Bio-Coated Graphitic Carbon Nitrides for Enhanced Nitrobenzene Degradation: Roles of Extracellular Electron Transfer
Abstract
:1. Introduction
2. Materials and Methods
3. Results
3.1. Surface Morphology and Photo-Chemical Analysis
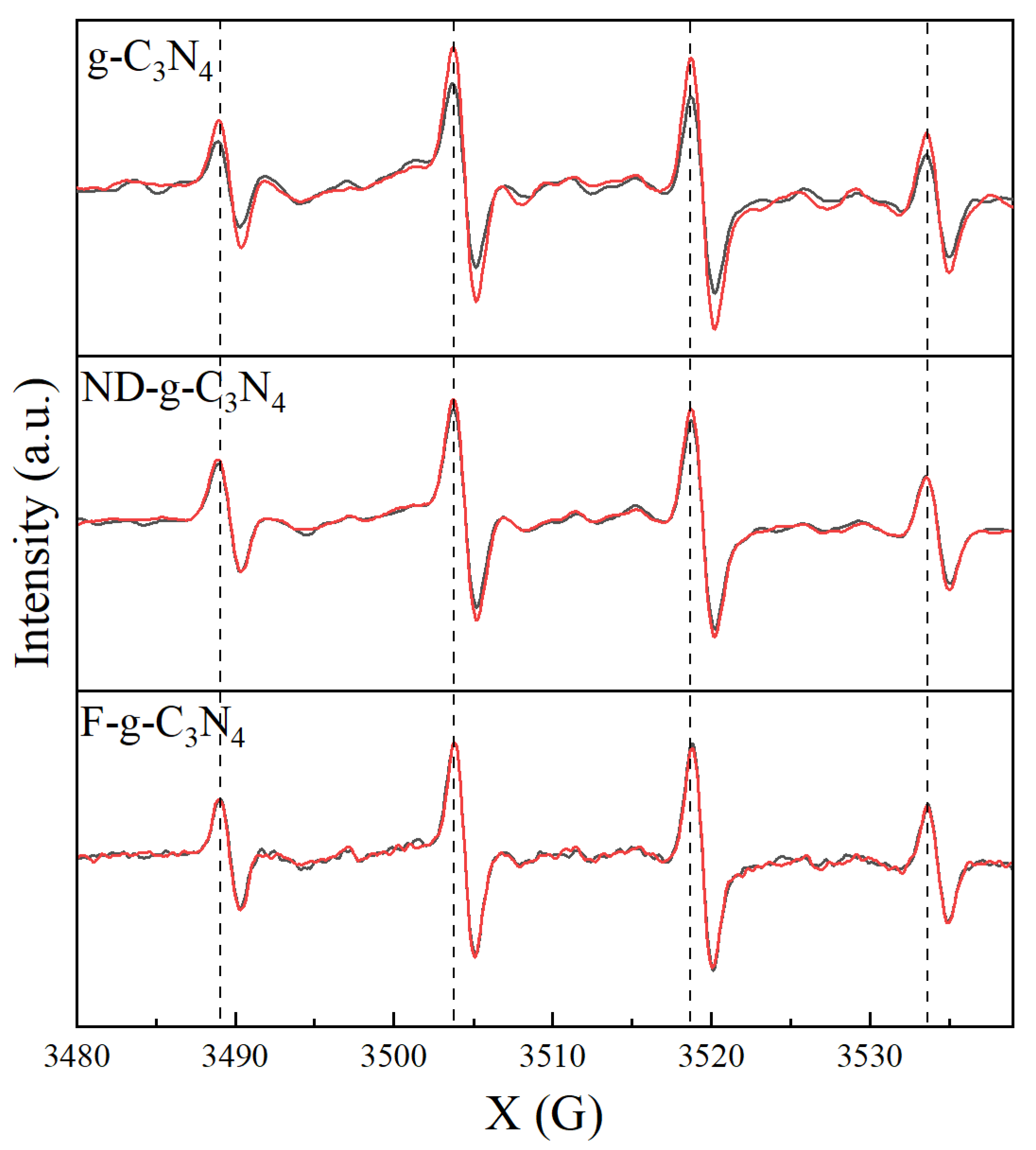
3.2. Molecular Characterization
3.3. Electrochemical Impedance Spectroscopy (EIS) Analysis
3.4. Photocatalytic Performance
3.4.1. Role of Dissolved Oxygen
3.4.2. Reactive Oxygen Species Generation
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Li, L.; Liu, Q.; Wang, Y.X.; Zhao, H.Q.; He, C.S.; Yang, H.Y.; Gong, L.; Mu, Y.; Yu, H.Q. Facilitated biological reduction of nitroaromatic compounds by reduced graphene oxide and the role of its surface characteristics. Sci. Rep. 2016, 6, 30082. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, C.; Shuai, D.; Naraginti, S.; Wang, D.; Zhang, W. Visible-light-driven photocatalytic inactivation of MS2 by metal-free g-C3N4: Virucidal performance and mechanism. Water Res. 2016, 106, 249–258. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Yu, H.; Quan, X.; Chen, S.; Zhang, Y.; Zhao, H.; Wang, H. Fabrication of atomic single layer graphitic-C3N4 and its high performance of photocatalytic disinfection under visible light irradiation. Appl. Catal. B Environ. 2014, 152, 46–50. [Google Scholar] [CrossRef]
- Lin, T.; Song, Z.; Wu, Y.; Chen, L.; Wang, S.; Fu, F.; Guo, L. Boron- and phenyl-codoped graphitic carbon nitride with greatly enhanced light responsive range for photocatalytic disinfection. J. Hazard. Mater. 2018, 358, 62–68. [Google Scholar] [CrossRef] [PubMed]
- Qin, Z.; Zhao, Z.; Jiao, W.; Han, Z.; Xia, L.; Fang, Y.; Wang, S.; Ji, L.; Jiang, Y. Phenanthrene removal and response of bacterial community in the combined system of photocatalysis and PAH-degrading microbial consortium in laboratory system. Bioresour. Technol. 2020, 301, 122736. [Google Scholar] [CrossRef] [PubMed]
- Akhavan, O.; Ghaderi, E.; Esfandiar, A. Wrapping bacteria by graphene nanosheets for isolation from environment, reactivation by sonication, and inactivation by near-infrared irradiation. J. Phys. Chem. B 2011, 115, 6279–6288. [Google Scholar] [CrossRef]
- Shao, W.; Liu, H.; Liu, X.; Wang, S.; Zhang, R. Anti-bacterial performances and biocompatibility of bacterial cellulose/graphene oxide composites. RSC Adv. 2015, 5, 4795–4803. [Google Scholar] [CrossRef]
- Zhang, X.; Wu, Y.; Xiao, G.; Tang, Z.; Wang, M.; Liu, F.; Zhu, X. Simultaneous photocatalytic and microbial degradation of dye-containing wastewater by a novel g-C3N4-P25/photosynthetic bacteria composite. PLoS ONE 2017, 12, e0172747. [Google Scholar] [CrossRef]
- Yong, Y.C.; Yu, Y.Y.; Zhang, X.; Song, H. Highly active bidirectional electron transfer by a self-assembled electroactive reduced-graphene-oxide-hybridized biofilm. Angew. Chem. Int. Ed. Engl. 2014, 53, 4480–4483. [Google Scholar] [CrossRef]
