Posidonia Spheroids Intercepting Plastic Litter: Implications for Beach Clean-Ups
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Areas and Samplings
2.2. Plastic Isolation
2.3. Prevention Contamination
2.4. Data Analysis
3. Results
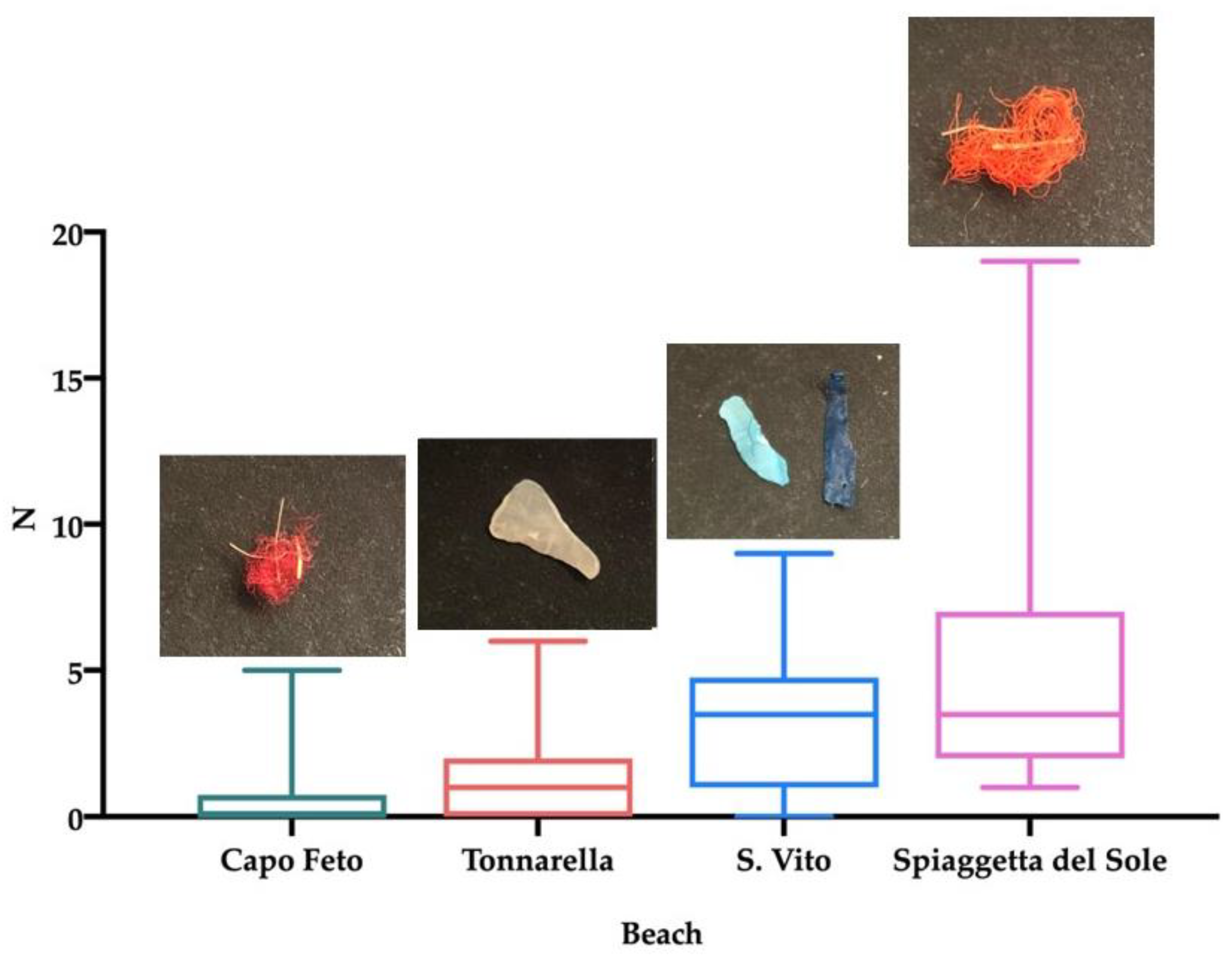
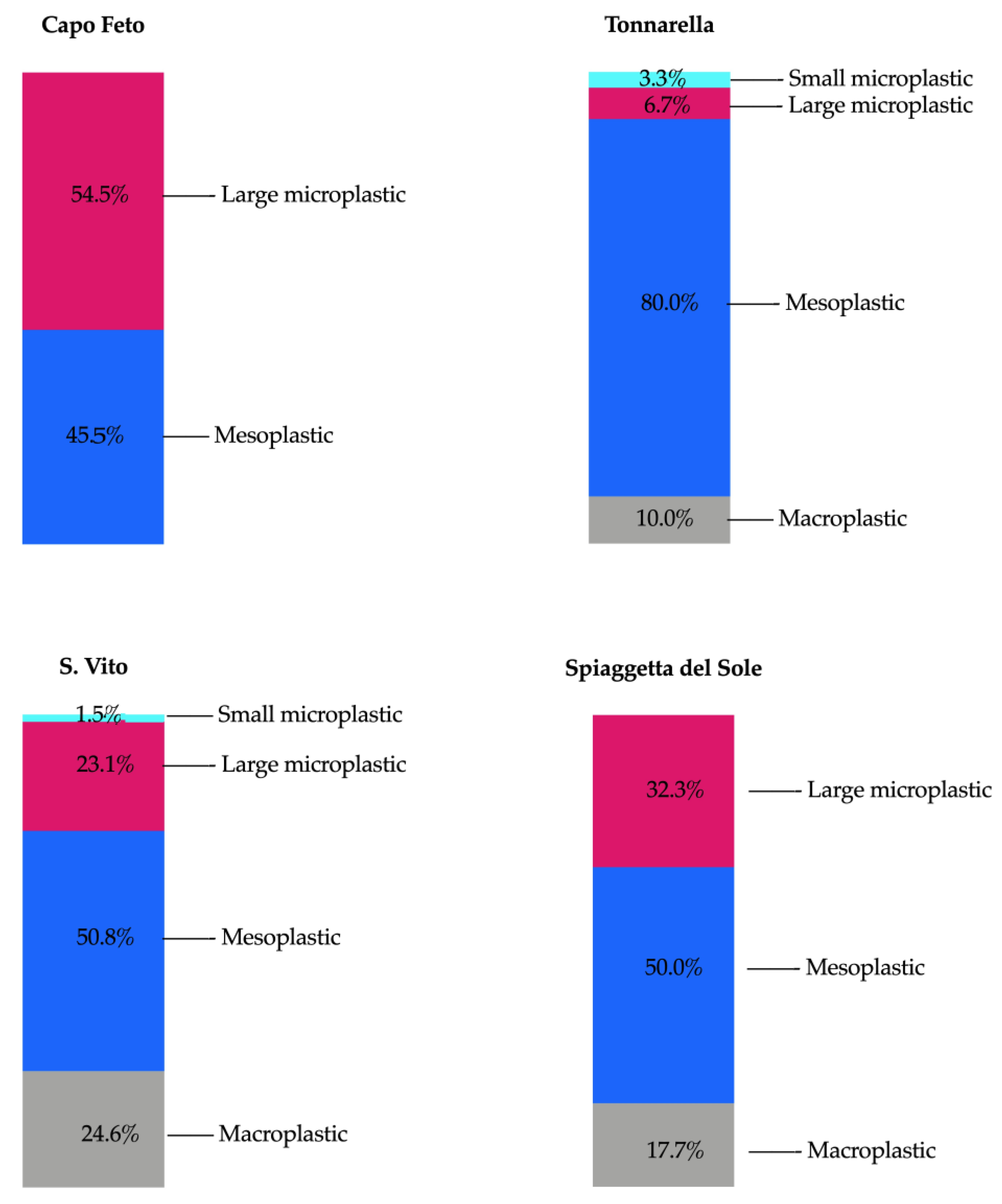
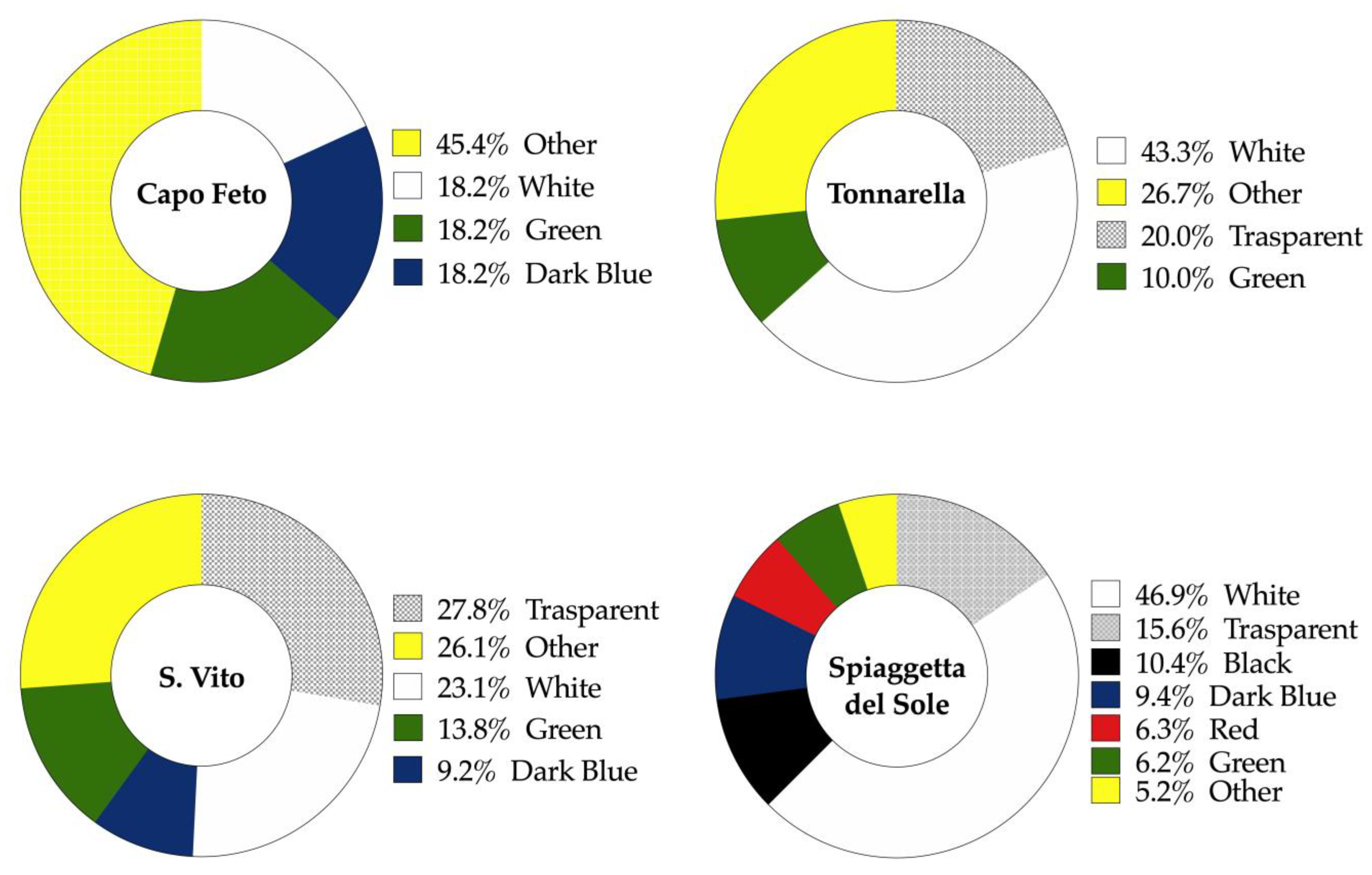
3.1. Plastic Abundance
3.2. Beach Comparison
4. Discussion
4.1. Beach Comparison
4.2. Implications for Cleaning Up
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ng, C.H.; Mistoh, M.A.; Teo, S.H.; Galassi, A.; Ibrahim, A.; Sipaut, C.S.; Foo, J.; Seay, J.; Taufiq-Yap, Y.H.; Janaun, J. Plastic Waste and Microplastic Issues in Southeast Asia. Front. Environ. Sci. 2023, 11, 427. [Google Scholar] [CrossRef]
- Lebreton, L.; Slat, B.; Ferrari, F.; Sainte-Rose, B.; Aitken, J.; Marthouse, R.; Hajbane, S.; Cunsolo, S.; Schwarz, A.; Levivier, A.; et al. Evidence that the Great Pacific Garbage Patch is rapidly accumulating plastic. Sci. Rep. 2018, 8, 4666. [Google Scholar] [CrossRef]
- Andrady, A.L. Microplastics in the Marine Environment. Mar. Poll. Bull. 2011, 62, 1596–1605. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.; Sharma, V.; Chatterjee, S. Microplastics in the Mediterranean Sea: Sources, Pollution Intensity, Sea Health, and Regulatory Policies. Front. Mar. Sci. 2021, 8, 634934. [Google Scholar] [CrossRef]
- Mathalon, A.; Hill, P. Microplastic Fibers in the Intertidal Ecosystem Surrounding Halifax Harbor, Nova Scotia. Mar. Pollut. Bull. 2014, 81, 69–79. [Google Scholar] [CrossRef] [PubMed]
- Barnes, D.K.A. Invasions by Marine Life on Plastic Debris. Nature 2002, 416, 808–809. [Google Scholar] [CrossRef]
- Jepsen, E.M.; de Bruyn, P.J.N. Pinniped Entanglement in Oceanic Plastic Pollution: A Global Review. Mar. Pollut. Bull. 2019, 145, 295–305. [Google Scholar] [CrossRef]
- Markic, A.; Gaertner, J.-C.; Gaertner-Mazouni, N.; Koelmans, A.A. Plastic Ingestion by Marine Fish in the Wild. Crit. Rev. Environ. Sci. Technol. 2020, 50, 657–697. [Google Scholar] [CrossRef]
- Ryan, P.G. Ingestion of Plastics by Marine Organisms. In Hazardous Chemicals Associated with Plastics in the Marine Environment; The Handbook of Environmental Chemistry; 2019; Springer: Cham, Switzerland, 2016; Volume 78, pp. 235–266. [Google Scholar] [CrossRef]
