Variation of Stem CO2 Efflux and Estimation of Its Contribution to the Ecosystem Respiration in an Even-Aged Pure Rubber Plantation of Hainan Island
Abstract
1. Introduction
2. Materials and Methods
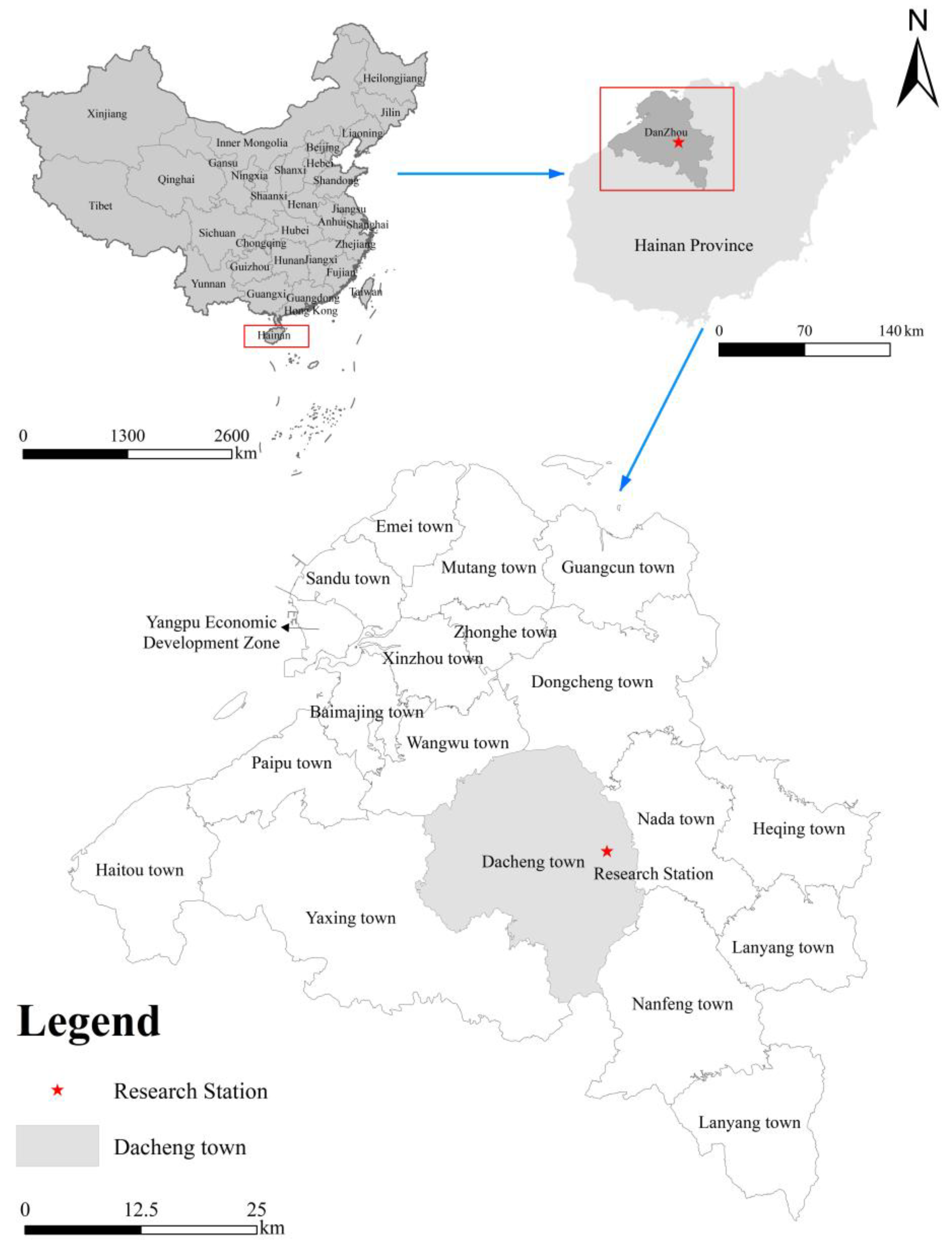
2.1. Study Area
2.2. Impacting Factor Measurements
2.3. Ecosystem Respiration Measurements
2.4. Stem CO2 Efflux Measurements
2.5. Two Extrapolation Methods for Estimating Stand Level of Stem CO2 Efflux
2.6. Statistics
3. Results
3.1. Seasonal Variation of Impacting Factors
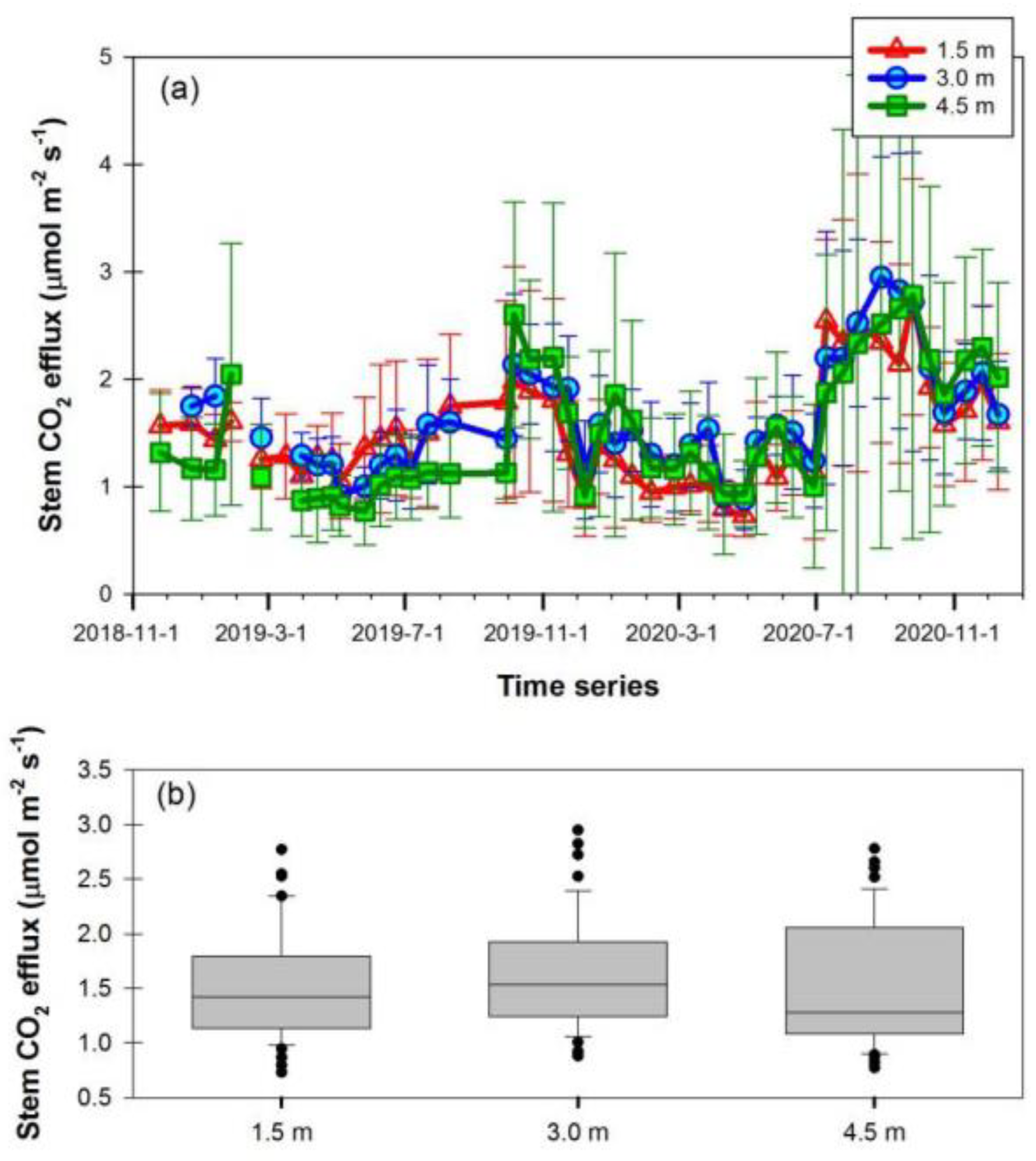
3.2. Seasonal Variation of Stem CO2 Efflux
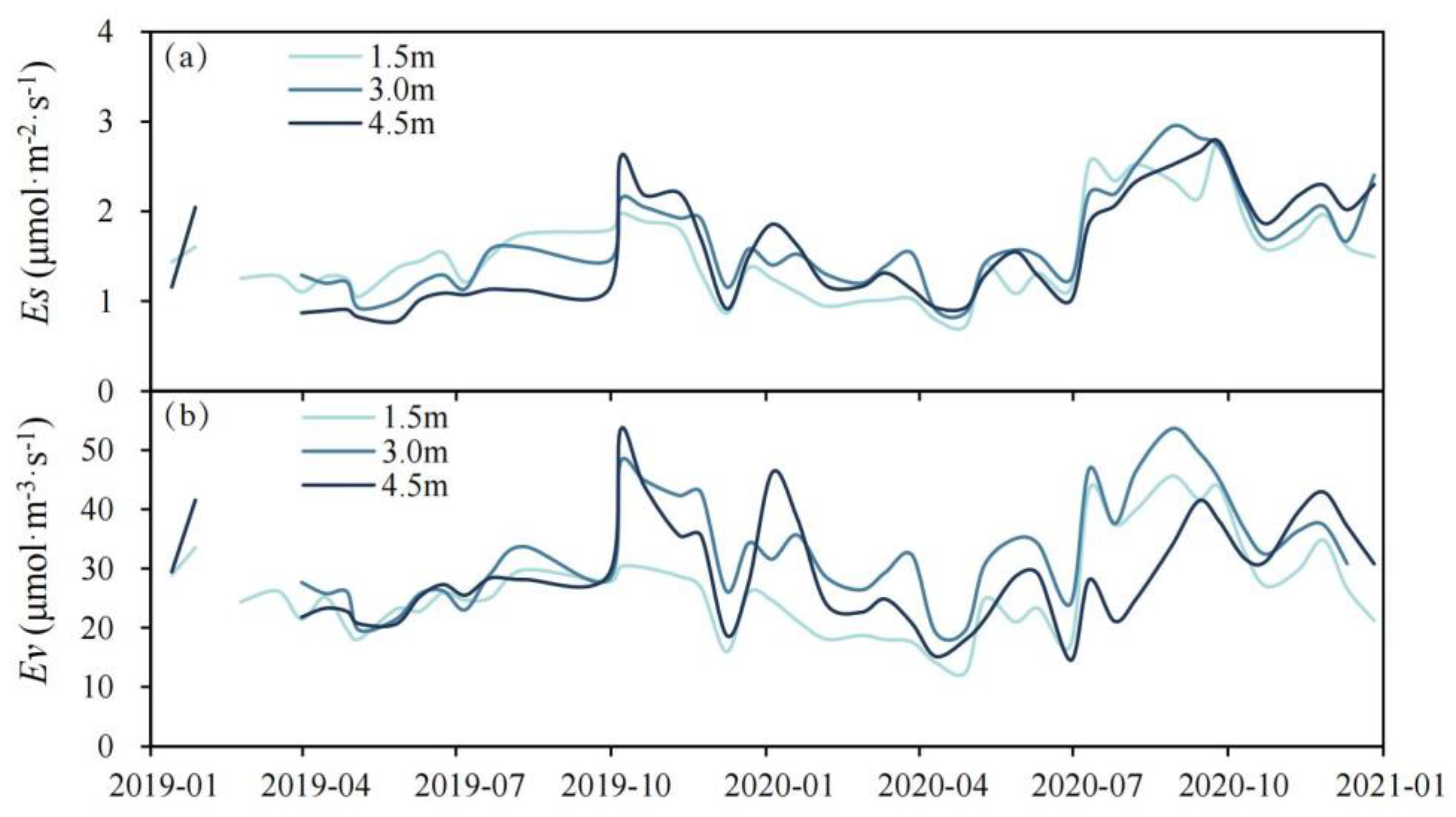
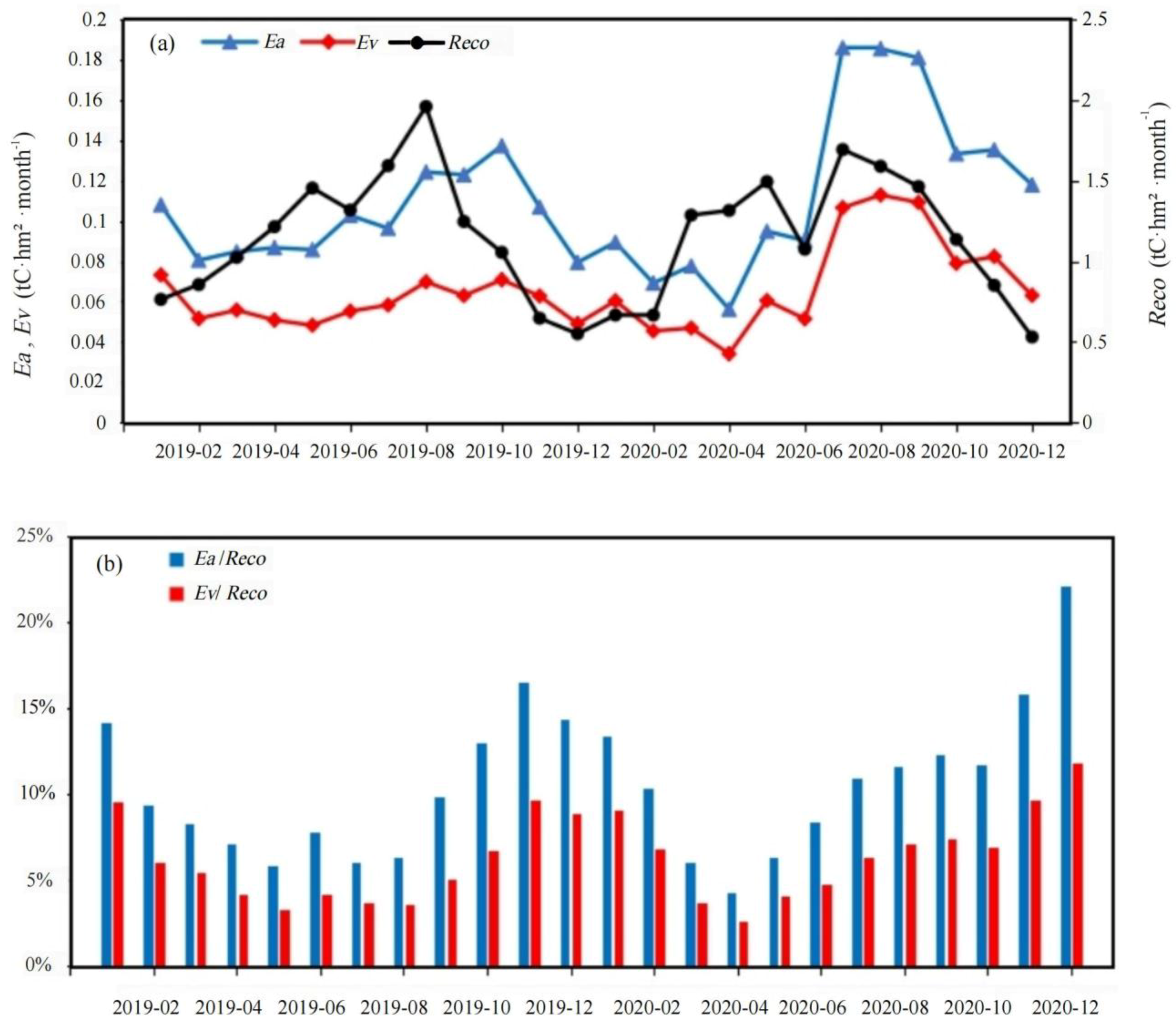
3.3. Findings on Ea and Ev
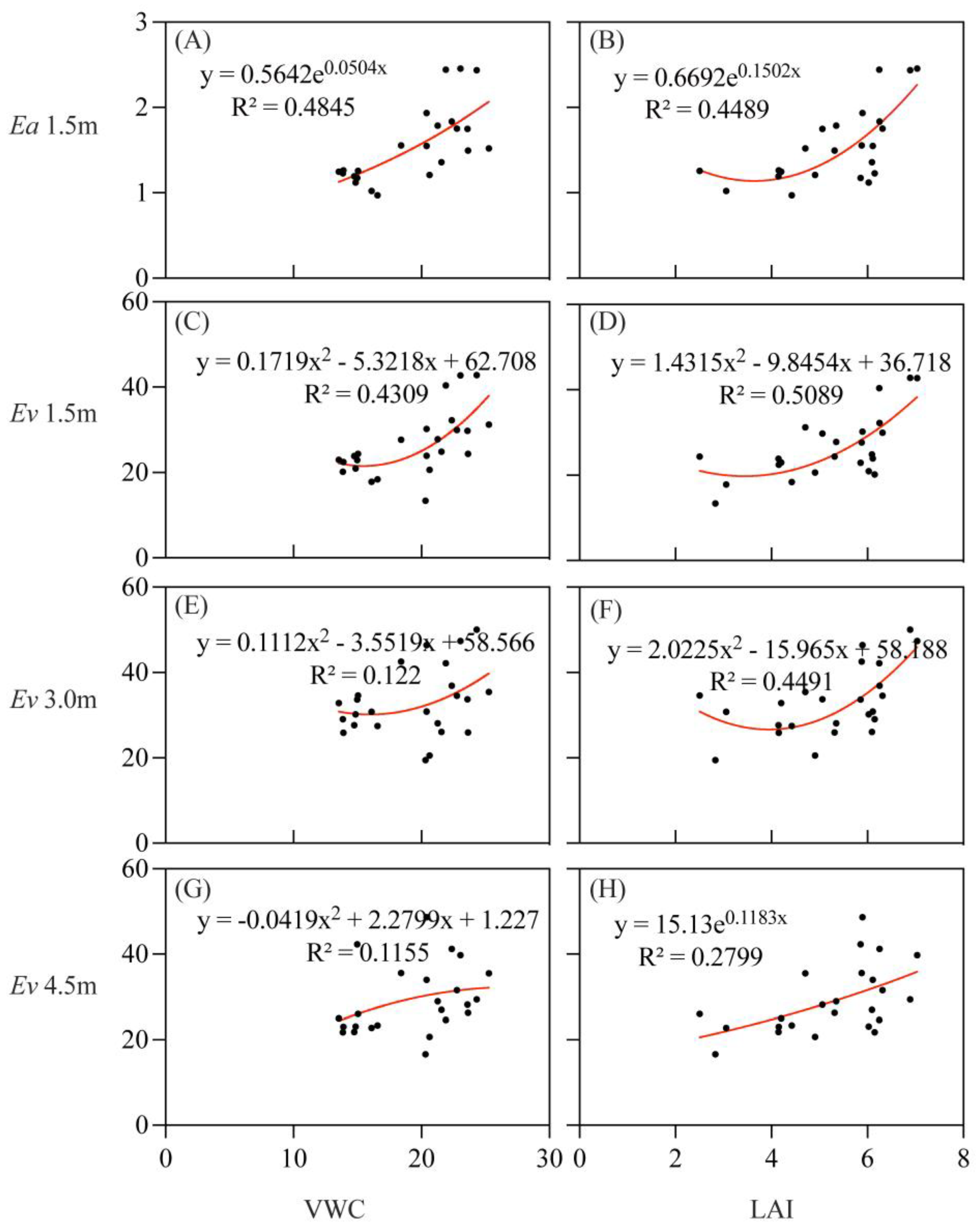
3.4. Correlation between Stem CO2 Efflux and Impacting Factors
3.5. Correlation between Stem CO2 Efflux and Ecosystem Respiration
4. Discussion
4.1. Seasonal Variations on Stem CO2 Efflux
4.2. Effect of Impacting Factors on Es in Rubber Plantation
4.3. Ea/Reco Annual Character in Rubber Plantation
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| Abbreviations | Definition | Units |
| ANOVA | One-way analysis of variance | |
| DBH | Diameter at breast height | cm |
| Ea | Area-based extrapolation method | μmol·m−3·s−1 |
| EC | Eddy covariance | |
| Es | Stem CO2 efflux | μmol·m−2·s−1 |
| Ev | Volume-based extrapolation method | μmol·m−3·s−1 |
| GPP | Gross primary productivity | |
| MDS | Marginal distribution sampling | |
| NEE | Net ecosystem carbon exchange | |
| LAI | Leaf area index | m/m |
| PCV | Polyvinyl chloride | |
| Reco | Ecosystem respiration | t C·hm−2·a−1 |
| Ta | Average atmospheric temperature | °C |
| Ts | Soil temperature | °C |
| VPD | Vapor pressure deficit | Kpa |
| VWC | Volumetric water content | % |
References
