Chemical Composition of Hydrophobic Coating Solutions and Its Impact on Carbonate Stones Protection and Preservation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.1.1. Stone Specimens
2.1.2. Hydrophobic Coatings
2.2. Methods
2.2.1. Sample Preparation
Stone Samples
Coatings
2.2.2. Analytical Methodologies and Techniques
Stone Specimens Characterisation
Coating Compatibility Assays
Hydrophobic Effectiveness
Durability Assessment
3. Results and Discussion
3.1. Characterisation of Stone Specimens
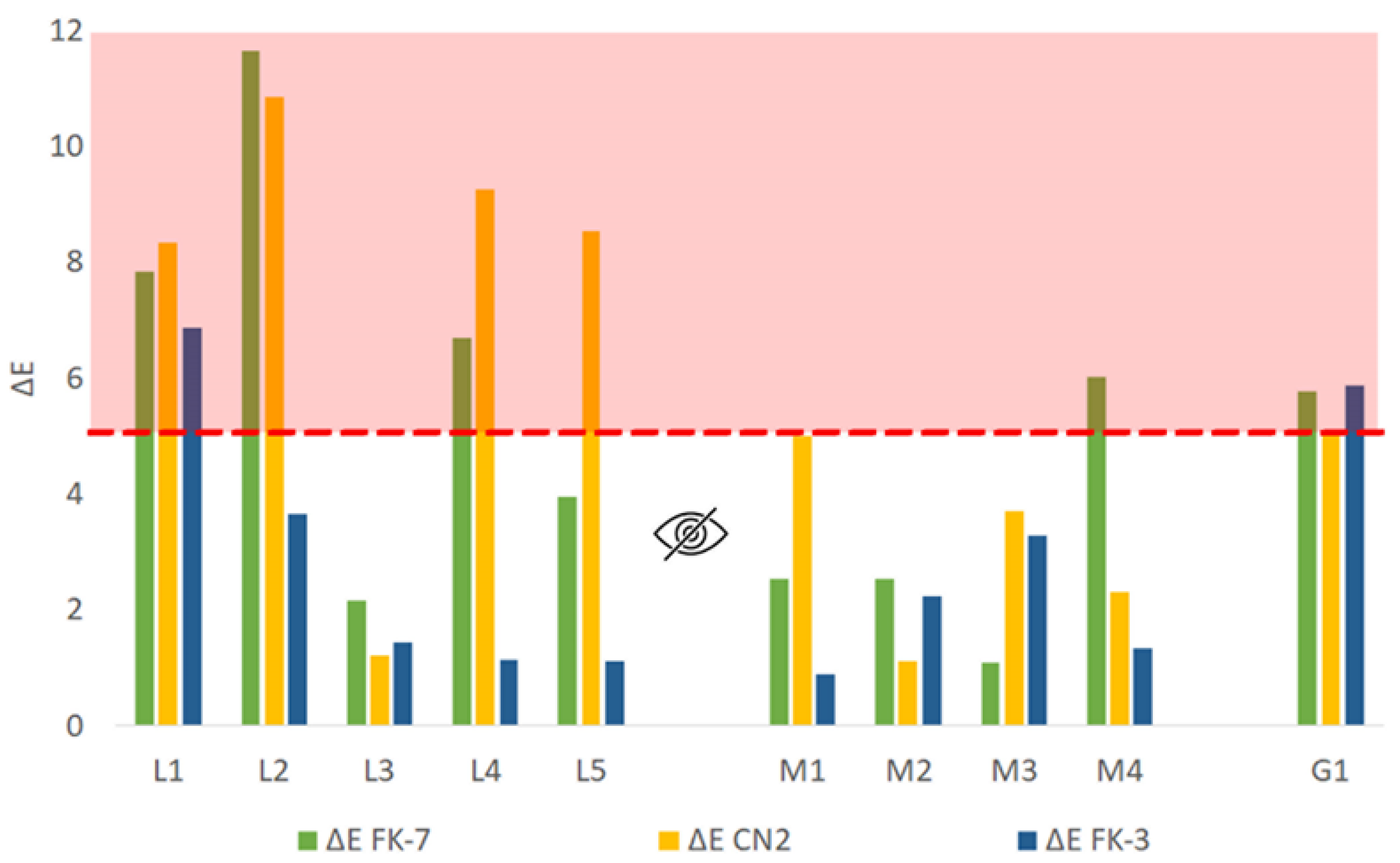
3.2. Chromatic Change Evaluation
3.2.1. Compatibility of Hydrophobic Changes
3.2.2. Durability of Hydrophobic Coatings
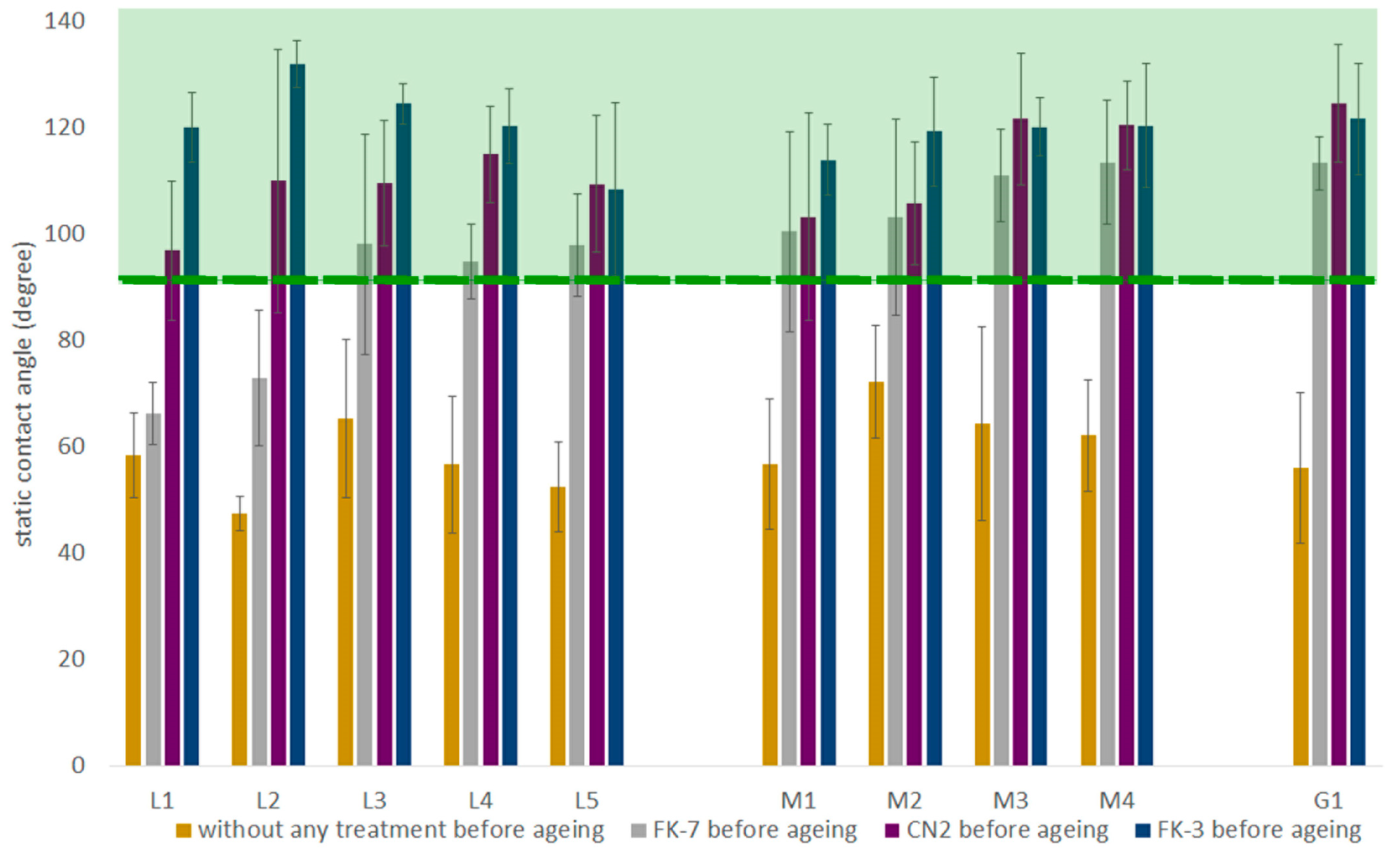
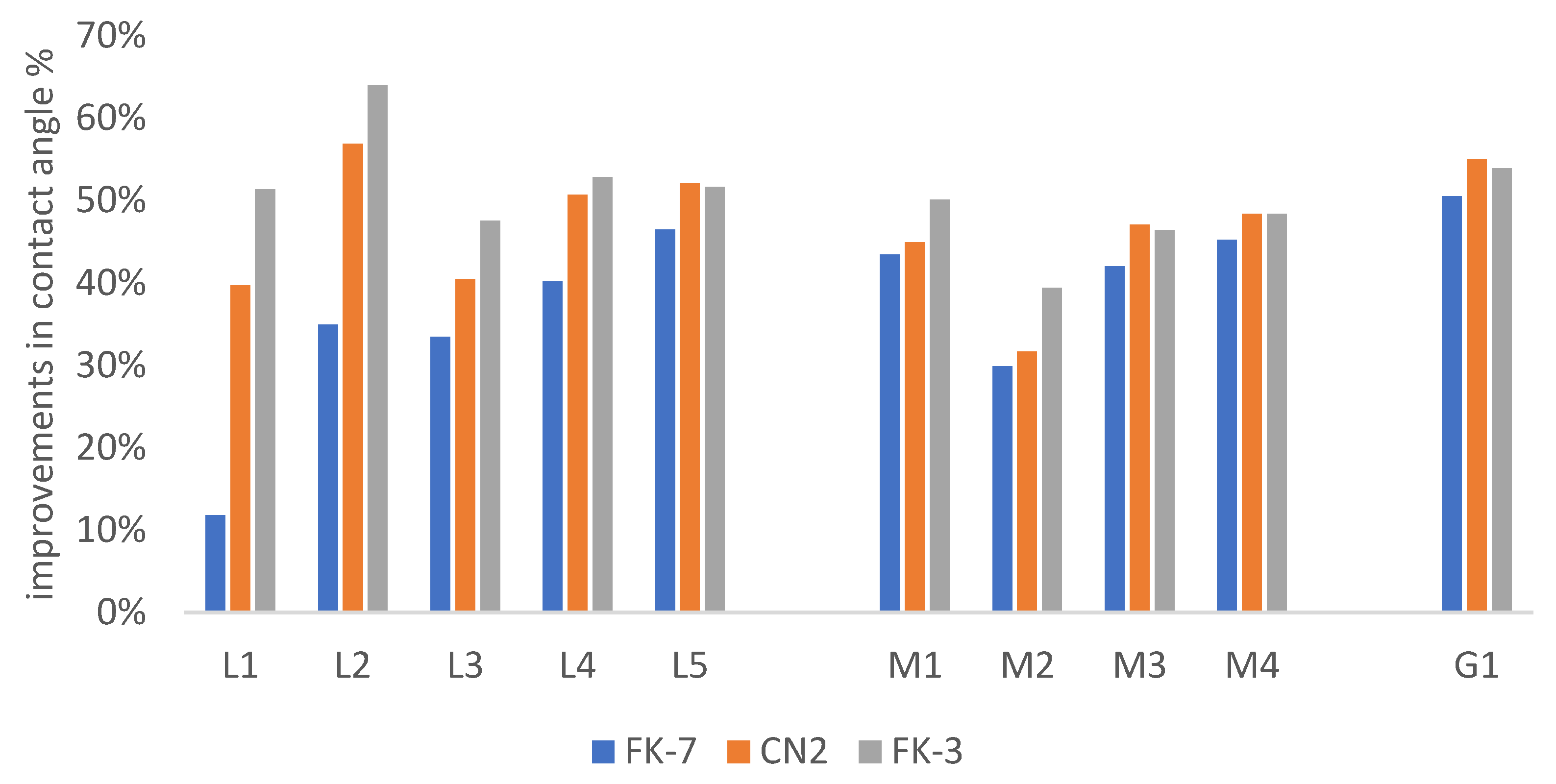
3.3. Hydrophobic Properties
3.3.1. Hydrophobic Effectiveness
3.3.2. Hydrophobics Durability
3.4. Breathability Measurements
3.4.1. Compatibility of Hydrophobic Coatings
3.4.2. Hydrophobic Coatings Performance
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Price, C.A.; Doehne, E. Stone Conservation: An Overview of Current Research, 2nd ed.; Getty Publications—Getty Conservation Institute: Los Angeles, CA, USA, 2011; ISBN 978-1-60606-046-9. [Google Scholar]
- Charola, A.E.; Price, C.A. Stone Conservation: An Overview of Current Research. J. Am. Inst. Conserv. 1998, 37, 223. [Google Scholar] [CrossRef]
- Charola, A.E. Stone Deterioration Characterization For Its Conservation. Geonomos 2016, 24, 16–20. [Google Scholar] [CrossRef]
- Lisci, C.; Pires, V.; Sitzia, F.; Mirão, J. Limestones Durability Study on Salt Crystallisation: An Integrated Approach. Case Stud. Constr. Mater. 2022, 17, e01572. [Google Scholar] [CrossRef]
- Pires, V.; Amaral, P.M.; Simão, J.A.R.; Galhano, C. Experimental Procedure for Studying the Degradation and Alteration of Limestone Slabs Applied on Exterior Cladding. Environ. Earth Sci. 2022, 81, 59. [Google Scholar] [CrossRef]