- Contino, M.; Ferruggia, G.; Pecoraro, R.; Scalisi, E.M.; Cavallaro, G.; Bonaccorso, C.; Fortuna, C.G.; Salvaggio, A.; Capparucci, F.; Bottari, T.; et al. Uptake Routes and Biodistribution of Polystyrene Nano-plastics on Zebrafish Larvae and Toxic Effects on Development. Fishes 2023, 8, 168. [Google Scholar] [CrossRef]
- Contino, M.; Ferruggia, G.; Indelicato, S.; Pecoraro, R.; Scalisi, E.M.; Salvaggio, A.; Brundo, M.V. Sublethal Effects of Polystyrene Nanoplastics on the Embryonic Development of Artemia salina (Linnaeus, 1758). Animals 2023, 13, 3152. [Google Scholar] [CrossRef]
- Porcino, N.; Bottari, T.; Mancuso, M. Is Wild Marine Biota Affected by Microplastics? Animals 2023, 13, 147. [Google Scholar] [CrossRef] [PubMed]
- Provencher, J.F.; Ammendolia, J.; Rochman, C.M.; Mallory, M.L. Assessing Plastic Debris in Aquatic Food Webs: What We Know and Don’t Know about Uptake and Trophic Transfer. Environ. Rev. 2019, 27, 304–317. [Google Scholar] [CrossRef]
- Carbery, M.; O’Connor, W.; Palanisami, T. Trophic Transfer of Microplastics and Mixed Contaminants in the Marine Food Web and Implications for Human Health. Environ. Int. 2018, 115, 400–409. [Google Scholar] [CrossRef] [PubMed]
- Mancuso, M.; Conti Nibali, V.; Porcino, N.; Branca, C.; Natale, S.; Smedile, F.; Azzaro, M.; D’angelo, G.; Bottari, T. Monitoring of Anthropogenic Microplastic Pollution in Antarctic Fish (Emerald Rockcod) from the Terranova Bay after a Quarter of Century. Sci. Total Environ. 2023, 904, 167244. [Google Scholar] [CrossRef] [PubMed]
- Mancuso, M.; Panarello, G.; Falco, F.; Di Paola, D.; Savoca, S.; Capillo, G.; Romeo, T.; Presti, G.; Gullotta, E.; Spanò, N.; et al. Investigating the Effects of Microplastic Ingestion in Scyliorhinus canicula from the South of Sicily. Sci. Total Environ. 2022, 850, 157875. [Google Scholar] [CrossRef]
- Brennecke, D.; Duarte, B.; Paiva, F.; Caçador, I.; Canning-Clode, J. Microplastics as Vector for Heavy Metal Contamination from the Marine Environment. Estuar. Coast. Shelf Sci. 2016, 178, 189–195. [Google Scholar] [CrossRef]
- Farrell, P.; Nelson, K. Trophic Level Transfer of Microplastic: Mytilus edulis (L.) to Carcinus maenas (L.). Environ. Pollut. 2013, 177, 1–3. [Google Scholar] [CrossRef]
