Multicomponent Adsorption of Pollutants from Wastewater Using Low-Cost Eco-Friendly Iron-Modified Rice Husk Biochar in the Era of Green Chemistry
Abstract
:1. Introduction
2. Methodology
2.1. Rice Husk Biochar Production
2.2. Modification of Biochar
2.3. Preparation of Stock Solution for Simulated Wastewater
2.4. Adsorption Experiment in Batches
2.5. Calculation for Adsorption Capacity Contaminants by Biochar
2.6. Adsorption Isotherm Models
2.7. Freundlich Adsorption Isotherm
2.8. Data Analysis
3. Results and Discussion
3.1. Surface Functional Analysis of Iron-Modified Biochar
3.2. Fourier-Transform Infrared Spectroscopy (FTIR) and Scanning Electron Microscopy (SEM) Analysis
3.3. Mono-Component System Adsorption of Contaminants onto Iron-Modified Rice Husk Biochar
3.4. Multi-Component (Binary and Quaternary) System Adsorption of Contaminants onto Iron-Modified Rice Husk Biochar
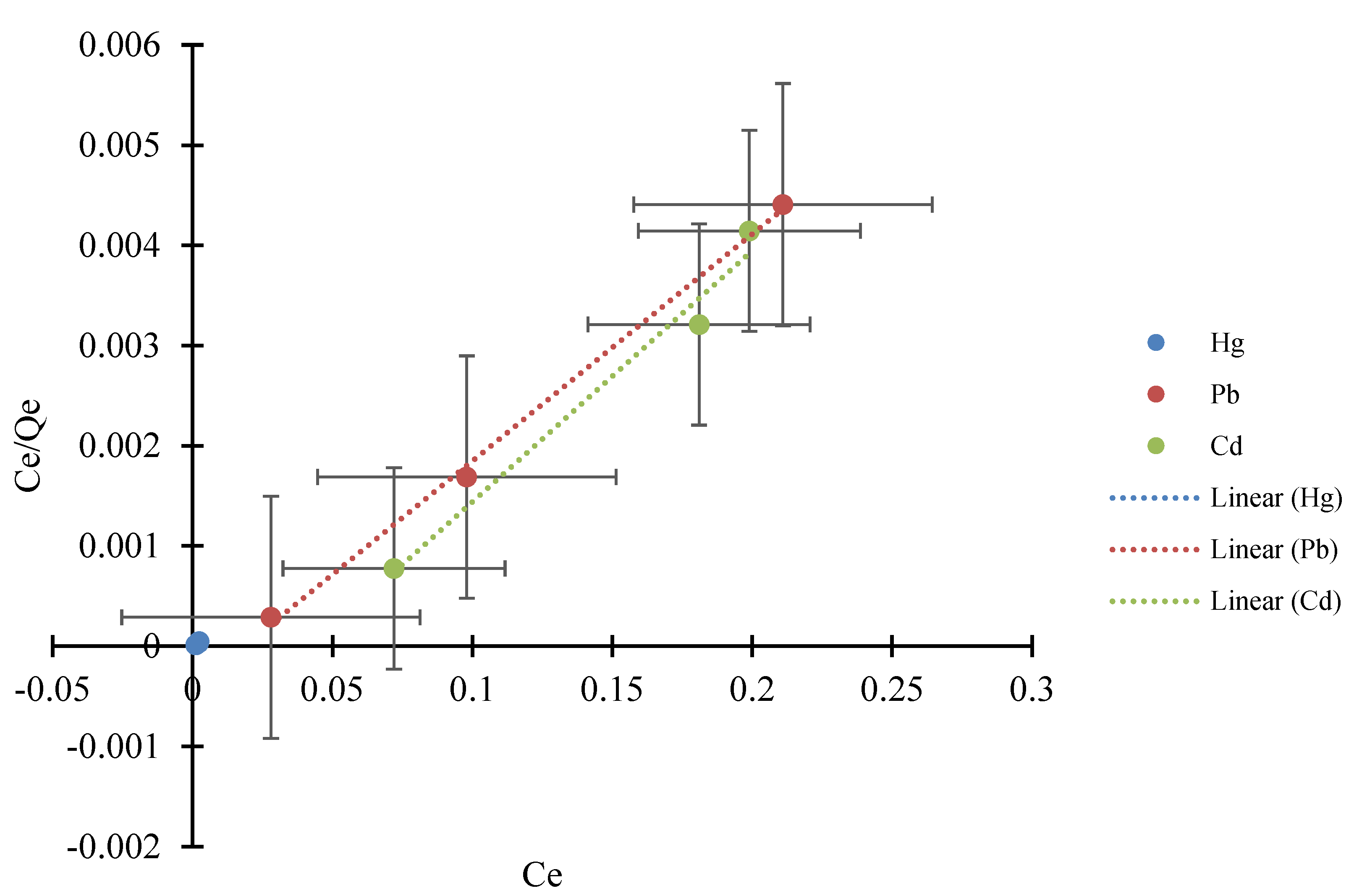
3.5. Adsorption Isotherm
3.5.1. Langmuir Adsorption Isotherm
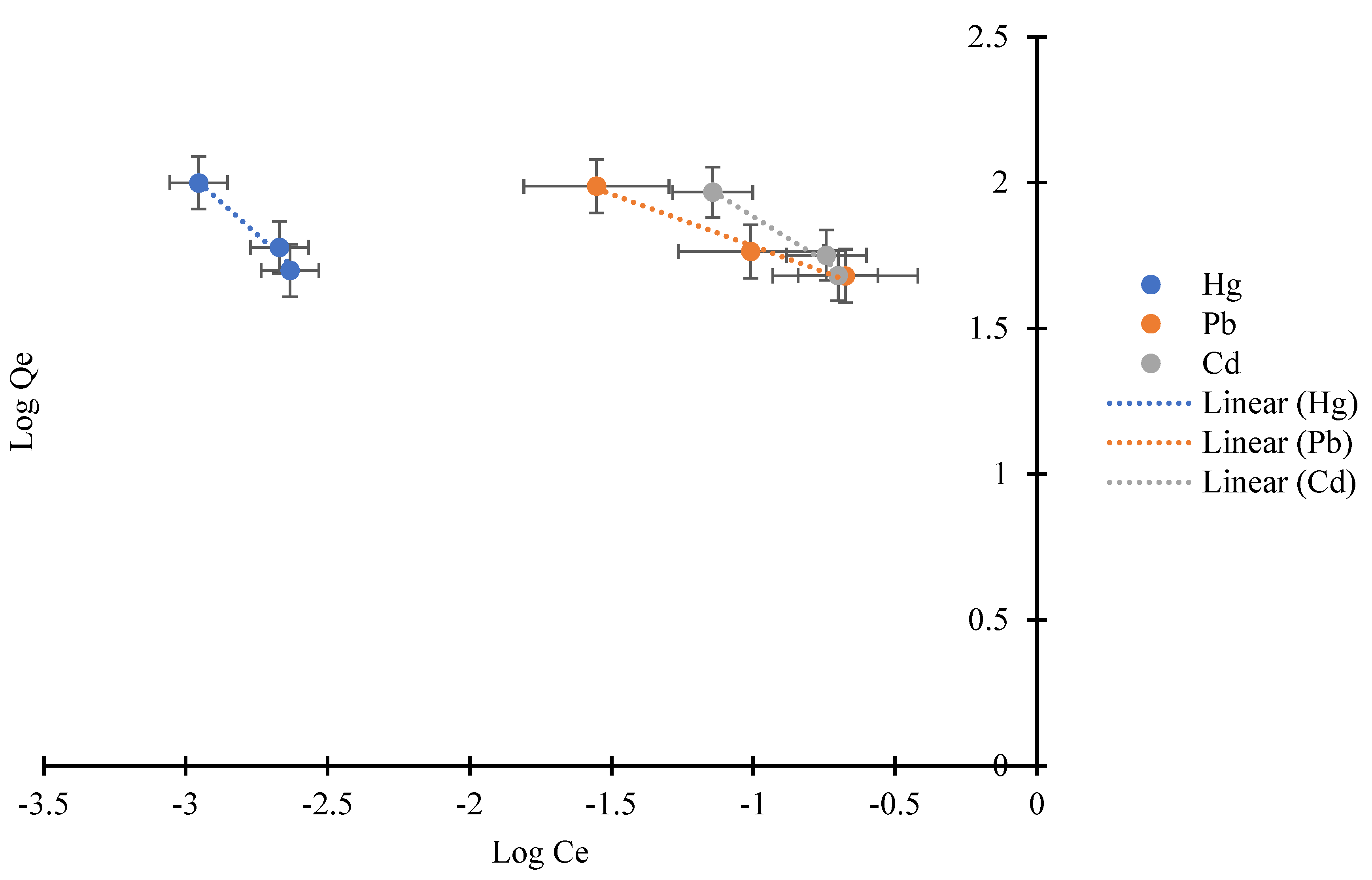
3.5.2. Freundlich Adsorption Isotherm
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mojiri, A.; Zhou, J.L.; Robinson, B.; Ohashi, A.; Ozaki, N.; Kindaichi, T.; Farraji, H.; Vakili, M. Pesticides in aquatic environments and their removal by adsorption methods. Chemosphere 2020, 253, 126646. [Google Scholar] [CrossRef]
