First Report of Microplastic Ingestion in Edible Fish along Moroccan Mediterranean Coasts
Abstract
:1. Introduction
2. Materials and Methods
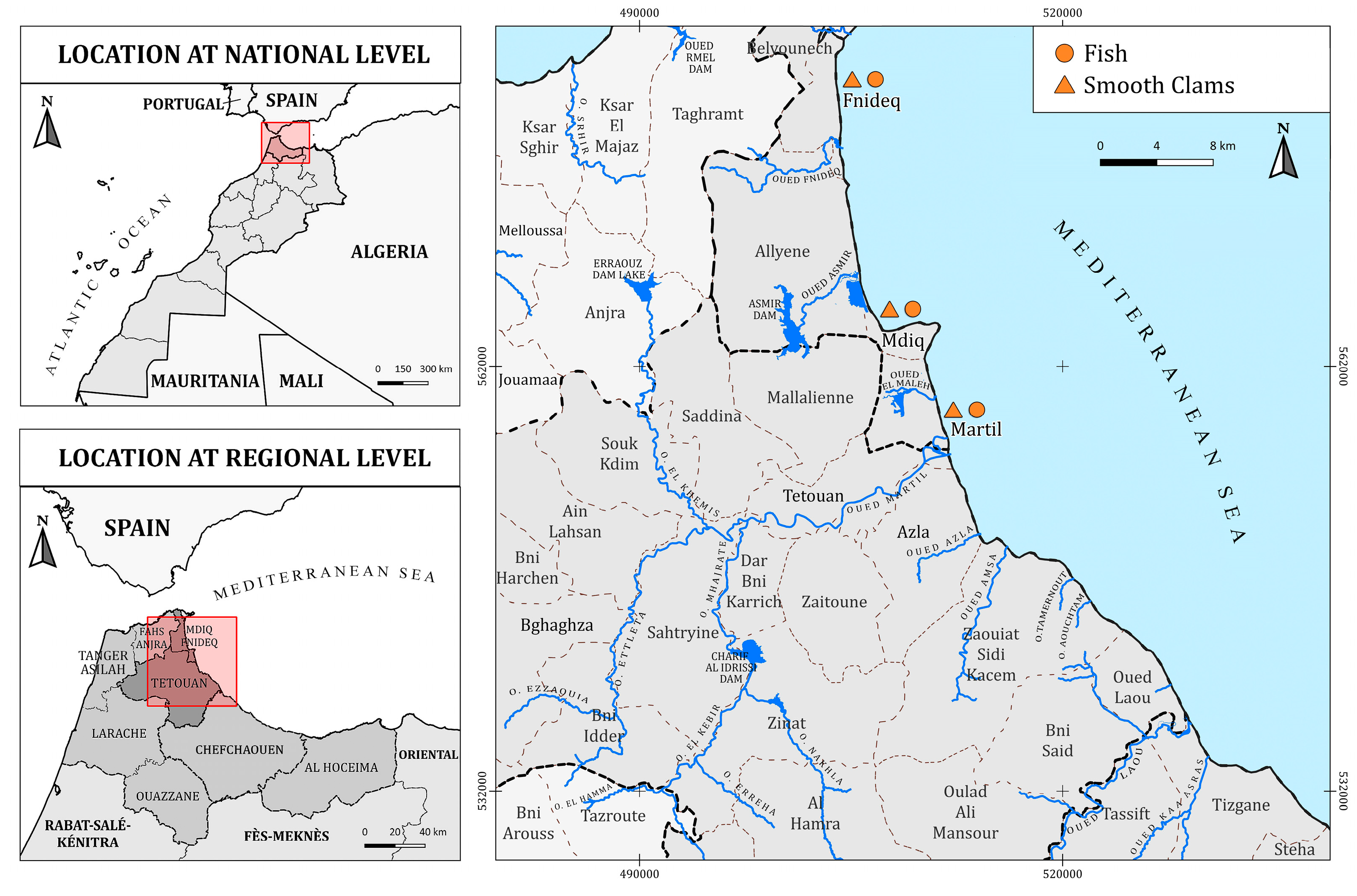
2.1. Study Area and Samplings
2.2. Microplastic Isolation
2.3. Quality Control and Preventing Contamination
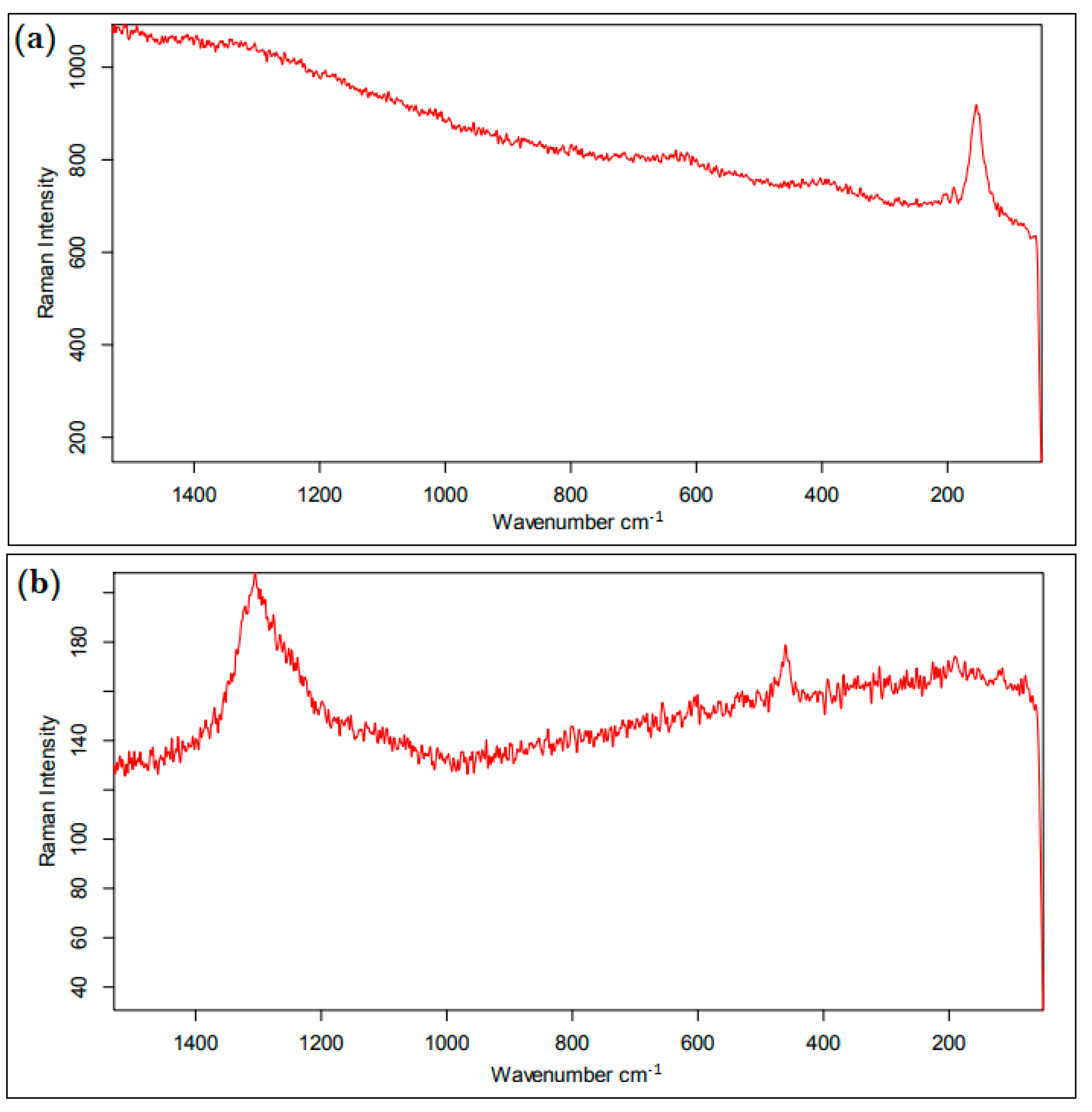
2.4. Polymer Identification
2.5. Polymer Hazard Index (PHI)
2.6. Data Analysis
3. Results
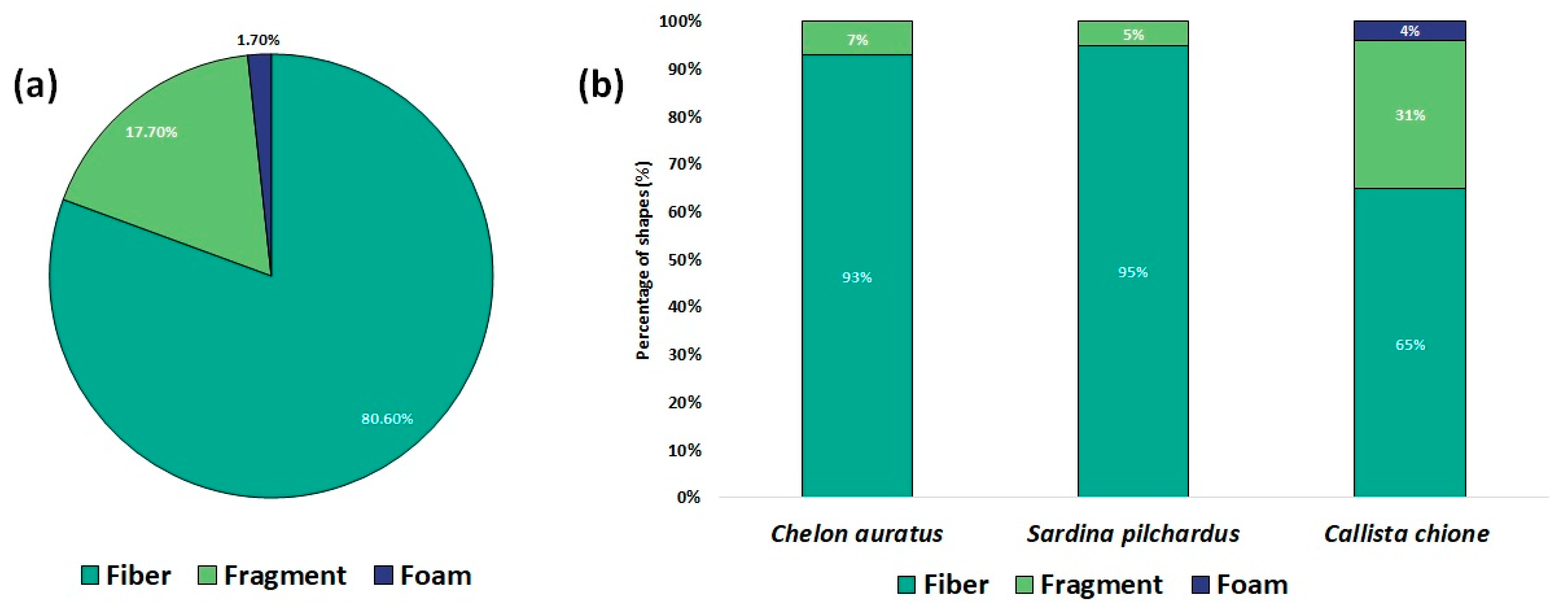
3.1. MPs Abundance
3.2. Polymer Composition and Risk Assessment
4. Discussion
| Sampling Area | Species | N | FO (%) | N Items/Individual | Polymer | Identification Method | References |
|---|---|---|---|---|---|---|---|
| Eastern Ionian Sea | Chelon auratus | 20 | 95 | 9.5 | - | - | Anastasopoulou et al. [57] |
| Northern Levant Sea | Chelon auratus | 39 | 36 | 3 | PE, PP | FT-IR | Güven et al. [51] |
| Northern Tunisia | Chelon auratus | 10 | - | 65.3 | PP, PE | FT-IR | Abidli et al. [53] |
| Northern Adriatic Sea | Sardina pilchardus | 10 | 25 | 1.4 | PVC, PS, PP | FT-IR | Avio et al. [60] |
| Alboran Sea; Northern Spain; Gulf of Lion | Sardina pilchardus | 105 | 33.3 | 0–0.5 | PET, PA, PE, cellophane, cotton, wool | FTIR | Compa et al. [61] |
| Northern Adriatic Sea | Sardina pilchardus | 99 | 19 | 1.8 | PE, PET, PS, PVC, Nylon | FT-IR | Avio et al. [62] |
| Northern Adriatic Sea | Sardina pilchardus | 80 | 96 | - | - | - | Renzi et al. [63] |
| Northern Adriatic Sea | Sardina pilchardus | 30 | 37 | 0.9 | - | - | Anastasopoulou et al. [57] |
| Eastern Ionian Sea | Sardina pilchardus | 36 | 47 | 0.8 | - | - | Anastasopoulou et al. [57] |
| Northern Levant Sea | Sardina pilchardus | 7 | - | 2.7 | PE, PP | FT-IR | Güven et al. [51] |
| Northern Spain | Sardina pilchardus | 15 | 87 | 1.8 | PE, PP | µRaman | Filgueiras et al. [54] |
| Northern Spain | Sardina pilchardus | 7 | - | 0.1 | - | - | Rios-Fuster et al. [47] |
| Northern Spain; Gulf of Lion | Sardina pilchardus | 104 | 58 | 1.1–1.8 | - | - | Pennino et al. [64] |
| Gulf of Lion | Sardina pilchardus | 13 | - | 0.5 | PE | FT-IR | Constant et al. [23] |
| North Aegean Sea | Sardina pilchardus | - | - | 2.5 | - | - | Kermenidou et al. [55] |
| Southern Tyrrhenian Sea | Sardina pilchardus | 19 | - | 0.5 | PP, PAN, PE, PA | Raman, FT-IR | Savoca et al. [56] |
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A
References
- Jambeck, J.R.; Geyer, R.; Wilcox, C.; Siegler, T.R.; Perryman, M.; Andrady, A.; Narayan, R.; Law, K.L. Plastic waste inputs from land into the ocean. Science 2015, 347, 768–771. [Google Scholar] [CrossRef] [PubMed]
- De-la-Torre, G.E.; Dioses-Salinas, D.C.; Pizarro-Ortega, C.I.; Santillàn, L. New plastic formations in the Anthropocene. Sci. Total Environ. 2021, 754, 142216. [Google Scholar] [CrossRef] [PubMed]
- Lau, W.W.Y.; Shiran, Y.; Bailey, R.M.; Cook, E.; Stuchtey, M.R.; Koskella, J.; Velis, C.A.; Godfrey, L.; Boucher, J.; Murphy, M.B.; et al. Evaluating scenarios toward zero plastic pollution. Science 2020, 369, 1455–1461. [Google Scholar] [CrossRef] [PubMed]