- Lisci, C.; Sitzia, F.; Pires, V.; Aniceto, M.; Mirão, J. Stone Endurance: A Comparative Analysis of Natural and Artificial Weathering on Stone Longevity. Heritage 2023, 6, 4593–4617. [Google Scholar] [CrossRef]
- Morillas, H.; Maguregui, M.; Gallego-Cartagena, E.; Marcaida, I.; Carral, N.; Madariaga, J.M. The Influence of Marine Environment on the Conservation State of Built Heritage: An Overview Study. Sci. Total Environ. 2020, 745, 140899. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.H.; Lee, M.S.; Suh, M.; Choi, S.-W. Weathering and Deterioration of Rock Properties of the Dabotap Pagoda (World Cultural Heritage), Republic of Korea. Environ. Geol. 2005, 47, 547–557. [Google Scholar] [CrossRef]
- Benavente, D.; de Jongh, M.; Cañaveras, J.C. Weathering Processes and Mechanisms Caused by Capillary Waters and Pigeon Droppings on Porous Limestones. Minerals 2020, 11, 18. [Google Scholar] [CrossRef]
- Liu, X.; Koestler, R.J.; Warscheid, T.; Katayama, Y.; Gu, J.-D. Microbial Deterioration and Sustainable Conservation of Stone Monuments and Buildings. Nat. Sustain. 2020, 3, 991–1004. [Google Scholar] [CrossRef]
- Ševčík, R.; Viani, A.; Machová, D.; Lanzafame, G.; Mancini, L.; Appavou, M.-S. Synthetic Calcium Carbonate Improves the Effectiveness of Treatments with Nanolime to Contrast Decay in Highly Porous Limestone. Sci. Rep. 2019, 9, 15278. [Google Scholar] [CrossRef]
- Steiger, M.; Charola, A.E.; Sterflinger, K. Stone in Architecture; Siegesmund, S., Snethlage, R., Eds.; Springer: Berlin/Heidelberg, Germany, 2014; ISBN 978-3-642-45154-6. [Google Scholar]
- Hosseini, M.; Karapanagiotis, I. Advanced Materials for the Conservation of Stone; Springer: Cham, Switzerland, 2018; ISBN 978-3-319-72259-7. [Google Scholar]
- Barnoos, V.; Shekofteh, A.; Oudbashi, O. Experimental Evaluation of the Consolidation Treatments of Low Porosity Limestone from the Historic Monument of the Anahita Temple of Kangavar, Iran. Archaeol. Anthropol. Sci. 2022, 14, 63. [Google Scholar] [CrossRef]