- Keiluweit, M.; Nico, P.S.; Johnson, M.G.; Kleber, M. Dynamic molecular structure of plant biomass-derived black carbon (biochar). Environ. Sci. Technol. 2010, 44, 1247–1253. [Google Scholar] [CrossRef]
- Iqbal, M.; Saeed, A.; Zafar, S.I. FTIR spectrophotometry, kinetics and adsorption isotherms modeling, ion exchange, and EDX analysis for understanding the mechanism of Cd2+ and Pb2+ removal by mango peel waste. J. Hazard. Mater. 2009, 164, 161–171. [Google Scholar] [CrossRef]
- Venkatesan, S.; Hassan, M.U.; Ryu, H.J. Adsorption and immobilization of radioactive ionic-corrosion-products using magnetic hydroxyapatite and cold-sintering for nuclear waste management applications. J. Nucl. Mater. 2019, 514, 40–49. [Google Scholar] [CrossRef]
- Sheng, P.X.; Ting, Y.-P.; Chen, J.; Hong, L. Sorption of lead, copper, cadmium, zinc, and nickel by marine algal biomass: Characterization of biosorptive capacity and investigation of mechanisms. J. Colloid Interface Sci. 2004, 275, 131–141. [Google Scholar] [CrossRef]
- Qi, J.; Yokoyama, W.; Masamba, K.G.; Majeed, H.; Zhong, F.; Li, Y. Structural and physicochemical properties of insoluble rice bran fibre: Effect of acid–base induced modifications. RSC Adv. 2015, 5, 79915–79923. [Google Scholar] [CrossRef]
- Zhang, S.; Huang, L.; Kong, W.; Zhou, S.; Fan, M. Agricultural waste materials as potential adsorbents for heavy metal ions from aqueous solution: A review. Bioresour. Technol. 2019, 289, 121647. [Google Scholar]