- Ryan, M.G.; Hubbard, R.M.; Clark, D.A.; Sanford, R.L., Jr. Woody—Tissue respiration for Simarouba amara and Minquartia guianensis, two tropical wet forest trees with different growth habits. Oeologia 1994, 100, 213–220. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Xiang, W.; Lei, P. Stem respiration and its controlling factors in forest ecosystems. J. Plant Ecol. 2007, 31, 403–412. [Google Scholar]
- Fan, H.; McGuire, M.A.; Teskey, R.O. Effects of stem size on stem respiration and its flux components in yellow-poplar (Liriodendron tulipifera L.) trees. Tree Physiol. 2017, 37, 1536–1545. [Google Scholar] [CrossRef]
- McGuire, M.A.; Teskey, R.O. Estimating stem respiration in trees by a mass balance approach that accounts for internal and external fluxes of CO2. Tree Physiol. 2004, 24, 571–578. [Google Scholar] [CrossRef]
- Bowman, W.P.; Barbour, M.M.; Turnbull, M.H.; Tissue, D.T.; Whitehead, D.; Griffin, K.L. Sap flow rates and sapwood density are critical factors in within- and between-tree variation in CO2 efflux from stems of mature Dacrydium cupressinum trees. New Phytol. 2005, 167, 815–828. [Google Scholar] [CrossRef]
- Teskey, R.; McGuire, M. Measurement of stem respiration of sycamore (Platanus occidentalis L.) trees involves internal and external fluxes of CO2 and possible transport of CO2 from roots. Plant Cell Environ. 2007, 30, 570–579. [Google Scholar] [CrossRef] [PubMed]
- Angert, A.; Muhr, J.; Negron Juarez, R.; Alegria Muñoz, W.; Kraemer, G.; Ramirez Santillan, J.; Barkan, E.; Mazeh, S.; Chambers, J.Q.; Trumbore, S.E. Internal respiration of Amazon tree stems greatly exceeds external CO2 efflux. Biogeosciences 2012, 9, 4979–4991. [Google Scholar] [CrossRef]
- Kinerson, R.S. Relationships between plant surface area and respiration in loblolly pine. J. Appl. Ecol. 1975, 12, 965–971. [Google Scholar] [CrossRef]
- Lavigne, M.B.; Franklin, S.E.; Hunt, E.R., Jr. Estimation stem maintenance respiration rates of dissimilar balsam fir stands. Tree Physiol. 1996, 16, 687–695. [Google Scholar] [CrossRef]