- MacArthur, D.E.; Waughray, D.; Stuchtey, M.R. The New Plastics Economy, Rethinking the Future of Plastics; World Economic Forum: Geneva, Switzerland; Ellen MacArthur Foundation: Isle of Wight, UK, 2016. [Google Scholar]
- Krelling, A.P.; Williams, A.T.; Turra, A. Differences in perception and reaction of tourist groups to beach marine debris that can influence a loss of tourism revenue in coastal areas. Mar. Policy 2017, 85, 87–99. [Google Scholar] [CrossRef]
- Beaumont, N.J.; Aanesen, M.; Austen, M.C.; Börger, T.; Clark, J.R.; Cole, M.; Hooper, T.; Lindeque, P.K.; Pascoe, C.; Wyles, K.J. Global ecological, social and economic impacts of marine plastic. Mar. Pollut. Bull. 2019, 142, 189–195. [Google Scholar] [CrossRef] [PubMed]
- Kühn, S.; van Franeker, J.A. Quantitative overview of marine debris ingested by marine megafauna. Mar. Pollut. Bull. 2020, 151, 110858. [Google Scholar] [CrossRef]
- Roman, L.; Schuyler, Q.; Wilcox, C.; Hardesty, B.D. Plastic pollution is killing marine megafauna, but how do we prioritize policies to reduce mortality? Conserv. Lett. 2021, 14, e12781. [Google Scholar] [CrossRef]
- Browne, M.A.; Crump, P.; Niven, S.J.; Teuten, E.; Tonkin, A.; Galloway, T.; Thompson, R. Accumulation of microplastic on shorelines woldwide: Sources and sinks. Environ. Sci. Technol. 2011, 45, 9175–9179. [Google Scholar] [CrossRef]
- Derraik, J.G.B. The Pollution of the Marine Environment by Plastic Debris: A Review. Mar. Pollut. Bull. 2002, 44, 842–852. [Google Scholar] [CrossRef]
- Bottari, T.; Mancuso, M.; Pedà, C.; De Domenico, F.; Laface, F.; Schirinzi, G.F.; Battaglia, P.; Consoli, P.; Spanò, N.; Greco, S.; et al. Microplastics in the bogue, Boops boops: A snapshot of the past from the southern Tyrrhenian Sea. J. Hazard. Mater. 2022, 424, 127669. [Google Scholar] [CrossRef]
- Rochman, C.M.; Kurobe, T.; Flores, I.; Teh, S.J. Early warning signs of endocrine disruption in adult fish from the ingestion of polyethylene with and without sorbed chemical pollutants from the marine environment. Sci. Total Environ. 2014, 493, 656–661. [Google Scholar] [CrossRef]
- Besseling, E.; Wang, B.; Lürling, M.; Koelmans, A.A. Nanoplastic affects growth of S. obliquus and reproduction of D. magna. Environ. Sci. Technol. 2014, 48, 12336–12343. [Google Scholar] [CrossRef] [PubMed]
- Welden, N.A.C.; Cowie, P.R. Long-term microplastic retention causes reduced body condition in the langoustine, Nephrops norvegicus. Environ. Pollut. 2016, 218, 895–900. [Google Scholar] [CrossRef] [PubMed]
- Contino, M.; Ferruggia, G.; Pecoraro, R.; Scalisi, E.M.; Cavallaro, G.; Bonaccorso, C.; Fortuna, C.G.; Salvaggio, A.; Capparucci, F.; Bottari, T.; et al. Uptake routes and biodistribution of polystyrene nanoplastics on zebrafish larvae and toxic effects on development. Fishes 2023, 8, 168. [Google Scholar] [CrossRef]