- Kumar, A.; Bhattacharya, T.; Mukherjee, S.; Sarkar, B. A perspective on biochar for repairing damages in the soil–plant system caused by climate change-driven extreme weather events. Biochar 2022, 4, 22. [Google Scholar] [CrossRef]
- Nguyen, T.D.; Ngo, H.H.; Guo, W.; Zhou, J.L.; Zhang, X. Adsorption of organochlorine pesticides from aqueous solution by iron-modified biochar. Environ. Technol. 2020, 41, 33–49. [Google Scholar]
- Rahman, Z.; Singh, V.P. The relative impact of toxic heavy metals (THMs) (arsenic (As), cadmium (Cd), chromium (Cr)(VI), mercury (Hg), and lead (Pb)) on the total environment: An overview. Environ. Monit. Assess. 2019, 191, 419. [Google Scholar] [CrossRef]
- Singh, D.; Singh, V.; Singh, J.; Singh, N.; Bahadur, L. Iron oxide nanoparticles impregnated chitosan composite for removal of As (III) from water: Batch and column studies. J. Environ. Chem. Eng. 2020, 8, 104218. [Google Scholar]
- Xiang, W.; Zhang, X.; Chen, K.; Fang, J.; He, F.; Hu, X.; Tsang, D.C.; Ok, Y.S.; Gao, B. Enhanced adsorption performance and governing mechanisms of ball-milled biochar for the removal of volatile organic compounds (VOCs). Chem. Eng. J. 2020, 385, 123842. [Google Scholar] [CrossRef]
- Duwiejuah, A. Eco-Friendly Biochars for the Adsorption of Heavy Metals from Aqueous Phase. 2017. Available online: http://www.udsspace.uds.edu.gh/handle/123456789/1637 (accessed on 17 July 2023).
