Novel Applications of Silk Proteins Based on Their Interactions with Metal Ions
Abstract
1. Introduction
2. Interaction of Silk Proteins with Metal Ions and the Factors Influencing the Interaction
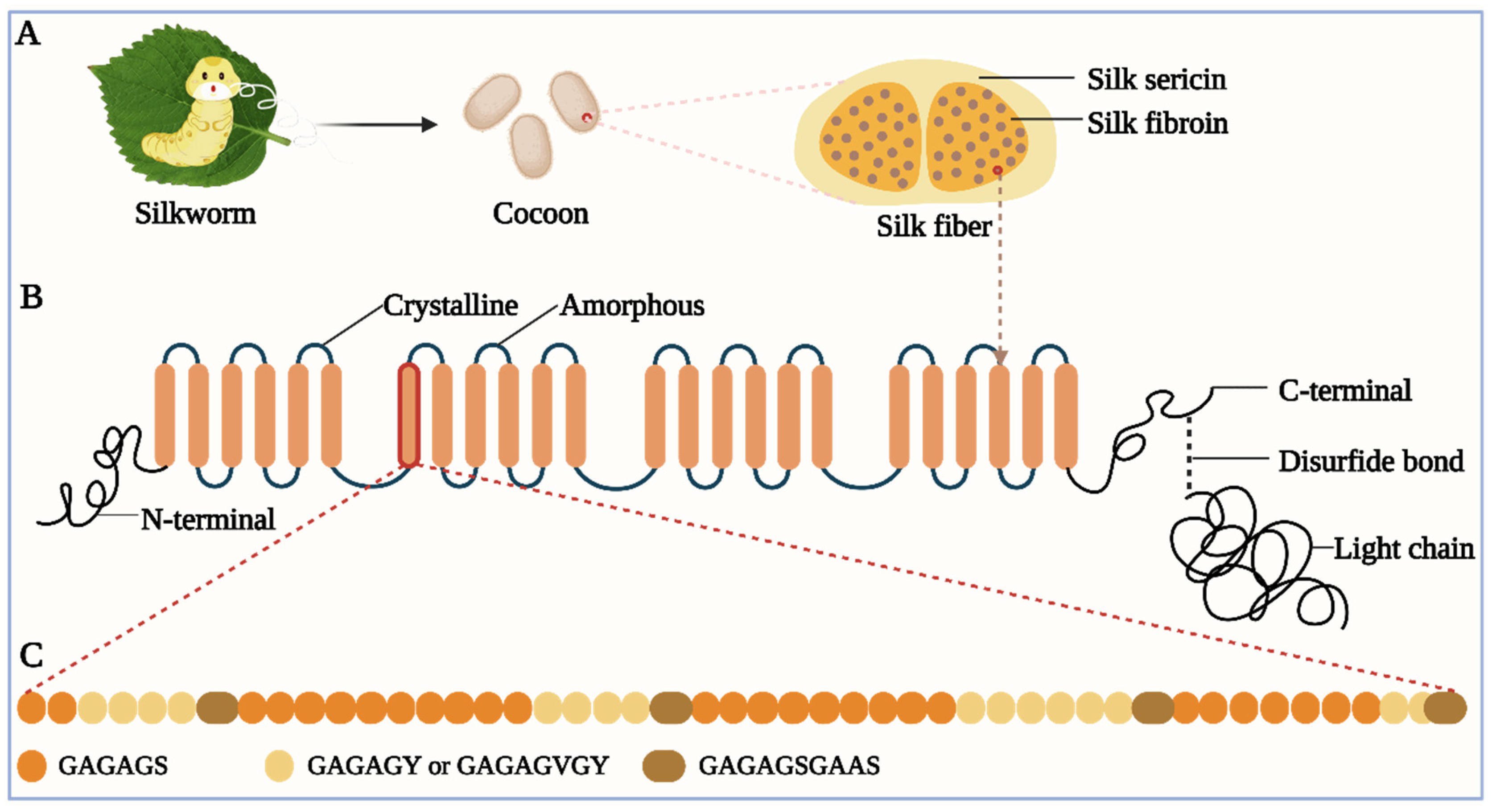
2.1. Amino Acid Composition
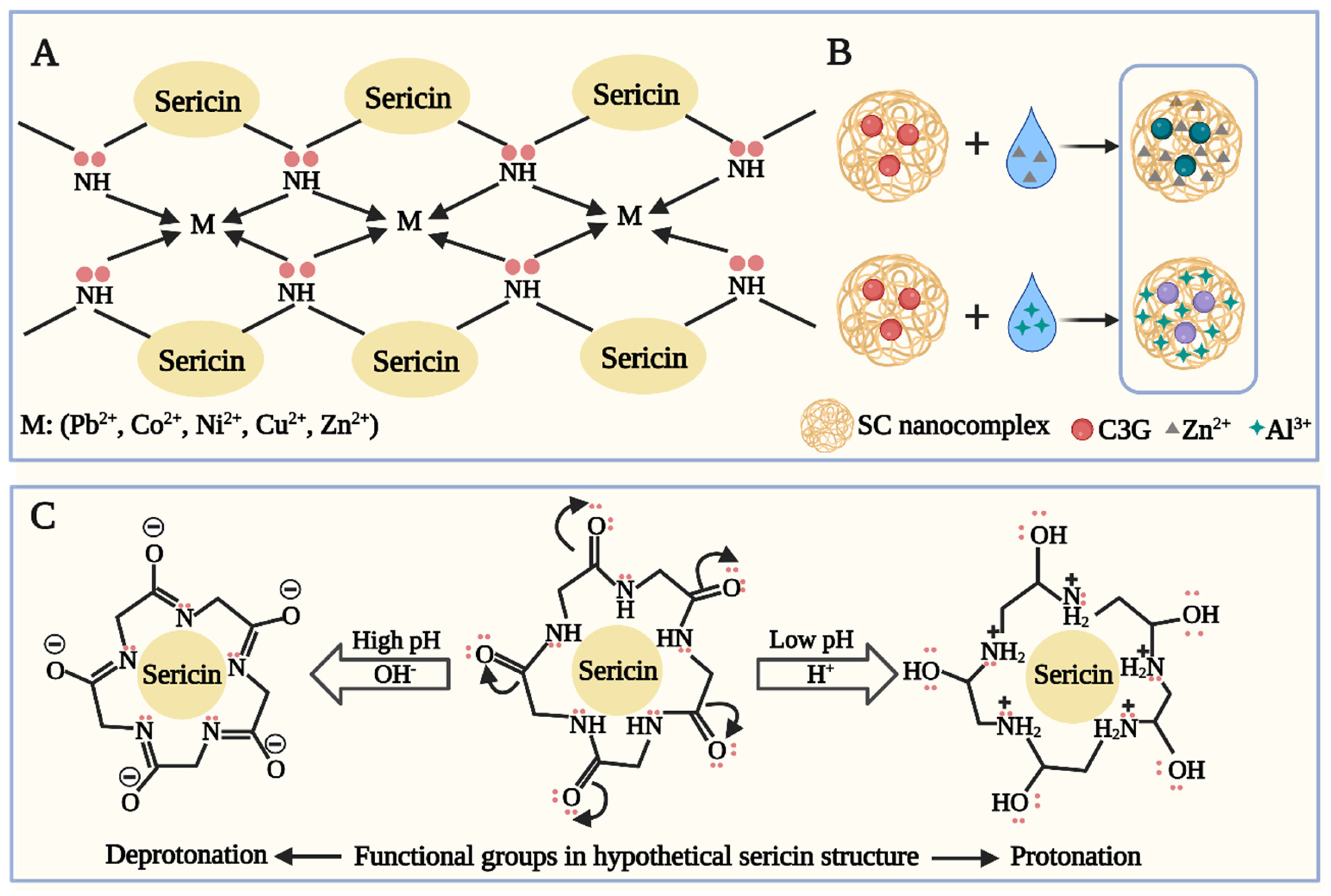
2.2. Interaction of Silk Proteins with Metal Ions
2.3. Factors Influencing the Interaction of Silk Proteins with Metal Ions
3. Favorable Properties of Silk Proteins for Application Expansion
3.1. Biocompatibility
3.2. Biodegradability
3.3. Mechanical Property and Processability
4. Application Based on the Interaction between Silk Proteins and Metal Ions
4.1. Performance Optimization of Silk Fabric
4.1.1. Mechanical Property Optimization
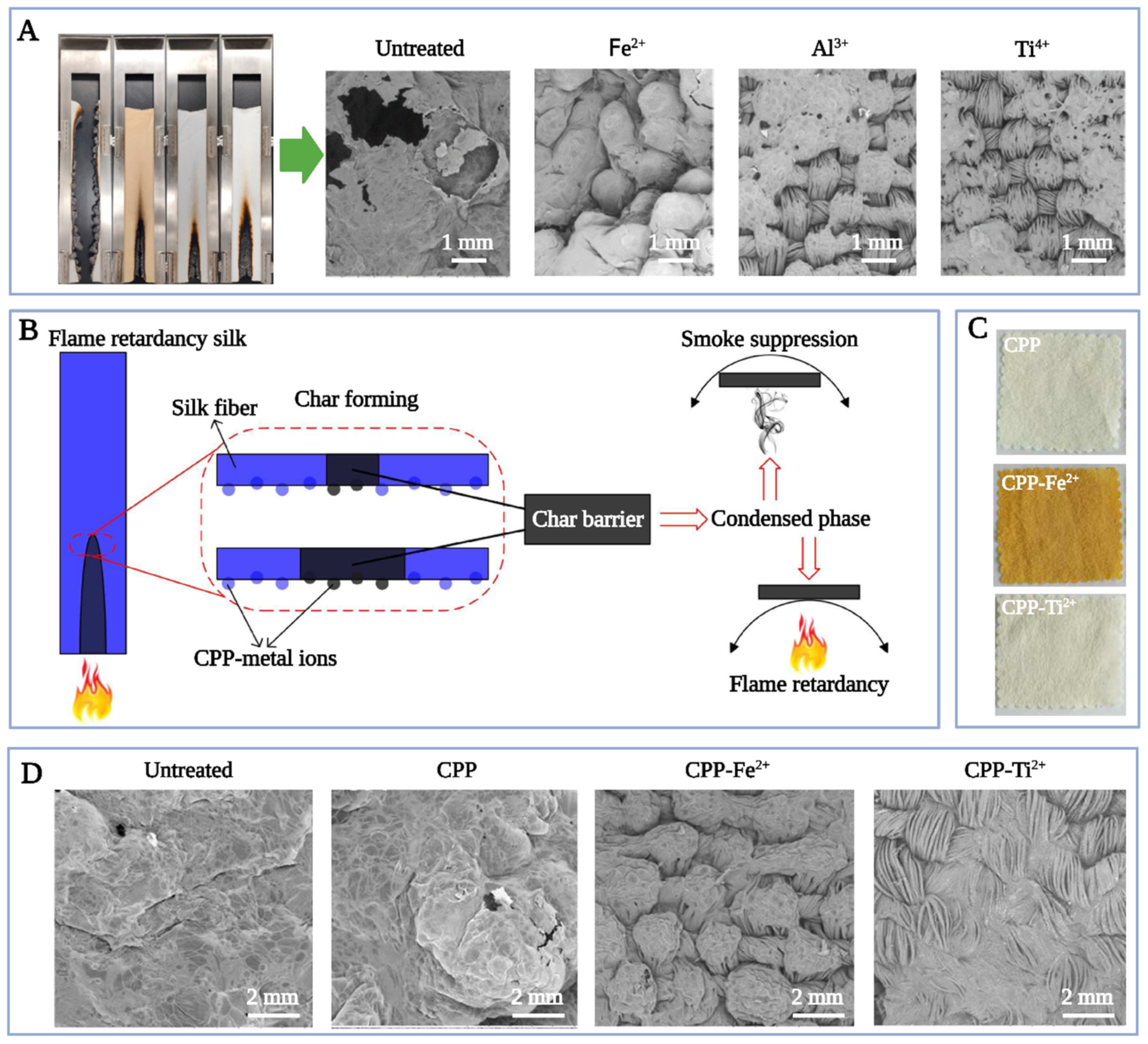
4.1.2. Flame Retardancy Enhancement
4.1.3. Color Fastness Improvement
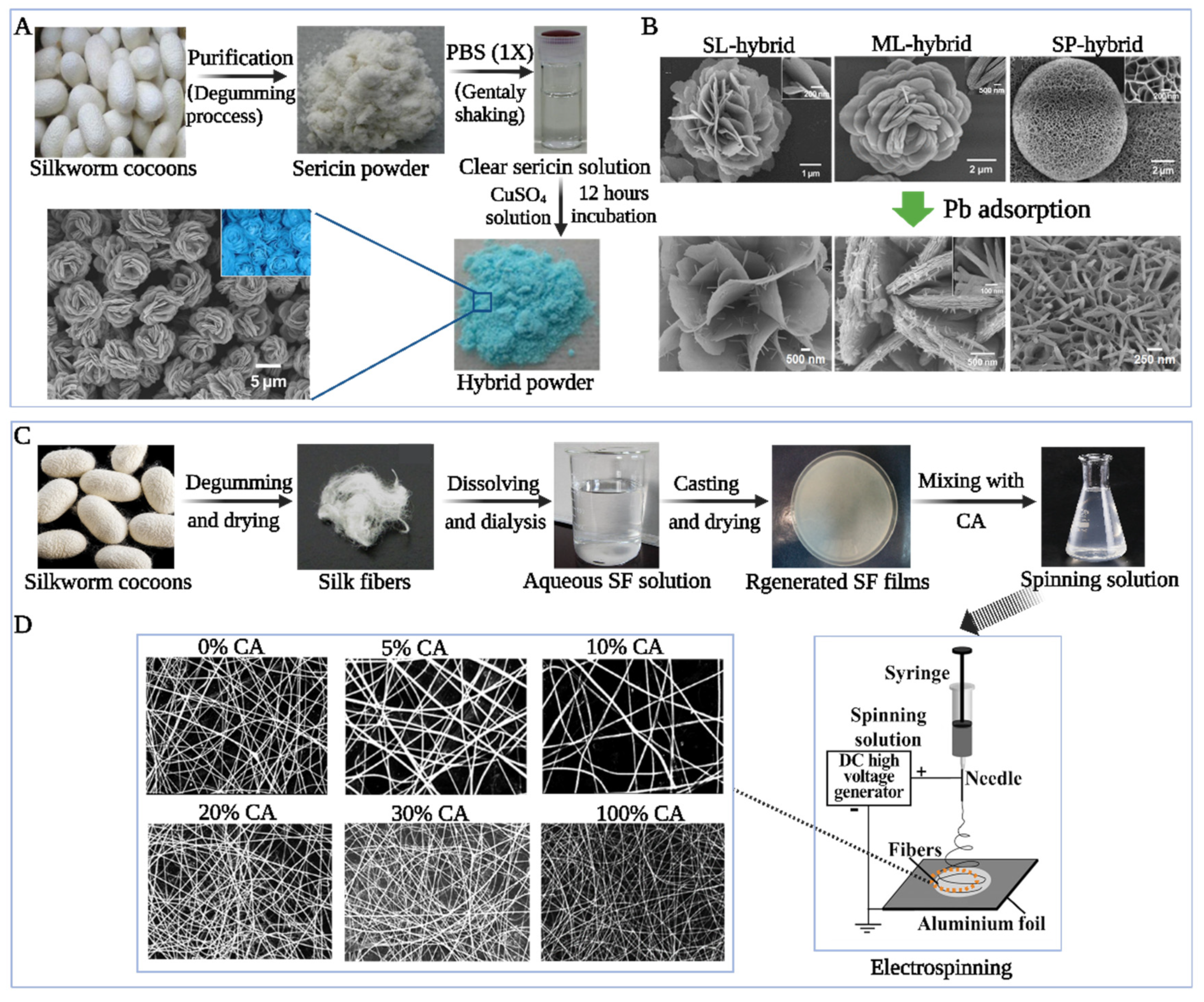
4.2. Heavy Metal-Contaminated Water Remediation
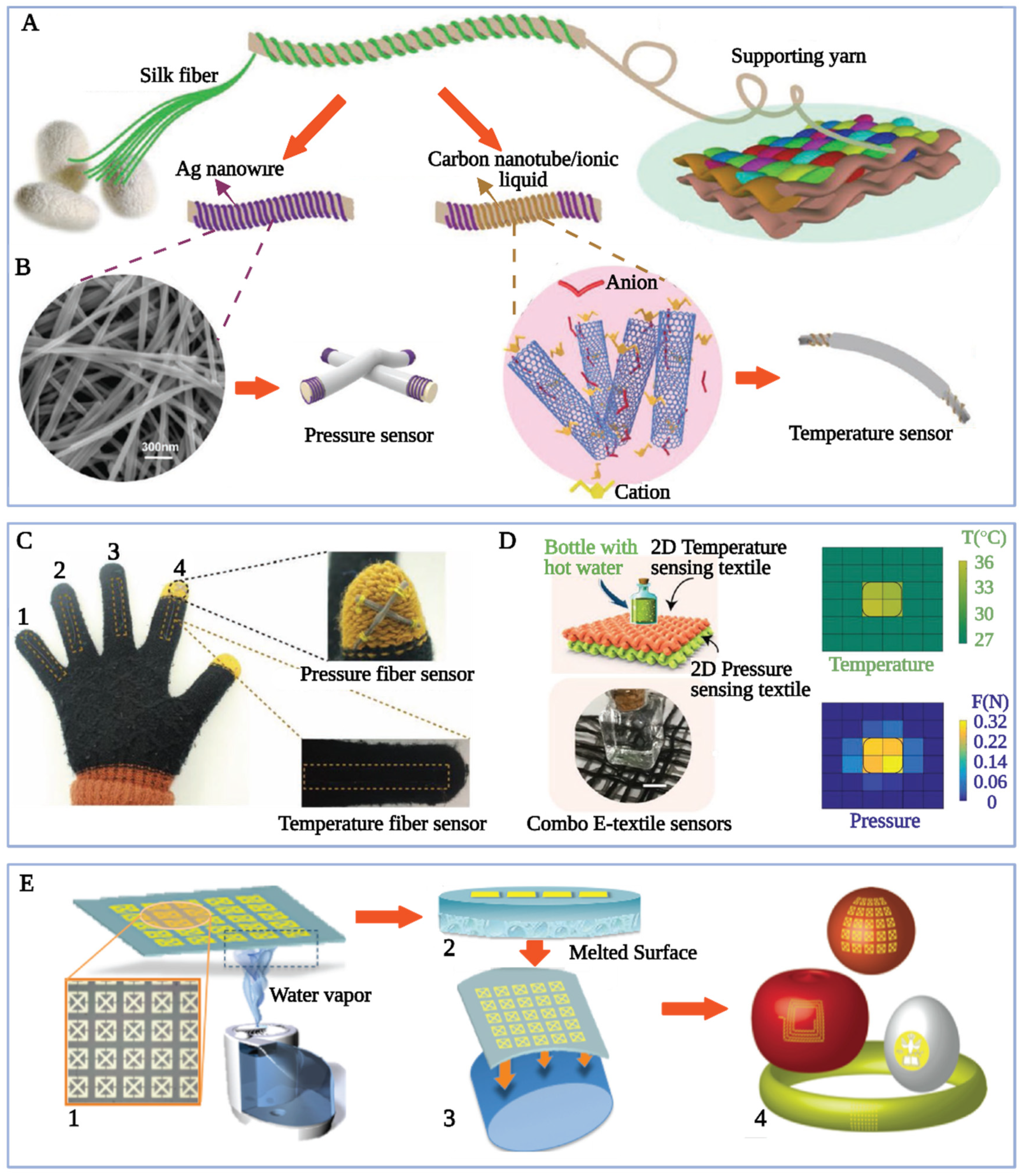
4.3. Silk-Based Biosensing Materials
4.3.1. Silk/Noble Metal Nanostructure Composites and In Vivo Micro Monitors
4.3.2. Silk/Base Metal Composite and Smart Environmental and Health Electronics
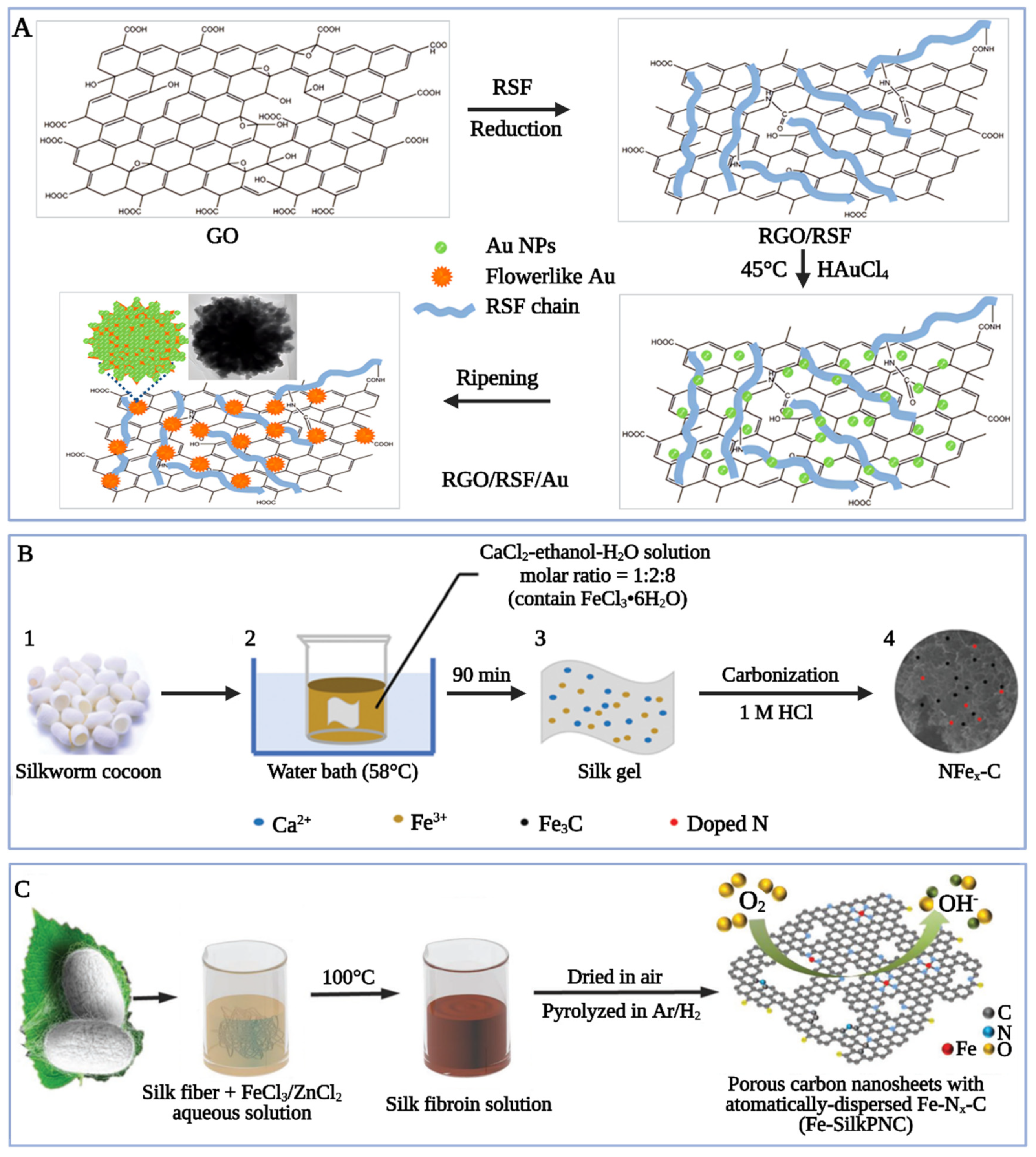
4.4. Silk-Based Electrochemical Materials
4.4.1. Energy-Storage Materials for Power Supply
4.4.2. Carbon-Based Electrocatalytic Composites
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| Au NPs/RGO | gold nanoparticles/reduced graphene oxide |
| BET | Brunauer–Emmett–Teller |
| BN/CA-NiCoFe-600 | boron (B), nitrogen (N) co-doped SF carbon aerogel that was anchored with nickel-cobalt-iron (NiCoFe) alloys |
| CA | cellulose acetate |
| CPP | casein phosphopeptide |
| CuCoFe2O4 | SF, cellulose, and β-cyclodextrin-based hydrogel modified by magnetic copper-doped cobalt ferrite |
| EDTA | ethylenediaminetetraacetic acid |
| EDX | energy dispersive X-ray spectroscopy |
| E1/2 | half-wave potential |
| FE-SEM | field emission scanning electron microscopy |
| Fe-SilkPNC | porous carbon nanosheets with automatically-dispersed Fe-Nx-C |
| HER | hydrogen evolution reaction |
| H-chain | heavy chain |
| LDI-MS | laser desorption/ionization mass spectrometry |
| LLZO CF–CSE | Li7La3Zr2O12 ceramic fabric composite solid electrolyte |
| LM/NaAlg | liquid metal alginate-coated NPs |
| LOI | limiting oxygen index |
| L-chain | light chain |
| MTT | 3-(4,5)-dimethylthiahiazo (-z-y1)-3,5-di- phenytetrazoliumromide |
| NC(s) | nanocluster(s) |
| NFex-C | nitrogen (N) and trace iron (Fe) co-doped 3D porous carbon |
| NP(s) | nanoparticle(s) |
| OER | oxygen evolution reaction |
| ORR | oxygen reduction reaction |
| PAA | poly-acrylic acid |
| PBS | phosphate-buffered saline |
| PDL(s) | poly dentate ligands |
| PdBMAP | Pd2+ tetramethacrylated benzoporphyrin |
| PEEK | polyetheretherketone |
| RGO | reduced graphene oxide |
| RSF | regenerated silk fibroin |
| S | sericin |
| SC | sericin/anthocyanin |
| SF | silk fibroin |
| STEM | scanning transmission electron microscopy |
| TEM | transmission electron microscope |
| UV (A) | ultraviolet (A) |
| UV-Vis | ultraviolet and visible |
| WK | wool keratose |
| 2D | two-dimensional |
| 3D | three-dimensional |
References
- Vainker, S.J. Chinese Silk: A Cultural History; Rutgers University Press: New Brunswick, NJ, USA, 2004. [Google Scholar]
- Slonimskiy, Y.B.; Egorkin, N.A.; Ashikhmin, A.A.; Friedrich, T.; Maksimov, E.G.; Sluchanko, N.N. Reconstitution of the functional Carotenoid-Binding Protein from silkworm in E. coli. Int. J. Biol. Macromol. 2022, 214, 664–671. [Google Scholar] [CrossRef] [PubMed]
- Dusi, G.G.; Marques, G.S.; Kienteca, M.L.; Gimenes, M.L.; Cerutti, M.L.M.N.; da Silva, V.R. Biosorption investigation of Cu(II) ions from aqueous solutions using sericin–alginate particles: Kinetic, equilibrium, and thermodynamic. Sustain. Chem. Pharm. 2022, 25, 100601. [Google Scholar] [CrossRef]
- Chen, K.; Li, Y.H.; Li, Y.B.; Pan, W.S.; Tan, G.X. Silk Fibroin Combined with Electrospinning as a Promising Strategy for Tissue Regeneration. Macromol. Biosci. 2023, 23, 2200380. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Matsumoto, H.; Moro-oka, Y.; Tanaka, M.; Miyahara, Y.; Suganami, T.; Matsumoto, A. Smart Microneedle Fabricated with Silk Fibroin Combined Semi-interpenetrating Network Hydrogel for Glucose-Responsive Insulin Delivery. ACS Biomater. Sci. Eng. 2019, 5, 5781–5789. [Google Scholar] [CrossRef] [PubMed]
- Cheng, T.-H.; Yang, Z.-Y.; Tang, R.-C.; Zhai, A.-D. Functionalization of silk by silver nanoparticles synthesized using the aqueous extract from tea stem waste. J. Mater. Res. Technol. 2020, 9, 4538–4549. [Google Scholar] [CrossRef]
- Li, G.; Li, Y.; Chen, G.; He, J.; Han, Y.; Wang, X.; Kaplan, D.L. Silk-Based Biomaterials in Biomedical Textiles and Fiber-Based Implants. Adv. Healthc. Mater. 2015, 4, 1134–1151. [Google Scholar] [CrossRef] [PubMed]
- Guo, Z.; Xie, W.; Gao, Q.; Wang, D.; Gao, F.; Li, S.; Zhao, L. In situ biomineralization by silkworm feeding with ion precursors for the improved mechanical properties of silk fiber. Int. J. Biol. Macromol. 2018, 109, 21–26. [Google Scholar] [CrossRef] [PubMed]
- He, H.; Cai, R.; Wang, Y.; Tao, G.; Guo, P.; Zuo, H.; Chen, L.; Liu, X.; Zhao, P.; Xia, Q. Preparation and characterization of silk sericin/PVA blend film with silver nanoparticles for potential antimicrobial application. Int. J. Biol. Macromol. 2017, 104, 457–464. [Google Scholar] [CrossRef]
- Liu, M.; Min, L.; Zhu, C.; Rao, Z.; Liu, L.; Xu, W.; Luo, P.; Fan, L. Preparation, characterization and antioxidant activity of silk peptides grafted carboxymethyl chitosan. Int. J. Biol. Macromol. 2017, 104, 732–738. [Google Scholar] [CrossRef]
- Liu, Q.; Wang, X.; Tan, X.; Xie, X.; Dong, H.; Li, X.; Li, Y.; Zhao, P.; Xia, Q. Disruption of the Metal Ion Environment by EDTA for Silk Formation Affects the Mechanical Properties of Silkworm Silk. Int. J. Mol. Sci. 2019, 20, 3026. [Google Scholar] [CrossRef]
- Chen, J.; Venkatesan, H.; Hu, J. Chemically Modified Silk Proteins. Adv. Eng. Mater. 2018, 20, 1700961. [Google Scholar] [CrossRef]
- Sun, J.; Su, J.; Ma, C.; Göstl, R.; Herrmann, A.; Liu, K.; Zhang, H. Fabrication and Mechanical Properties of Engineered Protein-Based Adhesives and Fibers. Adv. Mater. 2020, 32, 1906360. [Google Scholar] [CrossRef] [PubMed]
- Guo, C.; Zhang, J.; Jordan, J.S.; Wang, X.; Henning, R.W.; Yarger, J.L. Structural Comparison of Various Silkworm Silks: An Insight into the Structure–Property Relationship. Biomacromolecules 2018, 19, 906–917. [Google Scholar] [CrossRef] [PubMed]
- Teramoto, H.; Kojima, K.; Iga, M.; Yoshioka, T. Unique Material Properties of Bombyx mori Silk Fiber Incorporated with 3-Azidotyrosine. Biomacromolecules 2023, 24, 4208–4217. [Google Scholar] [CrossRef]
- Wang, X.; Zhao, P.; Li, Y.; Yi, Q.; Ma, S.; Xie, K.; Chen, H.; Xia, Q. Modifying the mechanical properties of silk fiber by genetically disrupting the ionic environment for silk formation. Biomacromolecules 2015, 16, 3119–3125. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Wang, X.; Tan, X.; Xie, X.; Li, Y.; Zhao, P.; Xia, Q. A strategy for improving the mechanical properties of silk fiber by directly injection of ferric ions into silkworm. Mater. Des. 2018, 146, 134–141. [Google Scholar] [CrossRef]
- Wu, Y.X.; Huang, Y.T.; Guan, J.P.; Cheng, X.W.; Xu, J.T.; Chen, G.Q. Facile preparation of effective flame retardant silk fabric by the metal salt adsorption approach. Polym. Degrad. Stab. 2020, 182, 109378. [Google Scholar] [CrossRef]
- Zhou, Y.; Tang, R.-C.; Xing, T.; Guan, J.-P.; Shen, Z.-H.; Zhai, A.-D. Flavonoids-metal salts combination: A facile and efficient route for enhancing the flame retardancy of silk. Ind. Crops Prod. 2019, 130, 580–591. [Google Scholar] [CrossRef]
- Malucelli, G.; Bosco, F.; Alongi, J.; Carosio, F.; Di Blasio, A.; Mollea, C.; Cuttica, F.; Casale, A. Biomacromolecules as novel green flame retardant systems for textiles: An overview. RSC Adv. 2014, 4, 46024–46039. [Google Scholar] [CrossRef]
