Mitigating Greenhouse Gas Emissions from Crop Production and Management Practices, and Livestock: A Review
Abstract
:1. Introduction
2. Major Sources of Greenhouse Gases in Agriculture
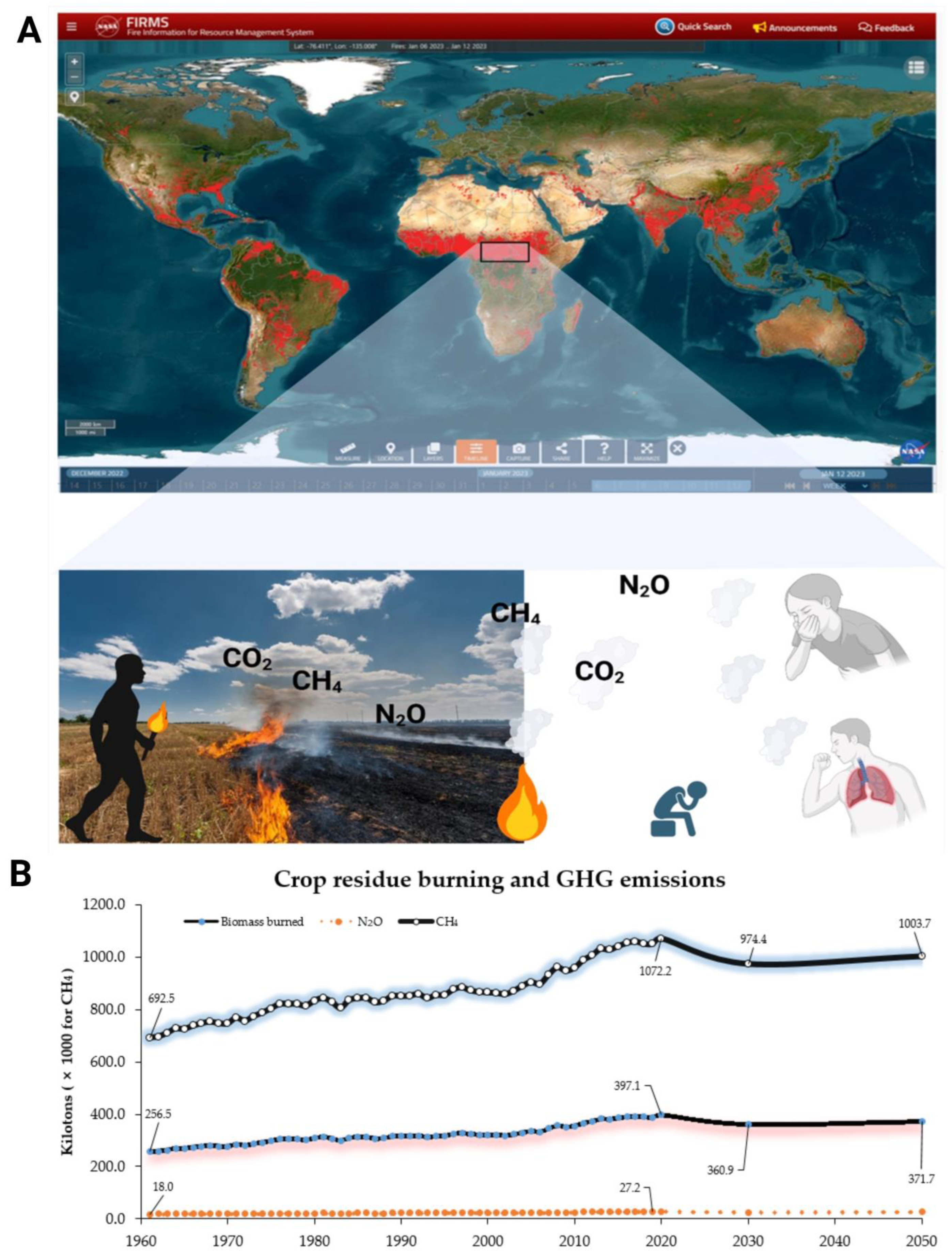
2.1. Agricultural Land Use and Management of Crop Residues
2.2. Farming Practices and Fertilizers
2.3. Livestock Production
3. Mechanisms of Greenhouse Gases Production and Emission from Crop Cultivation
3.1. Methanogenesis and Methanotrophy
3.2. Nitrous Oxide Generation and Emission
3.3. Carbon Dioxide Emission and Sequestration
4. Mechanisms of GHG Production and Emission from Livestock
5. Approaches to Reduce GHG Emissions in Agriculture
5.1. Improving Management of Crop Residues
5.2. Enhancing Nitrogen Use Efficiency in Plants
5.3. Improving Abiotic Stress Tolerance in Plants
5.4. Exploring Radial Oxygen Loss and Intermittent Drainage
5.5. Biochar Reduces Mineral Fertilizers Use, Improves Soil Properties and Mitigate Ghg Emissions
5.6. Enhancing Sink Strength
5.7. Use of Nitrification Inhibitors or Low GHG-Emitting Crop Cultivars
5.8. Improving Livestock Production and Feeding Efficiency
6. Conclusions and Future Prospects
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Clapp, J.; Cohen, M.J. The Global Food Crisis: Governance Challenges and Opportunities; Wilfrid Laurier University Press: Waterloo, Canada, 2009. [Google Scholar]
- Singh, D.; Seager, R.; Cook, B.I.; Cane, M.; Ting, M.; Cook, E.; Davis, M. Climate and the Global Famine of 1876–1878. J. Clim. 2018, 31, 9445–9467. [Google Scholar] [CrossRef]
- Gooding, P. ENSO, IOD, Drought, and Floods in Equatorial Eastern Africa, 1876–1878. In Droughts, Floods, and Global Climatic Anomalies in the Indian Ocean World; Springer: Berlin/Heidelberg, Germany, 2022; pp. 259–287. [Google Scholar]
- Holden, E.; Linnerud, K.; Banister, D. Sustainable development: Our common future revisited. Glob. Environ. Change 2014, 26, 130–139. [Google Scholar] [CrossRef]
- Sam, S.J.E. The people, planet, prosperity, peace and partnership: Why the sustainable development goals should matter to everyone. Ecodate 2016, 30, 7–12. [Google Scholar]
- Mpabanga, D.; Sesa, L. Imperatives: The Five P’s: People, Planet, Prosperity, Peace and Partnerships and Sustainable Development Goals-The Need to Transform Public Administration and Management. Afr. J. Public Adm. Manag. 2020, 17, 44–58. [Google Scholar]
- Papoulis, D.; Kaika, D.; Bampatsou, C.; Zervas, E.J.C. Public perception of climate change in a period of economic crisis. Climate 2015, 3, 715–726. [Google Scholar] [CrossRef]
- Adger, W.N.; Kelly, P.M. Social vulnerability to climate change and the architecture of entitlements. Mitig. Adapt. Strateg. Glob. Change 1999, 4, 253–266. [Google Scholar] [CrossRef]
- Otto, I.M.; Reckien, D.; Reyer, C.P.; Marcus, R.; Le Masson, V.; Jones, L.; Norton, A.; Serdeczny, O. Social vulnerability to climate change: A review of concepts and evidence. Reg. Environ. Change 2017, 17, 1651–1662. [Google Scholar] [CrossRef]
- Hasegawa, T.; Fujimori, S.; Havlík, P.; Valin, H.; Bodirsky, B.L.; Doelman, J.C.; Fellmann, T.; Kyle, P.; Koopman, J.F.; Lotze-Campen, H.; et al. Risk of increased food insecurity under stringent global climate change mitigation policy. Nat. Clim. Change 2018, 8, 699–703. [Google Scholar] [CrossRef]
- Ericksen, P.J.; Thornton, P.K.; Notenbaert, A.M.O.; Cramer, L.; Jones, P.G.; Herrero, M.T. Mapping hotspots of climate change and food insecurity in the global tropics. In CCAFS Report; CCAFS: Copenhagen, Denmark, 2011. [Google Scholar]
- Crutzen, P.J.J.N. Methane’s sinks and sources. Nature 1991, 350, 380–381. [Google Scholar] [CrossRef]
- Reay, D. Greenhouse Gas Sinks; CABI: Wallingford, UK, 2007. [Google Scholar]
- Reay, D.; Smith, P.; Van Amstel, A. Methane sources and the global methane budget. In Methane and Climate Change; Earthscan: London, UK; Washington, DC, USA, 2010; pp. 1–13. [Google Scholar]
- Ming, T.; Davies, P.; Liu, W.; Caillol, S. Removal of non-CO2 greenhouse gases by large-scale atmospheric solar photocatalysis. Prog. Energy Combust. Sci. 2017, 60, 68–96. [Google Scholar]
- Schütz, H.; Holzapfel-Pschorn, A.; Conrad, R.; Rennenberg, H.; Seiler, W. A 3-year continuous record on the influence of daytime, season, and fertilizer treatment on methane emission rates from an Italian rice paddy. J. Geophys. Res. Atmos. 1989, 94, 16405–16416. [Google Scholar]
- Colmer, T.D. Long-distance transport of gases in plants: A perspective on internal aeration and radial oxygen loss from roots. Plant Cell Environ. 2003, 26, 17–36. [Google Scholar] [CrossRef]
- Schiermeier, Q. Study fails to catch plants making methane. Nature 2009. [Google Scholar] [CrossRef]
- Nisbet, R.E.R.; Fisher, R.; Nimmo, R.H.; Bendall, D.S.; Crill, P.M.; Gallego-Sala, A.V.; Hornibrook, E.R.C.; López-Juez, E.; Lowry, D.; Nisbet, P.B.R.; et al. Emission of methane from plants. Proc. R. Soc. B Biol. Sci. 2009, 276, 1347–1354. [Google Scholar] [CrossRef]
- Nouchi, I.; Mariko, S.; Aoki, K. Mechanism of methane transport from the rhizosphere to the atmosphere through rice plants. Plant Physiol. 1990, 94, 59–66. [Google Scholar] [CrossRef]
- Wassmann, R.; Aulakh, M.S. The role of rice plants in regulating mechanisms of methane missions. Biol. Fertil. Soils 2000, 31, 20–29. [Google Scholar] [CrossRef]
- Garnet, K.N.; Megonigal, J.P.; Litchfield, C.; Taylor, G.E., Jr. Physiological control of leaf methane emission from wetland plants. Aquat. Bot. 2005, 81, 141–155. [Google Scholar] [CrossRef]
- McLeod, A.R.; Fry, S.C.; Loake, G.J.; Messenger, D.J.; Reay, D.S.; Smith, K.A.; Yun, B.W. Ultraviolet radiation drives methane emissions from terrestrial plant pectins. New Phytol. 2008, 180, 124–132. [Google Scholar] [CrossRef]
- Putkinen, A.; Siljanen, H.M.; Laihonen, A.; Paasisalo, I.; Porkka, K.; Tiirola, M.; Haikarainen, I.; Tenhovirta, S.; Pihlatie, M. New insight to the role of microbes in the methane exchange in trees: Evidence from metagenomic sequencing. New Phytol. 2021, 231, 524–536. [Google Scholar] [CrossRef]
- Li, L.; Wei, S.; Shen, W. The role of methane in plant physiology: A review. Plant Cell Rep. 2020, 39, 171–179. [Google Scholar] [CrossRef] [PubMed]
- Wang, N.; Huang, D.; Li, C.; Deng, Y.; Li, W.; Yao, Y.; Liao, W. Regulatory roles of methane in plants. Sci. Hortic. 2020, 272, 109492. [Google Scholar] [CrossRef]
- Olhoff, A.; Christensen, J.M. Emissions Gap Report 2020; UNEP DTU Partnership: Nairobi, Kenya, 2020. [Google Scholar]
- Sun, B.F.; Zhao, H.; Lü, Y.Z.; Fei, L.U.; Wang, X.K. The effects of nitrogen fertilizer application on methane and nitrous oxide emission/uptake in Chinese croplands. J. Integr. Agric. 2016, 15, 440–450. [Google Scholar] [CrossRef]
- Xu, X.; Wu, Z.; Dong, Y.; Zhou, Z.; Xiong, Z. Effects of nitrogen and biochar amendment on soil methane concentration profiles and diffusion in a rice-wheat annual rotation system. Sci. Rep. 2016, 6, 38688. [Google Scholar] [CrossRef]
- Kabange, N.R.; Lee, S.M.; Shin, D.; Lee, J.Y.; Kwon, Y.; Kang, J.W.; Cha, J.K.; Park, H.; Alibu, S.; Lee, J.H. Multiple Facets of Nitrogen: From Atmospheric Gas to Indispensable Agricultural Input. Life 2022, 12, 1272. [Google Scholar] [CrossRef]
- Nunes-Nesi, A.; Fernie, A.R.; Stitt, M. Metabolic and signaling aspects underpinning the regulation of plant carbon nitrogen interactions. Mol. Plant 2010, 3, 973–996. [Google Scholar] [CrossRef] [PubMed]
- Stokstad, E. The nitrogen fix. Science 2016, 353, 1225–1227. [Google Scholar] [CrossRef]
- Tokarz, E.; Urban, D. Soil redox potential and its impact on microorganisms and plants of wetlands. J. Ecol. Eng. 2015, 16, 20–30. [Google Scholar] [CrossRef]
- Wilmoth, J.L.; Schaefer, J.K.; Schlesinger, D.R.; Roth, S.W.; Hatcher, P.G.; Shoemaker, J.K.; Zhang, X. The role of oxygen in stimulating methane production in wetlands. Glob. Change Biol. 2021, 27, 5831–5847. [Google Scholar] [CrossRef]
- Morard, P.; Silvestre, J. Plant injury due to oxygen deficiency in the root environment of soilless culture: A review. Plant Soil 1996, 184, 243–254. [Google Scholar] [CrossRef]