- Ogawa, M.; Okimori, Y. Pioneering works in biochar research, Japan. Soil Res. 2010, 48, 489–500. [Google Scholar] [CrossRef]
- Abbey, C.Y.B.; Duwiejuah, A.B.; Quianoo, A.K. Removal of toxic metals from aqueous phase using cacao pod husk biochar in the era of green chemistry. Appl. Water Sci. 2023, 13, 57. [Google Scholar] [CrossRef]
- Duwiejuah, A.B.; Amadu, Y.; Gameli, B.H.R.; Bawa, A.-A.; Imoro, Z.A.; Alidu, S.M.; Imoro, A.Z. Spent Chinese Green Tea as an Adsorbent for Simultaneous Removal of Potentially Toxic Metals from Aqueous Solution. Chem. Afr. 2022, 5, 2107–2114. [Google Scholar] [CrossRef]
- Guo, X.; Fang, F.; Du, L.; Yang, M.; Chen, J.; Xie, T. Facile synthesis of magnetic bentonite for lead ion adsorption. J. Hazard. Mater. 2016, 309, 219–227. [Google Scholar]
- He, S.; Li, Y.; Weng, L.; Wang, J.; He, J.; Liu, Y.; Zhang, K.; Wu, Q.; Zhang, Y.; Zhang, Z. Competitive adsorption of Cd2+, Pb2+ and Ni2+ onto Fe3+-modified argillaceous limestone: Influence of pH, ionic strength and natural organic matters. Sci. Total. Environ. 2018, 637–638, 69–78. [Google Scholar] [CrossRef] [PubMed]
- Hou, D.; O’connor, D.; Igalavithana, A.D.; Alessi, D.S.; Luo, J.; Tsang, D.C.W.; Sparks, D.L.; Yamauchi, Y.; Rinklebe, J.; Ok, Y.S. Metal contamination and bioremediation of agricultural soils for food safety and sustainability. Nat. Rev. Earth Environ. 2020, 1, 366–381. [Google Scholar] [CrossRef]
- Brewer, C.E.; Chuang, V.J.; Masiello, C.A.; Gonnermann, H.; Gao, X.; Dugan, B.; Driver, L.E.; Panzacchi, P.; Zygourakis, K.; Davies, C.A. New approaches to measuring biochar density and porosity. Biomass Bioenergy 2014, 66, 176–185. [Google Scholar] [CrossRef]
- Ambaye, T.G.; Vaccari, M.; van Hullebusch, E.D.; Amrane, A.; Rtimi, S. Mechanisms and adsorption capacities of biochar for the removal of organic and inorganic pollutants from industrial wastewater. Int. J. Environ. Sci. Technol. 2021, 18, 3273–3294. [Google Scholar] [CrossRef]
- Sayin, F.; Akar, S.T.; Akar, T. From green biowaste to water treatment applications: Utilization of modified new biochar for the efficient removal of ciprofloxacin. Sustain. Chem. Pharm. 2021, 24, 100522. [Google Scholar] [CrossRef]
- Khan, S.; Naushad, M.; Govarthanan, M.; Iqbal, J.; Alfadul, S.M. Emerging contaminants of high concern for the environment: Current trends and future research. Environ. Res. 2022, 207, 112609. [Google Scholar] [CrossRef]
