Effects of Drainage on Carbon Stock in Hemiboreal Forests: Insights from a 54-Year Study
Abstract
:1. Introduction
2. Materials and Methods
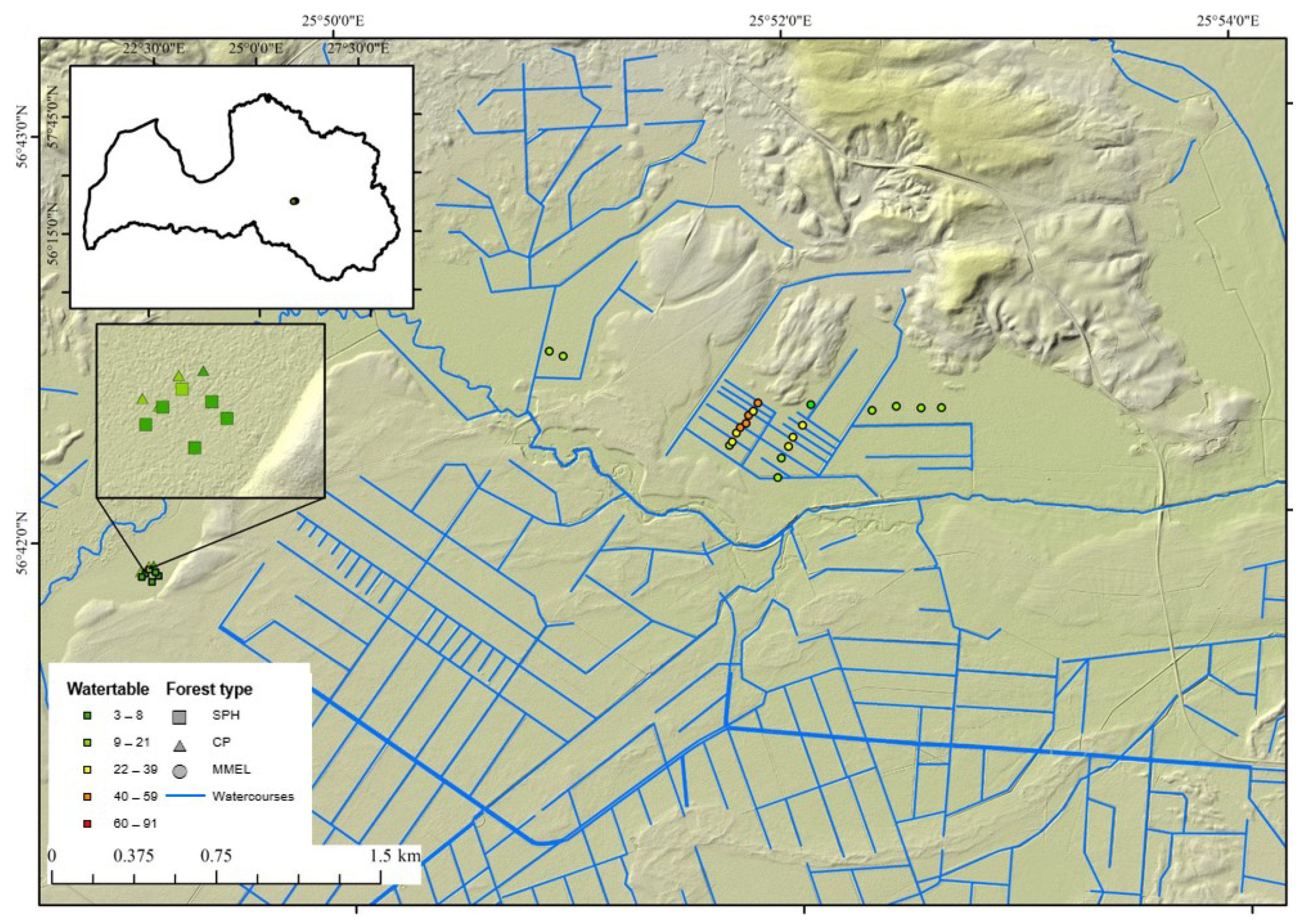
2.1. Location of Sample Plots
2.2. Field Survey
2.3. Sample Preparation and Measurements
2.4. Calculations and Data Analyses
3. Results
3.1. Stand Parameters
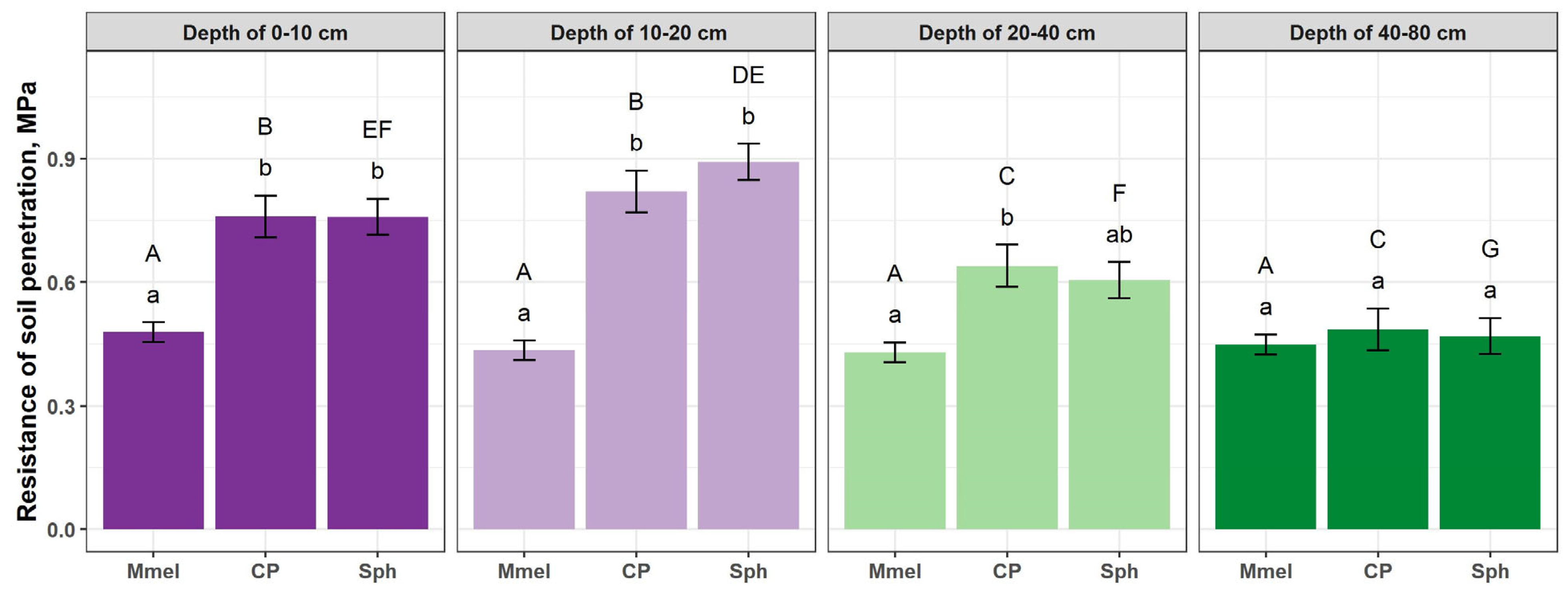
3.2. Soil Parameters
3.3. Soil Characteristics
3.4. Carbon Stock
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Bradshaw, C.J.A.; Warkentin, I.G. Global estimates of boreal forest carbon stocks and flux. Glob. Planet. Chang. 2015, 128, 24–30. [Google Scholar] [CrossRef]
- Jauhiainen, J.; Alm, J.; Bjarnadottir, B.; Callesen, I.; Christiansen, J.R.; Clarke, N.; Dalsgaard, L.; He, H.; Jordan, S.; Kazanavičiūtė, V.; et al. Reviews and syntheses: Greenhouse gas exchange data from drained organic forest soils—A review of current approaches and recommendations for future research. Biogeosciences 2019, 16, 4687–4703. [Google Scholar] [CrossRef]
- Barthelmes, H.; Couwenberg, J.; Risager, M.; Tegetmeyer, C.; Joosten, H. Peatlands and Climate in a Ramsar Context: A Nordic-Baltic Perspective; Nordic Council of Ministers: Copenhagen, Denmark, 2015.