- Becerra, J.; Zaderenko, A.P.; Ortiz, R.; Karapanagiotis, I.; Ortiz, P. Comparison of the Performance of a Novel Nanolime Doped with ZnO Quantum Dots with Common Consolidants for Historical Carbonate Stone Buildings. Appl. Clay Sci. 2020, 195, 105732. [Google Scholar] [CrossRef]
- Lakhani, R.; Sharma, R.K. Strategies for the Restoration of Heritage Buildings: Material Issues; CSIR-CBRI: New Delhi, India, 2018. [Google Scholar]
- Karapanagiotis, I.; Manoudis, P.N. Superhydrophobic and Superamphiphobic Materials for the Conservation of Natural Stone: An Overview. Constr. Build. Mater. 2022, 320, 126175. [Google Scholar] [CrossRef]
- Parkin, I.P.; Palgrave, R.G. Self-Cleaning Coatings. J. Mater. Chem. 2005, 15, 1689. [Google Scholar] [CrossRef]
- Wang, Z.; Li, Y.; Jiang, L.; Qi, B.; Zhou, L. Relationship between Secondary Structure and Surface Hydrophobicity of Soybean Protein Isolate Subjected to Heat Treatment. J. Chem. 2014, 2014, 475389. [Google Scholar] [CrossRef]
- Chindaprasirt, P.; Rattanasak, U. Fabrication of Self-Cleaning Fly Ash/Polytetrafluoroethylene Material for Cement Mortar Spray-Coating. J. Clean. Prod. 2020, 264, 121748. [Google Scholar] [CrossRef]
- Liu, Q.; Zhang, B.-J. Syntheses of a Novel Nanomaterial for Conservation of Historic Stones Inspired by Nature. Mater. Lett. 2007, 61, 4976–4979. [Google Scholar] [CrossRef]
- Melo, M.; Bracci, S.; Camaiti, M.; Chiantore, O.; Piacenti, F. Photodegradation of Acrylic Resins Used in the Conservation of Stone. Polym. Degrad. Stab. 1999, 66, 23–30. [Google Scholar] [CrossRef]
- Wendler, E.; von Plehwe-Leisen, E. Water Repellent Treatment of Porous Materials. A New Edition of the WTA Leaflet. In Proceedings of the 5th International Conference on Water Repellent Treatment of Building Materials; Brussels, Belgium, 15–16 April 2008, Aedificatio Publishers: Freiburg, Germany, 2008; pp. 155–168. [Google Scholar]