- Tang, B.; Poma, G.; Bastiaensen, M.; Yin, S.-S.; Luo, X.-J.; Mai, B.-X.; Covaci, A. Bioconcentration and biotransformation of organophosphorus flame retardants (PFRs) in common carp (Cyprinus carpio). Environ. Int. 2019, 126, 512–522. [Google Scholar] [CrossRef]
- Vorkamp, K.; Balmer, J.; Hung, H.; Letcher, R.J.; Rigét, F.F.; de Wit, C.A. Current-use halogenated and organophosphorous flame retardants: A review of their presence in Arctic ecosystems. Emerg. Contam. 2019, 5, 179–200. [Google Scholar] [CrossRef]
- Mohsin, M.; Ahmad, S.W.; Khatri, A.; Zahid, B. Performance enhancement of fire retardant finish with environment friendly bio cross-linker for cotton. J. Clean. Prod. 2013, 51, 191–195. [Google Scholar] [CrossRef]
- Song, P.; Fang, Z.; Tong, L.; Jin, Y.; Lu, F. Effects of metal chelates on a novel oligomeric intumescent flame retardant system for polypropylene. J. Anal. Appl. Pyrolysis 2008, 82, 286–291. [Google Scholar] [CrossRef]
- Zhang, W.; Yang, Z.Y.; Tang, R.C.; Guan, J.P.; Qiao, Y.F. Application of tannic acid and ferrous ion complex as eco-friendly flame retardant and antibacterial agents for silk. J. Clean. Prod. 2020, 250, 119545. [Google Scholar] [CrossRef]
- Teli, M.; Paul, R. Novel natural dye from coffee seed coat. Int. Dyer 2006, 191, 29–32. [Google Scholar]
- Tehrani, M.; Ghaheh, F.S.; Beni, Z.T.; Rahimi, M. Extracted dyes’ stability as obtained from spent coffee grounds on silk fabrics using eco-friendly mordants. Environ. Sci. Pollut. Res. 2023, 30, 68625–68635. [Google Scholar] [CrossRef] [PubMed]
- Yin, Y.; Fei, L.; Wang, C. Optimization of natural dye extracted from phytolaccaceae berries and its mordant dyeing properties on natural silk fabric. J. Nat. Fibers 2018, 15, 69–79. [Google Scholar] [CrossRef]
- Bhavsar, P.; Dalla Fontana, G.; Tonin, C.; Patrucco, A.; Zoccola, M. Superheated water hydrolyses of waste silkworm pupae protein hydrolysate: A novel application for natural dyeing of silk fabric. Dyes Pigm. 2020, 183, 108678. [Google Scholar] [CrossRef]
- Gupta, S.; Nighojkar, A.; Mayilswamy, N.; Kandasubramanian, B. Recent Trends in the Application of Silk-Based Composites for Remediation of Toxic Contaminants from Wastewater. J. Polym. Environ. 2022, 31, 2243–2272. [Google Scholar] [CrossRef]
- Chen, B.; Ma, Q.; Tan, C.; Lim, T.T.; Huang, L.; Zhang, H. Carbon-based sorbents with three-dimensional architectures for water remediation. Small 2015, 11, 3319–3336. [Google Scholar] [CrossRef]
- Jain, M.; Yadav, M.; Kohout, T.; Lahtinen, M.; Garg, V.K.; Sillanpää, M. Development of iron oxide/activated carbon nanoparticle composite for the removal of Cr(VI), Cu(II) and Cd(II) ions from aqueous solution. Water Resour. Ind. 2018, 20, 54–74. [Google Scholar] [CrossRef]
- Aydin, D. A novel turn on fluorescent probe for the determination of Al3+ and Zn2+ ions and its cells applications. Talanta 2020, 210, 120615. [Google Scholar] [CrossRef] [PubMed]
- Zhou, W.; He, J.; Cui, S.; Gao, W. Preparation of electrospun silk fibroin/Cellulose Acetate blend nanofibers and their applications to heavy metal ions adsorption. Fibers Polym. 2011, 12, 431–437. [Google Scholar] [CrossRef]
- Li, N.; Zhang, L.; Chen, Y.; Fang, M.; Zhang, J.; Wang, H. Highly Efficient, Irreversible and Selective Ion Exchange Property of Layered Titanate Nanostructures. Adv. Funct. Mater. 2012, 22, 835–841. [Google Scholar] [CrossRef]
- Singh, S.P.; Rathinam, K.; Kasher, R.; Arnusch, C.J. Hexavalent chromium ion and methyl orange dye uptake via a silk protein sericin–chitosan conjugate. RSC Adv. 2018, 8, 27027–27036. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Ning, H.; Hu, N.; Huang, K.; Weng, S.; Wu, X.; Wu, L.; Liu, J.; Alamusi. Preparation and characterization of graphene oxide/silk fibroin hybrid aerogel for dye and heavy metal adsorption. Compos. Part B Eng. 2019, 163, 716–722. [Google Scholar] [CrossRef]
- Zhang, Y.; Peng, S.; Li, X.; Wang, X.; Jiang, J.; Liu, X.; Wang, L. Design and function of lignin/silk fibroin-based multilayer water purification membranes for dye adsorption. Int. J. Biol. Macromol. 2023, 253, 126863. [Google Scholar] [CrossRef] [PubMed]
- Babu Busi, K.; Palanivel, M.; Kanta Ghosh, K.; Basu Ball, W.; Gulyás, B.; Padmanabhan, P.; Chakrabortty, S. The Multifarious Applications of Copper Nanoclusters in Biosensing and Bioimaging and Their Translational Role in Early Disease Detection. Nanomaterials 2022, 12, 301. [Google Scholar] [CrossRef]
- Yang, X.; Sun, J.-K.; Kitta, M.; Pang, H.; Xu, Q. Encapsulating highly catalytically active metal nanoclusters inside porous organic cages. Nat. Catal. 2018, 1, 214–220. [Google Scholar] [CrossRef]
- Xiao, Y.; Wu, Z.; Yao, Q.; Xie, J. Luminescent metal nanoclusters: Biosensing strategies and bioimaging applications. Aggregate 2021, 2, 114–132. [Google Scholar] [CrossRef]
- Zhao, R.; Xiang, J.; Wang, B.; Chen, L.; Tan, S. Recent Advances in the Development of Noble Metal NPs for Cancer Therapy. Bioinorg. Chem. Appl. 2022, 2022, 2444516. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Liu, Y.; Xiong, Y.; Wei, D.; Peng, J.; Mahmud, S.; Liu, H. Konjac glucomannan reduced-stabilized silver nanoparticles for mono-azo and di-azo contained wastewater treatment. Inorganica Chim. Acta 2021, 515, 120058. [Google Scholar] [CrossRef]
- Restrepo, C.V.; Villa, C.C. Synthesis of silver nanoparticles, influence of capping agents, and dependence on size and shape: A review. Environ. Nanotechnol. Monit. Manag. 2021, 15, 100428. [Google Scholar] [CrossRef]
- Nguyen, N.T.-P.; Nguyen, L.V.-H.; Thanh, N.T.; Toi, V.V.; Ngoc Quyen, T.; Tran, P.A.; David Wang, H.-M.; Nguyen, T.-H. Stabilization of silver nanoparticles in chitosan and gelatin hydrogel and its applications. Mater. Lett. 2019, 248, 241–245. [Google Scholar] [CrossRef]
- Dong, Q.; Su, H.L.; Zhang, D. In situ depositing silver nanoclusters on silk fibroin fibers supports by a novel biotemplate redox technique at room temperature. J. Phys. Chem. B 2005, 109, 17429–17434. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.M.; Gao, W.R.; Xu, S.P.; Xu, W.Q. Luminescent fibers: In situ synthesis of silver nanoclusters on silk via ultraviolet light-induced reduction and their antibacterial activity. Chem. Eng. J. 2012, 210, 585–589. [Google Scholar] [CrossRef]
- Wang, Z.; Li, J.; Shao, C.; Lin, X.; Yang, Y.; Chen, N.; Wang, Y.; Qu, L. Moisture power in natural polymeric silk fibroin flexible membrane triggers efficient antibacterial activity of silver nanoparticles. Nano Energy 2021, 90, 106529. [Google Scholar] [CrossRef]
- Ru, M.; Hai, A.M.; Wang, L.; Yan, S.; Zhang, Q. Recent progress in silk-based biosensors. Int. J. Biol. Macromol. 2023, 224, 422–436. [Google Scholar] [CrossRef]
- Lv, L.; Wu, X.; Yang, Y.; Hang, X.; Mezzenga, R.; Li, C. Trans-Scale 2D Synthesis of Millimeter-Large Au Single Crystals via Silk Fibroin Templates. ACS Sustain. Chem. Eng. 2018, 6, 12419–12425. [Google Scholar] [CrossRef]
- Choi, J.H.; Choi, M.; Kang, T.; Ho, T.S.; Choi, S.H.; Byun, K.M. Combination of Porous Silk Fibroin Substrate and Gold Nanocracks as a Novel SERS Platform for a High-Sensitivity Biosensor. Biosensors 2021, 11, 441. [Google Scholar] [CrossRef]
- Xu, Z.; Shi, L.; Yang, M.; Zhu, L. Preparation and biomedical applications of silk fibroin-nanoparticles composites with enhanced properties—A review. Mater. Sci. Eng. C 2019, 95, 302–311. [Google Scholar] [CrossRef]
- Zhang, G.; Geng, F.; Zhao, T.; Zhou, F.; Zhang, N.; Zhang, S.; Deng, C. Biocompatible symmetric Na-ion microbatteries with sphere-in-network heteronanomat electrodes realizing high reliability and high energy density for implantable bioelectronics. ACS Appl. Mater. Interfaces 2018, 10, 42268–42278. [Google Scholar] [CrossRef] [PubMed]
- He, H.-Z.; Zhang, Y.; Li, Y.; Wang, P. Recent innovations of silk-derived electrocatalysts for hydrogen evolution reaction, oxygen evolution reaction and oxygen reduction reaction. Int. J. Hydrog. Energy 2021, 46, 7848–7865. [Google Scholar] [CrossRef]