- Duyen, D.V.; Kwon, Y.; Kabange, N.R.; Lee, J.Y.; Lee, S.M.; Kang, J.W.; Park, H.; Cha, J.K.; Cho, J.H.; Shin, D.; et al. Novel QTL Associated with Aerenchyma-Mediated Radial Oxygen Loss (ROL) in Rice (Oryza sativa L.) under Iron (II) Sulfide. Plants 2022, 11, 788. [Google Scholar] [CrossRef]
- Shan, Y.H.; Johnson-Beebout, S.E.; Buresh, R.J. Crop residue management for lowland rice-based cropping systems in Asia. Adv. Agron. 2008, 98, 117–199. [Google Scholar]
- Dobermann, A.T.H.F.; Fairhurst, T.H. Rice straw management. Better Crops Int. 2002, 16, 7–11. [Google Scholar]
- Singh, D.; Dhiman, S.K.; Kumar, V.; Babu, R.; Shree, K.; Priyadarshani, A.; Singh, A.; Shakya, L.; Nautiyal, A.; Saluja, S. Residue Burning and Its Relationship between Health, Agriculture Value Addition, and Regional Finance. Atmosphere 2022, 13, 1405. [Google Scholar] [CrossRef]
- Andini, A.; Bonnet, S.; Rousset, P.; Hasanudin, U. Impact of open burning of crop residues on air pollution and climate change in Indonesia. Curr. Sci. 2018, 115, 2259–2266. [Google Scholar] [CrossRef]
- Romasanta, R.R.; Sander, B.O.; Gaihre, Y.K.; Alberto, M.C.; Gummert, M.; Quilty, J.; Castalone, A.G.; Balingbing, C.; Sandro, J.; Correa, T., Jr.; et al. How does burning of rice straw affect CH4 and N2O emissions? A comparative experiment of different on-field straw management practices. Agric. Ecosyst. Environ. 2017, 239, 143–153. [Google Scholar] [CrossRef]
- Baggs, E.M.; Philippot, L. Microbial terrestrial pathways to nitrous oxide. In Nitrous Oxide and Climate Change; Routledge: London, UK, 2010; p. 256. [Google Scholar]
- Khalil, K.; Mary, B.; Renault, P. Nitrous oxide production by nitrification and denitrification in soil aggregates as affected by O2 concentration. Soil Biol. Biochem. 2004, 36, 687–699. [Google Scholar] [CrossRef]
- Dalal, R.C.; Wang, W.; Robertson, G.P.; Parton, W.J. Nitrous oxide emission from Australian agricultural lands and mitigation options: A review. Soil Res. 2003, 41, 165–195. [Google Scholar] [CrossRef]
- Dalal, R.C.; Allen, D.E.; Livesley, S.J.; Richards, G. Magnitude and biophysical regulators of methane emission and consumption in the Australian agricultural, forest, and submerged landscapes: A review. Plant Soil 2008, 309, 43–76. [Google Scholar] [CrossRef]
- Thangarajan, R.; Bolan, N.S.; Tian, G.; Naidu, R.; Kunhikrishnan, A. Role of organic amendment application on greenhouse gas emission from soil. Sci. Total Environ. 2013, 465, 72–96. [Google Scholar] [CrossRef]
- Rayne, N.; Aula, L. Livestock manure and the impacts on soil health: A review. Soil Syst. 2020, 4, 64. [Google Scholar] [CrossRef]
- Risse, L.; Cabrera, M.; Franzluebbers, A.; Gaskin, J.; Gilley, J.E.; Killorn, R.; Radcliffe, D.; Tollner, W.; Zhang, H. Land Application of Manure for Beneficial Reuse; American Society of Agricultural and Biological Engineers: St. Joseph, MI, USA, 2006. [Google Scholar]
- Bhunia, S.; Bhowmik, A.; Mallick, R.; Mukherjee, J. Agronomic efficiency of animal-derived organic fertilizers and their effects on biology and fertility of soil: A review. Agronomy 2021, 11, 823. [Google Scholar] [CrossRef]
- Jensen, E.S.; Hauggaard-Nielsen, H. How can increased use of biological N2 fixation in agriculture benefit the environment? Plant Soil 2003, 252, 177–186. [Google Scholar] [CrossRef]
- Tao, R.; Liang, Y.; Wakelin, S.A.; Chu, G. Supplementing chemical fertilizer with an organic component increases soil biological function and quality. Appl. Soil Ecol. 2015, 96, 42–51. [Google Scholar] [CrossRef]
- Zhang, S.; Sun, L.; Wang, Y.; Fan, K.; Xu, Q.; Li, Y.; Ma, Q.; Wang, J.; Ren, W.; Ding, Z. Cow manure application effectively regulates the soil bacterial community in tea plantation. BMC Microbiol. 2020, 20, 190. [Google Scholar] [CrossRef]
- Zhou, M.; Zhu, B.; Wang, S.; Zhu, X.; Vereecken, H.; Brüggemann, N. Stimulation of N2O emission by manure application to agricultural soils may largely offset carbon benefits: A global meta-analysis. Glob. Change Biol. 2017, 23, 4068–4083. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Sharma, P.; Shu, S.; Lin, T.; Ciais, P.; Tubiello, F.; Smith, P.; Campbell, N.; Jain, A. Global greenhouse gas emissions from animal-based foods are twice those of plant-based foods. Nat. Food 2021, 2, 724–732. [Google Scholar] [CrossRef]
- Bridgham, S.D.; Cadillo-Quiroz, H.; Keller, J.K.; Zhuang, Q. Methane emissions from wetlands: Biogeochemical, microbial, and modeling perspectives from local to global scales. Glob. Change Biol. 2013, 19, 1325–1346. [Google Scholar] [CrossRef]
- Lyu, Z.; Shao, N.; Akinyemi, T.; Whitman, W.B. Methanogenesis. Curr. Biol. 2018, 28, R727–R732. [Google Scholar] [CrossRef]
- Fournier, G.P.; Gogarten, J.P. Evolution of acetoclastic methanogenesis in Methanosarcina via horizontal gene transfer from cellulolytic Clostridia. J. Bacteriol. 2008, 190, 1124–1127. [Google Scholar] [CrossRef]
- Tveit, A.T.; Hestnes, A.G.; Robinson, S.L.; Schintlmeister, A.; Dedysh, S.N.; Jehmlich, N.; von Bergen, M.; Herbold, C.; Wagner, M.; Richter, A.; et al. Widespread soil bacterium that oxidizes atmospheric methane. Proc. Natl. Acad. Sci. USA 2019, 116, 8515–8524. [Google Scholar] [CrossRef]
- Hanson, R.S.; Hanson, T.E. Methanotrophic bacteria. Microbiol. Rev. 1996, 60, 439–471. [Google Scholar] [CrossRef] [PubMed]
- Kirschke, S.; Bousquet, P.; Ciais, P.; Saunois, M.; Canadell, J.G.; Dlugokencky, E.J.; Bergamaschi, P.; Bergmann, D.; Blake, D.R.; Bruhwiler, L.; et al. Three decades of global methane sources and sinks. Nat. Geosci. 2013, 6, 813–823. [Google Scholar] [CrossRef]
- Hink, L.; Nicol, G.W.; Prosser, J.I. Archaea produce lower yields of N2O than bacteria during aerobic ammonia oxidation in soil. Environ. Microbiol. 2017, 19, 4829–4837. [Google Scholar] [CrossRef]
- Hink, L.; Lycus, P.; Gubry-Rangin, C.; Frostegård, Å.; Nicol, G.W.; Prosser, J.I.; Bakken, L.R. Kinetics of NH3-oxidation, NO-turnover, N2O-production and electron flow during oxygen depletion in model bacterial and archaeal ammonia oxidisers. Environ. Microbiol. 2017, 19, 4882–4896. [Google Scholar] [CrossRef]
- Schmidt-Rohr, K. Oxygen is the high-energy molecule powering complex multicellular life: Fundamental corrections to traditional bioenergetics. ACS Omega 2020, 5, 2221–2233. [Google Scholar] [CrossRef]
- Murrell, J. The aerobic methane oxidizing bacteria (methanotrophs). In Handbook of Hydrocarbon and Lipid Microbiology; Springer: Berlin/Heidelberg, Germany, 2010. [Google Scholar]
- Reim, A.; Lüke, C.; Krause, S.; Pratscher, J.; Frenzel, P. One millimetre makes the difference: High-resolution analysis of methane-oxidizing bacteria and their specific activity at the oxic–anoxic interface in a flooded paddy soil. ISME J. 2012, 6, 2128–2139. [Google Scholar] [CrossRef] [PubMed]
- Stein, L.Y.; Roy, R.; Dunfield, P.F. Aerobic methanotrophy and nitrification: Processes and connections. In eLS; John Wiley & Sons: Hoboken, NJ, USA, 2012. [Google Scholar]
- Walkiewicz, A.; Brzezińska, M.; Bieganowski, A. Methanotrophs are favored under hypoxia in ammonium-fertilized soils. Biol. Fertil. Soils 2018, 54, 861–870. [Google Scholar] [CrossRef]
- Ravishankara, A.R.; Daniel, J.S.; Portmann, R.W. Nitrous oxide (N2O): The dominant ozone-depleting substance emitted in the 21st century. Science 2009, 326, 123–125. [Google Scholar] [CrossRef]
- Thomson, A.J.; Giannopoulos, G.; Pretty, J.; Baggs, E.M.; Richardson, D.J. Biological sources and sinks of nitrous oxide and strategies to mitigate emissions. Philos. Trans. R. Soc. B Biol. Sci. 2012, 367, 1157–1168. [Google Scholar] [CrossRef]
- Bange, H.W.; Freing, A.; Kock, A.; Löscher, C.R. Marine pathways to nitrous oxide. Nitrous Oxide Clim. Change 2010, 2, 36–62. [Google Scholar]
- Inatomi, M.; Hajima, T.; Ito, A. Fraction of nitrous oxide production in nitrification and its effect on total soil emission: A meta-analysis and global-scale sensitivity analysis using a process-based model. PLoS ONE 2019, 14, e0219159. [Google Scholar] [CrossRef]