- Alvarez de Buergo Ballester, M.; Fort González, R. Basic Methodology for the Assessment and Selection of Water-Repellent Treatments Applied on Carbonatic Materials. Prog. Org. Coat. 2001, 43, 258–266. [Google Scholar] [CrossRef]
- Munafò, P.; Goffredo, G.B.; Quagliarini, E. TiO2-Based Nanocoatings for Preserving Architectural Stone Surfaces: An Overview. Constr. Build. Mater. 2015, 84, 201–218. [Google Scholar] [CrossRef]
- Artesani, A.; Di Turo, F.; Zucchelli, M.; Traviglia, A. Recent Advances in Protective Coatings for Cultural Heritage–An Overview. Coatings 2020, 10, 217. [Google Scholar] [CrossRef]
- Ferrari, A.; Pini, M.; Neri, P.; Bondioli, F. Nano-TiO2 Coatings for Limestone: Which Sustainability for Cultural Heritage? Coatings 2015, 5, 232–245. [Google Scholar] [CrossRef]
- Aslanidou, D.; Karapanagiotis, I.; Lampakis, D. Waterborne Superhydrophobic and Superoleophobic Coatings for the Protection of Marble and Sandstone. Materials 2018, 11, 585. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhang, W.; Li, D.; Sun, Y.; Wang, Z.; Hou, C.; Chen, L.; Cao, Y.; Liu, Y. Mechanical and Anticorrosive Properties of Graphene/Epoxy Resin Composites Coating Prepared by in-Situ Method. Int. J. Mol. Sci. 2015, 16, 2239–2251. [Google Scholar] [CrossRef] [PubMed]
- Frascá, M.H.B.O.; Yamamoto, J.K. Ageing Tests for Dimension Stone-Experimental Studies of Granitic Rocks from Brazil. In Proceedings the 10th IAEG International Congress; Nottingham, UK, 6–10 September 2006, The Geological Society of London: London, UK, 2006. [Google Scholar]
- Zhao, J.; Gao, X.; Chen, S.; Lin, H.; Li, Z.; Lin, X. Hydrophobic or Superhydrophobic Modification of Cement-Based Materials: A Systematic Review. Compos. Part B Eng. 2022, 243, 110104. [Google Scholar] [CrossRef]
- ISO 4287:1997; Geometrical Product Specifications (GPS) Surface Texture: Profile Method—Terms, Definitions and Surface Texture Parameters. ISO: Geneva, Switzerland, 1997.
