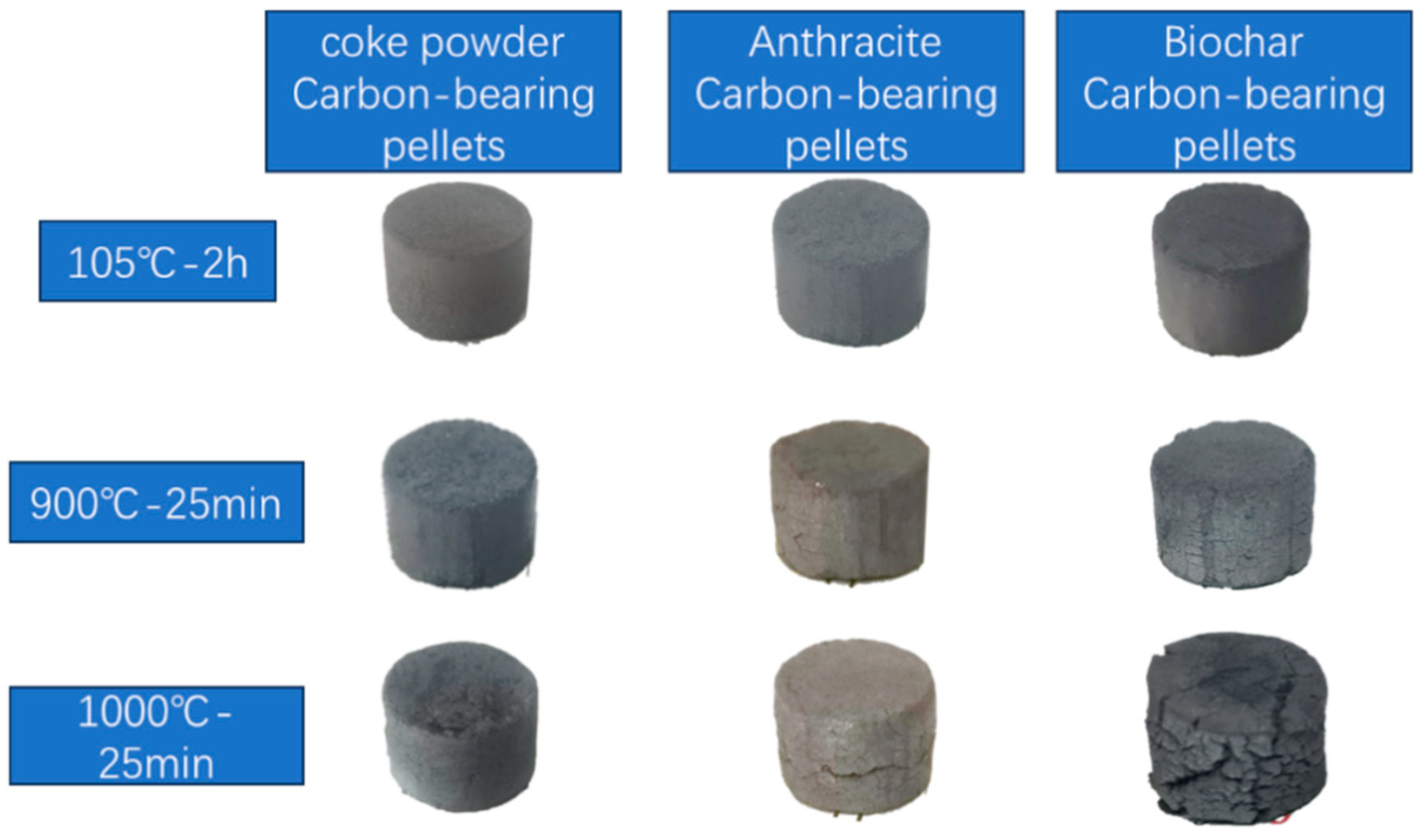
A preliminary test was first conducted on carbon-bearing pellets. The biochar with 8% water was added as a reducing agent [
13]. To ensure the wet and dry pellet strength, 1% of CMC (carboxymethyl cellulose) was added as a binder. To investigate the usage of bentonite binder, different amounts of bentonite (1%, 3%, and 5%) were added. The pellets were then subjected to calcination at 900 °C and 1000 °C for 25 min. Results show that when 1% and 3% of bentonite were added, the carbon-bearing pellets with biochar as the reducing agent underwent severe swelling during calcination, resulting in a loose and distorted shape. Due to ignorable strength, it is difficult to remove from the crucible. When the addition ratio reached 5%, the pellets still maintained their basic spherical shape after calcination but experienced some degree of swelling, as shown in
Figure 1. Compressive strength testing was carried out, and the pellets exhibited a compressive strength of 23 N after calcination at 900 °C and 57 N at 1000 °C, which still shows a relatively low strength. Note that the same proportion of binder was added when coke powder and anthracite were used as reducing agents to prepare carbon-bearing pellets to facilitate comparison.