- Liu, R.; Hu, H.; Suter, H.; Hayden, H.L.; He, J.; Mele, P.; Chen, D. Nitrification is a primary driver of nitrous oxide production in laboratory microcosms from different land-use soils. Front. Microbiol. 2016, 7, 1373. [Google Scholar] [CrossRef] [PubMed]
- Codispoti, L.; Christensen, J. Nitrification, denitrification and nitrous oxide cycling in the eastern tropical South Pacific Ocean. Mar. Chem. 1985, 16, 277–300. [Google Scholar] [CrossRef]
- Ji, Q.; Buitenhuis, E.; Suntharalingam, P.; Sarmiento, J.L.; Ward, B.B. Global nitrous oxide production determined by oxygen sensitivity of nitrification and denitrification. Glob. Biogeochem. Cycles 2018, 32, 1790–1802. [Google Scholar] [CrossRef]
- Scala, D.J.; Kerkhof, L.J. Nitrous oxide reductase (nosZ) gene-specific PCR primers for detection of denitrifiers and three nosZ genes from marine sediments. FEMS Microbiol. Lett. 1998, 162, 61–68. [Google Scholar] [CrossRef]
- Bouwman, A.; Stehfest, E.; van Kessel, C. Nitrous oxide emissions from the nitrogen cycle in arable agriculture: Estimation and mitigation. In Nitrous Oxide and Climate Change; Routledge: London, UK, 2010; pp. 85–106. [Google Scholar]
- Ji, Q.; Babbin, A.R.; Jayakumar, A.; Oleynik, S.; Ward, B.B. Nitrous oxide production by nitrification and denitrification in the Eastern Tropical South Pacific oxygen minimum zone. Geophys. Res. Lett. 2015, 42, 10–755. [Google Scholar] [CrossRef]
- Wrage, N.; Velthof, G.L.; Van Beusichem, M.L.; Oenema, O. Role of nitrifier denitrification in the production of nitrous oxide. Soil Biol. Biochem. 2001, 33, 1723–1732. [Google Scholar] [CrossRef]
- Zhang, J.; Mueller, C.; Cai, Z. Heterotrophic nitrification of organic N and its contribution to nitrous oxide emissions in soils. Soil Biol. Biochem. 2015, 84, 199–209. [Google Scholar] [CrossRef]
- Livesley, S.J.; Kiese, R.; Graham, J.; Weston, C.J.; Butterbach-Bahl, K.; Arndt, S.K. Trace gas flux and the influence of short-term soil water and temperature dynamics in Australian sheep grazed pastures of differing productivity. Plant Soil 2008, 309, 89–103. [Google Scholar] [CrossRef]
- Jørgensen, C.J.; Elberling, B. Effects of flooding-induced N2O production, consumption and emission dynamics on the annual N2O emission budget in wetland soil. Soil Biol. Biochem. 2012, 53, 9–17. [Google Scholar] [CrossRef]
- Mørkved, P.T.; Dörsch, P.; Bakken, L.R. The N2O product ratio of nitrification and its dependence on long-term changes in soil pH. Soil Biol. Biochem. 2007, 39, 2048–2057. [Google Scholar] [CrossRef]
- de Klein, C.A.; Eckard, R.J.; van der Weerden, T.J. Nitrous oxide emissions from the nitrogen cycle in livestock agriculture: Estimation and mitigation. In Nitrous Oxide and Climate Change; Routledge: London, UK, 2010; pp. 107–144. [Google Scholar]
- Conen, F.; Neftel, A. Nitrous oxide emissions from land-use and land-management change. In Nitrous Oxide and Climate Change; Routledge: London, UK, 2010; pp. 143–159. [Google Scholar]
- Well, R.; Butterbach-Bahl, K. Indirect emissions of nitrous oxide from nitrogen deposition and leaching of agricultural nitrogen. In Nitrous Oxide and Climate Change; Smith, K., Ed.; Routledge: London, UK, 2010; p. 162. [Google Scholar]
- Gubry-Rangin, C.; Nicol, G.W.; Prosser, J.I. Archaea rather than bacteria control nitrification in two agricultural acidic soils. FEMS Microbiol. Ecol. 2010, 74, 566–574. [Google Scholar] [CrossRef] [PubMed]
- Stein, L.Y. Insights into the physiology of ammonia-oxidizing microorganisms. Curr. Opin. Chem. Biol. 2019, 49, 9–15. [Google Scholar] [CrossRef] [PubMed]
- Di, H.J.; Cameron, K.C.; Shen, J.P.; Winefield, C.S.; O’Callaghan, M.; Bowatte, S.; He, J.Z. Nitrification driven by bacteria and not archaea in nitrogen-rich grassland soils. Nat. Geosci. 2009, 2, 621–624. [Google Scholar] [CrossRef]
- Di, H.J.; Cameron, K.C.; Sherlock, R.R.; Shen, J.P.; He, J.Z.; Winefield, C.S. Nitrous oxide emissions from grazed grassland as affected by a nitrification inhibitor, dicyandiamide, and relationships with ammonia-oxidizing bacteria and archaea. J. Soils Sediments 2010, 10, 943–954. [Google Scholar] [CrossRef]
- Treusch, A.H.; Leininger, S.; Kletzin, A.; Schuster, S.C.; Klenk, H.P.; Schleper, C. Novel genes for nitrite reductase and Amo-related proteins indicate a role of uncultivated mesophilic crenarchaeota in nitrogen cycling. Environ. Microbiol. 2005, 7, 1985–1995. [Google Scholar] [CrossRef]
- Leininger, S.; Urich, T.; Schloter, M.; Schwark, L.; Qi, J.; Nicol, G.W.; Prosser, J.I.; Schuster, S.C.; Schleper, C. Archaea predominate among ammonia-oxidizing prokaryotes in soils. Nature 2006, 442, 806–809. [Google Scholar] [CrossRef]
- Offre, P.; Prosser, J.I.; Nicol, G.W. Growth of ammonia-oxidizing archaea in soil microcosms is inhibited by acetylene. FEMS Microbiol. Ecol. 2009, 70, 99–108. [Google Scholar] [CrossRef]
- He, J.Z.; Shen, J.P.; Zhang, L.M.; Zhu, Y.G.; Zheng, Y.M.; Xu, M.G.; Di, H. Quantitative analyses of the abundance and composition of ammonia-oxidizing bacteria and ammonia-oxidizing archaea of a Chinese upland red soil under long-term fertilization practices. Environ. Microbiol. 2007, 9, 2364–2374. [Google Scholar] [CrossRef]
- Shen, J.P.; Zhang, L.M.; Zhu, Y.G.; Zhang, J.B.; He, J.Z. Abundance and composition of ammonia-oxidizing bacteria and ammonia-oxidizing archaea communities of an alkaline sandy loam. Environ. Microbiol. 2008, 10, 1601–1611. [Google Scholar] [CrossRef]
- Freitag, A.; Rudert, M.; Bock, E. Growth of Nitrobacter by dissimilatoric nitrate reduction. FEMS Microbiol. Lett. 1987, 48, 105–109. [Google Scholar] [CrossRef]
- Daims, H.; Lebedeva, E.V.; Pjevac, P.; Han, P.; Herbold, C.; Albertsen, M.; Jehmlich, N.; Palatinszky, M.; Vierheilig, J.; Bulaev, A.; et al. Complete nitrification by Nitrospira bacteria. Nature 2015, 528, 504–509. [Google Scholar] [CrossRef] [PubMed]
- Van Kessel, M.A.; Speth, D.R.; Albertsen, M.; Nielsen, P.H.; Op den Camp, H.J.; Kartal, B.; Jetten, M.S.; Lücker, S.v. Complete nitrification by a single microorganism. Nature 2015, 528, 555–559. [Google Scholar] [CrossRef] [PubMed]
- Zou, J.; Huang, Y.A.O.; Qin, Y.; Liu, S.; Shen, Q.; Pan, G.; Lu, Y.; Liu, Q. Changes in fertilizer-induced direct N2O emissions from paddy fields during rice-growing season in China between 1950s and 1990s. Glob. Change Biol. 2009, 15, 229–242. [Google Scholar] [CrossRef]
- Ma, J.; Li, X.L.; Xu, H.; Han, Y.; Cai, Z.C.; Yagi, K. Effects of nitrogen fertiliser and wheat straw application on CH4 and N2O emissions from a paddy rice field. Soil Res. 2007, 45, 359–367. [Google Scholar] [CrossRef]
- Davidson, E.A. The contribution of manure and fertilizer nitrogen to atmospheric nitrous oxide since 1860. Nat. Geosci. 2009, 2, 659–662. [Google Scholar] [CrossRef]
- Syakila, A.; Kroeze, C. The global nitrous oxide budget revisited. Greenh. Gas Meas. Manag. 2011, 1, 17–26. [Google Scholar] [CrossRef]
- Prather, M.J.; Holmes, C.D.; Hsu, J. Reactive greenhouse gas scenarios: Systematic exploration of uncertainties and the role of atmospheric chemistry. Geophys. Res. Lett. 2012, 39. [Google Scholar] [CrossRef]
- Manzano, P.; del Prado, A.; Pardo, G. Comparable GHG emissions from animals in wildlife and livestock-dominated savannas. NPJ Clim. Atmos. Sci. 2023, 6, 27. [Google Scholar] [CrossRef]
- Gerber, P.J.; Steinfeld, H.; Henderson, B.; Mottet, A.; Opio, C.; Dijkman, J.; Falcucci, A.; Tempio, G. Tackling Climate Change through Livestock: A Global Assessment of Emissions and Mitigation Opportunities; Food and Agriculture Organization of the United Nations (FAO): Rome, Italy, 2013. [Google Scholar]
- Chang, J.; Ciais, P.; Gasser, T.; Smith, P.; Herrero, M.; Havlík, P.; Obersteiner, M.; Guenet, B.; Goll, D.S.; Li, W.; et al. Climate warming from managed grasslands cancels the cooling effect of carbon sinks in sparsely grazed and natural grasslands. Nat. Commun. 2021, 12, 118. [Google Scholar] [CrossRef]
- Adopted, I.P.C.C. Climate Change 2014 Synthesis Report; IPCC: Geneva, Szwitzerland, 2014.