- Jia, X.; Wang, C.; Ranganathan, V.; Napier, B.; Yu, C.; Chao, Y.; Forsyth, M.; Omenetto, F.G.; MacFarlane, D.R.; Wallace, G.G. A Biodegradable Thin-Film Magnesium Primary Battery Using Silk Fibroin–Ionic Liquid Polymer Electrolyte. ACS Energy Lett. 2017, 2, 831–836. [Google Scholar] [CrossRef]
- Zheng, S.-M.; Yuan, Z.-H.; Dionysiou, D.D.; Zhong, L.-B.; Zhao, F.; Yang, J.-C.E.; Zheng, Y.-M. Silkworm cocoon waste-derived nitrogen-doped hierarchical porous carbon as robust electrode materials for efficient capacitive desalination. Chem. Eng. J. 2023, 458, 141471. [Google Scholar] [CrossRef]
- He, H.; Zhang, Y.; Zhang, W.; Li, Y.; Wang, Y.; Wang, P.; Hu, D. Dual Metal-Loaded Porous Carbon Materials Derived from Silk Fibroin as Bifunctional Electrocatalysts for Hydrogen Evolution Reaction and Oxygen Evolution Reaction. ACS Appl. Mater. Interfaces 2021, 13, 30678–30692. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Wei, L.; Wang, H.; Lan, G.; Yang, H.; Shen, J. Silk gel-based N self-doped porous activated carbon as an efficient electrocatalyst in neutral, alkaline and acidic medium. Fuel 2021, 287, 119485. [Google Scholar] [CrossRef]
- Xi, R.; Li, Y.; Zhang, Y.; Wang, P.; Hu, D. Effects of activation method on biomass carbon-based materials used for electrochemical hydrogen evolution reaction catalyst. Int. J. Hydrog. Energy 2023, in press. [Google Scholar] [CrossRef]
- Padamwar, M.N.; Pawar, A.P.; Daithankar, A.V.; Mahadik, K.R. Silk sericin as a moisturizer: An in vivo study. J. Cosmet. Dermatol. 2005, 4, 250–257. [Google Scholar] [CrossRef]
- Tomeh, M.A.; Hadianamrei, R.; Zhao, X. Silk Fibroin as a Functional Biomaterial for Drug and Gene Delivery. Pharmaceutics 2019, 11, 494. [Google Scholar] [CrossRef]
- Inoue, S.; Tanaka, K.; Arisaka, F.; Kimura, S.; Ohtomo, K.; Mizuno, S. Silk fibroin of Bombyx mori is secreted, assembling a high molecular mass elementary unit consisting of H-chain, L-chain, and P25, with a 6:6:1 molar ratio. J. Biol. Chem. 2000, 275, 40517–40528. [Google Scholar] [CrossRef]
- Tanaka, K.; Inoue, S.; Mizuno, S. Hydrophobic interaction of P25, containing Asn-linked oligosaccharide chains, with the H-L complex of silk fibroin produced by Bombyx mori. Insect Biochem. Mol. Biol. 1999, 29, 269–276. [Google Scholar] [CrossRef] [PubMed]
- Rastogi, S.; Kandasubramanian, B. Progressive trends in heavy metal ions and dyes adsorption using silk fibroin composites. Environ. Sci. Pollut. Res. 2020, 27, 210–237. [Google Scholar] [CrossRef] [PubMed]
- Hao, Z.; Long, D.; Zhang, Y.; Umuhoza, D.; Dai, J.; Xu, Z.; Zhang, G.; Meng, W.; Xiang, Z.; Zhao, A. New insight into the mechanism of in vivo fibroin self-assembly and secretion in the silkworm, Bombyx mori. Int. J. Biol. Macromol. 2021, 169, 473–479. [Google Scholar] [CrossRef] [PubMed]
- Fedič, R.; Žurovec, M.; Sehnal, F. Correlation between Fibroin Amino Acid Sequence and Physical Silk Properties. J. Biol. Chem. 2003, 278, 35255–35264. [Google Scholar] [CrossRef] [PubMed]
- Kundu, B.; Rajkhowa, R.; Kundu, S.C.; Wang, X. Silk fibroin biomaterials for tissue regenerations. Adv. Drug Deliv. Rev. 2013, 65, 457–470. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, K.; Kajiyama, N.; Ishikura, K.; Waga, S.; Kikuchi, A.; Ohtomo, K.; Takagi, T.; Mizuno, S. Determination of the site of disulfide linkage between heavy and light chains of silk fibroin produced by Bombix mori. Biochim. Biophys. Acta Protein Struct. Mol. Enzymol. 1999, 1432, 92–103. [Google Scholar] [CrossRef]
- Mori, K.; Tanaka, K.; Kikuchi, Y.; Waga, M.; Waga, S.; Mizuno, S. Production of a chimeric fibroin light-chain polypeptide in a fibroin secretion-deficient naked pupa mutant of the silkworm Bombyx mori. J. Mol. Biol. 1995, 251, 217–228. [Google Scholar] [CrossRef] [PubMed]
- Takei, F.; Kikuchi, Y.; Kikuchi, A.; Mizuno, S.; Shimura, K. Further evidence for importance of the subunit combination of silk fibroin in its efficient secretion from the posterior silk gland cells. J. Cell Biol. 1987, 105, 175–180. [Google Scholar] [CrossRef]
- Sun, W.; Gregory, D.A.; Tomeh, M.A.; Zhao, X. Silk Fibroin as a Functional Biomaterial for Tissue Engineering. Int. J. Mol. Sci. 2021, 22, 1499. [Google Scholar] [CrossRef]
- Qi, Y.; Wang, H.; Wei, K.; Yang, Y.; Zheng, R.-Y.; Kim, I.S.; Zhang, K.-Q. A Review of Structure Construction of Silk Fibroin Biomaterials from Single Structures to Multi-Level Structures. Int. J. Mol. Sci. 2017, 18, 237. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Yao, L.; Zhang, L. Silk sericin-based biomaterials shine in food and pharmaceutical industries. Smart Mater. Med. 2023, 4, 447–459. [Google Scholar] [CrossRef]
- Rong, H.H.; Zhang, M.C.; Liang, X.; Liu, C.; Saadi, M.; Chen, X.Y.; Yao, L.; Zhang, Y.R.; He, N.; Hu, E.R.; et al. Demonstration of electronic synapses using a sericin-based bio-memristor. Appl. Phys. Express 2023, 16, 031007. [Google Scholar] [CrossRef]
- Lee, K.H. Silk sericin retards the crystallization of silk fibroin. Macromol. Rapid Commun. 2004, 25, 1792–1796. [Google Scholar] [CrossRef]
- Kundu, S.C.; Dash, B.C.; Dash, R.; Kaplan, D.L. Natural protective glue protein, sericin bioengineered by silkworms: Potential for biomedical and biotechnological applications. Prog. Polym. Sci. 2008, 33, 998–1012. [Google Scholar] [CrossRef]
- Zhang, Y.-Q. Applications of natural silk protein sericin in biomaterials. Biotechnol. Adv. 2002, 20, 91–100. [Google Scholar] [CrossRef] [PubMed]
- Suryawanshi, R.; Kanoujia, J.; Parashar, P.; Saraf, S.A. Sericin: A Versatile Protein Biopolymer with Therapeutic Significance. Curr. Pharm. Des. 2020, 26, 5414–5429. [Google Scholar] [CrossRef]
- Zhou, K.; Peters, R.J. Investigating the conservation pattern of a putative second terpene synthase divalent metal binding motif in plants. Phytochemistry 2009, 70, 366–369. [Google Scholar] [CrossRef]
- Paul, J.J.; Kircus, S.R.; Sorrell, T.N.; Ropp, P.A.; Thorp, H.H. Effects of coordinating metal ions on the mediated inhibition of trypsin by bis(benzimidazoles) and related compounds. Inorg. Chem. 2006, 45, 5126–5135. [Google Scholar] [CrossRef]
- Qiao, X.Y.; Miller, R.; Schneck, E.; Sun, K. Influence of pH on the surface and foaming properties of aqueous silk fibroin solutions. Soft Matter 2020, 16, 3695–3704. [Google Scholar] [CrossRef]
- Wang, Z.; Zhang, Y.; Zhang, J.; Huang, L.; Liu, J.; Li, Y.; Zhang, G.; Kundu, S.C.; Wang, L. Exploring natural silk protein sericin for regenerative medicine: An injectable, photoluminescent, cell-adhesive 3D hydrogel. Sci. Rep. 2014, 4, 7064. [Google Scholar] [CrossRef] [PubMed]
- Khosa, M.A.; Shah, S.S.; Feng, X.S. Metal sericin complexation and ultrafiltration of heavy metals from aqueous solution. Chem. Eng. J. 2014, 244, 446–456. [Google Scholar] [CrossRef]
- Zhong, L.-S.; Hu, J.-S.; Liang, H.-P.; Cao, A.-M.; Song, W.-G.; Wan, L.-J. Self-Assembled 3D Flowerlike Iron Oxide Nanostructures and Their Application in Water Treatment. Adv. Mater. 2006, 18, 2426–2431. [Google Scholar] [CrossRef]
- Cao, C.-Y.; Qu, J.; Yan, W.-S.; Zhu, J.-F.; Wu, Z.-Y.; Song, W.-G. Low-Cost Synthesis of Flowerlike α-Fe2O3 Nanostructures for Heavy Metal Ion Removal: Adsorption Property and Mechanism. Langmuir 2012, 28, 4573–4579. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Sun, Z.; Guo, C. Chemical Modification of Silk Proteins: Current Status and Future Prospects. Adv. Fiber Mater. 2022, 4, 705–719. [Google Scholar] [CrossRef]
- Zheng, H.; Zuo, B. Functional silk fibroin hydrogels: Preparation, properties and applications. J. Mater. Chem. B 2021, 9, 1238–1258. [Google Scholar] [CrossRef] [PubMed]
- Bartlett, G.J.; Woolfson, D.N. On the satisfaction of backbone-carbonyl lone pairs of electrons in protein structures. Protein Sci. 2016, 25, 887–897. [Google Scholar] [CrossRef]
- He, H.; Zhang, Y.; Wang, P.; Hu, D. Preparation of sponge-cake-like N-doped porous carbon materials derived from silk fibroin by chemical activation. Microporous Mesoporous Mater. 2021, 317, 110998. [Google Scholar] [CrossRef]