- Tang, H.; Cao, T.; Liang, X.; Wang, A.; Salley, S.O.; McAllister, J.; Ng, K.Y.S. Influence of Silicone Surface Roughness and Hydrophobicity on Adhesion and Colonization of Staphylococcus Epidermidis. J. Biomed. Mater. Res. Part A 2009, 88A, 454–463. [Google Scholar] [CrossRef] [PubMed]
- Vázquez, P.; Luque, A.; Alonso, F.J.; Grossi, C.M. Surface Changes on Crystalline Stones Due to Salt Crystallisation. Environ. Earth Sci. 2013, 69, 1237–1248. [Google Scholar] [CrossRef]
- Popov, Y.A.; Pribnow, D.F.C.; Sass, J.H.; Williams, C.F.; Burkhardt, H. Characterization of Rock Thermal Conductivity by High-Resolution Optical Scanning. Geothermics 1999, 28, 253–276. [Google Scholar] [CrossRef]
- Zuena, M.; Ruggiero, L.; Della Ventura, G.; Bemporad, E.; Ricci, M.A.; Sodo, A. Effectiveness and Compatibility of Nanoparticle Based Multifunctional Coatings on Natural and Man-Made Stones. Coatings 2021, 11, 480. [Google Scholar] [CrossRef]
- EN EN 1936; Natural Stone Test Method. Determination of Real Density and Apparent Density, and of Total and Open Porosity. British Standards Institution: London, UK, 2006.
- Li, Y.; Dong, W.; Li, H.; Li, Z. Method of Vacuum Water Absorption to Determine the Porosity of Hardened Concrete. Int. J. Struct. Civ. Eng. Res. 2015, 4, 282–286. [Google Scholar] [CrossRef]
- ASTM E96/E96M-14; Standard Test Methods for Water Vapor Transmission of Materials. ASTM International: Kansas City, MO, USA, 2014.
- Andreotti, S.; Franzoni, E.; Degli Esposti, M.; Fabbri, P. Poly(Hydroxyalkanoate)s-Based Hydrophobic Coatings for the Protection of Stone in Cultural Heritage. Materials 2018, 11, 165. [Google Scholar] [CrossRef]
- Sesana, E.; Gagnon, A.S.; Ciantelli, C.; Cassar, J.; Hughes, J.J. Climate Change Impacts on Cultural Heritage: A Literature Review. WIREs Clim. Chang. 2021, 12, e710. [Google Scholar] [CrossRef]
- EN 15886:2010; Conservation of Cultural Property—Test Methods—Colour Measurement of Surfaces. European Standard: Brussels, Belgium, 2010.
- Prieto, B.; Sanmartín, P.; Silva, B.; Martínez-Verdú, F. Measuring the Color of Granite Rocks: A Proposed Procedure. Color Res. Appl. 2010, 35, 368–375. [Google Scholar] [CrossRef]
- Law, K.-Y. Definitions for Hydrophilicity, Hydrophobicity, and Superhydrophobicity: Getting the Basics Right. J. Phys. Chem. Lett. 2014, 5, 686–688. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Elimelech, M.; Lin, S. Environmental Applications of Interfacial Materials with Special Wettability. Environ. Sci. Technol. 2016, 50, 2132–2150. [Google Scholar] [CrossRef] [PubMed]
- Facio, D.S.; Mosquera, M.J. Simple Strategy for Producing Superhydrophobic Nanocomposite Coatings In Situ on a Building Substrate. ACS Appl. Mater. Interfaces 2013, 5, 7517–7526. [Google Scholar] [CrossRef] [PubMed]
- De Ferri, L.; Lottici, P.P.; Lorenzi, A.; Montenero, A.; Salvioli-Mariani, E. Study of Silica Nanoparticles—Polysiloxane Hydrophobic Treatments for Stone-Based Monument Protection. J. Cult. Herit. 2011, 12, 356–363. [Google Scholar] [CrossRef]
- Manoudis, P.N.; Karapanagiotis, I. Modification of the Wettability of Polymer Surfaces Using Nanoparticles. Prog. Org. Coatings 2014, 77, 331–338. [Google Scholar] [CrossRef]
- EN 15802:2010; Conservation of Cultural Property—Test Methods—Determination of Static Contact Angle. European Standard: Brussels, Belgium, 2010.
- Esposito Corcione, C.; De Simone, N.; Santarelli, M.L.; Frigione, M. Protective Properties and Durability Characteristics of Experimental and Commercial Organic Coatings for the Preservation of Porous Stone. Prog. Org. Coatings 2017, 103, 193–203. [Google Scholar] [CrossRef]
- Yu, N.; Xiao, X.; Ye, Z.; Pan, G. Facile Preparation of Durable Superhydrophobic Coating with Self-Cleaning Property. Surf. Coatings Technol. 2018, 347, 199–208. [Google Scholar] [CrossRef]
- ASTM G154C7; Resistance of a Nonmetallic Material to Simulated Sunlight and Moisture Exposure. ASTM International: West Conshohocken, PA, USA, 2006.