- Poore, J.; Nemecek, T. Reducing food’s environmental impacts through producers and consumers. Science 2018, 360, 987–992. [Google Scholar] [CrossRef] [PubMed]
- Casper, J.K. Agriculture: The Food We Grow and Animals We Raise; Infobase Publishing: New York, NY, USA, 2007. [Google Scholar]
- Bhuvaneshwari, S.; Hettiarachchi, H.; Meegoda, J.N. Crop residue burning in India: Policy challenges and potential solutions. Int. J. Environ. Res. Public Health 2019, 16, 832. [Google Scholar] [CrossRef] [PubMed]
- Horwath, W. Greenhouse gas emissions from rice cropping systems. In Understanding Greenhouse Gas Emissions from Agricultural Management; ACS Publications: Washington, DC, USA, 2011; pp. 67–89. [Google Scholar]
- Hoornweg, D.; Bhada-Tata, P. What a Waste: A Global Review of Solid Waste Management; World Bank: Washington, DC, USA, 2012. [Google Scholar]
- Rakotoson, T.; Dusserre, J.; Letourmy, P.; Frouin, J.; Ratsimiala, I.R.; Rakotoarisoa, N.V.; Vom Brocke, K.; Ramanantsoanirina, A.; Ahmadi, N.; Raboin, L.M. Genome-wide association study of nitrogen use efficiency and agronomic traits in upland rice. Rice Sci. 2021, 28, 379–390. [Google Scholar] [CrossRef]
- Bandyopadhyay, T.; Swarbreck, S.M.; Jaiswal, V.; Maurya, J.; Gupta, R.; Bentley, A.R.; Griffiths, H.; Prasad, M. GWAS identifies genetic loci underlying nitrogen responsiveness in the climate resilient C4 model Setaria italica (L.). J. Adv. Res. 2022, 42, 249–261. [Google Scholar] [CrossRef]
- Akhatar, J.; Goyal, A.; Kaur, N.; Atri, C.; Mittal, M.; Singh, M.P.; Kaur, R.; Rialch, I.; Banga, S.S. Genome wide association analyses to understand genetic basis of flowering and plant height under three levels of nitrogen application in Brassica juncea (L.) Czern & Coss. Sci. Rep. 2021, 11, 4278. [Google Scholar] [PubMed]
- Nazish, T.; Arshad, M.; Jan, S.U.; Javaid, A.; Khan, M.H.; Naeem, M.A.; Baber, M.; Ali, M. Transporters and transcription factors gene families involved in improving nitrogen use efficiency (NUE) and assimilation in rice (Oryza sativa L.). Transgenic Res. 2021, 31, 23–42. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Su, Q.; Nian, J.; Zhang, J.; Guo, M.; Dong, G.; Hu, J.; Wang, R.; Wei, C.; Li, G.; et al. The Ghd7 transcription factor represses ARE1 expression to enhance nitrogen utilization and grain yield in rice. Mol. Plant 2021, 14, 1012–1023. [Google Scholar] [CrossRef]
- Han, M.L.; Lv, Q.Y.; Zhang, J.; Wang, T.; Zhang, C.X.; Tan, R.J.; Wang, Y.L.; Zhong, L.Y.; Gao, Y.Q.; Chao, Z.F.; et al. Decreasing nitrogen assimilation under drought stress by suppressing DST-mediated activation of Nitrate Reductase 1.2 in rice. Mol. Plant 2022, 15, 167–178. [Google Scholar] [CrossRef]
- Zhang, Z.; Chu, C. Nitrogen-use divergence between indica and japonica rice: Variation at nitrate assimilation. Mol. Plant 2020, 13, 6–7. [Google Scholar] [CrossRef]
- Teng, W.; He, X.; Tong, Y. Genetic Control of Efficient Nitrogen Use for High Yield and Grain Protein Concentration in Wheat: A Review. Plants 2022, 11, 492. [Google Scholar] [CrossRef]
- Kabange, N.R.; Park, S.Y.; Shin, D.; Lee, S.M.; Jo, S.M.; Kwon, Y.; Cha, J.K.; Song, Y.C.; Ko, J.M.; Lee, J.H. Identification of a novel QTL for chlorate resistance in rice (Oryza sativa L.). Agriculture 2020, 10, 360. [Google Scholar] [CrossRef]
- Anas, M.; Liao, F.; Verma, K.K.; Sarwar, M.A.; Mahmood, A.; Chen, Z.-L.; Li, Q.; Zeng, X.-P.; Liu, Y.; Li, Y.-R. Fate of nitrogen in agriculture and environment: Agronomic, eco-physiological and molecular approaches to improve nitrogen use efficiency. Biol. Res. 2020, 53, 47. [Google Scholar] [CrossRef]
- Li, Q.; Lu, X.; Wang, C.; Shen, L.; Dai, L.; He, J.; Yang, L.; Li, P.; Hong, Y.; Zhang, Q.; et al. Genome-wide association study and transcriptome analysis reveal new QTL and candidate genes for nitrogen-deficiency tolerance in rice. Crop J. 2022, 10, 942–951. [Google Scholar] [CrossRef]
- Fan, X.; Naz, M.; Fan, X.; Xuan, W.; Miller, A.J.; Xu, G. Plant nitrate transporters: From gene function to application. J. Exp. Bot. 2017, 68, 2463–2475. [Google Scholar] [CrossRef]
- Lea, P.J.; Miflin, B.J. Glutamate synthase and the synthesis of glutamate in plants. Plant Physiol. Biochem. 2003, 41, 555–564. [Google Scholar] [CrossRef]
- Prasad, L.R.V.; Mailapalli, D.R. Evaluation of nitrogen fertilization patterns using DSSAT for enhancing grain yield and nitrogen use efficiency in rice. Commun. Soil Sci. Plant Anal. 2018, 49, 1401–1417. [Google Scholar] [CrossRef]
- Nakidakida, T.; Hayashi, H. Nitrogen recovery and nitrogen use efficiency of potatoes in an integrated compost fertilization system in an Andosol soil. In Proceedings of the III International Symposium on Organic Matter Management and Compost Use in Horticulture 1146, Murcia, Spain, 6 April 2015; pp. 41–48. [Google Scholar]
- Li, H.; Hu, B.; Chu, C. Nitrogen use efficiency in crops: Lessons from Arabidopsis and rice. J. Exp. Bot. 2017, 68, 2477–2488. [Google Scholar] [CrossRef] [PubMed]
- Duan, Y.H.; Zhang, Y.L.; Ye, L.T.; Fan, X.R.; Xu, G.H.; Shen, Q.R. Responses of rice cultivars with different nitrogen use efficiency to partial nitrate nutrition. Ann. Bot. 2007, 99, 1153–1160. [Google Scholar] [CrossRef]
- Djaman, K.; Mel, V.C.; Ametonou, F.Y.; El-Namaky, R.; Diallo, M.D.; Koudahe, K. Effect of nitrogen fertilizer dose and application timing on yield and nitrogen use efficiency of irrigated hybrid rice under semi-arid conditions. J. Agric. Sci. Food Res. 2018, 9, 223. [Google Scholar]
- Gallais, A.; Hirel, B. An approach to the genetics of nitrogen use efficiency in maize. J. Exp. Bot. 2004, 55, 295–306. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Hu, B.; Chu, C. Nitrogen assimilation in plants: Current status and future prospects. J. Genet. Genom. 2021, 49, 394–404. [Google Scholar] [CrossRef] [PubMed]
- Hirel, B.; Tétu, T.; Lea, P.J.; Dubois, F. Improving nitrogen use efficiency in crops for sustainable agriculture. Sustainability 2011, 3, 1452–1485. [Google Scholar] [CrossRef]
- Yamaya, T.; Obara, M.; Nakajima, H.; Sasaki, S.; Hayakawa, T.; Sato, T. Genetic manipulation and quantitative-trait loci mapping for nitrogen recycling in rice. J. Exp. Bot. 2002, 53, 917–925. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Zhu, L.; Shen, C.; Ji, Z.; Zhang, H.; Zhang, T.; Li, Y.; Yu, J.; Yang, N.; He, Y.; et al. Natural allelic variation in a modulator of auxin homeostasis improves grain yield and nitrogen use efficiency in rice. Plant Cell 2021, 33, 566–580. [Google Scholar] [CrossRef]
- Kiba, T.; Kudo, T.; Kojima, M.; Sakakibara, H. Hormonal control of nitrogen acquisition: Roles of auxin, abscisic acid, and cytokinin. J. Exp. Bot. 2011, 62, 1399–1409. [Google Scholar] [CrossRef]
- Séguéla, M.; Briat, J.F.; Vert, G.; Curie, C. Cytokinins negatively regulate the root iron uptake machinery in Arabidopsis through a growth-dependent pathway. Plant J. 2008, 55, 289–300. [Google Scholar] [CrossRef]
- Maruyama-Nakashita, A.; Nakamura, Y.; Yamaya, T.; Takahashi, H. A novel regulatory pathway of sulfate uptake in Arabidopsis roots: Implication of CRE1/WOL/AHK4-mediated cytokinin-dependent regulation. Plant J. 2004, 38, 779–789. [Google Scholar] [CrossRef]
- Wagner, B.M.; Beck, E. Cytokinins in the perennial herb Urtica dioica L. as influenced by its nitrogen status. Planta 1993, 190, 511–518. [Google Scholar] [CrossRef]
- Franco-Zorrilla, J.M.; Martin, A.C.; Solano, R.; Rubio, V.; Leyva, A.; Paz-Ares, J. Mutations at CRE1 impair cytokinin-induced repression of phosphate starvation responses in Arabidopsis. Plant J. 2002, 32, 353–360. [Google Scholar] [CrossRef]
- Nacry, P.; Canivenc, G.; Muller, B.; Azmi, A.; Van Onckelen, H.; Rossignol, M.; Doumas, P. A role for auxin redistribution in the responses of the root system architecture to phosphate starvation in Arabidopsis. Plant Physiol. 2005, 138, 2061–2074. [Google Scholar] [CrossRef]
- Vidal, E.A.; Araus, V.; Lu, C.; Parry, G.; Green, P.J.; Coruzzi, G.M.; Gutiérrez, R.A. Nitrate-responsive miR393/AFB3 regulatory module controls root system architecture in Arabidopsis thaliana. Proc. Natl. Acad. Sci. USA 2010, 107, 4477–4482. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.-E.; Chen, H.-Y.; Tseng, C.-S.; Tsay, Y.-F. Improving nitrogen use efficiency by manipulating nitrate remobilization in plants. Nat. Plants 2020, 6, 1126–1135. [Google Scholar] [CrossRef]
- Ueda, Y.; Yanagisawa, S. Transcription factor-based genetic engineering to increase nitrogen use efficiency. In Engineering Nitrogen Utilization in Crop Plants; Springer: Berlin/Heidelberg, Germany, 2018; pp. 37–55. [Google Scholar]