- Li, Y.; Yao, L.; Zhang, L.; Zhang, Y.; Zheng, T.; Liu, L.; Zhang, L. Enhanced physicochemical stabilities of cyanidin-3-O-glucoside via combination with silk fibroin. Food Chem. 2021, 355, 129479. [Google Scholar] [CrossRef]
- Yao, L.; Xu, J.; Zhang, L.; Liu, L.; Zhang, L. Nanoencapsulation of Anthocyanin by an Amphiphilic Peptide for Stability Enhancement. Food Hydrocoll. 2021, 118, 106741. [Google Scholar] [CrossRef]
- Yao, L.; Xu, J.; Zhang, L.; Zheng, T.; Zhang, L. Physicochemical Stability-Increasing Effects of Anthocyanin via a Co-Assembly Approach with an Amphiphilic Peptide. Food Chem. 2021, 362, 130101. [Google Scholar] [CrossRef] [PubMed]
- Kwak, H.W.; Kim, Y.; Yun, N.K.; Lee, K.H. Silk sericin microparticles as a biosorbent for hexavalent chromium ion. Macromol. Res. 2014, 22, 788–795. [Google Scholar] [CrossRef]
- Elzoghby, A.O.; Elgohary, M.M.; Kamel, N.M. Chapter Six—Implications of Protein- and Peptide-Based Nanoparticles as Potential Vehicles for Anticancer Drugs. In Advances in Protein Chemistry and Structural Biology; Donev, R., Ed.; Academic Press: Cambridge, MA, USA, 2015; Volume 98, pp. 169–221. [Google Scholar]
- Barrera-Diaz, C.E.; Lugo-Lugo, V.; Bilyeu, B. A review of chemical, electrochemical and biological methods for aqueous Cr(VI) reduction. J. Hazard. Mater. 2012, 223, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Yao, L.; Hao, M.; Zhao, F.; Wang, Y.; Zhou, Y.; Liu, Z.; An, X.; Gao, Z.; Wang, J.; Zheng, T.; et al. Fabrication of silk sericin-anthocyanin nanocoating for chelating and saturation-visualization detection of metal ions. Nanoscale 2022, 14, 17277–17289. [Google Scholar] [CrossRef] [PubMed]
- Kwak, H.W.; Shin, M.; Yun, H.; Lee, K.H. Preparation of Silk Sericin/Lignin Blend Beads for the Removal of Hexavalent Chromium Ions. Int. J. Mol. Sci. 2016, 17, 1466. [Google Scholar] [CrossRef] [PubMed]
- Azizian, S. Kinetic models of sorption: A theoretical analysis. J. Colloid Interface Sci. 2004, 276, 47–52. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K. The Extraction of Silk Fibroin & Preparation, Modification and Application of Silk Fibroin Membrane; Dong Hua University: Shanghai, China, 2015. [Google Scholar]
- Bailey, S.E.; Olin, T.J.; Bricka, R.M.; Adrian, D.D. A review of potentially low-cost sorbents for heavy metals. Water Res. 1999, 33, 2469–2479. [Google Scholar] [CrossRef]
- Mehta, S.K.; Gaur, J.P. Use of Algae for Removing Heavy Metal Ions From Wastewater: Progress and Prospects. Crit. Rev. Biotechnol. 2005, 25, 113–152. [Google Scholar] [CrossRef]
- Dudev, T.; Lim, C. Competition among Metal Ions for Protein Binding Sites: Determinants of Metal Ion Selectivity in Proteins. Chem. Rev. 2014, 114, 538–556. [Google Scholar] [CrossRef]
- Khosa, M.A.; Wu, J.; Ullah, A. Chemical modification, characterization, and application of chicken feathers as novel biosorbents. RSC Adv. 2013, 3, 20800–20810. [Google Scholar] [CrossRef]
- Tai, H.-C.; Lim, C. Computational Studies of the Coordination Stereochemistry, Bonding, and Metal Selectivity of Mercury. J. Phys. Chem. A 2006, 110, 452–462. [Google Scholar] [CrossRef] [PubMed]
- Koley, P.; Sakurai, M.; Aono, M. Controlled Fabrication of Silk Protein Sericin Mediated Hierarchical Hybrid Flowers and Their Excellent Adsorption Capability of Heavy Metal Ions of Pb(II), Cd(II) and Hg(II). ACS Appl. Mater. Interfaces 2016, 8, 2380–2392. [Google Scholar] [CrossRef] [PubMed]
- Jiao, Z.Y.; Song, Y.; Jin, Y.; Zhang, C.; Peng, D.; Chen, Z.Z.; Chang, P.P.; Kundu, S.C.; Wang, G.B.; Wang, Z.; et al. In Vivo Characterizations of the Immune Properties of Sericin: An Ancient Material with Emerging Value in Biomedical Applications. Macromol. Biosci. 2017, 17, 1700229. [Google Scholar] [CrossRef] [PubMed]
- Kundu, B.; Kurland, N.E.; Bano, S.; Patra, C.; Engel, F.B.; Yadavalli, V.K.; Kundu, S.C. Silk proteins for biomedical applications: Bioengineering perspectives. Prog. Polym. Sci. 2014, 39, 251–267. [Google Scholar] [CrossRef]
- Gholipourmalekabadi, M.; Sapru, S.; Samadikuchaksaraei, A.; Reis, R.L.; Kaplan, D.L.; Kundu, S.C. Silk fibroin for skin injury repair: Where do things stand? Adv. Drug Deliv. Rev. 2020, 153, 28–53. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.K.; Kim, J.I.; Sim, B.R.; Khang, G. Bioengineered porous composite curcumin/silk scaffolds for cartilage regeneration. Mater. Sci. Eng. C Mater. Biol. Appl. 2017, 78, 571–578. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Wang, L.; Lou, Z.; Zheng, Y.; Wang, K.; Zhao, L.; Han, W.; Jiang, K.; Shen, G. Biomimetic, biocompatible and robust silk Fibroin-MXene film with stable 3D cross-link structure for flexible pressure sensors. Nano Energy 2020, 78, 105252. [Google Scholar] [CrossRef]
- Eivazzadeh-Keihan, R.; Ganjali, F.; Aliabadi, H.A.M.; Maleki, A.; Pouri, S.; Mahdavi, M.; Shalan, A.E.; Lanceros-Méndez, S. Synthesis and characterization of cellulose, β-cyclodextrin, silk fibroin-based hydrogel containing copper-doped cobalt ferrite nanospheres and exploration of its biocompatibility. J. Nanostruct. Chem. 2023, 13, 103–113. [Google Scholar] [CrossRef]
- Kwon, S.Y.; Chung, J.W.; Park, H.J.; Jiang, Y.Y.; Park, J.K.; Seo, Y.K. Silk and collagen scaffolds for tendon reconstruction. Proc. Inst. Mech. Eng. Part H J. Eng. Med. 2014, 228, 388–396. [Google Scholar] [CrossRef]
- Dewair, M.; Baur, X.; Ziegler, K. Use of immunoblot technique for detection of human IgE and IgG antibodies to individual silk proteins. J. Allergy Clin. Immunol. 1985, 76, 537–542. [Google Scholar] [CrossRef]
- Panilaitis, B.; Altman, G.H.; Chen, J.; Jin, H.-J.; Karageorgiou, V.; Kaplan, D.L. Macrophage responses to silk. Biomaterials 2003, 24, 3079–3085. [Google Scholar] [CrossRef]
- Boonrungsiman, S.; Thongtham, N.; Suwantong, O.; Wutikhun, T.; Soykeabkaew, N.; Nimmannit, U. An improvement of silk-based scaffold properties using collagen type I for skin tissue engineering applications. Polym. Bull. 2018, 75, 685–700. [Google Scholar] [CrossRef]
- Fu, Z.X.; Li, W.H.; Wei, J.J.; Yao, K.; Wang, Y.Q.; Yang, P.X.; Li, G.C.; Yang, Y.M.; Zhang, L.Z. Construction and Biocompatibility Evaluation of Fibroin/Sericin-Based Scaffolds. ACS Biomater. Sci. Eng. 2022, 8, 1494–1505. [Google Scholar] [CrossRef] [PubMed]
- Aramwit, P.; Palapinyo, S.; Srichana, T.; Chottanapund, S.; Muangman, P. Silk sericin ameliorates wound healing and its clinical efficacy in burn wounds. Arch. Dermatol. Res. 2013, 305, 585–594. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.R.; Lu, Q.Y.; Xiang, Y.J.; Yin, Y.L.; Li, J.Y.; Liu, Y.L.; Wu, X.C. Enhanced biocompatibility of silk sericin/caffeic acid nanoparticles by red blood cell membranes cloaking. Int. J. Biol. Macromol. 2023, 238, 124133. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Song, Y.-w.; Jin, L.; Wang, Z.-J.; Pu, D.-Y.; Lin, S.-Q.; Zhou, C.; You, H.-J.; Ma, Y.; Li, J.-M.; et al. Silk structure and degradation. Colloids Surf. B Biointerfaces 2015, 131, 122–128. [Google Scholar] [CrossRef]
- Wang, Y.Q.; Yao, D.Y.; Li, L.H.; Qian, Z.Y.; He, W.; Ding, R.; Liu, H.F.; Fan, Y.B. Effect of Electrospun Silk Fibroin-Silk Sericin Films on Macrophage Polarization and Vascularization. ACS Biomater. Sci. Eng. 2020, 6, 3502–3512. [Google Scholar] [CrossRef] [PubMed]
- Cherng, J.H.; Chang, S.J.; Chiu, Y.K.; Chiu, Y.H.; Fang, T.J.; Chen, H.C. Low Molecular Weight Sericin Enhances the In Vitro of Immunological Modulation and Cell Migration. Front. Bioeng. Biotechnol. 2022, 10, 925197. [Google Scholar] [CrossRef]
- Jameson, J.F.; Pacheco, M.O.; Butler, J.E.; Stoppel, W.L. Estimating Kinetic Rate Parameters for Enzymatic Degradation of Lyophilized Silk Fibroin Sponges. Front. Bioeng. Biotechnol. 2021, 9, 664306. [Google Scholar] [CrossRef]
- Uddin, M.G.; Allardyce, B.J.; Rashida, N.; Rajkhowa, R. Mechanical, structural and biodegradation characteristics of fibrillated silk fibres and papers. Int. J. Biol. Macromol. 2021, 179, 20–32. [Google Scholar] [CrossRef]