- Collard, F.; Gilbert, B.; Compère, P.; Eppe, G.; Das, K.; Jauniaux, T.; Parmentier, E. Microplastics in Livers of European Anchovies (Engraulis encrasicolus, L.). Environ. Pollut. 2017, 229, 1000–1005. [Google Scholar] [CrossRef]
- Kennedy, H.; Beggins, J.; Duarte, C.M.; Fourqurean, J.W.; Holmer, M.; Marbà, N.; Middelburg, J.J. Seagrass Sediments as a Global Carbon Sink: Isotopic Constraints. Glob. Biogeochem. Cycles 2010, 24, GB4026. [Google Scholar] [CrossRef]
- Serrano, O.; Davis, G.; Lavery, P.S.; Duarte, C.M.; Martinez-Cortizas, A.; Mateo, M.A.; Masqué, P.; Arias-Ortiz, A.; Rozaimi, M.; Kendrick, G.A. RecDFonstruction of Centennial-Scale Fluxes of Chemical Elements in the Australian Coastal Environment Using Seagrass Archives. Sci. Total Environ. 2016, 541, 883–894. [Google Scholar] [CrossRef]
- de los Santos, C.B.; Krång, A.-S.; Infantes, E. Microplastic Retention by Marine Vegetated Canopies: Simulations with Seagrass Meadows in a Hydraulic Flume. Environ. Pollut. 2021, 269, 116050. [Google Scholar] [CrossRef]
- Huang, Y.; Xiao, X.; Xu, C.; Perianen, Y.D.; Hu, J.; Holmer, M. Seagrass Beds Acting as a Trap of Microplastics—Emerging Hotspot in the Coastal Region? Environ. Pollut. 2020, 257, 113450. [Google Scholar] [CrossRef] [PubMed]
- Jones, K.; Hartl, M.; Bell, M.; Capper, A. Microplastic Accumulation in a Zostera marina L. Bed at Deerness Sound, Orkney, Scotland. Mar. Pollut. Bull. 2020, 152, 110883. [Google Scholar] [CrossRef] [PubMed]
- Tahir, A.; Samawi, M.F.; Sari, K.; Hidayat, R.; Nimzet, R.; Wicaksono, E.A.; Asrul, L.; Werorilangi, S. Studies on Microplastic Contamination in Seagrass Beds at Spermonde Archipelago of Makassar Strait, Indonesia. J. Phys. Conf. Ser. 2019, 1341, 22008. [Google Scholar] [CrossRef]
- Datu, S.; Supriadi, S.; Tahir, A. Microplastic in Cymodocea Rotundata Seagrass Blades. IJEAB 2019, 4, 1758–1761. [Google Scholar] [CrossRef]
- Dahl, M.; Bergman, S.; Björk, M.; Diaz-Almela, E.; Granberg, M.; Gullström, M.; Leiva-Dueñas, C.; Magnusson, K.; Marco-Méndez, C.; Piñeiro-Juncal, N.; et al. A Temporal Record of Microplastic Pollution in Mediterranean Seagrass Soils. Environ. Pollut. 2021, 273, 116451. [Google Scholar] [CrossRef] [PubMed]
- Menicagli, V.; Balestri, E.; Vallerini, F.; De Battisti, D.; Lardicci, C. Plastics and Sedimentation Foster the Spread of a Non-Native Macroalga in Seagrass Meadows. Sci. Total Environ. 2021, 757, 143812. [Google Scholar] [CrossRef]