- Lisci, C.; Sitzia, F.; Pires, V.; Mirão, J. Building Stones Durability by UVA Radiation, Moisture and Spray Accelerated Weathering. J. Build. Pathol. Rehabil. 2022, 7, 60. [Google Scholar] [CrossRef]
- Tabor, D. Mohs’s Hardness Scale—A Physical Interpretation. Proc. Phys. Soc. Sect. B 1954, 67, 249–257. [Google Scholar] [CrossRef]
- Zeng, X.; Xiao, Y.; Ji, X.; Wang, G. Mineral Identification Based on Deep Learning That Combines Image and Mohs Hardness. Minerals 2021, 11, 506. [Google Scholar] [CrossRef]
- Wahab, G.M.A.; Gouda, M.; Ibrahim, G. Study of Physical and Mechanical Properties for Some of Eastern Desert Dimension Marble and Granite Utilized in Building Decoration. Ain Shams Eng. J. 2019, 10, 907–915. [Google Scholar] [CrossRef]
- Wang, Y.; Zhou, Q.; Li, K.; Zhong, Q.; Bui, Q.B. Preparation of Ni–W–SiO2 Nanocomposite Coating and Evaluation of Its Hardness and Corrosion Resistance. Ceram. Int. 2015, 41, 79–84. [Google Scholar] [CrossRef]
- Wenzel, R.N. Resistance of Solid Surfaces to Wetting by Water. Ind. Eng. Chem. 1936, 28, 988–994. [Google Scholar] [CrossRef]
- Hu, Y.-Z.; Ma, T.-B. Tribology of Nanostructured Surfaces. In Comprehensive Nanoscience and Technology; Elsevier: Amsterdam, The Netherlands, 2011; pp. 383–418. [Google Scholar]
- Amaral, P.; Correia, A.; Lopes, L.; Rebola, P.; Pinho, A.; Carrilho Lopes, J. On the Use of Thermal Properties for Characterizing Dimension Stones. Key Eng. Mater. 2013, 548, 231–238. [Google Scholar] [CrossRef]
- Zhang, L. Aspects of Rock Permeability. Front. Struct. Civ. Eng. 2013, 7, 102–116. [Google Scholar] [CrossRef]
- Costa, M.; Rousaki, A.; Lycke, S.; Saelens, D.; Tack, P.; Sánchez, A.; Tuñón, J.; Ceprián, B.; Amate, P.; Montejo, M.; et al. Comparison of the Performance of Two Handheld XRF Instruments in the Study of Roman Tesserae from Cástulo (Linares, Spain). Eur. Phys. J. Plus 2020, 135, 647. [Google Scholar] [CrossRef]
- Gomes, E.M.C.; Fonseca, P.E. Eventos Metamórfico/Metassomáticos Tardi-Variscos Na Regiao de Alvito (Alentejo, Sul de Portugal). Cad. Do Lab. Xeolóxico Laxe Rev. Xeol. Galega E Do Hercínico Penins. 2006, 31, 67–86. [Google Scholar]
- Mavromatis, V.; Goetschl, K.E.; Grengg, C.; Konrad, F.; Purgstaller, B.; Dietzel, M. Barium Partitioning in Calcite and Aragonite as a Function of Growth Rate. Geochim. Cosmochim. Acta 2018, 237, 65–78. [Google Scholar] [CrossRef]
- Astilleros, J.M.; Pina, C.M.; Fernández-Díaz, L.; Putnis, A. The Effect of Barium on Calcite {104} Surfaces during Growth. Geochim. Cosmochim. Acta 2000, 64, 2965–2972. [Google Scholar] [CrossRef]
- Malavasi, I.; Bernagozzi, I.; Antonini, C.; Marengo, M. Assessing Durability of Superhydrophobic Surfaces. Surf. Innov. 2015, 3, 49–60. [Google Scholar] [CrossRef]
- Bayer, I.S. Superhydrophobic Coatings from Ecofriendly Materials and Processes: A Review. Adv. Mater. Interfaces 2020, 7. [Google Scholar] [CrossRef]