- Lei, L.; Wu, S.; Lu, S.; Liu, M.; Song, Y.; Fu, Z.; Shi, H.; Raley-Susman, K.M.; He, D. Microplastic particles cause intestinal damage and other adverse effects in zebrafish Danio rerio and nematode Caenorhabditis elegans. Sci. Total Environ. 2018, 619–620, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Mallik, A.; Xavier, K.M.; Naidu, B.C.; Nayak, B.B. Ecotoxicological and Physiological Risks of Microplastics on Fish and Their 511 Possible Mitigation Measures. Sci. Total Environ. 2021, 779, 146433. [Google Scholar] [CrossRef]
- Amelia, T.S.M.; Khalik, W.M.; Ong, M.C.; Shao, Y.T.; Pan, H.; Bhubalan, K. Marine microplastics as vectors of major ocean pollutants and its hazards to the marine ecosystem and humans. Prog. Earth Planet Sci. 2021, 8, 12. [Google Scholar] [CrossRef]
- Cózar, A.; Sanz-Martín, M.; Martí, E.; González-Gordillo, J.I.; Ubeda, B.Á.; Gálvez, J.; Irigoien, X.; Duarte, C.M. Plastic accumulation in the Mediterranean Sea. PLoS ONE 2015, 10, e0121762. [Google Scholar] [CrossRef]
- Suaria, G.; Avio, C.G.; Mineo, A.; Lattin, G.L.; Magaldi, M.G.; Belmonte, G.; Moore, C.J.; Regoli, F.; Aliani, S. The Mediterranean plastic soup: Synthetic polymers in Mediterranean surface waters. Sci. Rep. 2020, 6, 37551. [Google Scholar] [CrossRef]
- Eriksen, M.; Lebreton, L.C.M.; Carson, H.S.; Thiel, M.; Moore, C.J.; Borerro, J.C.; Galgani, F.; Ryan, P.G.; Reisser, J. Plastic pollution in the world’s oceans: More than 5 trillion plastic pieces weighing over 250,000 tons afloat at sea. PLoS ONE 2014, 9, e111913. [Google Scholar] [CrossRef]
- Fossi, M.C.; Pedà, C.; Compa, M.; Tsangaris, C.; Alomar, C.; Claro, F.; Ioakeimidis, C.; Galgani, F.; Hema, T.; Deudero, S.; et al. Bioindicators for monitoring marine litter ingestion and its impacts on Mediterranean biodiversity. Environ. Pollut. 2018, 237, 1023–1040. [Google Scholar] [CrossRef] [PubMed]
- Constant, M.; Reynaud, M.; Weiss, L.; Ludwig, W.; Kerhervé, P. Ingested Microplastics in 18 Local Fish Species from the Northwestern Mediterranean Sea. Microplastics 2021, 1, 186–197. [Google Scholar] [CrossRef]
- Alshawafi, A.; Analla, M.; Alwashali, E.; Aksissou, M. Assessment of marine debris on the coastal wetland of Martil in the North-East of Morocco. Mar. Pollut. Bull. 2017, 117, 302–310. [Google Scholar] [CrossRef] [PubMed]
- Loulad, S.; Houssa, R.; El Ouamari, N.; Rhinane, H. Quantity and spatial distribution of seafloor marine debris in the Moroccan Mediterranean Sea. Mar. Pollut. Bull. 2019, 139, 163–173. [Google Scholar] [CrossRef] [PubMed]
- Bouzekry, A.; Mghili, B.; Aksissou, M. Addressing the challenge of marine plastic litter in the Moroccan Mediterranean: A citizen science project with schoolchildren. Mar. Pollut. Bull. 2022, 184, 114167. [Google Scholar] [CrossRef] [PubMed]
- Mghili, B.; Keznine, M.; Hasni, S.; Aksissou, M. Abundance, composition and sources of benthic marine litter trawled-up in the fishing grounds on the Moroccan Mediterranean coast. Reg. Stud. Mar. Sci. 2023, 63, 103002. [Google Scholar] [CrossRef]