- Damesin, C.; Ceschia, E.; Foff, N.L.; Ottorini, J.M.; Dufrene, E. Stem and branch respiration of beech: From tree measurements to estimations at the stand level. New Phytol. 2002, 153, 159–172. [Google Scholar] [CrossRef]
- Kim, M.H.; Nakane, K.; Lee, J.T.; Bang, H.S.; Na, Y.E. Stem/branch maintenance respiration of Japanese red pine stand. For. Ecol. Manag. 2007, 243, 283–290. [Google Scholar] [CrossRef]
- Oohata, S.; Yamakura, T.; Saito, H.; Shidei, T. A study on the vertical distribution of respiratory activity of a 40-year-old stand of Chamecyparis obtusa. Bull. Kyoto Univ. For. 1971, 42, 103–116. [Google Scholar]
- Negisi, K. Diurnal fluctuation of CO2 release from the stem bark of standing young Pinus densiflora trees. J. Jpn. For. Soc. 1975, 57, 375–383. [Google Scholar]
- Mori, S.; Hagihara, A. Respiration in stems of hinoki (Chamaecyparis obtusa) trees. J. Jpn. For. Soc. 1988, 70, 187–481. [Google Scholar]
- McCree, K.J. An equation for the rate of dark respiration of white cover plants grown under controlled conditions. In Prediction and Measurement of Photosynthetic Productivity; Setlik, I., Ed.; Pudoc: Wageningen, The Netherlands, 1970; pp. 221–229. [Google Scholar]
- Levy, P.E.; Meir, P.; Allen, S.J.; Jarvis, P.G. The effect of aqueous transport of CO2 in xylem sap on gas exchange in woody plants. Tree Physiol. 1999, 19, 53–58. [Google Scholar] [CrossRef]
- McGuire, M.A.; Cerasoli, S.; Teskey, R.O. CO2 fluxes and respiration of branch segments of sycamore (Platanus occidentalis L.) examined at different sap velocities, branch diameters and temperatures. J. Exp. Bot. 2007, 58, 2159–2168. [Google Scholar] [CrossRef]
- Saveyn, A.; Steppe, K.; McGuire, M.A.; Lemeur, R.; Teskey, R.O. Stem respiration and carbon dioxide efflux of young Populus deltoides trees in relation to temperature and xylem carbon dioxide concentration. Oecologia 2008, 154, 637–649. [Google Scholar] [CrossRef]
- Teskey, R.; Saveyn, A.; Steppe, K.; McGuire, M.A. Origin, fate and significance of CO2 in tree stems. New Phytol. 2008, 177, 17–32. [Google Scholar] [CrossRef]