- Chen, K.; Zhou, S.; Yang, S.; Wu, L. Fabrication of All-Water-Based Self-Repairing Superhydrophobic Coatings Based on UV-Responsive Microcapsules. Adv. Funct. Mater. 2015, 25, 1035–1041. [Google Scholar] [CrossRef]
- Lettieri, M.; Masieri, M.; Aquaro, M.; Dilorenzo, D.; Frigione, M. Eco-Friendly Protective Coating to Extend the Life of Art-Works and Structures Made in Porous Stone Materials. Coatings 2021, 11, 1270. [Google Scholar] [CrossRef]
- Scheerer, S.; Ortega-Morales, O.; Gaylarde, C. Chapter 5 Microbial Deterioration of Stone Monuments—An Updated Overview. In Advances in Applied Microbiology; Elsevier: Amsterdam, The Netherlands, 2009; pp. 97–139. [Google Scholar]
- Roncon, R.; Borsoi, G.; Parracha, J.L.; Flores-Colen, I.; Veiga, R.; Nunes, L. Impact of Water-Repellent Products on the Moisture Transport Properties and Mould Susceptibility of External Thermal Insulation Composite Systems. Coatings 2021, 11, 554. [Google Scholar] [CrossRef]
- Castelvetro, V.; Aglietto, M.; Ciardelli, F.; Chiantore, O.; Lazzari, M.; Toniolo, L. Structure Control, Coating Properties, and Durability of Fluorinated Acrylic-Based Polymers. J. Coatings Technol. 2002, 74, 57–66. [Google Scholar] [CrossRef]
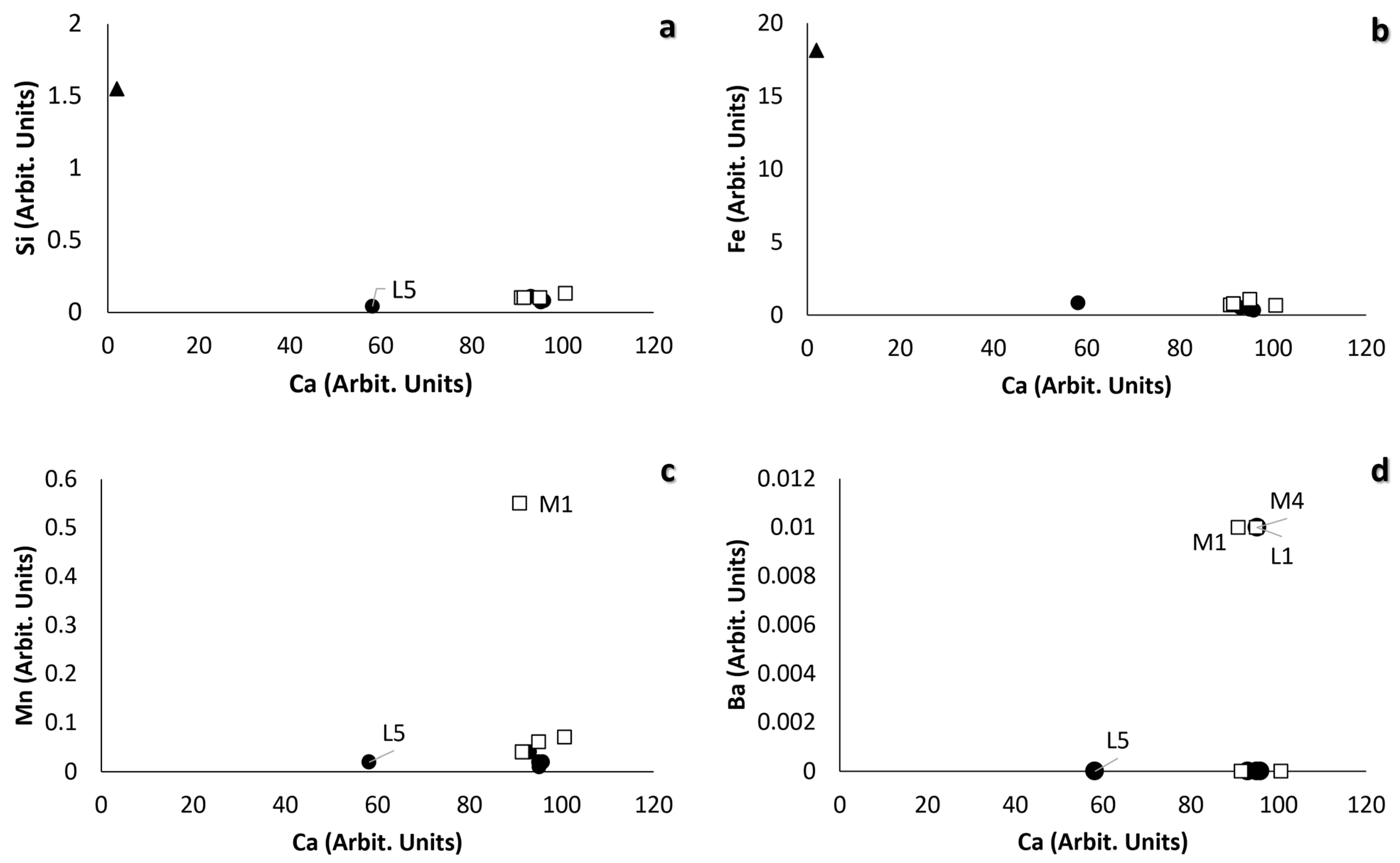




| Ref N. | Typology | Hardness (HLD) | Roughness Ra (μm) | Thermal Conductivity λ (wm−1k−1) | Open Porosity (%) | Water Vapour Permeability WVP (g m−1 s−1 Pa−1) | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| Mean | Stdev | Mean | Stdev | Mean | Stdev | Mean | Stdev | |||
| L1 | Limestone | 535.5 | 8.6 | 5.9 | 1.6 | 2.1 | 0.0 | 13.3 | 0.2 | 6.0 × 10−12 |
| L2 | Limestone | 478.8 | 21.0 | 3.5 | 1.2 | 2.0 | 0.0 | 11.3 | 0.3 | 6.3 × 10−12 |
| L3 | Limestone | 696.2 | 6.6 | 2.5 | 0.8 | 2.5 | 0.0 | 8.5 | 0.1 | 2.3 × 10−12 |
| L4 | Limestone | 570.7 | 36.8 | 3.4 | 1.0 | 2.4 | 0.0 | 0.4 | 0.0 | 8.9 × 10−12 |
| L5 | Calcitic dolomite | 665.3 | 15.2 | 2.7 | 0.3 | 2.4 | 0.0 | 1.2 | 0.1 | 7.7 × 10−12 |
| M1 | Marble | 459.2 | 54.0 | 3.3 | 0.6 | 2.5 | 0.0 | 0.2 | 0.0 | 2.2 × 10−12 |
| M2 | Marble | 533.7 | 47.2 | 2.3 | 0.2 | 2.3 | 0.0 | 0.5 | 0.0 | 4.7 × 10−12 |
| M3 | Marble | 573.7 | 33.1 | 4.3 | 0.1 | 2.5 | 0.0 | 0.2 | 0.0 | 5.1 × 10−12 |
| M4 | Marble | 554.8 | 30.1 | 4.9 | 0.9 | 2.2.6 | 0.0 | 0.3 | 0.0 | 3.8 × 10−12 |
| G1 | Granitoid | 862.0 | 25.9 | 4.0 | 1.1 | 2.5 | 0.0 | 0.7 | 0.1 | 5.5 × 10−12 |
| Ref N. | Typology | Mineral Phases | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Calcite | Dolomite | Quartz | Amphibole | Plagioclase | K-Feldspar | Illite/Muscovite | Biotite | Chlorite | ||
| L1 | Limestone | +++ | - | tr | - | - | - | - | - | - |
| L2 | Limestone | +++ | - | tr | - | - | - | - | - | - |
| L3 | Limestone | +++ | - | +/tr | - | - | - | - | - | - |
| L4 | Limestone | +++ | - | tr | - | - | - | - | - | - |
| L5 | Calcitic dolomite | ++ | +++ | - | - | - | - | - | - | - |