- Bulut, Y.; Aydın, H. A kinetics and thermodynamics study of methylene blue adsorption on wheat shells. Desalination 2006, 194, 259–267. [Google Scholar] [CrossRef]
- Mishra, S.; Bharagava, R.N.; More, N.; Yadav, A.; Zainith, S.; Mani, S.; Chowdhary, P. Heavy Metal Contamination: An Alarming Threat to Environment and Human Health. In Environmental Biotechnology: For Sustainable Future; Springer: Singapore, 2019; pp. 103–125. [Google Scholar] [CrossRef]
- Adelaja, O.A.; Bankole, A.C.; Oladipo, M.E.; Lene, D.B. Biosorption of Hg(II) ions, Congo red and their binary mixture using raw and chemically activated mango leaves. Int. J. Energy Water Resour. 2019, 3, 1–12. [Google Scholar] [CrossRef]
- Senthilkumaar, S.; Manivannan, S.; Varadarajan, P.R. Mercury removal from synthetic wastewater by iron-modified biochar derived from rice straw. J. Environ. Chem. Eng. 2019, 7, 103167. [Google Scholar] [CrossRef]
- Gao, X.; Li, M.; Zhao, Y.; Zhang, Y. Mechanistic study of selective adsorption of Hg2+ ion by porous alginate beads. Chem. Eng. J. 2019, 378, 122096. [Google Scholar] [CrossRef]
- Zhao, X.; Zhang, H.; Chen, Y.; Chen, X.; Yu, S. Removal of lead from aqueous solution using iron-modified rice husk biochar. Environ. Sci. Pollut. Res. 2019, 26, 21834–21843. [Google Scholar]
- Zhu, L.; Wang, Z.; Gao, H.; Zhao, Q.; Zhang, J. Adsorption of aldrin from aqueous solutions by graphene. J. Hazard. Mater. 2011, 185, 140–148. [Google Scholar] [CrossRef]
- Ali, I.; Alharbi, O.M.L.; Ahmed, S. Heavy metal removal from wastewater using various adsorbents: A review. J. Water Process Eng. 2019, 30, 100436. [Google Scholar]
- Zhuang, Z.; Wang, L.; Tang, J. Efficient removal of volatile organic compound by ball-milled biochars from different preparing conditions. J. Hazard. Mater. 2021, 406, 124676. [Google Scholar] [CrossRef]
- Sarwar, A.; Wang, J.; Khan, M.S.; Farooq, U.; Riaz, N.; Nazir, A.; Mahmood, Q.; Hashem, A.; Al-Arjani, A.B.F.; Alqarawi, A.A.; et al. Iron oxide (Fe3o4)-supported sio2 magnetic nanocomposites for efficient adsorption of fluoride from drinking water: Synthesis, characterization, and adsorption isotherm analysis. Water 2021, 13, 1514. [Google Scholar] [CrossRef]