- Wu, J.; Zhang, Z.S.; Xia, J.Q.; Alfatih, A.; Song, Y.; Huang, Y.J.; Wan, G.Y.; Sun, L.Q.; Tang, H.; Liu, Y. Rice NIN-LIKE PROTEIN 4 plays a pivotal role in nitrogen use efficiency. Plant Biotechnol. J. 2021, 19, 448–461. [Google Scholar] [CrossRef]
- Li, Q.; Ding, G.; Yang, N.; White, P.J.; Ye, X.; Cai, H.; Lu, J.; Shi, L.; Xu, F. Comparative genome and transcriptome analysis unravels key factors of nitrogen use efficiency in Brassica napus L. Plant Cell Environ. 2020, 43, 712–731. [Google Scholar] [PubMed]
- Heuermann, D.; Hahn, H.; Von Wirén, N. Seed yield and nitrogen efficiency in oilseed rape after ammonium nitrate or urea fertilization. Front. Plant Sci. 2021, 11, 2197. [Google Scholar] [CrossRef]
- Ruffel, S.; Krouk, G.; Ristova, D.; Shasha, D.; Birnbaum, K.D.; Coruzzi, G.M. Nitrogen economics of root foraging: Transitive closure of the nitrate–cytokinin relay and distinct systemic signaling for N supply vs. demand. Proc. Natl. Acad. Sci. USA 2011, 108, 18524–18529. [Google Scholar] [CrossRef]
- Gu, J.; Li, Z.; Mao, Y.; Struik, P.C.; Zhang, H.; Liu, L.; Wang, Z.; Yang, J. Roles of nitrogen and cytokinin signals in root and shoot communications in maximizing of plant productivity and their agronomic applications. Plant Sci. 2018, 274, 320–331. [Google Scholar] [CrossRef] [PubMed]
- Azarakhsh, M.; Lebedeva, M.A.; Lutova, L.A. Identification and expression analysis of Medicago truncatula isopentenyl transferase genes (IPTs) involved in local and systemic control of nodulation. Front. Plant Sci. 2018, 9, 304. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.; Roswanjaya, Y.P.; Kohlen, W.; Stougaard, J.; Reid, D. Nitrate restricts nodule organogenesis through inhibition of cytokinin biosynthesis in Lotus japonicus. Nat. Commun. 2021, 12, 6544. [Google Scholar] [CrossRef] [PubMed]
- Sasaki, T.; Suzaki, T.; Soyano, T.; Kojima, M.; Sakakibara, H.; Kawaguchi, M. Shoot-derived cytokinins systemically regulate root nodulation. Nat. Commun. 2014, 5, 4983. [Google Scholar] [CrossRef]
- Singh, B.N.; Dwivedi, P.; Sarma, B.K.; Singh, G.S.; Singh, H.B. A novel function of N-signaling in plants with special reference to Trichoderma interaction influencing plant growth, nitrogen use efficiency, and cross talk with plant hormones. 3 Biotech 2019, 9, 109. [Google Scholar] [CrossRef]
- Mundim, F.M.; Pringle, E.G. Whole-plant metabolic allocation under water stress. Front. Plant Sci. 2018, 9, 852. [Google Scholar] [CrossRef] [PubMed]
- Rolly, N.K.; Yun, B.-W. Regulation of Nitrate (NO3) Transporters and Glutamate Synthase-Encoding Genes under Drought Stress in Arabidopsis: The Regulatory Role of AtbZIP62 Transcription Factor. Plants 2021, 10, 2149. [Google Scholar] [CrossRef] [PubMed]
- Rolly, N.K.; Mun, B.-G.; Yun, B.-W. Insights into the Transcriptional Regulation of Branching Hormonal Signaling Pathways Genes under Drought Stress in Arabidopsis. Genes 2021, 12, 298. [Google Scholar] [CrossRef]
- Sun, L.; Di, D.-W.; Li, G.; Li, Y.; Kronzucker, H.J.; Shi, W. Transcriptome analysis of rice (Oryza sativa L.) in response to ammonium resupply reveals the involvement of phytohormone signaling and the transcription factor OsJAZ9 in reprogramming of nitrogen uptake and metabolism. J. Plant Physiol. 2020, 246, 153137. [Google Scholar] [CrossRef] [PubMed]
- Zhong, L.; Chen, D.; Min, D.; Li, W.; Xu, Z.; Zhou, Y.; Li, L.; Chen, M.; Ma, Y. AtTGA4, a bZIP transcription factor, confers drought resistance by enhancing nitrate transport and assimilation in Arabidopsis thaliana. Biochem. Biophys. Res. Commun. 2015, 457, 433–439. [Google Scholar] [CrossRef]
- Gupta, K.J. Plant Respiration and Internal Oxygen: Methods and Protocols; Springer: Berlin/Heidelberg, Germany, 2017. [Google Scholar]
- Armstrong, W.; Armstrong, J. Plant internal oxygen transport (diffusion and convection) and measuring and modelling oxygen gradients. In Low-Oxygen Stress in Plants; Springer: Berlin/Heidelberg, Germany, 2014; pp. 267–297. [Google Scholar]
- Kosmacz, M.; Weits, D.A. Oxygen perception in plants. In Low-Oxygen Stress in Plants; Springer: Berlin/Heidelberg, Germany, 2014; pp. 3–17. [Google Scholar]
- Guan, B.; Lin, Z.; Liu, D.; Li, C.; Zhou, Z.; Mei, F.; Li, J.; Deng, X. Effect of waterlogging-induced autophagy on programmed cell death in Arabidopsis roots. Front. Plant Sci. 2019, 10, 468. [Google Scholar] [CrossRef]
- Armstrong, W. Advances in Botanical Research, Volume 7. Aeration in Higher Plants; Woolhouse, H.W., Ed.; Academic Press: Cambridge, MA, USA, 1979. [Google Scholar]
- Sifton, H.B. Air-space tissue in plants. Bot. Rev. 1945, 11, 108–143. [Google Scholar] [CrossRef]
- Roland, J.C. Cell wall differentiation and stages involved with intercellular gas space opening. J. Cell Sci. 1978, 32, 325–336. [Google Scholar] [CrossRef]
- Jeffree, C.; Dale, J.; Fry, S.C. The genesis of intercellular spaces in developing leaves of Phaseolus vulgaris L. Protoplasma 1986, 132, 90–98. [Google Scholar] [CrossRef]
- Raven, J.A. Into the voids: The distribution, function, development and maintenance of gas spaces in plants. Ann. Bot. 1996, 78, 137–142. [Google Scholar] [CrossRef]
- Wegner, L.H. Oxygen transport in waterlogged plants. In Waterlogging Signalling and Tolerance in Plants; Springer: Berlin/Heidelberg, Germany, 2010; pp. 3–22. [Google Scholar]
- Lai, W.-L.; Zhang, Y.; Chen, Z.-H. Radial oxygen loss, photosynthesis, and nutrient removal of 35 wetland plants. Ecol. Eng. 2012, 39, 24–30. [Google Scholar] [CrossRef]
- Mohammed, U.; Caine, R.; Atkinson, J.; Harrison, E.; Wells, D.; Chater, C.; Gray, J.; Swarup, R.; Murchie, E.H. Rice plants overexpressing OsEPF1 show reduced stomatal density and increased root cortical aerenchyma formation. Sci. Rep. 2019, 9, 5584. [Google Scholar] [CrossRef]
- Scheehle, E.A.; Kruger, D. Global anthropogenic methane and nitrous oxide emissions. Energy J. 2006. [Google Scholar] [CrossRef]
- Denmead, O.; Freney, J.; Simpson, J.R. Nitrous oxide emission during denitrification in a flooded field. Soil Sci. Soc. Am. J. 1979, 43, 716–718. [Google Scholar] [CrossRef]
- Bodelier, P.L.; Pérez, G.; Veraart, A.J.; Krause, S. Methanotroph ecology, environmental distribution and functioning. In Methanotrophs; Springer: Berlin/Heidelberg, Germany, 2019; pp. 1–38. [Google Scholar]
- Trotsenko, Y.A.; Murrell, J.C. Metabolic aspects of aerobic obligate methanotrophy⋆. Adv. Appl. Microbiol. 2008, 63, 183–229. [Google Scholar]
- Saggar, S.; Tate, K.; Giltrap, D.; Singh, J. Soil-atmosphere exchange of nitrous oxide and methane in New Zealand terrestrial ecosystems and their mitigation options: A review. Plant Soil 2008, 309, 25–42. [Google Scholar] [CrossRef]
- Schütz, H.; Seiler, W.; Rennenberg, H. Soil and land use related sources and sinks of methane (CH4) in the context of the global methane budget. In Soils and the Greenhouse Effect; John Wiley and Sons Ltd.: Hoboken, NJ, USA, 1990; pp. 269–285. [Google Scholar]
- Colmer, T.D.; Kotula, L.; Malik, A.I.; Takahashi, H.; Konnerup, D.; Nakazono, M.; Pedersen, O. Rice acclimation to soil flooding: Low concentrations of organic acids can trigger a barrier to radial oxygen loss in roots. Plant Cell Environ. 2019, 42, 2183–2197. [Google Scholar] [CrossRef] [PubMed]
- Ejiri, M.; Shiono, K. Prevention of radial oxygen loss is associated with exodermal suberin along adventitious roots of annual wild species of Echinochloa. Front. Plant Sci. 2019, 10, 254. [Google Scholar] [CrossRef]
- Abiko, T.; Kotula, L.; Shiono, K.; Malik, A.I.; Colmer, T.D.; Nakazono, M. Enhanced formation of aerenchyma and induction of a barrier to radial oxygen loss in adventitious roots of Zea nicaraguensis contribute to its waterlogging tolerance as compared with maize (Zea mays ssp. mays). Plant Cell Environ. 2012, 35, 1618–1630. [Google Scholar] [CrossRef]
- Ejiri, M.; Fukao, T.; Miyashita, T.; Shiono, K. A barrier to radial oxygen loss helps the root system cope with waterlogging-induced hypoxia. Breed. Sci. 2021, 71, 40–50. [Google Scholar] [CrossRef] [PubMed]
- Peralta Ogorek, L.L.; Pellegrini, E.; Pedersen, O. Novel functions of the root barrier to radial oxygen loss–radial diffusion resistance to H2 and water vapour. New Phytol. 2021, 231, 1365–1376. [Google Scholar] [CrossRef]
- Foyer, C.H.; Noctor, G.; Hodges, M. Respiration and nitrogen assimilation: Targeting mitochondria-associated metabolism as a means to enhance nitrogen use efficiency. J. Exp. Bot. 2011, 62, 1467–1482. [Google Scholar] [CrossRef] [PubMed]
- Maier, R.J. Nitrogen fixation and respiration: Two processes linked by the energetic demands of Nitrogenase. In Respiration in Archaea and Bacteria; Springer: Berlin/Heidelberg, Germany, 2004; pp. 101–120. [Google Scholar]