- Mghili, B.; Keznine, M.; Analla, M.; Aksissou, M. The impacts of abandoned, discarded and lost fishing gear on marine biodiversity in Morocco. Ocean Coast Manag. 2023, 239, 106593. [Google Scholar] [CrossRef]
- Mghili, B.; Lamine, I.; Bouzekry, A.; Gunasekaran, K.; Aksissou, M. Cigarette butt pollution in popular beaches of Morocco: Abundance, distribution, and mitigation measures. Mar. Pollut. Bull. 2023, 195, 115530. [Google Scholar] [CrossRef]
- MPM. Rapport d’activité Ministère des Pêches Maritimes Maroc; Ministère de l’Agriculture, de la Pêche Maritime, du Développement Rural et des Eaux et Forêts: Rabat, Morocco, 2018. [Google Scholar]
- Chen, C.-T.; Carlotti, F.; Harmelin-Vivien, M.; Bănaru, D. Temporal variation in prey selection by adult European sardine (Sardina pilchardus) in the NW Mediterranean Sea. Prog. Oceanogr. 2021, 196, 102617. [Google Scholar] [CrossRef]
- Costalago, D.; Palomera, I. Feeding of European pilchard (Sardina pilchardus) in the northwestern Mediterranean: From late larvae to adults. Sci. Mar. 2014, 78, 41–54. [Google Scholar] [CrossRef]
- Kesiktaş, M.; Yemişken, E.; Yildiz, T.; Eryilmaz, L. Age, growth and reproduction of the golden grey mullet, Chelon auratus (Risso, 1810) in the Golden Horn Estuary, Istanbul. J. Mar. Biolog. Assoc. U. K. 2020, 100, 989–995. [Google Scholar] [CrossRef]
- Bouzaidi, H.; Maatouk, M.; El Moumni, B.; Haroufi, O.; Saber, M.A.; AbouElmaaty, E.E.; Daoudi, M. Population structure, age and growth of Callista chione (Bivalvia: Veneridae) in Martil Coast of the western Mediterranean. Reg. Stud. Mar. Sci. 2021, 48, 101996. [Google Scholar] [CrossRef]
- Baeta, M.; Solís, M.; Ramon, M.; Ballesteros, M. Effects of fishing closure and mechanized clam dredging on a Callista chione bed in the western Mediterranean Sea. Reg. Stud. Mar. Sci. 2021, 48, 102063. [Google Scholar] [CrossRef]
- Dehaut, A.; Hermabessiere, L.; Duflos, G. Current frontiers and recommendations for the study of microplastics in seafood. Trac. Trends Anal. Chem. 2019, 116, 346–359. [Google Scholar] [CrossRef]
- Lithner, D.; Larsson, Å.; Dave, G. Environmental and health hazard ranking and assessment of plastic polymers based on chemical composition. Sci. Total Environ. 2011, 409, 3309–3324. [Google Scholar] [CrossRef] [PubMed]
- Nithin, A.; Sundaramanickam, A.; Iswarya, P.; Babu, O.G. Hazard index of microplastics contamination in various fishes collected off Parangipettai, Southeast coast of India. Chemosphere 2022, 307, 136037. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Xu, J.; Zhu, L.; Peng, G.; Jabeen, K.; Wang, X.; Li, D. Seasonal distributions of microplastics and estimation of the microplastic load ingested by wild caught fish in the East China Sea. J. Hazard. Mater. 2021, 419, 126456. [Google Scholar] [CrossRef]
- Capo, X.; Rubio, M.; Solomando, A.; Alomar, C.; Compa, M.; Sureda, A.; Deudero, S. Microplastic intake and enzymatic responses in Mytilus galloprovincialis reared at the vicinities of an aquaculture station. Chemosphere 2021, 280, 130575. [Google Scholar] [CrossRef]