- Xiang, J.; Lin, Q.; Cheng, S.; Guo, J.; Yao, X.; Liu, Q.; Yin, G.; Liu, D. Enhanced adsorption of Cd(II) from aqueous solution by a magnesium oxide–rice husk biochar composite. Environ. Sci. Pollut. Res. 2018, 25, 14032–14042. [Google Scholar] [CrossRef] [PubMed]
- Sun, C.; Chen, T.; Huang, Q.; Wang, J.; Lu, S.; Yan, J. Enhanced adsorption for Pb(II) and Cd(II) of magnetic rice husk biochar by KMnO4 modification. Environ. Sci. Pollut. Res. 2019, 26, 8902–8913. [Google Scholar] [CrossRef]
- Isak, N.; Xhaxhiu, K. A review on the adsorption of diuron, carbaryl, and alachlor using natural and activated clays. Remediat. J. 2023, 33, 339–353. [Google Scholar] [CrossRef]
- Srivastav, A.L.; Pham, T.D.; Izah, S.C.; Singh, N.; Singh, P.K. Biochar Adsorbents for Arsenic Removal from Water Environment: A Review. Bull. Environ. Contam. Toxicol. 2022, 108, 616–628. [Google Scholar] [CrossRef]
- Wu, Y.; Wu, G.; Zhang, X.; He, Y.; Zhou, X. Adsorption of lead (II) from aqueous solutions by iron-modified rice husk biochar. J. Environ. Chem. Eng. 2020, 8, 104087. [Google Scholar]


| Batch | Contaminants | Conc. (Onefold) (mg/L) at 1 g of Dosage | Conc. (Threefold) (mg/L) at 5 g of Dosage | Conc. (Fivefold) (mg/L) at 10 g of Dosage |
|---|---|---|---|---|
| Mono | Hg2+ Pb2+ Cd2+ Aldrin | 1.00 1.00 1.00 1.00 | 3.00 3.00 3.00 3.00 | 5.00 5.00 5.00 5.00 |
| Binary | Hg2+:Pb2+ Hg2+:Cd2+ Cd2+:Pb2+ Aldrin:Hg2+ Aldrin:Pb2+ Aldrin:Cd2+ | 1.00:1.00 1.00:1.00 1.00:1.00 1.00:1.00 1.00:1.00 1.00:1.00 | 3.00:3.00 3.00:3.00 3.00:3.00 3.00:3.00 3.00:3.00 3.00:3.00 | 5.00:5.00 5.00:5.00 5.00:5.00 5.00:5.00 5.00:5.00 5.00:5.00 |
| Quaternary | Aldrin:Cd2+:Hg2+:Pb2+ | 1.00:1.00:1.00:1.00 | 3.00:3.00:3.00:3.00 | 5.00:5.00:5.00:5.00 |
| Pollutant | Adsorbent Dosage (G) | pH | Initial Conc. (mg/L) | Final Conc. (mg/L) | Removal Efficiency (%) |
|---|---|---|---|---|---|
| Hg2+ | 1 | 7.34 | 1 | 0.00111 | 99.89 |
| 5 | 7.26 | 3 | 0.00214 | 99.93 | |
| 10 | 7.35 | 5 | 0.00233 | 99.95 | |
| Pb2+ | 1 | 7.45 | 1 | 0.028 | 97.20 |
| 5 | 7.47 | 3 | 0.098 | 96.73 | |
| 10 | 7.51 | 5 | 0.211 | 95.78 | |
| Cd2+ | 1 | 7.67 | 1 | 0.072 | 92.80 |
| 5 | 7.64 | 3 | 0.181 | 93.97 | |
| 10 | 7.65 | 5 | 0.199 | 96.02 | |
| Aldrin | 1 | 7.22 | 1 | 0 | 100 |
| 5 | 7.25 | 3 | 0 | 100 | |
| 10 | 7.60 | 5 | 0 | 100 |
| Binary Component | Pollutant | Adsorbent Dosage | pH | Initial Conc. | Removal Efficiency |