- Brown, K.A.; Harris, D.F.; Wilker, M.B.; Rasmussen, A.; Khadka, N.; Hamby, H.; Keable, S.; Dukovic, G.; Peters, J.W.; Seefeldt, L.C.; et al. Light-driven dinitrogen reduction catalyzed by a CdS:nitrogenase MoFe protein biohybrid. Science 2016, 352, 448–450. [Google Scholar] [CrossRef]
- Ding, R.; Yan, W.; Wu, Y.; Xiao, Y.; Gang, H.; Wang, S.; Chen, L.; Zhao, F. Light-excited photoelectrons coupled with bio-photocatalysis enhanced the degradation efficiency of oxytetracycline. Water Res. 2018, 143, 589–598. [Google Scholar] [CrossRef]
- Sakimoto, K.K.; Wong, A.B.; Yang, P. Self-photosensitization of nonphotosynthetic bacteria for solar-to-chemical production. Science 2016, 351, 74–77. [Google Scholar] [CrossRef]
- Ma, D.; Zou, D.; Zhou, D.; Li, T.; Dong, S.; Xu, Z.; Dong, S. Phenol removal and biofilm response in coupling of visible-light-driven photocatalysis and biodegradation: Effect of hydrothermal treatment temperature. Int. Biodeterior. Biodegrad. 2015, 104, 178–185. [Google Scholar] [CrossRef]
- Li, Y.; Tian, X.; Chen, L.; Li, J.; Zhao, F. Enhanced interfacial electron transfer between semiconductor and non-photosynthetic microorganism under visible light. Bioelectrochemistry 2022, 147, 108195. [Google Scholar] [CrossRef]
- Liu, Y.; Shen, Y.; Sun, L.; Li, J.; Liu, C.; Ren, W.; Li, F.; Gao, L.; Chen, J.; Liu, F.; et al. Elemental superdoping of graphene and carbon nanotubes. Nat. Commun. 2016, 7, 10921. [Google Scholar] [CrossRef]
- Pilarczyk, K.; Lewandowska, K.; Mech, K.; Kawa, M.; Gajewska, M.; Barszcz, B.; Bogucki, A.; Podborska, A.; Szacilowski, K. Charge transfer tuning in TiO2 hybrid nanostructures with acceptor-acceptor systems. J. Mater. Chem. C 2017, 5, 2415–2424. [Google Scholar] [CrossRef]
- Shiraishi, Y.; Shiota, S.; Hirakawa, H.; Tanaka, S.; Ichikawa, S.; Hirai, T. Titanium Dioxide/Reduced Graphene Oxide Hybrid Photocatalysts for Efficient and Selective Partial Oxidation of Cyclohexane. ACS Catal. 2016, 7, 293–300. [Google Scholar] [CrossRef]
- Su, R.; Tiruvalam, R.; He, Q.; Dimitratos, N.; Kesavan, L.; Hammond, C.; Lopez-Sanchez, J.A.; Bechstein, R.; Kiely, C.J.; Hutchings, G.J.; et al. Promotion of phenol photodecomposition over TiO2 using Au, Pd, and Au-Pd nanoparticles. ACS Nano 2012, 6, 6284–6292. [Google Scholar] [CrossRef] [PubMed]
- Tsukamoto, D.; Shiraishi, Y.; Sugano, Y.; Ichikawa, S.; Tanaka, S.; Hirai, T. Gold nanoparticles located at the interface of anatase/rutile TiO2 particles as active plasmonic photocatalysts for aerobic oxidation. J. Am. Chem. Soc. 2012, 134, 6309–6315. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Zhao, J.; Shang, E.; Xia, X.; Niu, J.; Crittenden, J. Effects of Chloride Ions on Dissolution, ROS Generation, and Toxicity of Silver Nanoparticles under UV Irradiation. Environ. Sci. Technol. 2018, 52, 4842–4849. [Google Scholar] [CrossRef] [PubMed]