- Nalbone, L.; Cincotta, F.; Giarratana, F.; Ziino, G.; Panebianco, A. Microplastics in fresh and processed mussels sampled from fish shops and large retail chains in Italy. Food Control 2021, 125, 108003. [Google Scholar] [CrossRef]
- Atici, A.A. The first evidence of microplastic uptake in natural freshwater mussel, Unio stevenianus from Karasu River, Turkey. Biomarkers 2022, 27, 118–126. [Google Scholar] [CrossRef]
- Naidu, B.C.; Xavier, K.A.M.; Shukla, S.P.; Jaiswar, A.K.; Nayak, B.B. Comparative study on the microplastics abundance, characteristics, and possible sources in yellow clams of different demographic regions of the northwest coast of India. J. Hazard. Mater. Lett. 2022, 3, 100051. [Google Scholar] [CrossRef]
- Wu, Y.; Yang, J.; Li, Z.; He, H.; Wang, Y.; Wu, H.; Xie, L.; Chen, D.; Wang, L. How does bivalve size influence microplastics accumulation? Environ. Res. 2022, 214, 113847. [Google Scholar] [CrossRef] [PubMed]
- Abbasi, S.; Soltani, N.; Keshavarzi, B.; Moore, F.; Turner, A.; Hassanaghaei, M. Microplastics in different tissues of fish and prawn from the Musa Estuary, Persian Gulf. Chemosphere 2018, 205, 80–87. [Google Scholar] [CrossRef] [PubMed]
- Thanh, N.D.; Thu, V.A.; Thom, D.T.; Manh, D.T.; Tuan, P.M.; Ngo, V.D.; Van Tuyen, T. Investigation of microplastics existence in mussel (Perna viridis) from Ha Long bay, Viet Nam. Vietnam J. Sci. Technol. 2022, 60, 1–10. [Google Scholar]
- Rios-Fuster, B.; Alomar, C.; Compa, M.; Guijarro, B.; Deudero, S. Anthropogenic particles ingestion in fish species from two areas of the western Mediterranean Sea. Mar. Pollut. Bull. 2019, 144, 325–333. [Google Scholar] [CrossRef] [PubMed]
- Wakkaf, T.; El Zrelli, R.; Kedzierski, M.; Balti, R.; Shaiek, M.; Mansour, L.; Tlig-Zouari, S.; Bruzaud, S.; Rabaoui, L. Microplastics in edible mussels from a southern Mediterranean lagoon: Preliminary results on seawater-mussel transfer and implications for environmental protection and seafood safety. Mar. Pollut. Bull. 2020, 158, 111355. [Google Scholar] [CrossRef] [PubMed]
- Horton, A.A.; Jürgens, M.D.; Lahive, E.; van Bodegom, P.M.; Vijver, M.G. The influence of exposure and physiology on microplastic ingestion by the freshwater fish Rutilus rutilus (roach) in the River Thames, UK. Environ. Pollut. 2018, 236, 188–194. [Google Scholar] [CrossRef]
- Miller, M.E.; Hamann, M.; Kroon, F.J. Bioaccumulation and biomagnification of microplastics in marine organisms: A review and meta-analysis of current data. PLoS ONE 2020, 15, e0240792. [Google Scholar] [CrossRef]
- Güven, O.; Gökdağ, K.; Jovanović, B.; Kıdeyş, A.E. Microplastic litter composition of the Turkish territorial waters of the Mediterranean Sea, and its occurrence in the gastrointestinal tract of fish. Environ. Pollut. 2017, 223, 286–294. [Google Scholar] [CrossRef]