- Seng, N.; Lai, S.; Fong, J.; Saleh, M.F.; Cheng, C.; Cheok, Z.; Todd, P. Early Evidence of Microplastics on Seagrass and Macroalgae. Mar. Freshwater Res. 2020, 71, 922. [Google Scholar] [CrossRef]
- Gutow, L.; Eckerlebe, A.; Gimenez, L.; Saborowski, R. Experimental Evaluation of Seaweeds as a Vector for Microplastics into Marine Food Webs. J. Environ. Sci. Technol. 2015, 50, 915–923. [Google Scholar] [CrossRef]
- Remy, F.; Collard, F.; Gilbert, B.; Compère, P.; Eppe, G.; Lepoint, G. When Microplastic Is Not Plastic: The Ingestion of Artificial Cellulose Fibers by Macrofauna Living in Seagrass Macrophytodetritus. J. Environ. Sci. Technol. 2015, 49, 11158–11166. [Google Scholar] [CrossRef]
- Ganesapandian, S.; Manikandan, S.; Kumaraguru, A.K. Marine Litter in the Northern Part of Gulf of Mannar, Southeast Coast of India. Res. J. Environ. Sci. 2011, 5, 471–478. [Google Scholar] [CrossRef]
- Abreo, N.A.; Macusi, E.; Jimenez, L. A Survey of Subtidal Anthropogenic Marine Debris (AMD) in Mayo Bay, Mati City, Davao Oriental, Philippines. Philipp. J. Sci. 2018, 147, 599–602. [Google Scholar]
- Cozzolino, L.; Nicastro, K.R.; Zardi, G.I.; de los Santos, C.B. Species-Specific Plastic Accumulation in the Sediment and Canopy of Coastal Vegetated Habitats. Sci. Total Environ. 2020, 723, 138018. [Google Scholar] [CrossRef] [PubMed]
- Balestri, E.; Menicagli, V.; Vallerini, F.; Lardicci, C. Biodegradable Plastic Bags on the Seafloor: A Future Threat for Seagrass Meadows? Sci. Total Environ. 2017, 605, 755–763. [Google Scholar] [CrossRef] [PubMed]
- Trache, D.; Tarchoun, A.F.; De Vita, D.; Kennedy, J.F. Posidonia oceanica (L.) Delile: A Mediterranean Seagrass with Potential Applications but Regularly and Erroneously Referred to as an Algal Species. Int. J. Biol. Macromol. 2023, 230, 122624. [Google Scholar] [CrossRef]
- Bellissimo, G.; Sirchia, B.; Ruvolo, V. Monitoring of Posidonia oceanica Meadows in the Sicilian Coasts under the Water Framework Directive (WFD). In Eighth International Symposium “Monitoring of Mediterranean Coastal Areas. Problems and Measurement Techniques”. 2020, pp. 510–518, ISBN 978-88-5518-146-4. Available online: https://library.oapen.org/bitstream/id/1dbb1e33-6c4b-4fdb-a246-253478891c43/14814.pdf (accessed on 1 May 2023).