|---|---|---|---|---|---|
| Hg2+–Pb2+ | |||||
| Hg2+ | 1 | 7.85 | 1 | 99.80 | |
| 5 | 7.92 | 3 | 99.90 | ||
| 10 | 7.95 | 5 | 99.92 | ||
| Pb2+ | 1 | 7.85 | 1 | 98.80 | |
| 5 | 7.92 | 3 | 97.90 | ||
| 10 | 7.95 | 5 | 97.58 | ||
| Hg2+–Cd2+ | |||||
| Hg2+ | 1 | 7.96 | 1 | 99.88 | |
| 5 | 7.97 | 3 | 99.93 | ||
| 10 | 8.01 | 5 | 99.95 | ||
| Cd2+ | 1 | 7.96 | 1 | 78.90 | |
| 5 | 7.97 | 3 | 92.23 | ||
| 10 | 8.01 | 5 | 93.92 | ||
| Pb2+–Cd2+ | |||||
| Pb2+ | 1 | 8.07 | 1 | 88.90 | |
| 5 | 8.1 | 3 | 98.53 | ||
| 10 | 8.15 | 5 | 99.56 | ||
| Cd2+ | 1 | 8.07 | 1 | 99.88 | |
| 5 | 8.1 | 3 | 99.96 | ||
| 10 | 8.15 | 5 | 99.98 | ||
| Aldrin–Hg2+ | |||||
| Aldrin | 1 | 7.8 | 1 | 100.00 | |
| 5 | 7.3 | 3 | 100.00 | ||
| 10 | 7.16 | 5 | 100.00 | ||
| Hg2+ | 1 | 7.8 | 1 | 99.89 | |
| 5 | 7.3 | 3 | 99.95 | ||
| 10 | 7.16 | 5 | 99.96 | ||
| Aldrin–Pb2+ | |||||
| Aldrin | 1 | 6.8 | 1 | 100 | |
| 5 | 6.88 | 3 | 100 | ||
| 10 | 7.01 | 5 | 100 | ||
| Pb2+ | 1 | 6.8 | 1 | 98.80 | |
| 5 | 6.88 | 3 | 95.97 | ||
| 10 | 7.01 | 5 | 98.24 | ||
| Aldrin–Cd2+ | |||||
| Aldrin | 1 | 7.71 | 1 | 100.00 | |
| 5 | 7.45 | 3 | 100.00 | ||
| 10 | 7.19 | 5 | 100.00 | ||
| Cd2+ | 1 | 7.71 | 1 | 98.20 | |
| 5 | 7.45 | 3 | 99.03 | ||
| 10 | 7.19 | 5 | 99.34 | ||
| Quaternary Component | |||||
| Aldrin–Hg2+–Pb2+–Cd2+ | |||||
| Aldrin | 1 | 7.15 | 1 | 100.00 | |
| 5 | 7.14 | 3 | 100.00 | ||
| 10 | 6.76 | 5 | 100.00 | ||
| Hg2+ | 1 | 7.15 | 1 | 99.88 | |
| 5 | 7.14 | 3 | 99.95 | ||
| 10 | 6.76 | 5 | 99.97 | ||
| Pb2+ | 1 | 7.15 | 1 | 98.20 | |
| 5 | 7.14 | 3 | 99.27 | ||
| 10 | 6.76 | 5 | 99.44 | ||
| Cd2+ | 1 | 7.15 | 1 | 98.90 | |
| 5 | 7.14 | 3 | 99.53 | ||
| 10 | 6.76 | 5 | 99.46 | ||
| Mono-Component | Qmax (mg/g) | KL (mg/L) | RL | R2 |
|---|---|---|---|---|
| Hg2+ | 36.4964 | −7.30 × 10−4 | 9.99 × 10−1 | 0.9746 |
| Pb2+ | 44.2478 | −1.77 × 10−2 | 9.47 × 10−1 | 0.9977 |
| Cd2+ | 39.8406 | −4.38 × 10−2 | 7.81 × 10−1 | 0.9803 |
| Binary Component | ||||
| Hg2+ *–Pb2+ | 34.4828 | −1.38 × 10−3 | 9.99 × 10−1 | 0.9985 |
| Hg2+–Pb2+ * | 46.0829 | −9.22 × 10−3 | 9.72 × 10−1 | 0.9945 |
| Hg2+ *–Cd2+ | 35.3357 | −7.07 × 10−4 | 9.96 × 10−1 | 0.9993 |
| Hg2+–Cd2+ * | 25.9067 | −1.35 × 10−1 | 8.65 × 10−1 | 0.9661 |
| Pb2+ *–Cd2+ | 114.9425 | 3.45 × 10−2 | 1.10 | 0.9804 |
| Pb2+–Cd2+ * | 19.0840 | 1.53 × 10−3 | 1.01 | 0.2172 |