- Sayed, A.E.H.; Hamed, M.; Badrey, A.E.A.; Ismail, R.F.; Osman, Y.A.A.; Osman, A.G.M.; Soliman, H.A.M. Microplastic distribution, abundance, and composition in the sediments, water, and fishes of the Red and Mediterranean seas, Egypt. Mar. Pollut. Bull. 2021, 173, 112966. [Google Scholar] [CrossRef]
- Abidli, S.; Akkari, N.; Lahbib, Y.; Trigui El Menif, N. First evaluation of microplastics in two commercial fish species from the lagoons of Bizerte and Ghar El Melh (Northern Tunisia). Reg. Stud. Mar. Sci. 2021, 41, 101581. [Google Scholar] [CrossRef]
- Filgueiras, A.V.; Preciado, I.; Cartón, A.; Gago, J. Microplastic ingestion by pelagic and benthic fish and diet composition: A case study in the NW Iberian shelf. Mar. Pollut. Bull. 2020, 160, 111623. [Google Scholar] [CrossRef]
- Kermenidou, M.; Frydas, I.S.; Moschoula, E.; Kousis, D.; Christofilos, D.; Karakitsios, S.; Sarigiannis, D. Quantification and characterization of microplastics in the Thermaic Gulf, in the North Aegean Sea. Sci. Total Environ. 2023, 892, 164299. [Google Scholar] [CrossRef] [PubMed]
- Savoca, S.; Bottari, T.; Fazio, E.; Bonsignore, M.; Mancuso, M.; Luna, G.M.; Romeo, T.; D’Urso, L.; Capillo, G.; Panarello, G.; et al. Plastics occurrence in juveniles of Engraulis encrasicolus and Sardina pilchardus in the Southern Tyrrhenian Sea. Sci. Total Environ. 2020, 718, 137457. [Google Scholar] [CrossRef]
- Anastasopoulou, A.; Viršek, M.K.; Varezic, D.B.; Digka, N.; Fortibuoni, T.; Koren, S.; Mandic, M.; Mytilineou, C.; Pesic, A.; Ronchi, F.; et al. Assessment on marine litter ingested by fish in the Adriatic and NE Ionian Sea macro-region (Mediterranean). Mar. Pollut. Bull. 2018, 133, 841–851. [Google Scholar] [CrossRef]
- Trani, A.; Mezzapesa, G.; Piscitelli, L.; Mondelli, D.; Nardelli, L.; Belmonte, G.; Toso, A.; Piraino, S.; Panti, C.; Baini, M.; et al. Microplastics in water surface and in the gastrointestinal tract of target marine organisms in Salento coastal seas (Italy, Southern Puglia). Environ. Pollut. 2023, 316, 120702. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Guerrero-Hernández, M.J.; González-Fernández, D.; Sendra, M.; Ramos, F.; Yeste, M.P.; González-Ortegón, E. Contamination from microplastics and other anthropogenic particles in the digestive tracts of the commercial species Engraulis encrasicolus and Sardina pilchardus. Sci. Total Environ. 2023, 860, 160451. [Google Scholar] [CrossRef] [PubMed]
- Avio, C.G.; Pittura, L.; d’Errico, G.; Abel, S.; Amorello, S.; Marino, G.; Gorbi, S.; Regoli, F. Distribution and characterization of microplastic particles and textile microfibers in Adriatic food webs: General insights for biomonitoring strategies. Environ. Pollut. 2020, 258, 113766. [Google Scholar] [CrossRef]
- Compa, M.; Ventero, A.; Iglesias, M.; Deudero, S. Ingestion of microplastics and natural fibres in Sardina pilchardus (Walbaum, 1792) and Engraulis encrasicolus (Linnaeus, 1758) along the Spanish Mediterranean coast. Mar. Pollut. Bull. 2018, 128, 89–96. [Google Scholar] [CrossRef]