- Rajkhowa, R.; Hu, X.; Tsuzuki, T.; Kaplan, D.L.; Wang, X. Structure and Biodegradation Mechanism of Milled Bombyx mori Silk Particles. Biomacromolecules 2012, 13, 2503–2512. [Google Scholar] [CrossRef] [PubMed]
- Cao, F.; Zeng, B.; Zhu, Y.; Yu, F.; Wang, M.; Song, X.; Cheng, X.; Chen, L.; Wang, X. Porous ZnO modified silk sutures with dual light defined antibacterial, healing promotion and controlled self-degradation capabilities. Biomater. Sci. 2020, 8, 250–255. [Google Scholar] [CrossRef]
- Wang, H.-Y.; Zhang, Y.-Q.; Wei, Z.-G. Characterization of undegraded and degraded silk fibroin and its significant impact on the properties of the resulting silk biomaterials. Int. J. Biol. Macromol. 2021, 176, 578–588. [Google Scholar] [CrossRef] [PubMed]
- Sun, M.; Li, Q.; Yu, H.; Cheng, J.; Wu, N.; Shi, W.; Zhao, F.; Shao, Z.; Meng, Q.; Chen, H.; et al. Cryo-self-assembled silk fibroin sponge as a biodegradable platform for enzyme-responsive delivery of exosomes. Bioact. Mater. 2022, 8, 505–514. [Google Scholar] [CrossRef] [PubMed]
- Gholipourmalekabadi, M.; Mozafari, M.; Gholipourmalekabadi, M.; Nazm Bojnordi, M.; Hashemi-soteh, M.B.; Salimi, M.; Rezaei, N.; Sameni, M.; Samadikuchaksaraei, A.; Ghasemi Hamidabadi, H. In vitro and in vivo evaluations of three-dimensional hydroxyapatite/silk fibroin nanocomposite scaffolds. Biotechnol. Appl. Biochem. 2015, 62, 441–450. [Google Scholar] [CrossRef]
- Umuhoza, D.; Yang, F.; Long, D.; Hao, Z.; Dai, J.; Zhao, A. Strategies for Tuning the Biodegradation of Silk Fibroin-Based Materials for Tissue Engineering Applications. ACS Biomater. Sci. Eng. 2020, 6, 1290–1310. [Google Scholar] [CrossRef] [PubMed]
- Feng, Y.F.; Li, X.F.; Zhang, Q.; Ye, D.Z.; Li, M.Z.; You, R.C.; Xu, W.L. Fabrication of porous silk fibroin/cellulose nanofibril sponges with hierarchical structure using a lithium bromide solvent system. Cellulose 2019, 26, 1013–1023. [Google Scholar] [CrossRef]
- Li, X.; Li, N.; Fan, Q.; Yan, K.; Zhang, Q.; Wang, D.; You, R. Silk fibroin scaffolds with stable silk I crystal and tunable properties. Int. J. Biol. Macromol. 2023, 248, 125910. [Google Scholar] [CrossRef]
- Guan, Y.; You, H.; Cai, J.; Zhang, Q.; Yan, S.; You, R. Physically crosslinked silk fibroin/hyaluronic acid scaffolds. Carbohydr. Polym. 2020, 239, 116232. [Google Scholar] [CrossRef]
- Kambe, Y.; Mizoguchi, Y.; Kuwahara, K.; Nakaoki, T.; Hirano, Y.; Yamaoka, T. Beta-sheet content significantly correlates with the biodegradation time of silk fibroin hydrogels showing a wide range of compressive modulus. Polym. Degrad. Stab. 2020, 179, 109240. [Google Scholar] [CrossRef]
- Guo, C.; Li, C.; Kaplan, D.L. Enzymatic Degradation of Bombyx mori Silk Materials: A Review. Biomacromolecules 2020, 21, 1678–1686. [Google Scholar] [CrossRef] [PubMed]
- Wongpinyochit, T.; Johnston, B.F.; Seib, F.P. Degradation Behavior of Silk Nanoparticles—Enzyme Responsiveness. ACS Biomater. Sci. Eng. 2018, 4, 942–951. [Google Scholar] [CrossRef] [PubMed]
- Vepari, C.; Kaplan, D.L. Silk as a biomaterial. Prog. Polym. Sci. 2007, 32, 991–1007. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Liu, M.; Huang, H.; Cheng, L.; Zhao, H.-P. Mechanical properties of Bombyx mori silkworm silk fibre and its corresponding silk fibroin filament: A comparative study. Mater. Des. 2019, 181, 108077. [Google Scholar] [CrossRef]
- Lujerdean, C.; Baci, G.M.; Cucu, A.A.; Dezmirean, D.S. The Contribution of Silk Fibroin in Biomedical Engineering. Insects 2022, 13, 286. [Google Scholar] [CrossRef] [PubMed]
- Altman, G.H.; Diaz, F.; Jakuba, C.; Calabro, T.; Horan, R.L.; Chen, J.; Lu, H.; Richmond, J.; Kaplan, D.L. Silk-based biomaterials. Biomaterials 2003, 24, 401–416. [Google Scholar] [CrossRef] [PubMed]
- Guo, C.C.; Li, C.M.; Mu, X.; Kaplan, D.L. Engineering silk materials: From natural spinning to artificial processing. Appl. Phys. Rev. 2020, 7, 011313. [Google Scholar] [CrossRef]
- Johari, N.; Moroni, L.; Samadikuchaksaraei, A. Tuning the conformation and mechanical properties of silk fibroin hydrogels. Eur. Polym. J. 2020, 134, 109842. [Google Scholar] [CrossRef]
- Vidya, M.; Rajagopal, S. Silk fibroin: A promising tool for wound healing and skin regeneration. Int. J. Polym. Sci. 2021, 2021, 9069924. [Google Scholar] [CrossRef]
- Park, C.J.; Ryoo, J.; Ki, C.S.; Kim, J.W.; Kim, I.S.; Bae, D.G.; Um, I.C. Effect of molecular weight on the structure and mechanical properties of silk sericin gel, film, and sponge. Int. J. Biol. Macromol. 2018, 119, 821–832. [Google Scholar] [CrossRef]
- Zhu, Z.; Ling, S.; Yeo, J.; Zhao, S.; Tozzi, L.; Buehler, M.J.; Omenetto, F.; Li, C.; Kaplan, D.L. High-Strength, Durable All-Silk Fibroin Hydrogels with Versatile Processability toward Multifunctional Applications. Adv. Funct. Mater. 2018, 28, 1704757. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Illeperuma, W.R.K.; Suo, Z.; Vlassak, J.J. Hybrid Hydrogels with Extremely High Stiffness and Toughness. ACS Macro Lett. 2014, 3, 520–523. [Google Scholar] [CrossRef] [PubMed]
- Nakayama, D.; Wu, F.; Mohanty, A.K.; Hirai, S.; Misra, M. Biodegradable Composites Developed from PBAT/PLA Binary Blends and Silk Powder: Compatibilization and Performance Evaluation. ACS Omega 2018, 3, 12412–12421. [Google Scholar] [CrossRef] [PubMed]
- Sahoo, J.K.; Hasturk, O.; Falcucci, T.; Kaplan, D.L. Silk chemistry and biomedical material designs. Nat. Rev. Chem. 2023, 7, 302–318. [Google Scholar] [CrossRef] [PubMed]
- Falcucci, T.; Presley, K.F.; Choi, J.; Fizpatrick, V.; Barry, J.; Kishore Sahoo, J.; Ly, J.T.; Grusenmeyer, T.A.; Dalton, M.J.; Kaplan, D.L. Degradable Silk-Based Subcutaneous Oxygen Sensors. Adv. Funct. Mater. 2022, 32, 2202020. [Google Scholar] [CrossRef]
- Gonzalez-Obeso, C.; Hartzell, E.J.; Scheel, R.A.; Kaplan, D.L. Delivering on the promise of recombinant silk-inspired proteins for drug delivery. Adv. Drug Deliv. Rev. 2023, 192, 114622. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; Shmelev, K.; Sun, L.; Gil, E.-S.; Park, S.-H.; Cebe, P.; Kaplan, D.L. Regulation of Silk Material Structure by Temperature-Controlled Water Vapor Annealing. Biomacromolecules 2011, 12, 1686–1696. [Google Scholar] [CrossRef]
- Wu, J.; Wang, J.; Zhang, J.; Zheng, Z.; Kaplan, D.L.; Li, G.; Wang, X. Oral Delivery of Curcumin Using Silk Nano- and Microparticles. ACS Biomater. Sci. Eng. 2018, 4, 3885–3894. [Google Scholar] [CrossRef]
- Reizabal, A.; Costa, C.M.; Pérez-Álvarez, L.; Vilas-Vilela, J.L.; Lanceros-Méndez, S. The New Silk Road: Silk Fibroin Blends and Composites for Next Generation Functional and Multifunctional Materials Design. Polym. Rev. 2023, 63, 1014–1077. [Google Scholar] [CrossRef]
- Holland, C.; Numata, K.; Rnjak-Kovacina, J.; Seib, F.P. The Biomedical Use of Silk: Past, Present, Future. Adv. Healthc. Mater. 2019, 8, 1800465. [Google Scholar] [CrossRef]
- Agudelo, W.; Montoya, Y.; Garcia-Garcia, A.; Restrepo-Osorio, A.; Bustamante, J. Electrochemical and Electroconductive Behavior of Silk Fibroin Electrospun Membrane Coated with Gold or Silver Nanoparticles. Membranes 2022, 12, 1154. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Wang, X.; Zhou, Y.; Tan, X.; Xie, X.; Li, Y.; Dong, H.; Tang, Z.; Zhao, P.; Xia, Q. Dynamic Changes and Characterization of the Metal Ions in the Silk Glands and Silk Fibers of Silkworm. Int. J. Mol. Sci. 2023, 24, 6556. [Google Scholar] [CrossRef]
- Gao, Z.F.; Zheng, L.L.; Fu, W.L.; Zhang, L.; Li, J.Z.; Chen, P. Feeding Alginate-Coated Liquid Metal Nanodroplets to Silkworms for Highly Stretchable Silk Fibers. Nanomaterials 2022, 12, 1177. [Google Scholar] [CrossRef] [PubMed]
- Shao, P.; Qiu, Q.; Xiao, J.; Zhu, Y.; Sun, P. Chemical Stability and in vitro release properties of β-carotene in emulsions stabilized by Ulva fasciata polysaccharide. Int. J. Biol. Macromol. 2017, 102, 225–231. [Google Scholar] [CrossRef] [PubMed]