3.1. Influence of Different Reduction Temperatures
Using coke powder, anthracite, and biochar as reducing agents, the pellets were subjected to calcination reduction under natural alkalinity conditions. The reduction temperature ranged from 400 to 1200 °C, and the reduction time was set at 25 min. The results of compressive strength testing after calcination are shown in
Figure 2. It can be observed that there are significant differences in the strength of carbon-bearing pellets prepared with different reducing agents. The compressive strength of pellets made with coke powder is the highest, followed by anthracite, while biochar exhibits the lowest strength. It is also shown that all three types of carbon-bearing pellets exhibit a similar trend in strength variation with different calcination temperatures. The strength initially decreases and then increases in the range of 400–1200 °C. The reason why the strength rapidly decreases from 400 to 900 °C is probably because the organic binder CMC gradually loses its effectiveness with increasing temperature, while the formation of iron phases has not yet occurred on a large scale, resulting in reduced strength. After 1000 °C, a significant amount of metallic iron is generated, leading to a rapid increase in strength, with the highest compressive strength exceeding 5000 N [
14,
15]. However, from
Figure 2, it can be seen that the compressive strength of biochar-containing pellets reaches a minimum of 900 °C and 1000 °C, measuring only 23 N and 57 N, respectively. Additionally, severe swelling in these pellets was observed, which fails to meet the requirements for multilayer charging during calcination in a rotary hearth furnace. Moreover, the swelling changes the space between the layers of material, thereby reducing the heat transfer efficiency of the rotary hearth furnace [
16,
17].
The metallization rate (
M) is an important indicator of the reduction effect of reactive pellets and was calculated using Equation (1), where MFe% is the metallic iron content of the carbon-containing pellet at a given moment, and TFe% is the total iron content of the carbon-containing pellet at a given moment.
Figure 3 shows the metallization rate of pellets reduced at different temperatures. As shown in
Figure 3, carbon-bearing pellets made with anthracite and biochar exhibit a similar trend with increasing reduction temperature. The metallization rate of the pellets shows a linear increase between 800 and 1100 °C. Above 1100 °C, although the metallization rate continues to rise with temperature, the rate of increase significantly slows down. For pellets reduced with anthracite as the reducing agent, the metallization rate reaches 82.53% when calcined at 1200 °C for 25 min, while the metallization rate reaches 86.66% for pellets reduced with biochar. Due to its lower reactivity compared to anthracite and biochar, coke powder exhibits a substantial increase in metallization rate only after calcination at 900–1000 °C, with a growth rate higher than the other two reducing agents, reaching 97.11% at 1200 °C. There is no significant difference between using biochar as a reducing agent and traditional reducing agents such as coke powder and anthracite. All three types of carbon-bearing pellets achieve a metallization rate above 80% under the conditions of calcination at 1200 °C for 25 min.
Figure 4 shows the scanning electron microscope (SEM) images of carbon-bearing pellets with biochar at different reduction temperatures, which are 400 °C and 1200 °C, with a reduction time of 25 min. The cross-sectional structures are shown in
Figure 4.