- Fenton, N.J.; Bergeron, Y. Facilitative succession in a boreal bryophyte community driven by changes in available moisture and light. J. Veg. Sci. 2006, 17, 65–76. [Google Scholar] [CrossRef]
- Simard, M.; Lecomte, N.; Bergeron, Y.; Bernier, P.Y.; Paré, D. Forest productivity decline caused by successional paludification of boreal soils. Ecol. Appl. 2007, 17, 1619–1637. [Google Scholar] [CrossRef] [PubMed]
- Kozlowski, T.T. Water supply and tree growth. Part II. Flooding. For. Abstr. 1982, 43, 145–161. [Google Scholar]
- Glenz, C.; Schlaepfer, R.; Iorgulescu, I.; Kienast, F. Flooding tolerance of Central European tree and shrub species. For. Ecol. Manag. 2006, 235, 1–13. [Google Scholar] [CrossRef]
- Gower, S.T.; Vogel, J.G.; Norman, J.M.; Kucharik, C.J.; Steele, S.J.; Stow, T.K. Carbon distribution and aboveground net primary production in aspen, jack pine, and black spruce stands in Saskatchewan and Manitoba, Canada. J. Geophys. Res. Atmos. 1997, 102, 29029–29041. [Google Scholar] [CrossRef]
- Schulze, E.D.; Lloyd, J.; Kelliher, F.M.; Wirth, C.; Rebmann, C.; Lühker, B.; Mund, M.; Knohl, A.; Milyukova, I.M.; Schulze, W.; et al. Productivity of forests in the Eurosiberian boreal region and their potential to act as a carbon sink—A synthesis. Glob. Chang. Biol. 1999, 5, 703–722. [Google Scholar] [CrossRef]
- Martin, J.L.; Gower, S.T.; Plaut, J.; Holmes, B. Carbon pools in a boreal mixedwood logging chronosequence. Glob. Chang. Biol. 2005, 11, 1883–1894. [Google Scholar] [CrossRef]
- Ķēniņa, L.; Jaunslaviete, I.; Liepa, L.; Zute, D.; Jansons, Ā. Carbon Pools in Old-Growth Scots Pine Stands in Hemiboreal Latvia. Forests 2019, 10, 911. [Google Scholar] [CrossRef]
- Ojanen, P.; Minkkinen, K.; Penttilä, T. The current greenhouse gas impact of forestry-drained boreal peatlands. For. Ecol. Manag. 2019, 289, 201–208. [Google Scholar] [CrossRef]
- Kasimir-Klemedtsson, Å.; Klemedtsson, L.; Berglund, K.; Martikainen, P.; Silvola, J.; Oenema, O. Greenhouse gas emissions from farmed organic soils: A review. Soil Use Manag. 1997, 13, 245–250. [Google Scholar] [CrossRef]
- Petrescu, A.M.R.; Lohila, A.; Tuovinen, J.P.; Baldocchi, D.D.; Desai, A.R.; Roulet, N.T.; Vesala, T.; Dolman, A.J.; Oechel, W.C.; Marcolla, B.; et al. The uncertain climate footprint of wetlands under human pressure. Proc. Natl. Acad. Sci. USA 2015, 112, 4594–4599. [Google Scholar] [CrossRef] [PubMed]
- Strack, M.; Waddington, J.M. Spatiotemporal variability in peatland subsurface methane dynamics. J. Geophys. Res. Biogeosci. 2018, 113, JG000472. [Google Scholar] [CrossRef]
- Holmgren, K.; Hagberg, L. Life Cycle Assessment of Climate Impact of Fischer-Tropsch Diesel Based on Peat and Biomass; IVL Svenska Miljöinstitutet: Stockholm, Sweden, 2009. [Google Scholar]
- Hagberg, L.; Holmgren, K. The Climate Impact of Future Energy Peat Production; IVL Svenska Miljöinstitutet: Stockholm, Sweden, 2009. [Google Scholar]
- Minkkinen, K.; Ojanen, P.; Penttilä, T.; Aurela, M.; Laurila, T.; Tuovinen, J.-P.; Lohila, A. Persistent carbon sink at a boreal drained bog forest. Biogeosciences 2018, 15, 3603–3624. [Google Scholar] [CrossRef]