| M1 | Marble | +++ | - | + | +/tr | - | - | ++ | - | - |
| M2 | Marble | +++ | - | +/tr | - | +/tr | - | ++ | - | - |
| M3 | Marble | +++ | - | tr | - | - | +/tr | ++ | - | - |
| M4 | Marble | +++ | - | tr | - | +/tr | - | - | - | - |
| G1 | Granitoid | - | - | ++ | - | +++ | + | - | +/tr | ++ |
| Ref N. | Typology | Before Treatment | After Treatment | Difference |
|---|---|---|---|---|
| (g m−1 s−1 Pa−1) | (g m−1 s−1 Pa−1) | % | ||
| L1 | Limestone | 6.0 × 10−12 | 5.1 × 10−12 | −15 |
| L2 | Limestone | 6.3 × 10−12 | 5.9 × 10−12 | −6 |
| L3 | Limestone | 3.0 × 10−12 | 1.7 × 10−12 | −42 |
| L4 | Limestone | 8.9 × 10−12 | 4.6 × 10−12 | −49 |
| L5 | Calcitic dolomite | 7.7 × 10−12 | 4.0 × 10−12 | −48 |
| M1 | Marble | 2.2 × 10−12 | 1.2 × 10−12 | −46 |
| M2 | Marble | 4.7 × 10−12 | 3.9 × 10−12 | −17 |
| M3 | Marble | 5.1 × 10−12 | 3.7 × 10−12 | −29 |
| M4 | Marble | 3.8 × 10−12 | 2.2 × 10−12 | −42 |
| G1 | Granitoid | 5.5 × 10−12 | 3.5 × 10−12 | −36 |
| Ref N. | Typology | After Treatment | After Ageing | Difference |
|---|---|---|---|---|
| (g m−1 s−1 Pa−1) | (g m−1 s−1 Pa−1) | % | ||
| L1 | Limestone | 5.10 × 10−12 | 4.80 × 10−12 | −6 |
| L2 | Limestone | 5.93 × 10−12 | 5.15 × 10−12 | −13 |
| L3 | Limestone | 1.71 × 10−12 | 3.39 × 10−12 | 98 |
| L4 | Limestone | 4.59 × 10−12 | 3.36 × 10−12 | −27 |
| L5 | Calcitic dolomite | 4.04 × 10−12 | 3.52 × 10−12 | −13 |
| M1 | Marble | 1.19 × 10−12 | 2.92 × 10−12 | 145 |
| M2 | Marble | 3.90 × 10−12 | 3.04 × 10−12 | −22 |
| M3 | Marble | 3.65 × 10−12 | 3.61 × 10−12 | −1 |
| M4 | Marble | 2.16 × 10−12 | 3.35 × 10−12 | 55 |
| G1 | Granitoid | 3.52 × 10−12 | 1.97 × 10−12 | −44 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Armal, F.; Dias, L.; Mirão, J.; Pires, V.; Sitzia, F.; Martins, S.; Costa, M.; Barrulas, P. Chemical Composition of Hydrophobic Coating Solutions and Its Impact on Carbonate Stones Protection and Preservation. Sustainability 2023, 15, 16135. https://doi.org/10.3390/su152216135
Armal F, Dias L, Mirão J, Pires V, Sitzia F, Martins S, Costa M, Barrulas P. Chemical Composition of Hydrophobic Coating Solutions and Its Impact on Carbonate Stones Protection and Preservation. Sustainability. 2023; 15(22):16135. https://doi.org/10.3390/su152216135
Chicago/Turabian StyleArmal, Forough, Luís Dias, José Mirão, Vera Pires, Fabio Sitzia, Sérgio Martins, Mafalda Costa, and Pedro Barrulas. 2023. "Chemical Composition of Hydrophobic Coating Solutions and Its Impact on Carbonate Stones Protection and Preservation" Sustainability 15, no. 22: 16135. https://doi.org/10.3390/su152216135
APA StyleArmal, F., Dias, L., Mirão, J., Pires, V., Sitzia, F., Martins, S., Costa, M., & Barrulas, P. (2023). Chemical Composition of Hydrophobic Coating Solutions and Its Impact on Carbonate Stones Protection and Preservation. Sustainability, 15(22), 16135. https://doi.org/10.3390/su152216135










