive Sewage Discharge Standards. China Standard Press: Beijing, China, 1996.
- Mukiza, E.; Liu, X.; Zhang, L.; Zhang, N. Preparation and characterization of a red mud-based road base material: Strength formation mechanism and leaching characteristics. Constr. Build. Mater. 2019, 220, 297–307. [Google Scholar] [CrossRef]
- Yin, L.; Boqiang, C.; Changxiang, W.; Yao, L.; Ning, J.; Zhanxin, L. Effect of Desulfurization Gypsum and Quicklime on Performance of Fly Ash Filling Paste. In Proceedings of the 9th China-Russia Symposium “Coal in the 21st Century: Mining, Intelligent Equipment and Environment Protection” (COAL 2018), Qingdao, China, 18–21 October 2018; Atlantis Press: Amsterdam, The Netherlands, 2018; pp. 262–269. [Google Scholar]
- Ruan, S.; Liu, L.; Zhu, M.; Shao, C.; Xie, L.; Hou, D. Application of desulfurization gypsum as activator for modified magnesium slag-fly ash cemented paste backfill material. Sci. Total Environ. 2023, 869, 161631. [Google Scholar] [CrossRef] [PubMed]







| Components (wt%) | Fe2O3 | Al2O3 | SiO2 | Na2O | TiO2 | CaO | SO3 | P2O5 | K2O |
|---|---|---|---|---|---|---|---|---|---|
| RM | 49.84 | 19.33 | 12.20 | 8.53 | 7.93 | 0.87 | 0.55 | 0.19 | 0.16 |
| CG | 6.02 | 22.09 | 61.26 | 0.95 | 1.55 | 2.69 | 0.23 | 0.15 | 3.42 |
| FGD | 0.00 | 0.03 | 0.04 | 0.06 | 0.00 | 49.19 | 50.64 | 0.00 | 0.00 |
| PC | 3.35 | 4.22 | 16.15 | 0.21 | 0.42 | 68.77 | 3.41 | 0.08 | 0.67 |
| Level | Factors | C1 | C2 | ||
|---|---|---|---|---|---|
| X1 | X2 | X3 | |||
| 1 | 8:0 | 1% | 0.47 | 1:4 | 1:1:3 |
| 2 | 8:0 | 2% | 0.49 | 1:4 | 1:1:3 |
| 3 | 8:0 | 3% | 0.51 | 1:4 | 1:1:3 |
| 4 | 8:0 | 4% | 0.53 | 1:4 | 1:1:3 |
| 5 | 7:1 | 1% | 0.49 | 1:4 | 1:1:3 |
| 6 | 7:1 | 2% | 0.47 | 1:4 | 1:1:3 |
| 7 | 7:1 | 3% | 0.53 | 1:4 | 1:1:3 |
| 8 | 7:1 | 4% | 0.51 | 1:4 | 1:1:3 |
| 9 | 6:2 | 1% | 0.51 | 1:4 | 1:1:3 |
| 10 | 6:2 | 2% | 0.53 | 1:4 | 1:1:3 |
| 11 | 6:2 | 3% | 0.47 | 1:4 | 1:1:3 |
| 12 | 6:2 | 4% | 0.49 | 1:4 | 1:1:3 |
| 13 | 5:3 | 1% | 0.53 | 1:4 | 1:1:3 |
| 14 | 5:3 | 2% | 0.51 | 1:4 | 1:1:3 |
| 15 | 5:3 | 3% | 0.49 | 1:4 | 1:1:3 |
| 16 | 5:3 | 4% | 0.47 | 1:4 | 1:1:3 |
| Level | Slump (mm) | Extension (mm) | Setting Time (min) | |
|---|---|---|---|---|
| Initial Time | Final Time | |||
| RMC1 | 12 | 109 | 125 | 234 |
| RMC 2 | 31 | 117 | 157 | 267 |
| RMC 3 | 189 | 151 | 173 | 327 |
| RMC 4 | 221 | 222 | 196 | 342 |
| RMC 5 | 232 | 205 | 237 | 332 |
| RMC 6 | 228 | 188 | 235 | 347 |
| RMC 7 | 250 | 246 | 249 | 373 |
| RMC 8 | 225 | 213 | 253 | 385 |
| RMC 9 | 255 | 338 | 246 | 363 |
| RMC 10 | 288 | 431 | 268 | 399 |
| RMC 11 | 245 | 214 | 281 | 380 |