- Weger, H.G.; Turpin, D.H. Mitochondrial respiration Can support NO3− and NO2− reduction during photosynthesis: Interactions between photosynthesis, respiration, and N assimilation in the N-limited green alga Selenastrum minutum. Plant Physiol. 1989, 89, 409–415. [Google Scholar] [CrossRef] [PubMed]
- De Laulanié, H. Intensive rice farming in Madagascar. Tropicultura 2011, 29, 183–187. [Google Scholar]
- Sinclair, T.R. Agronomic UFOs waste valuable scientific resources. Rice Today 2004, 3, 43. [Google Scholar]
- Pereira-Mora, L.; Terra, J.A.; Fernández-Scavino, A. Methanogenic community linked to organic acids fermentation from root exudates are affected by rice intensification in rotational soil systems. Appl. Soil Ecol. 2022, 176, 104498. [Google Scholar] [CrossRef]
- Xu, Z.; Chan, K. Biochar: Nutrient properties and their enhancement. Biochar Environ. Manag. 2009, 1, 67–84. [Google Scholar]
- Lehmann, J.; Cowie, A.; Masiello, C.A.; Kammann, C.; Woolf, D.; Amonette, J.E.; Cayuela, M.L.; Camps-Arbestain, M.; Whitman, T. Biochar in climate change mitigation. Nat. Geosci. 2021, 14, 883–892. [Google Scholar] [CrossRef]
- Roe, S.; Streck, C.; Beach, R.; Busch, J.; Chapman, M.; Daioglou, V.; Deppermann, A.; Doelman, J.; Emmet-Booth, J.; Engelmann, J.; et al. Land-based measures to mitigate climate change: Potential and feasibility by country. Glob. Change Biol. 2021, 27, 6025–6058. [Google Scholar] [CrossRef]
- Yang, W.; Feng, G.; Miles, D.; Gao, L.; Jia, Y.; Li, C.; Qu, Z. Impact of biochar on greenhouse gas emissions and soil carbon sequestration in corn grown under drip irrigation with mulching. Sci. Total Environ. 2020, 729, 138752. [Google Scholar] [CrossRef]
- Lyu, H.; Zhang, H.; Chu, M.; Zhang, C.; Tang, J.; Chang, S.X.; Mašek, O.; Ok, Y.S. Biochar affects greenhouse gas emissions in various environments: A critical review. Land Degrad. Dev. 2022, 33, 3327–3342. [Google Scholar] [CrossRef]
- Lin, X.; Xie, Z.; Zheng, J.; Liu, Q.; Bei, Q.; Zhu, J.G. Effects of biochar application on greenhouse gas emissions, carbon sequestration and crop growth in coastal saline soil. Eur. J. Soil Sci. 2015, 66, 329–338. [Google Scholar] [CrossRef]
- Lehmann, J.; Joseph, S. Biochar for environmental management: An introduction. In Biochar for Environmental Management; Routledge: London, UK, 2015; p. 1. [Google Scholar]
- Lehmann, J.; Czimczik, C.; Laird, D.; Sohi, S. Stability of biochar in soil. In Biochar for Environmental Management; Routledge: London, UK, 2009; pp. 183–206. [Google Scholar]
- Major, J.; Steiner, C.; Downie, A.; Lehmann, J. Biochar effects on nutrient leaching. In Biochar for Environmental Management; Routledge: London, UK, 2009; pp. 303–320. [Google Scholar]
- Turunen, M.; Hyväluoma, J.; Heikkinen, J.; Keskinen, R.; Kaseva, J.; Hannula, M.; Rasa, K. Quantifying the pore structure of different biochars and their impacts on the water retention properties of Sphagnum moss growing media. Biosyst. Eng. 2020, 191, 96–106. [Google Scholar] [CrossRef]
- Gaunt, J.; Cowie, A. Biochar, greenhouse gas accounting and emissions trading. In Biochar for Environmental Management: Science and Technology; Routledge: London, UK, 2009; pp. 317–340. [Google Scholar]
- Smernik, R.J. Biochar and sorption of organic compounds. In Biochar for Environmental Management; Routledge: London, UK, 2009; pp. 321–332. [Google Scholar]
- Thies, J.E.; Rillig, M.C. Characteristics of biochar: Biological properties. In Biochar for Environmental Management; Routledge: London, UK, 2012; pp. 117–138. [Google Scholar]
- DeLuca, T.H.; Gundale, M.J.; MacKenzie, M.D.; Jones, D.L. Biochar effects on soil nutrient transformations. In Biochar for Environmental Management: Science, Technology and Implementation; Routledge: London, UK, 2015; Volume 2, pp. 421–454. [Google Scholar]
- Rondon, M.A.; Molina, D.; Hurtado, M.; Ramirez, J.; Lehmann, J.; Major, J.; Amezquita, E. Enhancing the productivity of crops and grasses while reducing greenhouse gas emissions through bio-char amendments to unfertile tropical soils. In Proceedings of the 18th World Congress of Soil Science, Philadelphia, PA, USA, 9–15 July 2006; pp. 9–15. [Google Scholar]
- Yanai, Y.; Toyota, K.; Okazaki, M. Effects of charcoal addition on N2O emissions from soil resulting from rewetting air-dried soil in short-term laboratory experiments. Soil Sci. Plant Nutr. 2007, 53, 181–188. [Google Scholar] [CrossRef]
- Joseph, S.; Cowie, A.L.; Van Zwieten, L.; Bolan, N.; Budai, A.; Buss, W.; Cayuela, M.L.; Graber, E.R.; Ippolito, J.A.; Kuzyakov, Y.; et al. How biochar works, and when it doesn’t: A review of mechanisms controlling soil and plant responses to biochar. Gcb Bioenergy 2021, 13, 1731–1764. [Google Scholar] [CrossRef]
- Joseph, S.; Peacocke, C.; Lehmann, J.; Munroe, P. Developing a biochar classification and test methods. In Biochar for Environmental Management; Routledge: London, UK, 2009; Volume 1, pp. 139–158. [Google Scholar]
- Borchard, N.; Schirrmann, M.; Cayuela, M.L.; Kammann, C.; Wrage-Mönnig, N.; Estavillo, J.M.; Fuertes-Mendizábal, T.; Sigua, G.; Spokas, K.; Ippolito, J.A.; et al. Biochar, soil and land-use interactions that reduce nitrate leaching and N2O emissions: A meta-analysis. Sci. Total Environ. 2019, 651, 2354–2364. [Google Scholar] [CrossRef]
- Riley, W.; Ortiz-Monasterio, I.; Matson, P.A. Nitrogen leaching and soil nitrate, nitrite, and ammonium levels under irrigated wheat in Northern Mexico. Nutr. Cycl. Agroecosystems 2001, 61, 223–236. [Google Scholar] [CrossRef]
- Song, X.; Pan, G.; Zhang, C.; Zhang, L.; Wang, H. Effects of biochar application on fluxes of three biogenic greenhouse gases: A meta-analysis. Ecosyst. Health Sustain. 2016, 2, e01202. [Google Scholar] [CrossRef]
- He, Y.; Zhou, X.; Jiang, L.; Li, M.; Du, Z.; Zhou, G.; Shao, J.; Wang, X.; Xu, Z.; Hosseini Bai, S.; et al. Effects of biochar application on soil greenhouse gas fluxes: A meta-analysis. Gcb Bioenergy 2017, 9, 743–755. [Google Scholar] [CrossRef]
- Kalu, S.; Kulmala, L.; Zrim, J.; Peltokangas, K.; Tammeorg, P.; Rasa, K.; Kitzler, B.; Pihlatie, M.; Karhu, K. Potential of biochar to reduce greenhouse gas emissions and increase nitrogen use efficiency in boreal arable soils in the long-term. Front. Environ. Sci. 2022, 10, 914766. [Google Scholar] [CrossRef]
- Sass, R.L.; Cicerone, R.J. Photosynthate allocations in rice plants: Food production or atmospheric methane? Proc. Natl. Acad. Sci. USA 2002, 99, 11993–11995. [Google Scholar] [CrossRef] [PubMed]
- van Der Gon, H.D.; Kropff, M.; Van Breemen, N.; Wassmann, R.; Lantin, R.; Aduna, E.; Corton, T.; Van Laar, H.H. Optimizing grain yields reduces CH4 emissions from rice paddy fields. Proc. Natl. Acad. Sci. USA 2002, 99, 12021–12024. [Google Scholar] [CrossRef] [PubMed]
- Su, J.; Hu, C.; Yan, X.; Jin, Y.; Chen, Z.; Guan, Q.; Wang, Y.; Zhong, D.; Jansson, C.; Wang, F.; et al. Expression of barley SUSIBA2 transcription factor yields high-starch low-methane rice. Nature 2015, 523, 602–606. [Google Scholar] [CrossRef] [PubMed]
- Stein, L.Y.; Klotz, M.G. Nitrifying and denitrifying pathways of methanotrophic bacteria. Biochem. Soc. Trans. 2011, 39, 1826–1831. [Google Scholar] [CrossRef] [PubMed]
- Canarini, A.; Kaiser, C.; Merchant, A.; Richter, A.; Wanek, W. Root exudation of primary metabolites: Mechanisms and their roles in plant responses to environmental stimuli. Front. Plant Sci. 2019, 10, 157. [Google Scholar] [CrossRef] [PubMed]
- Rougier, M. Secretory activity of the root cap. In Plant Carbohydrates II: Extracellular Carbohydrates; Springer: Berlin/Heidelberg, Germany, 1981; pp. 542–574. [Google Scholar]
- Abbott, L.K.; Murphy, D.V. Soil Biological Fertility: A Key to Sustainable Land Use in Agriculture; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2003. [Google Scholar]
- Walker, T.S.; Bais, H.P.; Grotewold, E.; Vivanco, J.M. Root exudation and rhizosphere biology. Plant Physiol. 2003, 132, 44–51. [Google Scholar] [CrossRef]
- Nardi, S.; Concheri, G.; Pizzeghello, D.; Sturaro, A.; Rella, R.; Parvoli, G. Soil organic matter mobilization by root exudates. Chemosphere 2000, 41, 653–658. [Google Scholar] [CrossRef]
- Vives-Peris, V.; De Ollas, C.; Gómez-Cadenas, A.; Pérez-Clemente, R.M. Root exudates: From plant to rhizosphere and beyond. Plant Cell Rep. 2020, 39, 3–17. [Google Scholar] [CrossRef]
- De-la-Pena, C.; Badri, D.V.; Lei, Z.; Watson, B.S.; Brandao, M.M.; Silva-Filho, M.C.; Sumner, L.W.; Vivanco, J.M. Root secretion of defense-related proteins is development-dependent and correlated with flowering time. J. Biol. Chem. 2010, 285, 30654–30665. [Google Scholar] [CrossRef]
- Chai, Y.N.; Schachtman, D.P. Root exudates impact plant performance under abiotic stress. Trends Plant Sci. 2022, 27, 80–91. [Google Scholar] [CrossRef]