As shown in
Figure 4, after calcination at 400 °C, no reduction reaction occurs inside the pellets as the inner pellets consisting entirely of iron oxides and carbon was observed by the microscopic structure. Via the cross-sectional SEM images of the three types of carbon-bearing pellets, it can be observed that the metallic iron phase inside the pellets increases dramatically when the reduction temperature is increased to 1200 °C. Dendritic structures form in a specific area, and the amount of ferrite and residual carbon decreases. The metallic iron phase, ferrite, and slag phase are closely combined, while a large amount of low-melting-point slag phase fills the pores generated by carbon consumption. The internal pore size of the pellets shrinks and is evenly dispersed, resulting in a denser internal structure [
18,
19,
20].
Figure 5 shows the XRD analysis results of the pellets at different reduction temperatures. From
Figure 6, it can be observed that there is no significant difference in the reduction sequence of iron oxides in iron ore powder when coke powder, anthracite, or biochar is used as the reducing agent. At temperatures below 1000 °C, the main reduction reaction of iron oxides is “Fe
3O
4→FeO”, with a small amount of FeO being reduced to metallic iron. When the temperature exceeds 1000 °C, the reaction rate increases, and a significant amount of FeO is reduced to metallic iron. However, the XRD pattern of coke powder at 1100 °C still shows characteristic peaks of FeO, indicating that the reaction is incomplete at this temperature due to the poor reactivity of coke powder, consistent with the metallization rate detection results. When the temperature reaches 1200 °C, the metallization rates of all three types of pellets exceed 80%.
Based on the metallization rate, XRD diffraction analysis, and SEM spectrum analysis, biochar does not show significant differences as a reducing agent compared to coke powder and anthracite in the direct reduction in pellets. At 1200 °C, the metallization rates of the pellets can all reach above 80% after calcination for 25 min. Furthermore, biochar possesses renewable advantages that coke powder and anthracite lack, making it highly promising for development and utilization. However, the severe swelling and strength decrease in carbon-bearing pellets made with biochar at 900–1000 °C are currently urgent problems to be addressed.
3.2. Influence of Different Carbonto-Oxygen Ratios
The carbon-to-oxygen ratio is the molar ratio of carbon in the pellet to the oxygen content in the iron oxide and is an important factor affecting the performance of carbon-containing pellets. To investigate the effects of different carbon-to-oxygen ratios on the strength and metallization rate of carbon-bearing pellets, biochar was used as the reducing agent. Carbon-to-oxygen ratios of 0.5, 0.7, 0.9, and 1.1 were chosen. Biochar was mixed with iron ore powder and binder, and the mixture was then pressed into pellets. The prepared pellets were dried at 105 °C for 12 h and subjected to reduction experiments at a constant temperature of 1200 °C for 25 min to evaluate the impact of different carbon-to-oxygen ratios on the reduction in carbon-bearing pellets.
The compressive strength of the carbon-bearing pellets after calcination under different carbon-to-oxygen ratios is shown in
Figure 7. It can be seen that when the carbon-to-oxygen ratio is below 0.9, all the pellets exhibit compressive strengths exceeding 5000 N after calcination at 1200 °C for 25 min. However, when the carbon-to-oxygen ratio reaches 1.1, the pellets have no strength after calcination due to the possible existence of an appropriate range for the carbon content in the pellets. When the carbon content exceeds this range, the pyrolysis and volatilization of the reducing agent (biochar) indirectly hinder the formation of metallic iron. This is because the reducing agent carries away a significant amount of heat, while the formation of metallic iron dendrites determines the compressive strength of the carbon-bearing pellets. Therefore, once the carbon content exceeds a certain level, the strength of the carbon-bearing pellets begins to decline.