- Calvo, S.; Calvo, R.; Luzzu, F.; Raimondi, V.; Assenzo, M.; Cassetti, F.P.; Tomasello, A. Performance Assessment of Posidonia oceanica (L.) Delile Restoration Experiment on Dead Matte Twelve Years after Planting—Structural and Functional Meadow Features. Water 2021, 13, 724. [Google Scholar] [CrossRef]
- Kalogirou, S.; Corsini-Foka, M.; Sioulas, A.; Wennhage, H.; Pihl, L. Diversity, Structure and Function of Fish Assemblages Associated with Posidonia oceanica Beds in an Area of the Eastern Mediterranean Sea and the Role of Non-Indigenous Species. J. Fish Biol. 2010, 77, 2338–2357. [Google Scholar] [CrossRef]
- Ramírez, Á.; Urra, J.; Marina, P.; Rueda, J.; García Raso, J. Crustacean Decapod Assemblages Associated with Fragmented Posidonia oceanica Meadows in the Alboran Sea (Western Mediterranean Sea): Composition, Temporal Dynamics and Influence of Meadow Structure. Mar. Ecol. 2015, 37. [Google Scholar] [CrossRef]
- Pergent-Martini, C.; Pergent, G.; Monnier, B.; Boudouresque, C.F.; Mori, C.; Valette-Sansevin, A. Contribution of Posidonia oceanica meadows in the context of climate change mitigation in the Mediterranean Sea. Mar. Environ. Res. 2021, 165, 105236. [Google Scholar] [CrossRef]
- Sanchez-Vidal, A.; Canals, M.; de Haan, W.P.; Romero, J.; Veny, M. Seagrasses Provide a Novel Ecosystem Service by Trapping Marine Plastics. Sci. Rep. 2021, 11, 254. [Google Scholar] [CrossRef]
- Boudouresque, C.; Pergent, G.; Pergent-Martini, C.; Ruitton, S.; Thibaut, T.; Verlaque, M. The Necromass of the Posidonia oceanica Seagrass Meadow: Fate, Role, Ecosystem Services and Vulnerability. Hydrobiologia 2016, 781, 25–42. [Google Scholar] [CrossRef]
- Colombini, I.; Mateo, M.Á.; Serrano, O.; Fallaci, M.; Gagnarli, E.; Serrano, L.; Chelazzi, L. On the Role of Posidonia oceanica Beach Wrack for Macroinvertebrates of a Tyrrhenian Sandy Shore. Acta Oecol. 2009, 35, 32–44. [Google Scholar] [CrossRef]
- Del Vecchio, S.; Marbà, N.; Acosta, A.; Vignolo, C.; Traveset, A. Effects of Posidonia Oceanica Beach-Cast on Germination, Growth and Nutrient Uptake of Coastal Dune Plants. PLoS ONE 2013, 8, e70607. [Google Scholar] [CrossRef] [PubMed]
- Blondel, V.D.; Guillaume, J.L.; Lambiotte, R.; Lefebvre, E. Fast Unfolding of Communities in Large Networks. J. Stat. Mech.-Theory Exp. 2008, 2008, P10008. [Google Scholar] [CrossRef]
- Lefebvre, L.; Compère, P.; Gobert, S. The Formation of Aegagropiles from the Mediterranean Seagrass Posidonia oceanica (L.) Delile (1813): Plant Tissue Sources and Colonisation by Melanised Fungal Mycelium. Mar. Biol. 2023, 170, 19. [Google Scholar] [CrossRef]
- Pietrelli, L.; Di Gennaro, A.; Menegoni, P.; Lecce, F.; Poeta, G.; Acosta, A.T.R.; Battisti, C.; Iannilli, V. Pervasive Plastisphere: First Record of Plastics in Egagropiles (Posidonia Spheroids). Environ. Pollut. 2017, 229, 1032–1036. [Google Scholar] [CrossRef]
- Garofalo, G.; Quattrocchi, F.; Bono, G.; Di Lorenzo, M.; Di Maio, F.; Falsone, F.; Gancitano, V.; Geraci, M.L.; Lauria, V.; Massi, D.; et al. What Is in Our Seas? Assessing Anthropogenic Litter on the Seafloor of the Central Mediterranean Sea. Environ. Pollut. 2020, 266, 115213. [Google Scholar] [CrossRef]
- Dimech, M.; Camilleri, M.; Hiddink, J.; Kaiser, M.; Ragonese, S.; Schembri, P. Differences in Demersal Community Structure and Biomass Size Spectra within and Outside the Maltese Fishery Management Zone (FMZ). Sci. Mar. 2008, 72, 669–682. [Google Scholar] [CrossRef]
- Bottari, T.; Rinelli, P.; Bianchini, M.L.; Ragonese, S. Stock Identification of Raja clavata L. (Chondrichthyes, Rajidae) in Two Contiguous Areas of the Mediterranean. Hydrobiologia 2013, 703, 215–224. [Google Scholar] [CrossRef]
- Regione Sicilia. Available online: https://www.regione.sicilia.it/sites/default/files/2022-06/Relazione%20Generale.pdf (accessed on 1 May 2023).