- Lohila, A.; Minkkinen, K.; Aurela, M.; Tuovinen, J.-P.; Penttilä, T.; Ojanen, P.; Laurila, T. Greenhouse gas flux measurements in a forestry-drained peatland indicate a large carbon sink. Biogeosciences 2011, 8, 3203–3218. [Google Scholar] [CrossRef]
- Turetsky, M.R.; Donahue, W.F.; Benscoter, B.W. Experimental drying intensifies burning and carbon losses in a northern peatland. Nat. Commun. 2011, 2, 514. [Google Scholar] [CrossRef]
- Nieminen, M.; Koskinen, M.; Sarkkola, S.; Laurén, A.; Kaila, A.; Kiikkilä, O.; Nieminen, T.M.; Ukonmaanaho, L. Dissolved Organic Carbon Export from Harvested Peatland Forests with Differing Site Characteristics. Water Air Soil Pollut. 2015, 226, 181. [Google Scholar] [CrossRef]
- Peacock, M.; Granath, G.; Wallin, M.B.; Högbom, L.; Futter, M.N. Significant Emissions From Forest Drainage Ditches—An Unaccounted Term in Anthropogenic Greenhouse Gas Inventories? J. Geophys. Res. Biogeosci. 2021, 126, e2021JG006478. [Google Scholar] [CrossRef]
- Laiho, R.; Vasander, H.; Penttilä, T.; Laine, J. Dynamics of plant-mediated organic matter and nutrient cycling following water-level drawdown in boreal peatlands. Glob. Biogeochem. Cycles 2013, 17, 1–11. [Google Scholar] [CrossRef]
- Straková, P.; Penttilä, T.; Laine, J.; Laiho, R. Disentangling direct and indirect effects of water table drawdown on above- and belowground plant litter decomposition: Consequences for accumulation of organic matter in boreal peatlands. Glob. Chang. Biol. 2012, 18, 322–335. [Google Scholar] [CrossRef]
- Uri, V.; Kukumägi, M.; Aosaar, J.; Varik, M.; Becker, H.; Aun, K.; Lõhmus, K.; Soosaar, K.; Astover, A.; Uri, M.; et al. The dynamics of the carbon storage and fluxes in Scots pine (Pinus sylvestris) chronosequence. Sci. Total Environ. 2022, 817, 152973. [Google Scholar] [CrossRef] [PubMed]
- Lupikis, A.; Lazdins, A. Soil carbon stock changes in transitional mire drained for forestry in Latvia: A case study. Res. Rural Develop. 2017, 1, 55–61. [Google Scholar] [CrossRef]
- Ķēniņa, L.; Zute, D.; Jaunslaviete, I.; Samariks, V.; Jansons, Ā. Old-Growth Coniferous Stands on Fertile Drained Organic Soil: First Results of Tree Biomass and Deadwood Carbon Stocks in Hemiboreal Latvia. Forests 2022, 13, 279. [Google Scholar] [CrossRef]
- Bārdule, A.; Butlers, A.; Lazdiņš, A.; Līcīte, I.; Zvirbulis, U.; Putniņš, R.; Jansons, A.; Adamovičs, A.; Razma, Ģ. Evaluation of Soil Organic Layers Thickness and Soil Organic Carbon Stock in Hemiboreal Forests in Latvia. Forests 2021, 12, 840. [Google Scholar] [CrossRef]
- Butlers, A.; Lazdiņš, A.; Kalēja, S.; Bārdule, A. Carbon Budget of Undrained and Drained Nutrient-Rich Organic Forest Soil. Forests 2022, 13, 1790. [Google Scholar] [CrossRef]
- Butlers, A.; Lazdiņš, A.; Kalēja, S.; Purviņa, D.; Spalva, G.; Saule, G.; Bārdule, A. CH4 and N2O Emissions of Undrained and Drained Nutrient-Rich Organic Forest Soil. Forests 2023, 14, 1390. [Google Scholar] [CrossRef]
- Peacock, M.; Audet, J.; Bastviken, D.; Futter, M.N.; Gauci, V.; Grinham, A.; Harrison, J.A.; Kent, M.S.; Kosten, S.; Lovelock, C.E. Global importance of methane emissions from drainage ditches and canals. Environ. Res. Lett. 2021, 16, 044010. [Google Scholar] [CrossRef]