- Avio, C.G.; Gorbi, S.; Regoli, F. Experimental development of a new protocol for extraction and characterization of microplastics in fish tissues: First observations in commercial species from Adriatic Sea. Mar. Environ. Res. 2015, 111, 18–26. [Google Scholar] [CrossRef]
- Renzi, M.; Specchiulli, A.; Blašković, A.; Manzo, C.; Mancinelli, G.; Cilenti, L. Marine litter in stomach content of small pelagic fishes from the Adriatic Sea: Sardines (Sardina pilchardus) and anchovies (Engraulis encrasicolus). Environ. Sci. Pollut. Res. 2019, 26, 2771–2781. [Google Scholar] [CrossRef] [PubMed]
- Pennino, M.G.; Bachiller, E.; Lloret-Lloret, E.; Albo-Puigserver, M.; Esteban, A.; Jadaud, A.; Bellido, J.M.; Coll, M. Ingestion of microplastics and occurrence of parasite association in Mediterranean anchovy and sardine. Mar. Pollut. Bull. 2020, 158, 111399. [Google Scholar] [CrossRef] [PubMed]
- Rajan, K.; Khudsar, F.A.; Kumar, R. Spatio-temporal patterns of microplastic contamination in surface waters of Hooghly River Estuary: Causes and consequences. Reg. Stud. Mar. Sci. 2023, 65, 103111. [Google Scholar] [CrossRef]
- Qu, X.; Su, L.; Li, H.; Liang, M.; Shi, H. Assessing the relationship between the abundance and properties of microplastics in water and in mussels. Sci. Total Environ. 2018, 621, 679–686. [Google Scholar] [CrossRef] [PubMed]
- Lopes Costa, L.; da Costa, I.D.; da Silva Oliveira, A.; Rosental Zalmon, I. “Microplastic ecology”. Testing the influence of ecological traits and urbanization in microplastic ingestion by sandy beach fauna. Estuar. Coast. Shelf Sci. 2023, 290, 108406. [Google Scholar] [CrossRef]
- Bellas, J.; Martínez-Armental, J.; Martínez-Cámara, A.; Besada, V.; MartínezGómez, C. Ingestion of microplastics by demersal fish from the Spanish Atlantic and Mediterranean coasts. Mar. Pollut. Bull. 2016, 109, 55–60. [Google Scholar] [CrossRef]
- Lopes, C.; Raimundo, J.; Caetano, M.; Garrido, S. Microplastic ingestión and diet composition of planktivorous fish. Limnol. Oceanogr. Lett. 2020, 5, 103–112. [Google Scholar] [CrossRef]
- Neves, D.; Sobral, P.; Ferreira, J.L.; Pereira, T. Ingestion of microplastics by commercial fish off the Portuguese coast. Mar. Pollut. Bull. 2015, 101, 119–126. [Google Scholar] [CrossRef]
- Nie, H.; Wang, J.; Xu, K.; Huang, Y.; Yan, M. Microplastic pollution in water and fish samples around Nanxun Reef in Nansha Islands, South China Sea. Sci. Total Environ. 2019, 696, 134022. [Google Scholar] [CrossRef]
- Boucher, J.; Friot, D. Primary Microplastics in the Oceans: A Global Evaluation of Sources; IUCN: Gland, Switzerland, 2017; 43p. [Google Scholar]
- Hamed, M.; Martyniuk, C.J.; Lee, J.S.; Shi, H.; Sayed, A.E. Distribution, abundance, and composition of microplastics in market fishes from the Red and Mediterranean seas in Egypt. J. Sea Res. 2023, 194, 102407. [Google Scholar] [CrossRef]
- Kılıç, E. Microplastic ingestion evidence by economically important farmed fish species from Turkey. Mar. Pollut. Bull. 2022, 183, 114097. [Google Scholar] [CrossRef] [PubMed]