- Aubrey, D.P.; Teskey, R.O. Root-derived CO2 efflux via xylem stream rivals soil CO2 efflux. New Phytol. 2009, 184, 35–40. [Google Scholar] [CrossRef]
- Trumbore, S.E.; Angert, A.; Kunert, N.; Muhr, J.; Chambers, J.Q. What’s the flux? Unraveling how CO2 fluxes from trees reflect underlying physiological processes. New Phytol. 2013, 197, 353–355. [Google Scholar] [CrossRef]
- Hilman, B.; Angert, A. Measuring the ratio of CO2 efflux to O2 influx in tree stem respiration. Tree Physiol. 2016, 36, 1422–1431. [Google Scholar] [PubMed]
- Bloemen, J.; McGuire, M.A.; Aubrey, D.P.; Teskey, R.O.; Steppe, K. Transport of rootrespired CO2 via the transpiration stream impacts aboveground carbon assimilation and CO2 efflux in trees. New Phytol. 2013, 197, 555–565. [Google Scholar] [CrossRef] [PubMed]
- Roo, L.D.; Salomón, R.L.; Steppe, K. Woody tissue photosynthesis reduces stem CO2 efflux by half and remains unimpacted by drought stress in young Populus tremula trees. Plant Cell Environ. 2020, 43, 981–991. [Google Scholar] [CrossRef] [PubMed]
- Tarvainen, L.; Rntfors, M.; Wallin, G. Vertical gradients and seasonal variation in stem CO2 efflux within a Norway spruce stand. Tree Physiol. 2014, 34, 488–502. [Google Scholar] [CrossRef] [PubMed]
- Acosta, M.; Pokorny, R.; Janous, D.; Marek, M.V. Stem respiration of Norway spruce trees under elevated CO2 concentration. Biol. Plant. 2010, 54, 773–776. [Google Scholar] [CrossRef]
- Zhao, K.J.; Dong, B.Q.; Jia, Z.K.; Ma, L.Y. Effect of climatic factors on the temporal variation of stem respiration in Larix principis-rupprechtii Mayr. Agric. For. Meteorol. 2018, 248, 441–448. [Google Scholar] [CrossRef]
- Yan, Y.P.; Sha, L.Q.; Cao, M. Stem respiration rates of rubber (Hevea brasiliensis) plantations in Xishuangbanna. Acta Ecol. Sin. 2009, 29, 1840–1848. [Google Scholar]
- Bowman, W.P.; Turnbull, M.H.; Tissue, D.T.; Whitehead, D.; Griffin, K.L. Sapwood temperature gradients between lower stems and the crown do not influence estimates of stand-level stem CO2 efflux. Tree Physiol. 2008, 28, 1553–1559. [Google Scholar] [CrossRef]