- Bušs, K. Forest ecosystem classification in Latvia. Proc. Latv. Acad. Sci. Latv. 1997, 51, 204–218. [Google Scholar]
- Liepa, I. Tree Growth Study; LLU: Jelgava, Latvia, 1996. [Google Scholar]
- Köster, K.; Metslaid, M.; Engelhart, J.; Köster, E. Dead wood basic density, and the concentration of carbon and nitrogen for main tree species in managed hemiboreal forests. For. Ecol. Manag. 2015, 354, 35–42. [Google Scholar] [CrossRef]
- Liepiņš, J.; Lazdiņš, A.; Liepiņš, K. Equations for estimating above–and belowground biomass of Norway spruce, Scots pine, birch spp. and European aspen in Latvia. Scand. J. For. Res. 2017, 33, 1–43. [Google Scholar] [CrossRef]
- Neumann, M.; Moreno, A.; Mues, V.; Härkönen, S.; Mura, M.; Bouriaud, O.; Lang, M.; Achten, W.M.; Thivolle-Cazat, A.; Bronisz, K.; et al. Comparison of carbon estimation methods for European forests. For. Ecol. Manag. 2016, 361, 397–420. [Google Scholar] [CrossRef]
- Thomas, S.C.; Martin, A.R. Carbon Content of Tree Tissues: A Synthesis. Forests 2012, 3, 332–352. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2020. [Google Scholar]
- Bates, D.; Maechler, M.; Bolker, B.; Walker, S. Fitting Linear Mixed-Effects Models Using {lme4}. J. Stat. Softw. 2015, 67, 1–48. [Google Scholar] [CrossRef]
- Wickham, H.; François, R.; Henry, L.; Müller, K. dplyr: A Grammar of Data Manipulation. R Package Version 1.0.8. 2022. Available online: https://CRAN.R-project.org/package=dplyr (accessed on 10 October 2023).
- Rusell, V.L. emmeans: Estimated Marginal Means, Aka Least-Squares Means. R Package, 1.7.3. 2022. Available online: https://CRAN.R-project.org/package=emmeans (accessed on 10 October 2023).
- Skaggs, R.W.; Tian, S.; Chescheir, G.M.; Amatya, D.M.; Youssef, M.A. Forest Drainage; CABI Books: Wallingford, UK, 2023. [Google Scholar] [CrossRef]
- Hillman, G.R.; Roberts, J.J. Tamarack and Black Spruce Growth on a Boreal Fen in Central Alberta 9 Years after Drainage. New For. 2006, 31, 225–243. [Google Scholar] [CrossRef]
- Potapov, A.; Mehtätalo, L.; Kiviste, A.; Metslaid, S.; Kaart, T.; Stanturf, J.A.; Hordo, M. Basal area growth response of Scots pine to drainage: An analysis using a mixed-effects modeling approach. For. Ecol. Manag. 2023, 532, 120825. [Google Scholar] [CrossRef]
- Minkkinen, K.; Vasander, H.; Jauhiainen, S.; Karsisto, M.; Laine, J. Post-drainage changes in vegetation composition and carbon balance in Lakkasuo mire, Central Finland. Plant Soil 1999, 27, 107–120. [Google Scholar]
- Minkkinen, K.; Laine, J. Long-term effect of forest drainage on the peat carbon stores of pine mires in Finland. Can. J. For. Res. 1998, 28, 1267–1275. [Google Scholar] [CrossRef]
- Charman, D.J.; Aravena, R.; Warner, B.G. Carbon Dynamics in a Forested Peatland in North-Eastern Ontario, Canada. J. Ecol. 1994, 82, 55–62. [Google Scholar] [CrossRef]
- Domisch, T.; Finér, L.; Karsisto, M.; Laiho, R.; Laine, J. Relocation of carbon from decaying litter in drained peat soils. Soil Biol. Biochem. 1998, 30, 1529–1536. [Google Scholar] [CrossRef]