- Matluba, M.; Ahmed, M.K.; Chowdhury, K.M.A.; Khan, N.; Ashiq, M.A.; Islam, M.S. The pervasiveness of microplastic contamination in the gastrointestinal tract 563 of fish from the western coast of Bangladesh. Mar. Pollut. Bull. 2023, 193, 115145. [Google Scholar] [CrossRef] [PubMed]
- Erni-Cassola, G.; Zadjelovic, V.; Gibson, M.I.; Christie-Oleza, J.A. Distribution of plastic polymer types in the marine environment; A meta-analysis. J. Hazard Mater. 2019, 369, 691–698. [Google Scholar] [CrossRef] [PubMed]
- Andrady, A.L. Persistence of Plastic Litter in the Oceans. In Marine Anthropogenic Litter; Bergmann, M., Gutow, L., Klages, M., Eds.; Springer: Cham, Switzerland, 2015; pp. 57–72. [Google Scholar]




| Species | N | Habitat | Mean TL, cm | Mean TW, g | FO (%) | MPs/ind |
|---|---|---|---|---|---|---|
| Chelon auratus | 35 | Pelagic | 22.54 ± 1.70 | 91.22 ± 21.04 | 100 | 16.82 |
| Sardina pilchardus | 50 | Pelagic | 21.52 ± 1.11 | 74.35 ± 11.52 | 72 | 9.64 |
| Callista chione | 46 | Benthic | 6.90 ± 0.79 | 21.90 ± 9.00 | 100 | 19.19 |
| Polymer | Monomer | Hazard | Hazard Level | Score | PHI | Hazard Category | Risk Category |
|---|---|---|---|---|---|---|---|
| PP | Propylene | Harmful when inhaled. | I | 1 | 39.13 | III | High |
| PET | Ethylene glycol | Harmful if swallowed. | II | 4 | 104.32 | IV | Very High |
| PVC | Vinyl chloride | Extremely flammable gas; May cause cancer. | V | 10.5 | 183.46 | IV | Very High |
| PE | Ethylene | Extremely flammable gas; May cause drowsiness or dizziness. | II | 11 | 95.59 | III | High |
| PC | Bisphenol A | May cause an allergic skin reaction; Suspected of damaging fertility or the unborn child; May cause drowsiness or dizziness. | IV | 1.2 | 10.22 | II | Moderate |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bouzekry, A.; Mghili, B.; Bouadil, O.; Mancuso, M.; Ben-Haddad, M.; Bottari, T.; Aksissou, M. First Report of Microplastic Ingestion in Edible Fish along Moroccan Mediterranean Coasts. Sustainability 2023, 15, 16313. https://doi.org/10.3390/su152316313
Bouzekry A, Mghili B, Bouadil O, Mancuso M, Ben-Haddad M, Bottari T, Aksissou M. First Report of Microplastic Ingestion in Edible Fish along Moroccan Mediterranean Coasts. Sustainability. 2023; 15(23):16313. https://doi.org/10.3390/su152316313
Chicago/Turabian StyleBouzekry, Assia, Bilal Mghili, Oumayma Bouadil, Monique Mancuso, Mohamed Ben-Haddad, Teresa Bottari, and Mustapha Aksissou. 2023. "First Report of Microplastic Ingestion in Edible Fish along Moroccan Mediterranean Coasts" Sustainability 15, no. 23: 16313. https://doi.org/10.3390/su152316313
APA StyleBouzekry, A., Mghili, B., Bouadil, O., Mancuso, M., Ben-Haddad, M., Bottari, T., & Aksissou, M. (2023). First Report of Microplastic Ingestion in Edible Fish along Moroccan Mediterranean Coasts. Sustainability, 15(23), 16313. https://doi.org/10.3390/su152316313