- Du, Z.; Xu, N.; Yang, Y.; Li, G.; Tai, Z.; Li, N.; Sun, Y. Study on internal structure of casein micelles in reconstituted skim milk powder. Int. J. Biol. Macromol. 2023, 224, 437–452. [Google Scholar] [CrossRef]
- He, J.X.; Jia, G.X.; Cui, S.Z.; Wang, S.Y.; Gao, Y.Y. Chemical Modification of Bombyx mori Silk with Calcium-Salt Treatment and Subsequent Glycerin Triglycidyl Ether Crosslinking. J. Appl. Polym. Sci. 2010, 118, 3260–3268. [Google Scholar] [CrossRef]
- Drnovsek, N.; Kocen, R.; Gantar, A.; Drobnic-Kosorok, M.; Leonardi, A.; Krizaj, I.; Recnik, A.; Novak, S. Size of silk fibroin beta-sheet domains affected by Ca2+. J. Mater. Chem. B 2016, 4, 6597–6608. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Q.; Chen, G.; Tang, X. Optimal Technology of Silk Weighted by Dying With Dioscorea Cirrhosa Extract and Effect of Post-treatment With Metal Ions on its Performance. Acta Sericol. Sin. 2008, 34, 694–700. [Google Scholar] [CrossRef]
- Hua, J.S.; You, H.N.; Li, X.F.; You, R.C.; Ma, L.K. Cu(II) ion loading in silk fibroin scaffolds with silk I structure. Int. J. Biol. Macromol. 2020, 158, 275–281. [Google Scholar] [CrossRef]
- Zhao, Y.Y.; Zhou, L.X. Theoretical study on the interaction between metal cations and dimethyl phosphate anion. Chin. J. Struct. Chem. 2004, 23, 540–546. [Google Scholar] [CrossRef]
- Zhang, W.; Wang, M.; Guan, J.-P.; Tang, R.-C.; Qiao, Y.-F. Casein phosphopeptide-metal salts combination: A novel route for imparting the durable flame retardancy to silk. J. Taiwan Inst. Chem. Eng. 2019, 101, 1–7. [Google Scholar] [CrossRef]
- Boyle, A.L.; Rabe, M.; Crone, N.S.A.; Rhys, G.G.; Soler, N.; Voskamp, P.; Pannu, N.S.; Kros, A. Selective coordination of three transition metal ions within a coiled-coil peptide scaffold. Chem. Sci. 2019, 10, 7456–7465. [Google Scholar] [CrossRef] [PubMed]
- Zong, X.-H.; Zhou, P.; Shao, Z.-Z.; Chen, S.-M.; Chen, X.; Hu, B.-W.; Deng, F.; Yao, W.-H. Effect of pH and Copper(II) on the Conformation Transitions of Silk Fibroin Based on EPR, NMR, and Raman Spectroscopy. Biochemistry 2004, 43, 11932–11941. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Li, Y.; Liu, Q.S.; Chen, Q.M.; Xia, Q.Y.; Zhao, P. In vivo effects of metal ions on conformation and mechanical performance of silkworm silks. Biochim. Biophys. Acta Gen. Subj. 2017, 1861, 567–576. [Google Scholar] [CrossRef] [PubMed]
- Leal, S.S.; Botelho, H.M.; Gomes, C.M. Metal ions as modulators of protein conformation and misfolding in neurodegeneration. Coord. Chem. Rev. 2012, 256, 2253–2270. [Google Scholar] [CrossRef]
- Yan, B.; Zhou, Q.; Xing, T.; Chen, G. Dopamine-Dyed and Functionally Finished Silk with Rapid Oxidation Polymerization. Polymers 2018, 10, 728. [Google Scholar] [CrossRef] [PubMed]
- Yan, B.; Zhou, Q.; Zhu, X.; Guo, J.; Mia, S.; Yan, X.; Chen, G.; Xing, T. A superhydrophobic bionic coating on silk fabric with flame retardancy and UV shielding ability. Appl. Surf. Sci. 2019, 483, 929–939. [Google Scholar] [CrossRef]
- Yan, J.; Xia, D.; Zhou, W.; Li, Y.; Xiong, P.; Li, Q.; Wang, P.; Li, M.; Zheng, Y.; Cheng, Y. pH-responsive silk fibroin-based CuO/Ag micro/nano coating endows polyetheretherketone with synergistic antibacterial ability, osteogenesis, and angiogenesis. Acta Biomater. 2020, 115, 220–234. [Google Scholar] [CrossRef]
- Zheng, H.; Huang, Z.; Chen, T.; Sun, Y.; Chen, S.; Bu, G.; Guan, H. Gallium ions incorporated silk fibroin hydrogel with antibacterial efficacy for promoting healing of Pseudomonas aeruginosa-infected wound. Front. Chem. 2022, 10, 1017548. [Google Scholar] [CrossRef]
- Velmurugan, P.; Shim, J.; Bang, K.-S.; Oh, B.-T. Gold nanoparticles mediated coloring of fabrics and leather for antibacterial activity. J. Photochem. Photobiol. B Biol. 2016, 160, 102–109. [Google Scholar] [CrossRef]
- Kiro, A.; Bajpai, J.; Bajpai, A.K. Designing of silk and ZnO based antibacterial and noncytotoxic bionanocomposite films and study of their mechanical and UV absorption behavior. J. Mech. Behav. Biomed. Mater. 2017, 65, 281–294. [Google Scholar] [CrossRef]
- Yang, C.M.; Lee, J.; Lee, H.; Park, W.H. ZnO nanoparticle-embedded modified silk fibroin-tannin multifunctional hydrogel. Int. J. Biol. Macromol. 2022, 210, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Prakash, N.; Vendan, S.A.; Sudha, P.N.; Renganathan, N.G. Biodegradable Polymer-Based Ternary Blends for Adsorption of Heavy Metal From Simulated Industrial Wastewater. Synth. React. Inorg. Met. Org. Nano-Met. Chem. 2016, 46, 1664–1674. [Google Scholar] [CrossRef]
- Ki, C.S.; Gang, E.H.; Um, I.C.; Park, Y.H. Nanofibrous membrane of wool keratose/silk fibroin blend for heavy metal ion adsorption. J. Membr. Sci. 2007, 302, 20–26. [Google Scholar] [CrossRef]
- Baek, D.H.; Ki, C.S.; Um, I.C.; Park, Y.H. Metal ion adsorbability of electrospun wool keratose/silk fibroin blend nanofiber mats. Fibers Polym. 2007, 8, 271–277. [Google Scholar] [CrossRef]
- Ishikawa, S.-I.; Suyama, K.; Arihara, K.; Itoh, M. Selective recovery of uranium and thorium ions from dilute aqueous solutions by animal biopolymers. Biol. Trace Elem. Res. 2002, 86, 227–236. [Google Scholar] [CrossRef] [PubMed]
- Aslani, M.A.A.; Eral, M.; Akyil, S. Separation of thorium from aqueous solution using silk fibroin. J. Radioanal. Nucl. Chem. 1998, 238, 123–127. [Google Scholar] [CrossRef]
- Yalcin, E.; Gedikli, S.; Cabuk, A.; Karahaliloglu, Z.; Demirbilek, M.; Bayram, C.; Sam, M.; Saglam, N.; Denkbas, E.B. Silk fibroin/nylon-6 blend nanofilter matrix for copper removal from aqueous solution. Clean Technol. Environ. Policy 2015, 17, 921–934. [Google Scholar] [CrossRef]
- Denish, P.R.; Fenger, J.-A.; Powers, R.; Sigurdson, G.T.; Grisanti, L.; Guggenheim, K.G.; Laporte, S.; Li, J.; Kondo, T.; Magistrato, A.; et al. Discovery of a natural cyan blue: A unique food-sourced anthocyanin could replace synthetic brilliant blue. Sci. Adv. 2021, 7, 7871. [Google Scholar] [CrossRef]
- Pereira, C.D.; Techy, J.G.; Ganzarolli, E.M.; Quináia, S.P. Chromium fractionation and speciation in natural waters. J. Environ. Monit. 2012, 14, 1559–1564. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, M.; Niu, Q.Q.; Gao, P.F.; Zhang, G.M.; Dong, C.; Shuang, S.M. Gold nanoclusters as fluorescent sensors for selective and sensitive hydrogen sulfide detection. Talanta 2017, 171, 143–151. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.L.; Ren, X.J.; Gao, X.Y. Peptide or Protein-Protected Metal Nanoclusters for Therapeutic Application. Chin. J. Chem. 2022, 40, 267–274. [Google Scholar] [CrossRef]
- Yu, Y.; Lee, W.D.; Tan, Y.N. Protein-protected gold/silver alloy nanoclusters in metal-enhanced singlet oxygen generation and their correlation with photoluminescence. Mater. Sci. Eng. C Mater. Biol. Appl. 2020, 109, 110525. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.M.; Wang, R.R.; Shi, L.H.; Zhang, C.H.; Zhang, Y.; Zhou, Y.; Dong, C.; Li, G.; Shuang, S.M. Aggregation/assembly induced emission based on silk fibroin-templated fluorescent copper nanoclusters for “turn-on” detection of S2−. Sens. Actuators B Chem. 2019, 279, 361–368. [Google Scholar] [CrossRef]
- Zhang, G.M.; Xu, T.; Du, H.Z.; Qiao, Y.Y.; Guo, X.H.; Shi, L.H.; Zhang, Y.; Shuang, S.M.; Dong, C.; Ma, H.M. A reversible fluorescent pH-sensing system based on the one-pot synthesis of natural silk fibroin-capped copper nanoclusters. J. Mater. Chem. C 2016, 4, 3540–3545. [Google Scholar] [CrossRef]
- Yang, W.; Guo, W.; Zhang, B.; Chang, J. Synthesis of Noble Metal Nanoclusters Based on Protein and Peptide as a Template. Acta Chim. Sin. 2014, 72, 1209–1217. [Google Scholar] [CrossRef]
- Shiang, Y.-C.; Huang, C.-C.; Chen, W.-Y.; Chen, P.-C.; Chang, H.-T. Fluorescent gold and silver nanoclusters for the analysis of biopolymers and cell imaging. J. Mater. Chem. 2012, 22, 12972–12982. [Google Scholar] [CrossRef]
- Söptei, B.; Naszályi Nagy, L.; Baranyai, P.; Szabó, I.; Mező, G.; Hudecz, F.; Bóta, A. On the selection and design of proteins and peptide derivatives for the production of photoluminescent, red-emitting gold quantum clusters. Gold Bull. 2013, 46, 195–203. [Google Scholar] [CrossRef][Green Version]