- Aerts, R. Climate, leaf litter chemistry and leaf litter decomposition in terrestrial ecosystems: A triangular relationship. Oikos 1997, 79, 439–449. [Google Scholar] [CrossRef]
- Gholz, H.; Wedin, D.; Smitherman, S.M. Long-term dynamics of pine and hardwood litter in contrasting environments: Toward a global model of decomposition. Glob. Chang. Biol. 2000, 6, 750–756. [Google Scholar] [CrossRef]
- Von Arnold, K.; Hånell, B.; Stendahl, J.; Klemedtsson, L. Greenhouse gas fluxes from drained organic forestland in Sweden. Scand. J. For. Res. 2000, 20, 400–411. [Google Scholar] [CrossRef]
- Murphy, D.M.; Solomon, S.; Portmann, R.W.; Rosenlof, K.H.; Forster, P.M.; Wong, T. An observationally based energy balance for the Earth since 1950. J. Geophys. Res. Atmos. 2009, 114, JD012105. [Google Scholar] [CrossRef]



| Coefficient | Scots Pine | Norway Spruce | ||
|---|---|---|---|---|
| a | b | a | b | |
| Stem | 2.94 | 0.020 | 2.82 | 0.040 |
| Living branches | 1.576 | 0.201 | 1.639 | 0.336 |
| Dead branches | 2.102 | 0.006 | 3.289 | 0.000 |
| Stump | 2.442 | 0.007 | 2.698 | 0.004 |
| Coarse roots | 3.227 | 0.001 | 2.998 | 0.004 |
| Fine roots | 1.82 | 0.017 | 1.843 | 0.026 |
| Carbon Stock (Tons ha−1) | Mmel | CP | Sph |
|---|---|---|---|
| Stem | 53.54 ± 15.14 | 20.94 ± 10.95 | 6.08 ± 6.94 |
| Living branches | 16.20 ± 8.11 | 7.93 ± 3.21 | 6.28 ± 5.10 |
| Dead branches | 1.38 ± 0.43 | 0.91 ± 0.34 | 0.37 ± 0.55 |
| Coarse roots | 11.86 ± 4.76 | 3.55 ± 1.32 | 1.40 ± 1.67 |
| Fine roots | 12.04 ± 4.83 | 3.61 ± 1.34 | 1.42 ± 1.70 |
| Stump | 2.47 ± 1.04 | 1.31 ± 0.51 | 0.85 ± 0.78 |
| Total in biomass | 97.48 ± 27.41 | 38.27 ± 14.67 | 16.39 ± 16.12 |
| O horizon | 5.08 ± 2.06 | 0 | 0 |
| Soil depth 0–10 | 74.40 ± 16.34 | 41.80 ± 7.30 | 37.95 ± 4.52 |
| Soil depth 10–20 | 67.45 ± 15.01 | 42.12 ± 8.96 | 36.10 ± 5.29 |
| Soil depth 20–40 | 126.55 ± 28.33 | 85.87 ± 18.83 | 75.97 ± 16.65 |
| Soil depth 40–80 | 239.73 ± 52.12 | 208.12 ± 46.22 | 189.25 ± 40.31 |
| Total in soil | 513.21 ± 106.26 | 377.92 ± 79.51 | 339.28 ± 63.77 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dubra, S.; Samariks, V.; Līcīte, I.; Butlers, A.; Purviņa, D.; Lupiķis, A.; Jansons, Ā. Effects of Drainage on Carbon Stock in Hemiboreal Forests: Insights from a 54-Year Study. Sustainability 2023, 15, 16622. https://doi.org/10.3390/su152416622
Dubra S, Samariks V, Līcīte I, Butlers A, Purviņa D, Lupiķis A, Jansons Ā. Effects of Drainage on Carbon Stock in Hemiboreal Forests: Insights from a 54-Year Study. Sustainability. 2023; 15(24):16622. https://doi.org/10.3390/su152416622
Chicago/Turabian StyleDubra, Stefānija, Valters Samariks, Ieva Līcīte, Aldis Butlers, Dana Purviņa, Ainārs Lupiķis, and Āris Jansons. 2023. "Effects of Drainage on Carbon Stock in Hemiboreal Forests: Insights from a 54-Year Study" Sustainability 15, no. 24: 16622. https://doi.org/10.3390/su152416622
APA StyleDubra, S., Samariks, V., Līcīte, I., Butlers, A., Purviņa, D., Lupiķis, A., & Jansons, Ā. (2023). Effects of Drainage on Carbon Stock in Hemiboreal Forests: Insights from a 54-Year Study. Sustainability, 15(24), 16622. https://doi.org/10.3390/su152416622