| RMC 12 | 269 | 269 | 304 | 422 |
| RMC 13 | 245 | 385 | 278 | 392 |
| RMC 14 | 255 | 327 | 295 | 412 |
| RMC 15 | 263 | 252 | 312 | 437 |
| RMC 16 | 249 | 226 | 331 | 454 |
| Indicator | Slump/mm | Extension/mm | ||||
|---|---|---|---|---|---|---|
| X1 | X2 | X3 | X1 | X2 | X3 | |
| k1 | 113.25 | 186 | 183.5 | 149.75 | 259.25 | 184.25 |
| k2 | 233.75 | 200.5 | 198.75 | 213 | 265.75 | 210.75 |
| k3 | 264.25 | 236.75 | 231 | 313 | 215.75 | 257.25 |
| k4 | 253 | 241 | 251 | 297.5 | 232.5 | 321 |
| R | 151 | 55 | 67.5 | 163.25 | 50 | 136.75 |
| Indicator | Initial Setting Time/min | Final Setting Time/min | ||||
|---|---|---|---|---|---|---|
| X1 | X2 | X3 | X1 | X2 | X3 | |
| k1 | 162.75 | 221.5 | 243 | 292.5 | 330.25 | 353.75 |
| k2 | 243.5 | 238.75 | 252.5 | 359.25 | 356.25 | 364.5 |
| k3 | 274.75 | 253.75 | 241.75 | 391 | 379.25 | 371.75 |
| k4 | 304 | 271 | 247.75 | 423.75 | 400.75 | 376.5 |
| R | 141.25 | 49.5 | 10.75 | 131.25 | 70.5 | 22.75 |
| Sample | Leaching Ion (mg/L) | pH | Conductivity (ms/cm) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| As | Cd | Cr | Pb | Mn | Cu | Hg | |||
| RM | 0.0335 | <0.0012 | 0.145 | <0.0042 | 0.0172 | 0.0108 | 0.0000 | 12.34 | 1.9 |
| RMC3 | 0.0106 | <0.0012 | 0.133 | <0.0042 | <0.0036 | 0.0223 | 0.0005 | 10.37 | 4.2 |
| RMC8 | 0.0056 | <0.0012 | 0.0754 | <0.0042 | <0.0036 | 0.0118 | 0. 0004 | 10.55 | 3.7 |
| RMC11 | 0.0053 | <0.0012 | 0.0780 | <0.0042 | <0.0036 | 0.0078 | 0. 0001 | 10.34 | 3.2 |
| RMC15 | 0.0034 | <0.0012 | 0.0802 | <0.0042 | <0.0036 | 0.0060 | 0. 0001 | 10.17 | 3.0 |
| GB8978-1996 limits | 0.5 | 0.1 | 1.5 | 1.0 | 2.0 | 0.5 | 0.05 | / | / |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, J.; Yan, G.; Bai, X.; Kong, S.; Li, J.; Li, G.; Ge, Z.; Huang, J. Resource Utilization Potential of Red Mud: A Study on the Micro-Mechanism of the Synergistic Effect of Multiple Solid Waste Filling Materials. Sustainability 2023, 15, 15532. https://doi.org/10.3390/su152115532
Zhang J, Yan G, Bai X, Kong S, Li J, Li G, Ge Z, Huang J. Resource Utilization Potential of Red Mud: A Study on the Micro-Mechanism of the Synergistic Effect of Multiple Solid Waste Filling Materials. Sustainability. 2023; 15(21):15532. https://doi.org/10.3390/su152115532
Chicago/Turabian StyleZhang, Jiawei, Guochao Yan, Xuyang Bai, Shaoqi Kong, Jiajun Li, Gang Li, Zhiguo Ge, and Jinjing Huang. 2023. "Resource Utilization Potential of Red Mud: A Study on the Micro-Mechanism of the Synergistic Effect of Multiple Solid Waste Filling Materials" Sustainability 15, no. 21: 15532. https://doi.org/10.3390/su152115532