- Kapp, K.J.; Yeatman, E. Microplastic Hotspots in the Snake and Lower Columbia Rivers: A Journey from the Greater Yellowstone Ecosystem to the Pacific Ocean. Environ. Pollut. 2018, 241, 1082–1090. [Google Scholar] [CrossRef]
- Silva, A.B.; Bastos, A.S.; Justino, C.I.L.; da Costa, J.P.; Duarte, A.C.; Rocha-Santos, T.A.P. Microplastics in the Environment: Challenges in Analytical Chemistry—A Review. Anal. Chim. Acta 2018, 1017, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Wadell, H. Volume, Shape, and Roundness of Rock Particles. J. Geol. 1932, 40, 443–451. [Google Scholar] [CrossRef]
- Mghili, B.; Keznine, M.; Hasni, S.; Aksissou, M. Abundance, Composition and Sources of Benthic Marine Litter Trawled-up in the Fishing Grounds on the Moroccan Mediterranean Coast. Reg. Stud. Mar. Sci. 2023, 63, 103002. [Google Scholar] [CrossRef]
- Cau, A.; Franceschini, S.; Moccia, D.; Gorule, P.A.; Agus, B.; Bellodi, A.; Cannas, R.; Carugati, L.; Cuccu, D.; Dessì, C.; et al. Scattered Accumulation Hotspots of Macro-Litter on the Seafloor: Insights for Mitigation Actions. Environ. Pollut. 2021, 292, 118232. [Google Scholar] [CrossRef]
- Mghili, B.; Analla, M.; Aksissou, M.; Aissa, C. Marine Debris in Moroccan Mediterranean Beaches: An Assessment of Their Abundance, Composition and Sources. Mar. Pollut. Bull. 2020, 160, 111692. [Google Scholar] [CrossRef]
- Mghili, B.; De-la-Torre, G.E.; Analla, M.; Aksissou, M. Marine macroinvertebrates fouled in marine anthropogenic litter in the Moroccan Mediterranean. Mar. Pollut. Bull. 2022, 185, 114266. [Google Scholar] [CrossRef]
- Navarrete-Fernández, T.; Bermejo, R.; Hernández, I.; Deidun, A.; Andreu-Cazenave, M.; Cózar, A. The Role of Seagrass Meadows in the Coastal Trapping of Litter. Mar. Pollut. Bull. 2022, 174, 113299. [Google Scholar] [CrossRef]
- Ben-Haddad, M.; Abelouah, M.R.; Hajji, S.; Rangel-Buitrago, N.; Alla, A.A. The Halophyte Cakile maritima Scop. 1772 as a Trap of Plastic Litter on the Moroccan Coast. Mar. Pollut. Bull. 2023, 187, 114574. [Google Scholar] [CrossRef]
- De, K.; Sautya, S.; Dora, G.U.; Gaikwad, S.; Katke, D.; Salvi, A. Mangroves in the “Plasticene”: High Exposure of Coastal Mangroves to Anthropogenic Litter Pollution along the Central-West Coast of India. Sci. Total Environ. 2023, 858, 160071. [Google Scholar] [CrossRef]
- Mancuso, M.; Genovese, G.; Porcino, N.; Natale, S.; Crisafulli, A.; Spagnuolo, D.; Catalfamo, M.; Morabito, M.; Bottari, T. Psammophytes as Traps for Beach Litter in the Strait of Messina (Mediterranean Sea). Reg. Stud. Mar. Sci. 2023, 65, 103057. [Google Scholar] [CrossRef]
- Eionet Portal. Available online: https://www.eionet.europa.eu/etcs/etc-ce/products/etc-ce-products/etc-ce-report-1-2022-microplastic-pollution-from-textile-consumption-in-europe (accessed on 1 May 2023).
- Richardson, K.; Hardesty, B.D.; Wilcox, C. Estimates of Fishing Gear Loss Rates at a Global Scale: A Literature Review and Meta-Analysis. Fish Fish. 2019, 20, 1218–1231. [Google Scholar] [CrossRef]
- UN Environment Programme. UNEP Marine Plastic Debris and Microplastics—Global Lessons and Research to Inspire Action and Guide Policy Change; UN Environment Programme: Nairobi, Kenya, 2016. [Google Scholar]