- Chen, Y.; Wang, Y.; Wang, C.; Li, W.; Zhou, H.; Jiao, H.; Lin, Q.; Yu, C. Papain-directed synthesis of luminescent gold nanoclusters and the sensitive detection of Cu2+. J. Colloid Interface Sci. 2013, 396, 63–68. [Google Scholar] [CrossRef]
- Jiang, Y.K.; Xu, M.; Yadavalli, V.K. Silk Fibroin-Sheathed Conducting Polymer Wires as Organic Connectors for Biosensors. Biosensors 2019, 9, 103. [Google Scholar] [CrossRef]
- Cai, J.Y.; Wang, Q.S.; Li, X.F.; Guan, Y.P.; He, L.Y.; Yan, S.Q.; You, R.C.; Zhang, Q. Water-stable natural silk nanofibril composite films for electrical devices. Mater. Today Commun. 2020, 22, 100776. [Google Scholar] [CrossRef]
- Sasso, L.; Gerrard, J.A. Chapter 1—Self-Assembled Biological Nanofibers for Biosensor Applications. In Micro and Nanofabrication Using Self-Assembled Biological Nanostructures; Castillo-León, J., Svendsen, W.E., Eds.; William Andrew Publishing: Oxford, UK, 2015; pp. 1–20. [Google Scholar]
- Tao, H.; Kaplan, D.L.; Omenetto, F.G. Silk Materials–A Road to Sustainable High Technology. Adv. Mater. 2012, 24, 2824–2837. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.W.; Liang, H.; Li, Z.; Zhou, J.; Chen, X.; Bai, S.M.; Yang, H.H. Monodisperse phase transfer and surface bioengineering of metal nanoparticles via a silk fibroin protein corona. Nanoscale 2017, 9, 2695–2700. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Lan, J.; Wang, Y.; Xiong, Z.H.; Huang, C.Z. Luminescent golden silk and fabric through in situ chemically coating pristine-silk with gold nanoclusters. Biomaterials 2015, 36, 26–32. [Google Scholar] [CrossRef] [PubMed]
- Patil, A.C.; Bandla, A.; Liu, Y.-H.; Luo, B.; Thakor, N.V. Nontransient silk sandwich for soft, conformal bionic links. Mater. Today 2020, 32, 68–83. [Google Scholar] [CrossRef]
- Lee, M.; Jeon, H.; Kim, S. A Highly Tunable and Fully Biocompatible Silk Nanoplasmonic Optical Sensor. Nano Lett. 2015, 15, 3358–3363. [Google Scholar] [CrossRef] [PubMed]
- Benvidi, A.; Banaei, M.; Tezerjani, M.D.; Molahosseini, H.; Jahanbani, S. Impedimetric PSA aptasensor based on the use of a glassy carbon electrode modified with titanium oxide nanoparticles and silk fibroin nanofibers. Microchim. Acta 2017, 185, 50. [Google Scholar] [CrossRef] [PubMed]
- Kwon, H.; Kim, S. Chemically Tunable, Biocompatible, and Cost-Effective Metal–Insulator–Metal Resonators Using Silk Protein and Ultrathin Silver Films. ACS Photonics 2015, 2, 1675–1680. [Google Scholar] [CrossRef]
- Molinnus, D.; Drinic, A.; Iken, H.; Kröger, N.; Zinser, M.; Smeets, R.; Köpf, M.; Kopp, A.; Schöning, M.J. Towards a flexible electrochemical biosensor fabricated from biocompatible Bombyx mori silk. Biosens. Bioelectron. 2021, 183, 113204. [Google Scholar] [CrossRef]
- Promphet, N.; Phamonpon, W.; Karintrithip, W.; Rattanawaleedirojn, P.; Saengkiettiyut, K.; Boonyongmaneerat, Y.; Rodthongkum, N. Carbonization of self-reduced AuNPs on silk as wearable skin patches for non-invasive sweat urea detection. Int. J. Biol. Macromol. 2023, 242, 124757. [Google Scholar] [CrossRef]
- Sun, K.; Wang, C.; Tian, J.H.; Zhang, Z.; Zeng, N.; Yin, R.; Duan, W.X.; Hou, Q.; Zhao, Y.M.; Wu, H.K.; et al. Magnetic-Driven Broadband Epsilon-Near-Zero Materials at Radio Frequency. In Advanced Functional Materials; Wiley: New York, NY, USA, 2023. [Google Scholar]
- Wu, R.; Ma, L.; Hou, C.; Meng, Z.; Guo, W.; Yu, W.; Yu, R.; Hu, F.; Liu, X.Y. Silk Composite Electronic Textile Sensor for High Space Precision 2D Combo Temperature-Pressure Sensing. Small 2019, 15, 1901558. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Gui, Q.; Liao, S.; Jia, H.; Wang, Y. Coiled Fiber-Shaped Stretchable Thermal Sensors for Wearable Electronics. Adv. Mater. Technol. 2016, 1, 1600170. [Google Scholar] [CrossRef]
- Harada, S.; Kanao, K.; Yamamoto, Y.; Arie, T.; Akita, S.; Takei, K. Fully Printed Flexible Fingerprint-like Three-Axis Tactile and Slip Force and Temperature Sensors for Artificial Skin. ACS Nano 2014, 8, 12851–12857. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.-H.; Ma, S.-N.; Long, H.; Yuan, H.; Tang, C.Y.; Cheng, P.K.; Tsang, Y.H. Multifunctional Sensor Based on Porous Carbon Derived from Metal–Organic Frameworks for Real Time Health Monitoring. ACS Appl. Mater. Interfaces 2018, 10, 3986–3993. [Google Scholar] [CrossRef] [PubMed]
- Tao, H.; Brenckle, M.A.; Yang, M.; Zhang, J.; Liu, M.; Siebert, S.M.; Averitt, R.D.; Mannoor, M.S.; McAlpine, M.C.; Rogers, J.A.; et al. Silk-Based Conformal, Adhesive, Edible Food Sensors. Adv. Mater. 2012, 24, 1067–1072. [Google Scholar] [CrossRef] [PubMed]
- Mulyaningsih, R.D.; Pratiwi, R.; Hasanah, A.N. An Update on the Use of Natural Pigments and Pigment Nanoparticle Adducts for Metal Detection Based on Colour Response. Biosensors 2023, 13, 554. [Google Scholar] [CrossRef] [PubMed]
- Shobana, J.; Kalaivani, D.; Porchezhiyan, V.; Noorjahan, S.E. Syzgium cumini extract- a natural naked eye colorimetric sensor for selective and sensitive detection of Fe2+ in aqueous medium. Groundw. Sustain. Dev. 2023, 20, 100866. [Google Scholar] [CrossRef]
- Parizadeh, P.; Moeinpour, F.; Mohseni-Shahri, F.S. Anthocyanin-induced color changes in bacterial cellulose nanofibers for the accurate and selective detection of Cu(II) in water samples. Chemosphere 2023, 326, 138459. [Google Scholar] [CrossRef]
- Zhang, L.; Yao, L.; Zhao, F.; Yu, A.; Zhou, Y.; Wen, Q.; Wang, J.; Zheng, T.; Chen, P. Protein and Peptide-based Nanotechnology for Enhancing Stability, Bioactivity, and Delivery of Anthocyanins. Adv. Healthc. Mater. 2023, 12, 2300473. [Google Scholar] [CrossRef]
- Rahman, M.; Dutta, N.K.; Choudhury, N.R. Microroughness induced biomimetic coating for biodegradation control of magnesium. Mater. Sci. Eng. C Mater. Biol. Appl. 2021, 121, 111811. [Google Scholar] [CrossRef]
- Hu, M.M.; Hu, T.; Cheng, R.F.; Yang, J.X.; Cui, C.; Zhang, C.; Wang, X.H. MXene-coated silk-derive d carbon cloth toward flexible electrode for supercapacitor application. J. Energy Chem. 2018, 27, 161–166. [Google Scholar] [CrossRef]
- Zhou, J.; Zhang, R.; Xu, R.; Li, Y.; Tian, W.; Gao, M.; Wang, M.; Li, D.; Liang, X.; Xie, L.; et al. Super-Assembled Hierarchical Cellulose Aerogel-Gelatin Solid Electrolyte for Implantable and Biodegradable Zinc Ion Battery. Adv. Funct. Mater. 2022, 32, 2111406. [Google Scholar] [CrossRef]
- Khairy, M.; Kamal, R.; Mousa, M.A. Preparation and physical properties of conductive silk fabrics used in wearable clothes and flexible supercapacitors. J. Ind. Text. 2022, 52, 15280837221130512. [Google Scholar] [CrossRef]
- Cai, H.; Wang, Y.; Xu, M.; Cheng, L.; Liu, Z.; Li, Z.; Dai, F. Low cost, green and effective preparation of multifunctional flexible silk fabric electrode with ultra-high capacitance retention. Carbon 2022, 188, 197–208. [Google Scholar] [CrossRef]
- Badawy, I.M.; Ali, B.A.; Abbas, W.A.; Allam, N.K. Natural silk for energy and sensing applications: A review. Environ. Chem. Lett. 2021, 19, 2141–2155. [Google Scholar] [CrossRef]
- Zhang, H.; Li, Y.; Gao, G.; Song, T.Y.; Zhang, K.Y.; Yao, Y.C. Composite cathodes and modified separators based on corn-silk-based porous carbon for high performance lithium-sulfur batteries. J. Mater. Res. Technol. 2022, 19, 1590–1599. [Google Scholar] [CrossRef]
- Zhu, M.H.; Ran, Q.; Huang, H.H.; Xie, Y.F.; Zhong, M.X.; Lu, G.Y.; Bai, F.Q.; Lang, X.Y.; Jia, X.T.; Chao, D.M. Interface Reversible Electric Field Regulated by Amphoteric Charged Protein-Based Coating Toward High-Rate and Robust Zn Anode. Nano-Micro Lett. 2022, 14, 219. [Google Scholar] [CrossRef]
- Pan, P.; Zhang, M.; Cheng, Z.; Jiang, L.; Mao, J.; Ni, C.; Chen, Q.; Zeng, Y.; Hu, Y.; Fu, K. Garnet ceramic fabric-reinforced flexible composite solid electrolyte derived from silk template for safe and long-term stable All-Solid-State lithium metal batteries. Energy Storage Mater. 2022, 47, 279–287. [Google Scholar] [CrossRef]