- Steppe, K.; Saveyn, A.; McGuire, M.A. Resistance to radial CO2 diffusion contributes to between-tree variation in CO2 efflux of Populus deltoides stems. Funct. Plant Biol. 2007, 34, 785–792. [Google Scholar] [CrossRef]
- Yi, J.; Xie, G.; Wang, J.; Lan, G.; Wu, Z.; Yang, C. Stem Respiration of Rubber Tree of Different Strains and Different Girth. J. Anhui Agric. Sci. 2012, 40, 13433–13436. [Google Scholar]
- Duursma, R.A.; Kolari, P.; Peramaki, M.; Pulkkinen, M.; Makela, A.; Nikinmaa, E.; Hari, P.; Aurela, M.; Berbigier, P.; Bernhofer, C.H.; et al. Contributions of climate, leaf area index and leaf physiology to variation in gross primary production of six coniferous forests across Europe: A model-based analysis. Tree Physiol. 2009, 29, 621–639. [Google Scholar] [CrossRef] [PubMed]
- Bond-Lamberty, B.; Thomson, A. Temperature-associated increases in the global soil respiration record. Nature 2010, 464, 579–582. [Google Scholar] [CrossRef] [PubMed]
- Drake, J.E.; Davis, S.C.; Raetz, L.M.; DeLucia, E.H. Mechanisms of age-related changes in forest production: The influence of physiological and successional changes. Glob. Change Biol. 2011, 17, 1522–1535. [Google Scholar] [CrossRef]
- Meir, P.; Grace, J. Scaling relationships for woody tissue respiration in two tropical rain forests. Plant Cell Environ. 2002, 25, 963–973. [Google Scholar] [CrossRef]
- Meir, P.; Metcalfe, D.B.; Costa, A.C.; Fisher, R.A. The fate of assimilated carbon during drought: Impacts on respiration in Amazon rainforests. Philosophical Transactions of the Royal Society of London. Philos. Trans. R. Soc. B 2008, 363, 1849–1855. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Yang, C.; Gong, Y.; Zhang, J.; Song, B.; Wu, Z. Phenological Characteristics of Net Ecosystem Carbon Exchange in Hainan Rubber Forest Ecosystem. Chin. J. Trop. Crops 2022, 43, 1288–1296. [Google Scholar]