- Kohan-Baghkheirati, E.; Geisler-Lee, J. Gene Expression, Protein Function and Pathways of Arabidopsis thaliana Responding to Silver Nanoparticles in Comparison to Silver Ions, Cold, Salt, Drought, and Heat. Nanomaterials 2015, 5, 436–467. [Google Scholar] [CrossRef]
- Ma, F.; Sun, C.; Shao, Y.; Wu, Y.; Huang, B.; Hao, X. One-step exfoliation and fluorination of g-C3N4 nanosheets with enhanced photocatalytic activities. New J. Chem. 2017, 41, 3061–3067. [Google Scholar] [CrossRef]
- Sert, B.; Ozay, Y.; Harputlu, E.; Ozdemir, S.; Yalcin, M.S.; Ocakoglu, K.; Dizge, N. Improvement in performance of g-C3N4 nanosheets blended PES ultrafiltration membranes including biological properties. Colloids Surf. A Physicochem. Eng. Asp. 2021, 623, 126571. [Google Scholar] [CrossRef]
- Stager, J.L.; Zhang, X.; Logan, B.E. Addition of acetate improves stability of power generation using microbial fuel cells treating domestic wastewater. Bioelectrochemistry 2017, 118, 154–160. [Google Scholar] [CrossRef]
- Liu, X.; Iocozzia, J.; Wang, Y.; Cui, X.; Chen, Y.; Zhao, S.; Li, Z.; Lin, Z. Noble metal–metal oxide nanohybrids with tailored nanostructures for efficient solar energy conversion, photocatalysis and environmental remediation. Energy Environ. Sci. 2017, 10, 402–434. [Google Scholar] [CrossRef]
- Chen, J.; Hong, Z.; Chen, Y.; Lin, B.; Gao, B. One-step synthesis of sulfur-doped and nitrogen-deficient g-C3N4 photocatalyst for enhanced hydrogen evolution under visible light. Mater. Lett. 2015, 145, 129–132. [Google Scholar] [CrossRef]
- Malvankar, N.S.; Vargas, M.; Nevin, K.P.; Franks, A.E.; Leang, C.; Kim, B.C.; Inoue, K.; Mester, T.; Covalla, S.F.; Johnson, J.P.; et al. Tunable metallic-like conductivity in microbial nanowire networks. Nat. Nanotechnol. 2011, 6, 573–579. [Google Scholar] [CrossRef]
- Strycharz-Glaven, S.M.; Snider, R.M.; Guiseppi-Elie, A.; Tender, L.M. On the electrical conductivity of microbial nanowires and biofilms. Energy Environ. Sci. 2011, 4, 4366–4379. [Google Scholar] [CrossRef]
- Snider, R.M.; Strycharz-Glaven, S.M.; Tsoi, S.D.; Erickson, J.S.; Tender, L.M. Long-range electron transport in Geobacter sulfurreducens biofilms is redox gradient-driven. Proc. Natl. Acad. Sci. USA 2012, 109, 15467–15472. [Google Scholar] [CrossRef]
- Shi, L.; Dong, H.; Reguera, G.; Beyenal, H.; Lu, A.; Liu, J.; Yu, H.Q.; Fredrickson, J.K. Extracellular electron transfer mechanisms between microorganisms and minerals. Nat. Reviews. Microbiol. 2016, 14, 651–662. [Google Scholar] [CrossRef]
- An, X.; Yu, J.C. Graphene-based photocatalytic composites. RSC Adv. 2011, 1, 1426–1434. [Google Scholar] [CrossRef]
- Reguera, G.; McCarthy, K.D.; Mehta, T.; Nicoll, J.S.; Tuominen, M.T.; Lovley, D.R. Extracellular electron transfer via microbial nanowires. Nature 2005, 435, 1098–1101. [Google Scholar] [CrossRef]