- FAO. The State of World Fisheries and Aquaculture 2018—Meeting the Sustainable Development Goals; FAO: Rome, Italy, 2018. [Google Scholar]
- Wright, L.S.; Napper, I.E.; Thompson, R.C. Potential Microplastic Release from Beached Fishing Gear in Great Britain’s Region of Highest Fishing Litter Density. Mar. Pollut. Bull. 2021, 173, 113115. [Google Scholar] [CrossRef] [PubMed]
- Welden, N.A.C.; Cowie, P.R. Environment and Gut Morphology Influence Microplastic Retention in Langoustine, Nephrops norvegicus. Environ. Pollut. 2016, 214, 859–865. [Google Scholar] [CrossRef] [PubMed]
- Wójcik-Fudalewska, D.; Normant-Saremba, M.; Anastácio, P. Occurrence of Plastic Debris in the Stomach of the Invasive Crab Eriocheir sinensis. Mar. Pollut. Bull. 2016, 113, 306–311. [Google Scholar] [CrossRef] [PubMed]
- Carreras-Colom, E.; Constenla, M.; Soler-Membrives, A.; Cartes, J.E.; Baeza, M.; Padros, F.; Carrasson, M. Spatial Occurrence and Effects of Microplastic Ingestion on the Deep-Water Shrimp Aristeus antennatus. Mar. Pollut. Bull. 2018, 133, 44–52. [Google Scholar] [CrossRef]
- Yücel, N.; Kılıç, E. Microplastic Contamination in the Freshwater Crayfish Pontastacus leptodactylus (Eschscholtz, 1823). Mar. Pollut. Bull. 2022, 185, 114337. [Google Scholar] [CrossRef]
- Bono, G.; Falsone, F.; Francesca, F.; Di Maio, F.; Gabriele, M.; Gancitano, V.; Geraci, M.; Scannella, D.; Mancuso, M.; Okpala, C.; et al. Microplastics and Alien Black Particles as Contaminants of Deep-Water Rose Shrimp (Parapenaeus longistroris Lucas, 1846) in the Central Mediterranean Sea. J. Adv. Biotechnol. Bioeng. 2020, 8, 23–28. [Google Scholar] [CrossRef]
- Anastasopoulou, A.K.; Mytilineou, C.; Smith, C.; Papadopoulou, N. Plastic Debris Ingested by Deep-Water Fish of the Ionian Sea (Eastern Mediterranean). Deep Sea Res. Part I Oceanogr. Res. 2013, 74, 11–13. [Google Scholar] [CrossRef]
- Digka, N.; Tsangaris, C.; Torre, M.; Anastasopoulou, A.; Zeri, C. Microplastics in Mussels and Fish from the Northern Ionian Sea. Mar. Pollut. Bull. 2018, 135, 30–40. [Google Scholar] [CrossRef]
- Spedicato, M.T.; Zupa, W.; Carbonara, P.; Fiorentino, F.; Follesa, M.; Galgani, F.; Garcia, C.; Jadaud, A.; Ioakeimidis, C.; Lazarakis, G.; et al. Spatial Distribution of Marine Macro-Litter on the Seafloor in the Northern Mediterranean Sea: The MEDITS Initiative. Sci. Mar. 2019, 83, S1. [Google Scholar] [CrossRef]
- Mancia, A.; Chenet, T.; Bono, G.; Geraci, M.L.; Vaccaro, C.; Munari, C.; Mistri, M.; Cavazzini, A.; Pasti, L. Adverse Effects of Plastic Ingestion on the Mediterranean Small-Spotted Catshark (Scyliorhinus canicula). Mar. Environ. Res. 2020, 155, 104876. [Google Scholar] [CrossRef]
- Rotini, A.; Chiesa, S.; Manfra, L.; Borrello, P.; Piermarini, R.; Silvestri, C.; Cappucci, S.; Parlagreco, L.; Devoti, S.; Pisapia, M.; et al. Effectiveness of the “Ecological Beach” Model: Beneficial Management of Posidonia Beach Casts and Banquette. Water 2020, 12, 3238. [Google Scholar] [CrossRef]
- Restaino, O.F.; Giosafatto, C.V.L.; Mirpoor, S.F.; Cammarota, M.; Hejazi, S.; Mariniello, L.; Schiraldi, C.; Porta, R. Sustainable Exploitation of Posidonia oceanica Sea Balls (Egagropili): A Review. Int. J. Mol. Sci 2023, 24, 7301. [Google Scholar] [CrossRef] [PubMed]