- Wu, Z. Carbon Balance of the Rubber Plantation Ecosystem in Hainan Island. Ph.D. Thesis, Hainan University, Haikou, China, 2013. (In Chinese). [Google Scholar]
- Wu, Z.; Tao, Z.; Lan, G.; Wang, J.; Xie, G.; Zhou, Z. The net ecosystem carbon exchange and its environmental factors in a tropical rubber plantation ecosystem in Hainan island. Chin. J. Trop. Crops 2014, 35, 2099–2108. [Google Scholar]
- Wang, G. Study on the Dynamics of Rubber Tree’s Non-Structural Carbohydrate and the Effect of Stem Water Storage in Hainan Island. Master’s Thesis, Hainan University, Haikou, China, 2019. [Google Scholar]
- Reichstein, M.; Falge, E.; Baldocchi, D.; Papale, D.; Aubinet, M.; Berbigier, P.; Bernhofer, C.; Buchmann, N.; Gilmanov, T.; Granier, A.; et al. On the separation of net ecosystem exchange into assimilation and ecosystem respiration: Review and improved algorithm. Glob. Change Biol. 2005, 11, 1424–1439. [Google Scholar] [CrossRef]
- Zhao, G.; Liu, G.; Zhu, W. Estimating individual- and stand-level stem CO2 efux in a subalpine forest: Assessment of diferent extrapolation methods. Trees 2019, 33, 1603–1613. [Google Scholar] [CrossRef]
- Cerasoli, S.; McGuire, M.A.; Faria, J.; Mourato, M.; Schmidt, M.; Pereira, J.S.; Chaves, M.M.; Teskey, R.O. CO2 efflux, CO2 concentration and photosynthetic refixation in stems of Eucalyptus globulus (Labill.). J. Exp. Bot. 2009, 60, 99–105. [Google Scholar] [CrossRef]
- Chen, B.; Wu, Z.; Yang, C.; Qi, D.; Li, X.; Lan, G.; Xie, G.; Tao, Z.; Sun, R.; Xiao, X. Monthly Dynamics of Leaf Area Index of Rubber Plantation in Danzhou, Hainan Island, China. Chin. J. Trop. Agric. 2015, 35, 1–6. [Google Scholar]
- Zheng, G.; Monika, M.L. Retrieving Leaf Area Index (LAI) Using Remote Sensing: Theories, Methods and Sensors. Sensors 2009, 9, 2719–2745. [Google Scholar] [CrossRef]
- Wang, Q.; Tenhunen, J.; Dinh, N.Q. Evaluation of seasonal variation of MODIS derived leaf area index at two European deciduous broadleaf forest sites. Remote Sens. Environ. 2005, 96, 475–484. [Google Scholar] [CrossRef]