- Salem, M.A.; Wang, J.Y.; Al-Babili, S. Metabolomics of plant root exudates: From sample preparation to data analysis. Front. Plant Sci. 2022, 13, 5035. [Google Scholar] [CrossRef] [PubMed]
- Narula, N.; Kothe, E.; Behl, R.K. Role of root exudates in plant-microbe interactions. J. Appl. Bot. Food Qual. 2012, 82, 122–130. [Google Scholar]
- Kumar, R.; Bhatia, R.; Kukreja, K.; Behl, R.K.; Dudeja, S.S.; Narula, N. Establishment of Azotobacter on plant roots: Chemotactic response, development and analysis of root exudates of cotton (Gossypium hirsutum L.) and wheat (Triticum aestivum L.). J. Basic Microbiol. 2007, 47, 436–439. [Google Scholar] [CrossRef]
- Lu, Y.; Wassmann, R.; Neue, H.; Huang, C.; Bueno, C.S. Methanogenic responses to exogenous substrates in anaerobic rice soils. Soil Biol. Biochem. 2000, 32, 1683–1690. [Google Scholar] [CrossRef]
- Moscôso, J.S.C.; Silva, L.S.d.; Pujol, S.B.; Giacomini, S.J.; Severo, F.F.; Marzari, L.B.; Molin, G.D. Methane emission induced by short-chain organic acids in lowland soil. Rev. Bras. Ciência Solo 2019, 43. [Google Scholar] [CrossRef]
- Aulakh, M.S.; Wassmann, R.; Bueno, C.; Rennenberg, H. Impact of root exudates of different cultivars and plant development stages of rice (Oryza sativa L.) on methane production in a paddy soil. Plant Soil 2001, 230, 77–86. [Google Scholar] [CrossRef]
- Smith, M.R.; Rao, I.M.; Merchant, A. Source-sink relationships in crop plants and their influence on yield development and nutritional quality. Front. Plant Sci. 2018, 9, 1889. [Google Scholar] [CrossRef]
- Andersen, C.P. Source–sink balance and carbon allocation below ground in plants exposed to ozone. New Phytol. 2003, 157, 213–228. [Google Scholar] [CrossRef]
- Wingler, A.; Einig, W.; Schaeffer, C.; Wallenda, T.; Hampp, R.; Wallander, H.; Nylund, J.E. Influence of different nutrient regimes on the regulation of carbon metabolism in Norway spruce [Picea abies (L.) Karst.] seedlings. New Phytol. 1994, 128, 323–330. [Google Scholar] [CrossRef]
- Koch, K.E. Carbohydrate-modulated gene expression in plants. Annu. Rev. Plant Biol. 1996, 47, 509–540. [Google Scholar] [CrossRef]
- Mathur, J.; Hülskamp, M. Microtubules and microfilaments in cell morphogenesis in higher plants. Curr. Biol. 2002, 12, R669–R676. [Google Scholar] [CrossRef] [PubMed]
- Yao, Z.; Zhou, Z.; Zheng, X.; Xie, B.; Mei, B.; Wang, R.; Butterbach-Bahl, K.; Zhu, J. Effects of organic matter incorporation on nitrous oxide emissions from rice-wheat rotation ecosystems in China. Plant Soil 2010, 327, 315–330. [Google Scholar] [CrossRef]
- Zou, J.; Huang, Y.; Jiang, J.; Zheng, X.; Sass, R.L. A 3-year field measurement of methane and nitrous oxide emissions from rice paddies in China: Effects of water regime, crop residue, and fertilizer application. Glob. Biogeochem. Cycles 2005, 19. [Google Scholar] [CrossRef]
- Eagle, A.J.; Bird, J.A.; Horwath, W.R.; Linquist, B.A.; Brouder, S.M.; Hill, J.E.; van Kessel, C. Rice yield and nitrogen utilization efficiency under alternative straw management practices. Agron. J. 2000, 92, 1096–1103. [Google Scholar] [CrossRef]
- Li, X.; Zhang, G.; Xu, H.; Cai, Z.; Yagi, K. Effect of timing of joint application of hydroquinone and dicyandiamide on nitrous oxide emission from irrigated lowland rice paddy field. Chemosphere 2009, 75, 1417–1422. [Google Scholar] [CrossRef]
- Akiyama, H.; Yan, X.; Yagi, K. Evaluation of effectiveness of enhanced-efficiency fertilizers as mitigation options for N2O and NO emissions from agricultural soils: Meta-analysis. Glob. Change Biol. 2010, 16, 1837–1846. [Google Scholar] [CrossRef]
- Majumdar, D. Methane and nitrous oxide emission from irrigated rice fields: Proposed mitigation strategies. Curr. Sci. 2003, 84, 1317–1326. [Google Scholar]
- Mahesh, M.; Mohini, M. Crop residues for sustainable livestock production. J. Adv. Dairy Res. 2014, 2, e108. [Google Scholar]
- Ritchie, H.; Rosado, P.; Roser, M. Meat and Dairy Production. Our World Data 2017. Available online: https://ourworldindata.org/meat-production (accessed on 18 October 2023).
- Williams, P. Nutritional composition of red meat. Nutr. Diet. 2007, 64, S113–S119. [Google Scholar] [CrossRef]
- Porter, J.W.G. Milk as a source of lactose, vitamins and minerals. Proc. Nutr. Soc. 1978, 37, 225–230. [Google Scholar] [CrossRef] [PubMed]
- Shaheen, N.; Ahmed, M.K.; Islam, M.S.; Habibullah-Al-Mamun, M.; Tukun, A.B.; Islam, S.; MA Rahim, A.T. Health risk assessment of trace elements via dietary intake of ‘non-piscine protein source’foodstuffs (meat, milk and egg) in Bangladesh. Environ. Sci. Pollut. Res. 2016, 23, 7794–7806. [Google Scholar] [CrossRef] [PubMed]
- McAfee, A.J.; McSorley, E.M.; Cuskelly, G.J.; Moss, B.W.; Wallace, J.M.; Bonham, M.P.; Fearon, A.M. Red meat consumption: An overview of the risks and benefits. Meat Sci. 2010, 84, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Willett, W.C.; Ludwig, D.S. Milk and health. N. Engl. J. Med. 2020, 382, 644–654. [Google Scholar] [CrossRef] [PubMed]
- Tricarico, J.; de Haas, Y.; Hristov, A.; Kebreab, E.; Kurt, T.; Mitloehner, F.; Pitta, D. Symposium review: Development of a funding program to support research on enteric methane mitigation from ruminants. J. Dairy Sci. 2022, 105, 8535–8542. [Google Scholar] [CrossRef]
- United Nations Environment Programme. Emissions Gap Report 2022: The Closing Window—Climate Crisis Calls for Rapid Transformation of Societies; UN: New York, NY, USA, 2022. [Google Scholar]
- Frank, S.; Beach, R.; Havlík, P.; Valin, H.; Herrero, M.; Mosnier, A.; Hasegawa, T.; Creason, J.; Ragnauth, S.; Obersteiner, M. Structural change as a key component for agricultural non-CO2 mitigation efforts. Nat. Commun. 2018, 9, 1060. [Google Scholar] [CrossRef]
- Arndt, C.; Hristov, A.N.; Price, W.J.; McClelland, S.C.; Pelaez, A.M.; Cueva, S.F.; Oh, J.; Dijkstra, J.; Bannink, A.; Bayat, A.R.; et al. Full adoption of the most effective strategies to mitigate methane emissions by ruminants can help meet the 1.5 C target by 2030 but not 2050. Proc. Natl. Acad. Sci. USA 2022, 119, e2111294119. [Google Scholar] [CrossRef]
- Zhao, Y.; Zhao, G. Decreasing ruminal methane production through enhancing the sulfate reduction pathway. Anim. Nutr. 2022, 9, 320–326. [Google Scholar] [CrossRef]
- Haque, M.N. Dietary manipulation: A sustainable way to mitigate methane emissions from ruminants. J. Anim. Sci. Technol. 2018, 60, 15. [Google Scholar] [CrossRef]
- Newbold, C.; El Hassan, S.; Wang, J.; Ortega, M.; Wallace, R.J. Influence of foliage from African multipurpose trees on activity of rumen protozoa and bacteria. Br. J. Nutr. 1997, 78, 237–249. [Google Scholar] [CrossRef]
- Bodas, R.; Prieto, N.; García-González, R.; Andrés, S.; Giráldez, F.J.; López, S. Manipulation of rumen fermentation and methane production with plant secondary metabolites. Anim. Feed. Sci. Technol. 2012, 176, 78–93. [Google Scholar] [CrossRef]
- Patra, A.K.; Saxena, J. Dietary phytochemicals as rumen modifiers: A review of the effects on microbial populations. Antonie Van Leeuwenhoek 2009, 96, 363–375. [Google Scholar] [CrossRef]
- Patra, A.; Saxena, J. The effect and mode of action of saponins on the microbial populations and fermentation in the rumen and ruminant production. Nutr. Res. Rev. 2009, 22, 204–219. [Google Scholar] [CrossRef] [PubMed]
- Milich, L. The role of methane in global warming: Where might mitigation strategies be focused? Glob. Environ. Change 1999, 9, 179–201. [Google Scholar] [CrossRef]
- Benchaar, C.; Pomar, C.; Chiquette, J. Evaluation of dietary strategies to reduce methane production in ruminants: A modelling approach. Can. J. Anim. Sci. 2001, 81, 563–574. [Google Scholar] [CrossRef]
- Boadi, D.; Wittenberg, K.M. Methane production from dairy and beef heifers fed forages differing in nutrient density using the sulphur hexafluoride (SF6) tracer gas technique. Can. J. Anim. Sci. 2002, 82, 201–206. [Google Scholar] [CrossRef]
- Beauchemin, K.; Kreuzer, M.; O’mara, F.; McAllister, T.A. Nutritional management for enteric methane abatement: A review. Aust. J. Exp. Agric. 2008, 48, 21–27. [Google Scholar] [CrossRef]
- Tamminga, S.; Bannink, A.; Dijkstra, J.; Zom, R. Feeding Strategies to Reduce Methane Loss in Cattle; Animal Sciences Group: Wageningen, The Netherlands, 2007; pp. 1570–8616. [Google Scholar]
- O’mara, F.; Fitzgerald, J.; Murphy, J.; Rath, M. The effect on milk production of replacing grass silage with maize silage in the diet of dairy cows. Livest. Prod. Sci. 1998, 55, 79–87. [Google Scholar] [CrossRef]