- Zhang, L.; Zhang, L.; Cheng, S.; Zhou, X.; Shang, N.; Gao, S.; Wang, C. Pd supported on graphene modified g-C3N4 hybrid: A highly efficient catalyst for hydrogenation of nitroarenes. Appl. Organomet. Chem. 2020, 34, e5684. [Google Scholar] [CrossRef]
- El-Shishtawy, R.M.; Shawky, A.; Mohamed, R.M. An efficient visible-light-driven photoconversion of nitrobenzene to aniline over PtO-decorated WO3 nanocrystals prepared by a soft template-based method. J. Taiwan Inst. Chem. Eng. 2023, 142, 104634. [Google Scholar] [CrossRef]
- Akhtar, T.; Nasir, H.; Sitara, E.; Bukhari, S.A.B.; Ullah, S.; Iqbal, R.M.A. Efficient photocatalytic degradation of nitrobenzene by copper-doped TiO2: Kinetic study, degradation pathway, and mechanism. Environ. Sci. Pollut. Res. Int. 2022, 29, 49925–49936. [Google Scholar] [CrossRef] [PubMed]
- Challagulla, S.; Payra, S.; Chakraborty, C.; Roy, S. Determination of band edges and their influences on photocatalytic reduction of nitrobenzene by bulk and exfoliated g-C3N4. Phys. Chem. Chem. Phys. 2019, 21, 3174–3183. [Google Scholar] [CrossRef] [PubMed]
- Shanavas, S.; Ahamad, T.; Alshehri, S.M.; Acevedo, R.; Munusamy Anbarasan, P. Hydrothermal Assisted Synthesis of ZnFe2O4 Embedded g-C3N4 Nanocomposite with Enhanced Charge Transfer Ability for Effective Removal of Nitrobenzene and Cr(VI). ChemistrySelect 2020, 5, 5117–5127. [Google Scholar] [CrossRef]
- Hong, Z.; Shen, B.; Chen, Y.; Lin, B.; Gao, B. Enhancement of photocatalytic H2 evolution over nitrogen-deficient graphitic carbon nitride. J. Mater. Chem. A 2013, 1, 11754. [Google Scholar] [CrossRef]
- Wang, Y.; Li, Y.; Zhang, W.; Wang, Q.; Wang, D. Photocatalytic degradation and reactor modeling of 17alpha-ethynylestradiol employing titanium dioxide-incorporated foam concrete. Environ. Sci. Pollut. Res. Int. 2015, 22, 3508–3517. [Google Scholar] [CrossRef]
- Belhadj, H.; Hakki, A.; Robertson, P.K.; Bahnemann, D.W. In situ ATR-FTIR study of H2O and D2O adsorption on TiO2 under UV irradiation. Phys. Chem. Chem. Phys. PCCP 2015, 17, 22940–22946. [Google Scholar] [CrossRef]
- Daimon, T.; Nosaka, Y. Formation and Behavior of Singlet Molecular Oxygen in TiO2 Photocatalysis Studied by Detection of Near-Infrared Phosphorescence. J. Phys. Chem. C 2007, 111, 4420–4424. [Google Scholar] [CrossRef]
- Green, I.X.; Tang, W.; Neurock, M.; Yates, J.T., Jr. Insights into catalytic oxidation at the Au/TiO2 dual perimeter sites. Acc. Chem. Res. 2014, 47, 805–815. [Google Scholar] [CrossRef]