- Yang, J.; He, Y.; Aubrey, D.P.; Zhuang, Q.; Teskey, R.O. Global patterns and predictors of stem CO2 efflux in forest ecosystems. Glob. Change Biol. 2016, 22, 1433–1444. [Google Scholar] [CrossRef] [PubMed]
- Amthor, J.S. The role of maintenance respiration in plant growth. Plant Cell Environ. 1984, 7, 561–569. [Google Scholar] [CrossRef]
- Stockfors, J. Temperature variations and distribution of living cells within tree stems: Implications for stem respiration modeling and scale-up. Tree Physiol. 2000, 20, 1057–1062. [Google Scholar] [CrossRef]
- Epron, D.; Nouvellon, Y.; Roupsard, O.; Mouvondy, W.; Mabiala, A.; Saint-Andre’, L.; Joffre, R.; Jourdan, C.; Bonnefond, J.M.; Berbigier, P. Spatial and temporal variations of soil respiration in a Eucalyptus plantation in Congo. For. Ecol. Manag. 2004, 202, 149–160. [Google Scholar] [CrossRef]
- Sotta, E.D.; Veldkamp, E.; Guimaraes, B.R.; Paixao, R.K.; Ruivo, M.L.P.; Almeida, S.S. Landscape and climatic controls on spatial and temporal variation in soil CO2 efflux in an Eastern Amazonian Rainforest, Caxiuana, Brazil. For. Ecol. Manag. 2006, 237, 57–64. [Google Scholar] [CrossRef]
- Li, Y.; Xu, M.; Zou, X. Heterotrophic soil respiration in relation to environmental factors and microbial biomass in two wet tropical forests. Plant Soil. 2006, 281, 193–201. [Google Scholar] [CrossRef]
- Xu, L.; Baldocchi, D.D.; Tang, J. How soil moisture, rain pulses, and growth alter the response of ecosystem respiration to temperature. Glob. Biogeochem. Cycles 2004, 18, GB4002. [Google Scholar] [CrossRef]
- Salomon, R.; Valbuena-Carabana, M.; Gil, L.; McGuire, M.; Teskey, R.; Aubrey, D.; Gonzalez-Doncel, I.; Rodriguez-Calcerrada, J. Temporal and spatial patterns of internal and external stem CO2 fluxes in a sub-Mediterranean oak. Tree Physiol. 2016, 36, 1409–1421. [Google Scholar] [PubMed]