- Verhille, G.; Moulinet, S.; Vandenberghe, N.; Adda-Bedia, M.; Gal, P. Le Structure and Mechanics of Aegagropilae Fiber Network. Proc. Natl. Acad. Sci. USA 2017, 114, 4607–4612. [Google Scholar] [CrossRef] [PubMed]
- Pfeifer, L. “Neptune Balls” Polysaccharides: Disentangling the Wiry Seagrass Detritus. Polymers 2021, 13, 4285. [Google Scholar] [CrossRef]
- Rubio-Portillo, E.; Martin-Cuadrado, A.-B.; Ramos-Esplá, A.Á.; Antón, J. Metagenomics Unveils Posidonia Oceanica “Banquettes” as a Potential Source of Novel Bioactive Compounds and Carbohydrate Active Enzymes (CAZymes). Msystems 2021, 6, e0086621. [Google Scholar] [CrossRef]
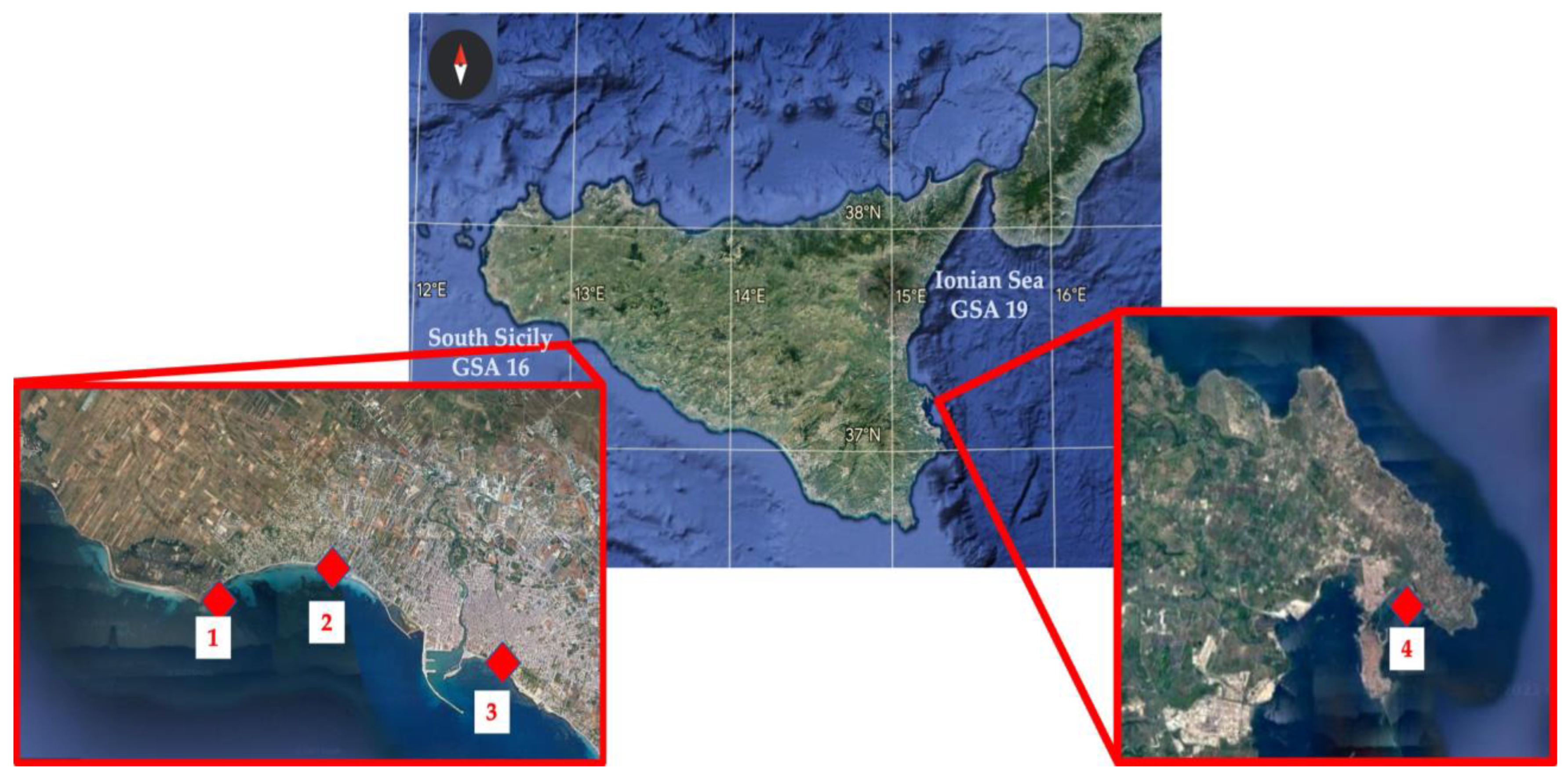


| Capo Feto | Tonnarella | S. Vito | Spiaggetta del Sole | |
|---|---|---|---|---|
| GPS Coordinates | 37°39′35.3″ N 12°31′40.6″ E | 37°39′36.5″ N 12°34′03.9″ E | 37°38′17.8″ N 12°36′38.7″ E | 37°14′26.0″ N 15°14′11.3″ E |
| Area | South of Sicily GSA 16 | South of Sicily GSA 16 | South of Sicily GSA 16 | Ionian Sea GSA 19 |
| Beach composition | Sand | Sand | Sand | Sand |
| Major usage beach | Protected area | Local and tourist people swimming. fishing and other activities | Local and tourist swimming | Local people. swimming. sunbathing. fishing. Surfing |
| Distance from the city (Km) | 4.5 | 1.6 | 0 | 1.3 |
| How often is the beach cleaned | Never | Once a year, during spring | Once a year, during spring | Once a year, before summer |
| Method used to clean | - | Manual | Mechanical | Mechanical |
| Beach | N | Length Range (mm) (mean ± SD) | Width Range (mm) (mean ± SD) | Height Range (mm) (mean ± SD) | Roundness (mean ± SD) | Weight Range (g) (mean ± SD) | N Items/Spheroid | N Items/kg |
|---|---|---|---|---|---|---|---|---|
| Capo Feto | 20 | 17.2–73.0 (50.6 ± 18.2) | 16.5–61.0 (45.8 ± 17.0) | 27.2–86.5 (56.6 ± 18.4) | 0.7–0.9 (0.9 ± 0.1) | 1–49 (20 ± 15) | 0.5 | 26.9 |
| Tonnarella | 20 | 21.8–87.4 (54.5 ± 23.0) | 15.4–72.3 (41.5 ± 17.8) | 29.1–98.3 (60.4 ± 19.8) | 0.5–0.9 (0.8 ± 0.1) | 3–73 (25 ± 22) | 1.5 | 58.8 |
| S. Vito | 20 | 20.0–85.4 (47.6 ± 18.6) | 17.5–57.6 (37.0 ± 14.0) | 30.5–106.7 (57.7 ± 24.7) | 0.5–09 (0.8 ± 0.1) | 1–71 (18 ± 21) | 3.2 | 175.7 |
| Spiaggetta del Sole | 20 | 23.7–79.2 (49.6 ± 14.6) | 13.7–30.9 (35.4 ± 10.1) | 28.2–114.4 (56.0 ± 20.4) | 0.6–0.9 (0.8 ± 0.1) | 1–34 (12 ± 9) | 4.8 | 408.5 |
| TOTAL | 80 | 17.2–87.4 (50.6 ± 15.3) | 13.7–72.3 (39.9 ± 20.6) | 27.2–114.4 (57.7 ± 20.6) | 0.5–0.9 (0.8 ± 0.1) | 1–73 (19.0 ± 17.9) | 2.5 | 132.5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Porcino, N.; Bottari, T.; Falco, F.; Natale, S.; Mancuso, M. Posidonia Spheroids Intercepting Plastic Litter: Implications for Beach Clean-Ups. Sustainability 2023, 15, 15740. https://doi.org/10.3390/su152215740
Porcino N, Bottari T, Falco F, Natale S, Mancuso M. Posidonia Spheroids Intercepting Plastic Litter: Implications for Beach Clean-Ups. Sustainability. 2023; 15(22):15740. https://doi.org/10.3390/su152215740
Chicago/Turabian StylePorcino, Nunziatina, Teresa Bottari, Francesca Falco, Sabrina Natale, and Monique Mancuso. 2023. "Posidonia Spheroids Intercepting Plastic Litter: Implications for Beach Clean-Ups" Sustainability 15, no. 22: 15740. https://doi.org/10.3390/su152215740