- Han, X.; Li, Y.; Li, D.; Liu, C. Role of Free Radicals/Reactive Oxygen Species in MeHg Photodegradation: Importance of Utilizing Appropriate Scavengers. Environ. Sci. Technol. 2017, 51, 3784–3793. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Li, Y.; Li, G.; Wang, C.; Zhang, W.; Wang, Q. Modeling of quantitative effects of water components on the photocatalytic degradation of 17α-ethynylestradiol in a modified flat plate serpentine reactor. J. Hazard. Mater. 2013, 254, 64–71. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, R.M.; Ibrahim, F.M. Visible light photocatalytic reduction of nitrobenzene using Ag/Bi2MoO6 nanocomposite. J. Ind. Eng. Chem. 2015, 22, 28–33. [Google Scholar] [CrossRef]
- Mohamed, R.M. Preparation of aniline from photocatalytic reduction of nitrobenzene using Pt/Nd2O3 nanocomposite. J. Alloys Compd. 2015, 648, 711–718. [Google Scholar] [CrossRef]
- Sheng, T.; Qi, Y.-J.; Lin, X.; Hu, P.; Sun, S.-G.; Lin, W.-F. Insights into the mechanism of nitrobenzene reduction to aniline over Pt catalyst and the significance of the adsorption of phenyl group on kinetics. Chem. Eng. J. 2016, 293, 337–344. [Google Scholar] [CrossRef]
- Wang, A.J.; Cheng, H.Y.; Liang, B.; Ren, N.Q.; Cui, D.; Lin, N.; Kim, B.H.; Rabaey, K. Efficient reduction of nitrobenzene to aniline with a biocatalyzed cathode. Environ. Sci. Technol. 2011, 45, 10186–10193. [Google Scholar] [CrossRef]








| Reaction Constant (k) Value | ||||||
|---|---|---|---|---|---|---|
| 5:5 * | 4:6 * | 3:7 * | 2:8 * | 1:9 * | Pristine | |
| g-C3N4 | 0.002 | 0.001833 | 0.001714 | 0.0015 | 0.001111 | 0.0009 |
| ND-g-C3N4 | 0.0023 | 0.00205 | 0.0015857 | 0.00135 | 0.0014556 | 0.0012 |
| F-g-C3N4 | 0.0022 | 0.0020 | 0.0014 | 0.12 | 0.001 | 0.001 |
| Adsorption Rate Value | ||||||
|---|---|---|---|---|---|---|
| 5:5 * | 4:6 * | 3:7 * | 2:8 * | 1:9 * | Pristine | |
| g-C3N4 | 0.528 | 0.363 | 0.339 | 0.307 | 0.312 | 0.250 |
| ND-g-C3N4 | 0.477 | 0.433 | 0.384 | 0.338 | 0.279 | 0.235 |
| F-g-C3N4 | 0.517 | 0.451 | 0.369 | 0.329 | 0.295 | 0.243 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Y.; Li, Y.; Wang, L.; Zhang, W.; Bürgi, T. Bio-Coated Graphitic Carbon Nitrides for Enhanced Nitrobenzene Degradation: Roles of Extracellular Electron Transfer. Sustainability 2023, 15, 16372. https://doi.org/10.3390/su152316372
Wang Y, Li Y, Wang L, Zhang W, Bürgi T. Bio-Coated Graphitic Carbon Nitrides for Enhanced Nitrobenzene Degradation: Roles of Extracellular Electron Transfer. Sustainability. 2023; 15(23):16372. https://doi.org/10.3390/su152316372
Chicago/Turabian StyleWang, Yuming, Yi Li, Longfei Wang, Wenlong Zhang, and Thomas Bürgi. 2023. "Bio-Coated Graphitic Carbon Nitrides for Enhanced Nitrobenzene Degradation: Roles of Extracellular Electron Transfer" Sustainability 15, no. 23: 16372. https://doi.org/10.3390/su152316372
APA StyleWang, Y., Li, Y., Wang, L., Zhang, W., & Bürgi, T. (2023). Bio-Coated Graphitic Carbon Nitrides for Enhanced Nitrobenzene Degradation: Roles of Extracellular Electron Transfer. Sustainability, 15(23), 16372. https://doi.org/10.3390/su152316372