- Rodríguez-Calcerrada, J.; Martin-StPaul, N.K.; Lempereur, M.; Ourcival, J.-M.; Rey, M.d.C.d.; Joffre, R.; Rambal, S. Stem CO2 efflux and its contribution to ecosystem CO2 efflux decrease with drought in a Mediterranean forest stand. Agric. For. Meteorol. 2014, 195–196, 61–72. [Google Scholar] [CrossRef][Green Version]


| Tree | Age (Years) | Diameter of 1.5 m Height (cm) | Diameter of 3.0 m Height (cm) | Diameter of 4.5 m Height (cm) | Height (m) | Tapped Years |
|---|---|---|---|---|---|---|
| TJ301 | 20 | 20.5 | 18.8 | 18.1 | 16.9 | 12 |
| TJ302 | 20 | 19.7 | 17.7 | 16.1 | 16.6 | 12 |
| TJ303 | 20 | 22.3 | 19.7 | 19.3 | 18.5 | 12 |
| TJ304 | 19 | 21.7 | 20.1 | 20.0 | 17.1 | 11 |
| Ta | Ts | VWC | LAI | ||
|---|---|---|---|---|---|
| Ea | r | −0.001 ** | 0.004 ** | 0.505 ** | 0.663 ** |
| Ev | r | −0.068 ** | −0.042 ** | 0.394 ** | 0.56 ** |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Song, B.; Wu, Z.; Dong, L.; Yang, C.; Yang, S. Variation of Stem CO2 Efflux and Estimation of Its Contribution to the Ecosystem Respiration in an Even-Aged Pure Rubber Plantation of Hainan Island. Sustainability 2023, 15, 16050. https://doi.org/10.3390/su152216050
Song B, Wu Z, Dong L, Yang C, Yang S. Variation of Stem CO2 Efflux and Estimation of Its Contribution to the Ecosystem Respiration in an Even-Aged Pure Rubber Plantation of Hainan Island. Sustainability. 2023; 15(22):16050. https://doi.org/10.3390/su152216050
Chicago/Turabian StyleSong, Bo, Zhixiang Wu, Lu Dong, Chuan Yang, and Siqi Yang. 2023. "Variation of Stem CO2 Efflux and Estimation of Its Contribution to the Ecosystem Respiration in an Even-Aged Pure Rubber Plantation of Hainan Island" Sustainability 15, no. 22: 16050. https://doi.org/10.3390/su152216050
APA StyleSong, B., Wu, Z., Dong, L., Yang, C., & Yang, S. (2023). Variation of Stem CO2 Efflux and Estimation of Its Contribution to the Ecosystem Respiration in an Even-Aged Pure Rubber Plantation of Hainan Island. Sustainability, 15(22), 16050. https://doi.org/10.3390/su152216050