- Zhi, J.; Li, S.; Han, M.; Chen, P. Biomolecule-guided cation regulation for dendrite-free metal anodes. Sci. Adv. 2020, 6, 1342. [Google Scholar] [CrossRef]
- Li, B.; Zhang, X.T.; Wang, T.L.; He, Z.X.; Lu, B.A.; Liang, S.Q.; Zhou, J. Interfacial Engineering Strategy for High-Performance Zn Metal Anodes. Nano-Micro Lett. 2022, 14, 6. [Google Scholar] [CrossRef]
- Han, J.S.; Zhang, L.J.; Duan, L.Q.; Liu, L.; Xiao, X.W.; Xin, X.; Li, X. Uniform Li-deposition induced by Ni-catalyzing graphitization of natural carbon materials in lithium metal batteries. ChemNanoMat 2023, 9, e202300194. [Google Scholar] [CrossRef]
- Li, X.; Yuan, L.; Liu, D.; Liao, M.; Chen, J.; Yuan, K.; Xiang, J.; Li, Z.; Huang, Y. Elevated Lithium Ion Regulation by a “Natural Silk” Modified Separator for High-Performance Lithium Metal Anode. Adv. Funct. Mater. 2021, 31, 2100537. [Google Scholar] [CrossRef]
- Lu, J.; Yang, J.; Zhang, Z.; Wang, C.; Xu, J.; Wang, T. Silk Fibroin Coating Enables Dendrite-free Zinc Anode for Long-Life Aqueous Zinc-Ion Batteries. ChemSusChem 2022, 15, e202200656. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Zhang, T.; Xin, W.; Peng, H.; Yan, Z.; Zhu, Z. Additive engineering for a hydrophilic/zincophilic polymeric layer towards dendrite-free zinc anode. Mater. Today Energy 2022, 29, 101130. [Google Scholar] [CrossRef]
- Dalwadi, S.; Goel, A.; Kapetanakis, C.; Salas-de la Cruz, D.; Hu, X. The Integration of Biopolymer-Based Materials for Energy Storage Applications: A Review. Int. J. Mol. Sci. 2023, 24, 3975. [Google Scholar] [CrossRef] [PubMed]
- Seo, Y.; Ha, H.; Cheong, J.Y.; Leem, M.; Darabi, S.; Matteini, P.; Muller, C.; Yun, T.G.; Hwang, B. Highly Reliable Yarn-Type Supercapacitor Using Conductive Silk Yarns with Multilayered Active Materials. J. Nat. Fibers 2022, 19, 835–846. [Google Scholar] [CrossRef]
- Sun, Y.; Xue, S.; Sun, J.; Li, X.; Ou, Y.; Zhu, B.; Demir, M. Silk-derived nitrogen-doped porous carbon electrodes with enhanced ionic conductivity for high-performance supercapacitors. J. Colloid Interface Sci. 2023, 645, 297–305. [Google Scholar] [CrossRef]
- Huang, W.; Zhang, A.; Liang, H.; Liu, R.; Cai, J.; Cui, L.; Liu, J. Novel fabrication of hollow and spinous NiCo2S4 nanotubes templated by natural silk for all-solid-state asymmetric supercapacitors. J. Colloid Interface Sci. 2019, 549, 140–149. [Google Scholar] [CrossRef]
- Song, P.; Tao, J.; He, X.; Sun, Y.; Shen, X.; Zhai, L.; Yuan, A.; Zhang, D.; Ji, Z.; Li, B. Silk-inspired stretchable fiber-shaped supercapacitors with ultrahigh volumetric capacitance and energy density for wearable electronics. Chem. Eng. J. 2020, 386, 124024. [Google Scholar] [CrossRef]
- Ju, Y.X.; Song, P.; Wang, P.Y.; Chen, X.X.; Chen, T.; Yao, X.H.; Zhao, W.G.; Zhang, D.Y. Cartilage structure-inspired elastic silk nanofiber network hydrogel for stretchable and high-performance supercapacitors. Int. J. Biol. Macromol. 2023, 242, 124912. [Google Scholar] [CrossRef]
- Lu, R.Q.; Sam, D.K.; Gong, S.H.; Wang, W.B.; Ge, D.D.; Liu, J.; Zhang, Y.J.; Gao, M.; Lv, X.M. Silk fibroin derived porous carbon aerogels confined hyperdispersed Rh nanoparticles to achieve electrocatalytic hydrogen evolution under high current density. Diam. Relat. Mater. 2022, 128, 109292. [Google Scholar] [CrossRef]
- Chen, T.; Peng, Y.; Qiu, M.; Yi, C.; Xu, Z. Protein-supported transition metal catalysts: Preparation, catalytic applications, and prospects. Int. J. Biol. Macromol. 2023, 230, 123206. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Liu, J.; Yang, W.; Pei, Y. Collagen and Silk Fibroin as Promising Candidates for Constructing Catalysts. Polymers 2023, 15, 375. [Google Scholar] [CrossRef] [PubMed]
- Xu, S.J.; Yong, L.; Wu, P.Y. One-Pot, Green, Rapid Synthesis of Flowerlike Gold Nanoparticles/Reduced Graphene Oxide Composite with Regenerated Silk Fibroin As Efficient Oxygen Reduction Electrocatalysts. ACS Appl. Mater. Interfaces 2013, 5, 654–662. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.T.; Wei, L.L.; Cao, C.; Zhang, F.T.; Lang, F.Z.; Wang, H.Q.; Yang, H.J.; Shen, J.Q. Salt-induced silk gel-derived N and trace Fe co-doped 3D porous carbon as an oxygen reduction catalyst in microbial fuel cells. Nanoscale 2019, 11, 13431–13439. [Google Scholar] [CrossRef]
- Wang, C.; Chen, W.; Xia, K.; Xie, N.; Wang, H.; Zhang, Y. Silk-Derived 2D Porous Carbon Nanosheets with Atomically-Dispersed Fe-Nx-C Sites for Highly Efficient Oxygen Reaction Catalysts. Small 2019, 15, 1804966. [Google Scholar] [CrossRef]
- Lu, R.; Sam, D.K.; Wang, W.; Gong, S.; Liu, J.; Durairaj, A.; Li, M.; Lv, X. Boron, nitrogen co-doped biomass-derived carbon aerogel embedded nickel-cobalt-iron nanoparticles as a promising electrocatalyst for oxygen evolution reaction. J. Colloid Interface Sci. 2022, 613, 126–135. [Google Scholar] [CrossRef] [PubMed]
- Sam, D.K.; Wang, W.; Gong, S.; Sam, E.K.; Lv, X.; Wang, J.; Liu, J. CO2 assisted synthesis of silk fibroin driven robust N-doped carbon aerogels coupled with nickel–cobalt particles as highly active electrocatalysts for HER. Int. J. Hydrog. Energy 2021, 46, 21525–21533. [Google Scholar] [CrossRef]
- Gong, S.H.; Xiao, X.X.; Wang, W.B.; Sam, D.K.; Lu, R.Q.; Xu, Y.G.; Liu, J.; Wu, C.D.; Lv, X.M. Silk fibroin-derived carbon aerogels embedded with copper nanoparticles for efficient electrocatalytic CO2-to-CO conversion. J. Colloid Interface Sci. 2021, 600, 412–420. [Google Scholar] [CrossRef]



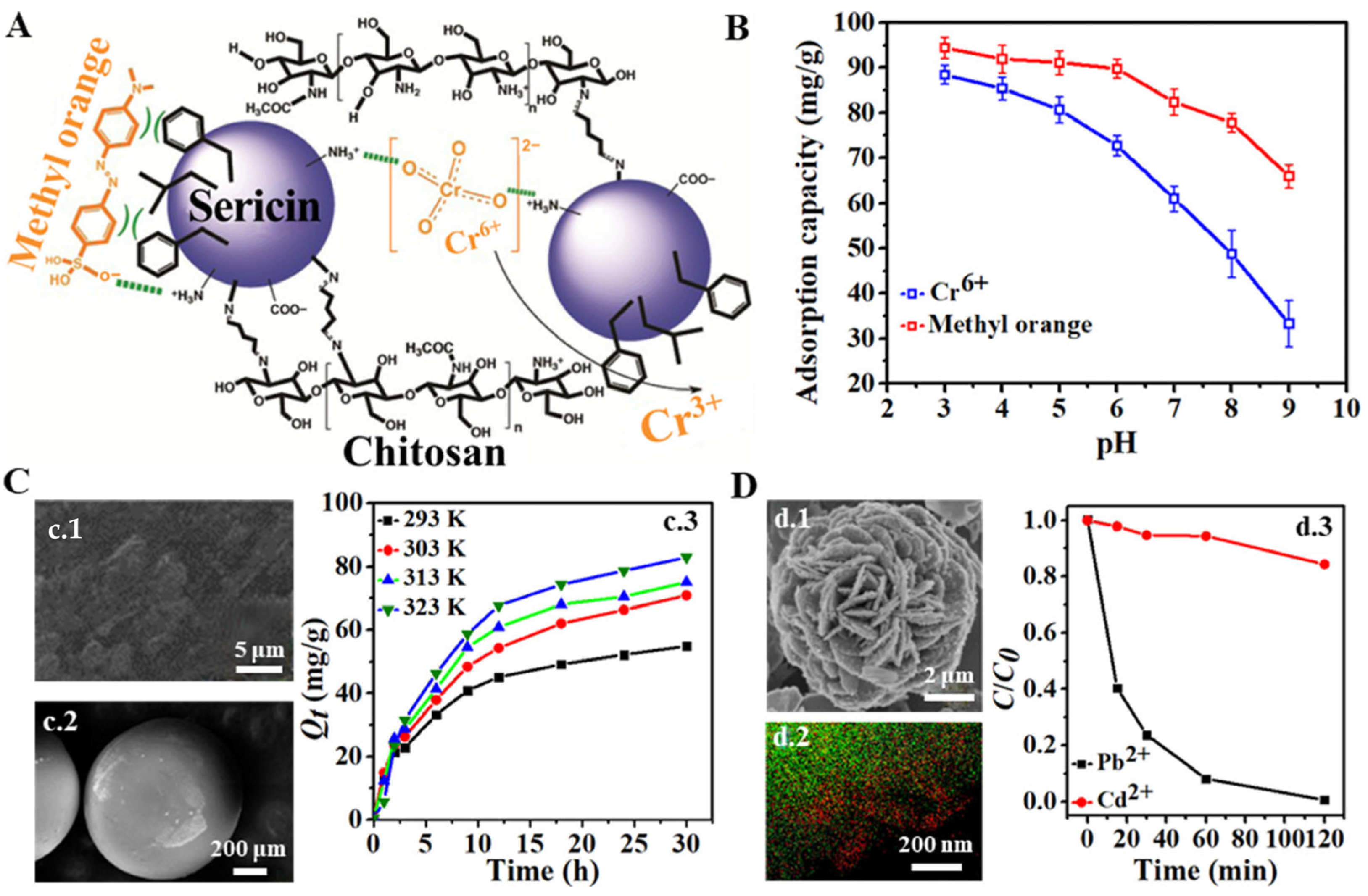
| Precursor Material | Biomaterial Form | Adsorbate | Adsorption Parameter | Adsorption Property | Ref. |
|---|---|---|---|---|---|
| Sericin, copper phosphate | Powder for batch method and film for continuous method | Pb2+, Cd2+, and Hg2+ | Sericin concentration (0.01, 0.1, and 1 mg/mL) |
| [105] |
| SF, cellulose acetate (CA) | Nanofibrous membrane | Cu2+ | CA content, Cu2+ concentration, and running time |
| [34] |
| Sericin and medium molecular weight chitosan | Powder for batch method | Cr6+ and methyl orange dye | Initial adsorbate concentration, contact time, pH, co-ion, and ionic strength |
| [36] |
| Sericin and kraft lignin | Bead | Cr6+ | Blend ratio, pH, initial Cr6+ concentration |
| [97] |
| Sericin | Microparticle | Cr6+ | pH and contact time |
| [93] |
| SF, chitosan, and starch | Film | Cr6+ | Contact time, pH, adsorbent dosage, |
| [176] |
| SF and wool keratose (WK) | Membrane | Cu2+ | - |
| [177] |
| SF and wool keratose | Mat | Cu2+ | WK/SF blend ratio and pH |
| [178] |
| SF | Powder | U6+ and Th4+ | pH, contact time, temperature |
| [179] |
| SF | Powder | Th4+ | pH, initial Th4+ concentration, SF dosage, solution volume, co-ion, retention time, and temperature |
| [180] |
| SF and nylon-6 | Nanofiber membrane | Cu2+ | pH, membrane number, flow rate, and initial Cu2+ concentration |
| [181] |
| Sericin and anthocyanin | Film (coating) | Zn2+, Al3+, and Cd2+ | Sericin dosage, adsorbate concentration, contact time, temperature, and co-ion |
| [96] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wen, Q.; Zhang, L.; Chen, Y.; Su, Y.; Yu, J.; Chen, P.; Zheng, T. Novel Applications of Silk Proteins Based on Their Interactions with Metal Ions. Sustainability 2023, 15, 16053. https://doi.org/10.3390/su152216053
Wen Q, Zhang L, Chen Y, Su Y, Yu J, Chen P, Zheng T. Novel Applications of Silk Proteins Based on Their Interactions with Metal Ions. Sustainability. 2023; 15(22):16053. https://doi.org/10.3390/su152216053
Chicago/Turabian StyleWen, Qingmei, Lei Zhang, Yilu Chen, Yi Su, Jingmou Yu, Pu Chen, and Tao Zheng. 2023. "Novel Applications of Silk Proteins Based on Their Interactions with Metal Ions" Sustainability 15, no. 22: 16053. https://doi.org/10.3390/su152216053
APA StyleWen, Q., Zhang, L., Chen, Y., Su, Y., Yu, J., Chen, P., & Zheng, T. (2023). Novel Applications of Silk Proteins Based on Their Interactions with Metal Ions. Sustainability, 15(22), 16053. https://doi.org/10.3390/su152216053