According to
Figure 7, the metallization rate of different C/O pellets after sintering varies with the carbon-to-oxygen ratio. It can be observed that as the carbon-to-oxygen ratio increases, the total iron content and metallic iron content in the pellets gradually increase, leading to an increase in the metallization rate consequently. Although the strength of the pellets is high when the carbon-to-oxygen ratio is 0.5, the metallization rate is relatively low at 54.86%. However, when the carbon-to-oxygen ratio is 0.7, the metallization rate reaches a relatively high level. Further increasing the carbon-to-oxygen ratio does not significantly affect the metallization rate. Therefore, it is recommended to choose a carbon-to-oxygen ratio ranging from 0.7 to 0.9.
3.3. Influence of Reduction Time
Based on the aforementioned discussion, pellets were subjected to different reduction times, namely 15 min, 25 min, and 35 min, at a temperature of 1200 °C to investigate the effects of reduction time on the strength and metallization rate of carbon-bearing pellets. In the experiments, biochar was chosen as the reducing agent. Biochar, iron ore powder, and binder were mixed and pressed into pellets with a carbon-to-oxygen ratio (C/O) of 0.7.
The mentalization rate of carbonized biochar pellets at different roasting times is shown in
Figure 8. It can be observed that with the extension of roasting time, the content of metallic iron gradually decreases, leading to a decrease in the metallization rate. This is probably because the reduction reaction is already completed before 25 min. A small amount of oxygen enters subsequently during the roasting process, leading to the oxidation of metallic iron under weak oxidizing conditions. However, at all three roasting times, the mentalization rate of the pellets remains above 80%. Due to the significant generation of metallic iron, the internal structure of the pellets becomes denser, resulting in a compressive strength of 5000 N.
Via research on different reduction temperatures, C/O ratios, and roasting times, it was found that using biochar as a reducing agent, a reduction temperature of 1200 °C, a C/O ratio of 0.7, and a roasting time of 15–25 min can achieve a mentalization rate of over 85% for the pellets, while maintaining the compressive strength within the quality requirements for raw materials in the blast furnace. Currently, there is not much difference in the low-temperature and high-temperature roasting strength between carbonized biochar pellets and coke powder-containing pellets. However, the strength of carbonized biochar pellets is undesirable at temperatures between 900 °C and 1000 °C due to the excessive expansion, making them unable to apply in rotary hearth furnaces.
3.5. Pellet Strength after Adding Type D Binder
Previous studies have indicated that a reduction temperature of 1200 °C, a carbon-to-oxygen atomic ratio of 0.7–0.9, and a roasting time of 15–25 min meet the requirements for strength and metallization rate. In this experiment, an 8% Type D binder was added with a carbon-to-oxygen atomic ratio of 0.8. The pellets were then roasted at temperatures of 400 °C, 600 °C, 800 °C, 900 °C, 1000 °C, 1100 °C, and 1200 °C, respectively. The morphology of the pellets after roasting is shown in
Figure 10, and the reduced pellet volume was measured, as shown in
Figure 11. It can be observed that the biochar carbon-bearing pellets did not show volume expansion during the roasting process. Instead, there was a significant reduction in volume due to the reduction reaction after 1000 °C, and the volume shrinkage rate reached 69.02% at 1200 °C.
After adding Type D binder, the compressive strength of biomass carbon-bearing pellets is shown in
Figure 12. From
Figure 12, it can be observed that the addition of 8% Type D binder significantly improves the compressive strength compared to the addition of bentonite. Furthermore, it can be seen that the pellet strength initially decreases, then increases, and finally decreases again after adding the Type D binder. The reason is that when the temperature rises to around 400 °C, the organic binder CMC loses its effectiveness, resulting in a decrease in strength. However, when the temperature reaches approximately 600 °C, the Type D binder becomes effective, ensuring the low-temperature strength of the pellets. After roasting at 900 °C, the strength increases from an initial 23 N to 1238 N, and after roasting at 1000 °C, the strength increases from 57 N to 814 N, meeting the requirements for production in the rotary hearth furnace.
After adding Type D binder, the results of metallization rates of pellets at different reduction temperatures are shown in
Figure 13. It can be seen that the metallization rate gradually increases with the increase in temperature. At 1200 °C, the metallization rate slightly decreases because the pellet reduction is already completed before 25 min, and subsequent slight oxidation occurs.