| Aldrin–Hg2+ * | 28.9017 | 8.67 × 10−4 | 1.00 | 0.9949 |
| Aldrin–Pb2+ * | 53.1915 | 2.66 × 10−3 | 1.01 | 0.9766 |
| Aldrin–Cd2+ * | 32.1543 | 1.29 × 10−2 | 1.06 | 0.9879 |
| Quaternary Component | ||||
| Al–Hg2+ *–Pb2+–Cd2+ | 23.0947 | 9.24 × 10−4 | 1.00 | 0.9826 |
| Al–Hg2+–Pb2+ *–Cd2+ | 26.6667 | 1.33 × 10−2 | 1.04 | 0.9894 |
| Al–Hg2+–Pb2+–Cd2+ * | 38.4615 | 7.69 × 10−3 | 1.04 | 0.9894 |
| 1/n | N | Kf | R2 | |
|---|---|---|---|---|
| Mono-Component | ||||
| Hg2+ | −1.14338 | −0.8746 | 0.261999 | 0.9773 |
| Pb2+ | −2.80505 | −0.3565 | 26.66245 | 0.9857 |
| Cd2+ | −1.65317 | −0.6049 | 18.99328 | 0.9772 |
| Binary Component | ||||
| Hg2+ *–Pb2+ | 1.071926 | 0.9329 | 3.486583 | 0.931 |
| Hg2+–Pb2+ * | −3.25839 | −0.3069 | 25.35712 | 0.9996 |
| Hg2+ *–Cd2+ | −1.11707 | −0.8952 | 0.239828 | 0.9848 |
| Hg2+–Cd2+ * | −0.79726 | −1.2543 | 10.17185 | 0.797 |
| Pb2+ *–Cd2+ | 2.75634 | 0.3628 | 193.1079 | 0.9782 |
| Pb2+–Cd2+ * | 0.19668 | 5.0844 | 5.57× 1016 | 0.3796 |
| Al2+–Hg2+ * | −0.73174 | −1.3666 | 0.009361 | 0.9997 |
| Al2+–Pb2+ * | −3.63504 | −0.2751 | 28.72764 | 0.8849 |
| Al2+–Cd2+ * | −0.90481 | −1.1052 | 1.163322 | 0.9972 |
| Quaternary Component | ||||
| Al–Hg2+ *– Pb2+–Cd2+ | −0.51467 | −1.943 | 0.000196 | 0.8931 |
| Al–Hg2+– Pb2+ *–Cd2+ | −0.66046 | −1.5141 | 0.209122 | 0.9025 |
| Al–Hg2+–Pb2+– Cd2+ * | 1.508068 | 0.6631 | 4.298332 | 0.749 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tichem, T.M.; Wang, Y.; Gameli, R.B.H.; Mbage, B.; Li, B. Multicomponent Adsorption of Pollutants from Wastewater Using Low-Cost Eco-Friendly Iron-Modified Rice Husk Biochar in the Era of Green Chemistry. Sustainability 2023, 15, 16348. https://doi.org/10.3390/su152316348
Tichem TM, Wang Y, Gameli RBH, Mbage B, Li B. Multicomponent Adsorption of Pollutants from Wastewater Using Low-Cost Eco-Friendly Iron-Modified Rice Husk Biochar in the Era of Green Chemistry. Sustainability. 2023; 15(23):16348. https://doi.org/10.3390/su152316348
Chicago/Turabian StyleTichem, Tibamba Matthew, Youbao Wang, Raphael B. H. Gameli, Bawa Mbage, and Bingbing Li. 2023. "Multicomponent Adsorption of Pollutants from Wastewater Using Low-Cost Eco-Friendly Iron-Modified Rice Husk Biochar in the Era of Green Chemistry" Sustainability 15, no. 23: 16348. https://doi.org/10.3390/su152316348