- Hassanat, F.; Gervais, R.; Julien, C.; Massé, D.; Lettat, A.; Chouinard, P.; Petit, H.; Benchaar, C. Replacing alfalfa silage with corn silage in dairy cow diets: Effects on enteric methane production, ruminal fermentation, digestion, N balance, and milk production. J. Dairy Sci. 2013, 96, 4553–4567. [Google Scholar] [CrossRef]
- Martin, C.; Morgavi, D.P.; Doreau, M. Methane mitigation in ruminants: From microbe to the farm scale. Animal 2010, 4, 351–365. [Google Scholar] [CrossRef] [PubMed]
- Ferris, C.; Gordon, F.; Patterson, D.; Porter, M.; Yan, T. The effect of genetic merit and concentrate proportion in the diet on nutrient utilization by lactating dairy cows. J. Agric. Sci. 1999, 132, 483–490. [Google Scholar] [CrossRef]
- Lovett, D.; Lovell, S.; Stack, L.; Callan, J.; Finlay, M.; Conolly, J.; O’Mara, F.P. Effect of forage/concentrate ratio and dietary coconut oil level on methane output and performance of finishing beef heifers. Livest. Prod. Sci. 2003, 84, 135–146. [Google Scholar] [CrossRef]
- Johnson, K.A.; Johnson, D.E. Methane emissions from cattle. J. Anim. Sci. 1995, 73, 2483–2492. [Google Scholar] [CrossRef] [PubMed]
- Murphy, M.; Baldwin, R.; Koong, L.J. Estimation of stoichiometric parameters for rumen fermentation of roughage and concentrate diets. J. Anim. Sci. 1982, 55, 411–421. [Google Scholar] [CrossRef] [PubMed]
- Hindrichsen, I.; Wettstein, H.; Machmüller, A.; Jörg, B.; Kreuzer, M. Effect of the carbohydrate composition of feed concentratates on methane emission from dairy cows and their slurry. Environ. Monit. Assess. 2005, 107, 329–350. [Google Scholar] [CrossRef]
- Hindrichsen, I.; Kreuzer, M. High methanogenic potential of sucrose compared with starch at high ruminal pH. J. Anim. Physiol. Anim. Nutr. 2009, 93, 61–65. [Google Scholar] [CrossRef]
- Castillo, C.; Benedito, J.; Méndez, J.; Pereira, V.; Lopez-Alonso, M.; Miranda, M.; Hernández, J. Organic acids as a substitute for monensin in diets for beef cattle. Anim. Feed. Sci. Technol. 2004, 115, 101–116. [Google Scholar] [CrossRef]
- McAllister, T.; Newbold, C.J. Redirecting rumen fermentation to reduce methanogenesis. Aust. J. Exp. Agric. 2008, 48, 7–13. [Google Scholar] [CrossRef]
- Beauchemin, K.; McGinn, S.M. Methane emissions from beef cattle: Effects of fumaric acid, essential oil, and canola oil. J. Anim. Sci. 2006, 84, 1489–1496. [Google Scholar] [CrossRef]
- Burt, S. Essential oils: Their antibacterial properties and potential applications in foods—A review. Int. J. Food Microbiol. 2004, 94, 223–253. [Google Scholar] [CrossRef] [PubMed]
- Benchaar, C.; Calsamiglia, S.; Chaves, A.V.; Fraser, G.; Colombatto, D.; McAllister, T.A.; Beauchemin, K.A. A review of plant-derived essential oils in ruminant nutrition and production. Anim. Feed. Sci. Technol. 2008, 145, 209–228. [Google Scholar] [CrossRef]
- Benchaar, C.; Greathead, H. Essential oils and opportunities to mitigate enteric methane emissions from ruminants. Anim. Feed. Sci. Technol. 2011, 166, 338–355. [Google Scholar] [CrossRef]
- Moss, A.R.; Jouany, J.-P.; Newbold, J. Methane production by ruminants: Its contribution to global warming. In Annales de Zootechnie; EDP Sciences: Les Ulis, France, 2000; pp. 231–253. [Google Scholar]
- Lopez, S.; McIntosh, F.; Wallace, R.; Newbold, C.J. Effect of adding acetogenic bacteria on methane production by mixed rumen microorganisms. Anim. Feed. Sci. Technol. 1999, 78, 1–9. [Google Scholar] [CrossRef]
- McAllister, T.; Beauchemin, K.; Alazzeh, A.; Baah, J.; Teather, R.; Stanford, K. The use of direct fed microbials to mitigate pathogens and enhance production in cattle. Can. J. Anim. Sci. 2011, 91, 193–211. [Google Scholar] [CrossRef]
- Newbold, C.J.; Rode, L. Dietary additives to control methanogenesis in the rumen. In International Congress Series; Elsevier: Amsterdam, The Netherlands, 2006; pp. 138–147. [Google Scholar]
- Beauchemin, K.; Colombatto, D.; Morgavi, D.; Yang, W.Z. Use of exogenous fibrolytic enzymes to improve feed utilization by ruminants. J. Anim. Sci. 2003, 81, E37–E47. [Google Scholar]
- Eun, J.-S.; Beauchemin, K.A. Assessment of the efficacy of varying experimental exogenous fibrolytic enzymes using in vitro fermentation characteristics. Anim. Feed. Sci. Technol. 2007, 132, 298–315. [Google Scholar] [CrossRef]
- Hook, S.E.; Northwood, K.S.; Wright, A.D.; McBride, B.W. Long-term monensin supplementation does not significantly affect the quantity or diversity of methanogens in the rumen of the lactating dairy cow. Appl. Environ. Microbiol. 2009, 75, 374–380. [Google Scholar] [CrossRef]
- Patra, A.K. Enteric methane mitigation technologies for ruminant livestock: A synthesis of current research and future directions. Environ. Monit. Assess. 2012, 184, 1929–1952. [Google Scholar] [CrossRef]
- McGinn, S.; Beauchemin, K.; Coates, T.; Colombatto, D. Methane emissions from beef cattle: Effects of monensin, sunflower oil, enzymes, yeast, and fumaric acid. J. Anim. Sci. 2004, 82, 3346–3356. [Google Scholar] [CrossRef]
- Guan, H.; Wittenberg, K.; Ominski, K.; Krause, D.O. Efficacy of ionophores in cattle diets for mitigation of enteric methane. J. Anim. Sci. 2006, 84, 1896–1906. [Google Scholar] [CrossRef]
- Clark, H.; Pinares-Patiño, C.; De Klein, C. Methane and nitrous oxide emissions from grazed grasslands. Grassland. A Glob. Resour. 2005, 279, 293. [Google Scholar]
- Madsen, J.; Lassen, J.; Hvelplund, T.; Weisbjerg, M.R. A fast, easy, reliable and cheap method to measure the methane production from ruminants. Eaap Publ. 2010, 127, 121–122. [Google Scholar]
- Eckard, R.; Grainger, C.; De Klein, C.A.M. Options for the abatement of methane and nitrous oxide from ruminant production: A review. Livest. Sci. 2010, 130, 47–56. [Google Scholar] [CrossRef]
- Sherman, R. Large-Scale Organic Materials Composting. North Carolina Cooperative Extension Service. 2005; North Carolina State Extension Publications: Raleigh, NC, USA, 2011. [Google Scholar]
- Kelly, F.; Michael, L. Storing Manure on Small Horse and Livestock Farms; The State University of New Jersey: Rutgers, NJ, USA, 2014. [Google Scholar]
- Ghaly, A.; MacDonald, K.N. An effective passive solar dryer for thin layer drying of poultry manure. Am. J. Eng. Appl. Sci. 2012, 5, 136–150. [Google Scholar]
- Endres, M.I.; Janni, K.A. Compost bedded pack barns for dairy cows. Univ. Neb.-Linc. 2008, 1, 1–9. [Google Scholar]
- Bewley, J.; Taraba, J.; Day, G.; Black, R.; Damasceno, F.A. Compost Bedded Pack Barn Design Features and Management Considerations; Cooperative Extension Publ. ID-206, Cooperative Extension Service; University of Kentucky College of Agriculture: Lexington, KY, USA, 2012. [Google Scholar]
- Aguirre-Villegas, H.; Larson, R.A.; Ruark, M.D. Solid-Liquid Separation of Manure and Effects on Greenhouse Gas and Ammonia Emissions; University of Wisconsin-Extension, Cooperative Extension: Madison, WI, USA, 2017; Available online: https://learningstore.extension.wisc.edu/products/solid-liquid-separation-of-manure-and-effects-on-greenhouse-gas-and-ammonia-emissions-p1844 (accessed on 23 October 2023).








| Advantages | Prerequisite | Application | Outcomes |
|---|---|---|---|
| Nitrogen use efficiency | |||
|
| Can be improved through the following:
|
|
| Radial oxygen loss (ROL) | |||
|
| Can be improved using the following:
|
|
| Application of biochar | |||
|
|
|
|
| Best Agricultural Practices | |||
|
|
|
|
| Improving ruminant feeding efficiency and enhancing target-specific ruminal bacterial activity | |||
|
|
|
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kabange, N.R.; Kwon, Y.; Lee, S.-M.; Kang, J.-W.; Cha, J.-K.; Park, H.; Dzorkpe, G.D.; Shin, D.; Oh, K.-W.; Lee, J.-H. Mitigating Greenhouse Gas Emissions from Crop Production and Management Practices, and Livestock: A Review. Sustainability 2023, 15, 15889. https://doi.org/10.3390/su152215889
Kabange NR, Kwon Y, Lee S-M, Kang J-W, Cha J-K, Park H, Dzorkpe GD, Shin D, Oh K-W, Lee J-H. Mitigating Greenhouse Gas Emissions from Crop Production and Management Practices, and Livestock: A Review. Sustainability. 2023; 15(22):15889. https://doi.org/10.3390/su152215889
Chicago/Turabian StyleKabange, Nkulu Rolly, Youngho Kwon, So-Myeong Lee, Ju-Won Kang, Jin-Kyung Cha, Hyeonjin Park, Gamenyah Daniel Dzorkpe, Dongjin Shin, Ki-Won Oh, and Jong-Hee Lee. 2023. "Mitigating Greenhouse Gas Emissions from Crop Production and Management Practices, and Livestock: A Review" Sustainability 15, no. 22: 15889. https://doi.org/10.3390/su152215889
APA StyleKabange, N. R., Kwon, Y., Lee, S.-M., Kang, J.-W., Cha, J.-K., Park, H., Dzorkpe, G. D., Shin, D., Oh, K.-W., & Lee, J.-H. (2023). Mitigating Greenhouse Gas Emissions from Crop Production and Management Practices, and Livestock: A Review. Sustainability, 15(22), 15889. https://doi.org/10.3390/su152215889








