Organomineral Fertilization Associated with Inoculation of Rhizobium tropici and Co-Inoculation of Azospirillum brasilense in Common Bean
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Area Description
2.2. Origin and Characterization of Organic Waste
2.3. Formulation of the Organomineral Fertilizer
2.4. Experimental Design and Treatments
2.5. Experimental Unit and Crop Treatments
2.6. Development Analysis
2.7. Analysis of Yield and Its Components
2.8. Statistical Analysis
3. Results and Discussion
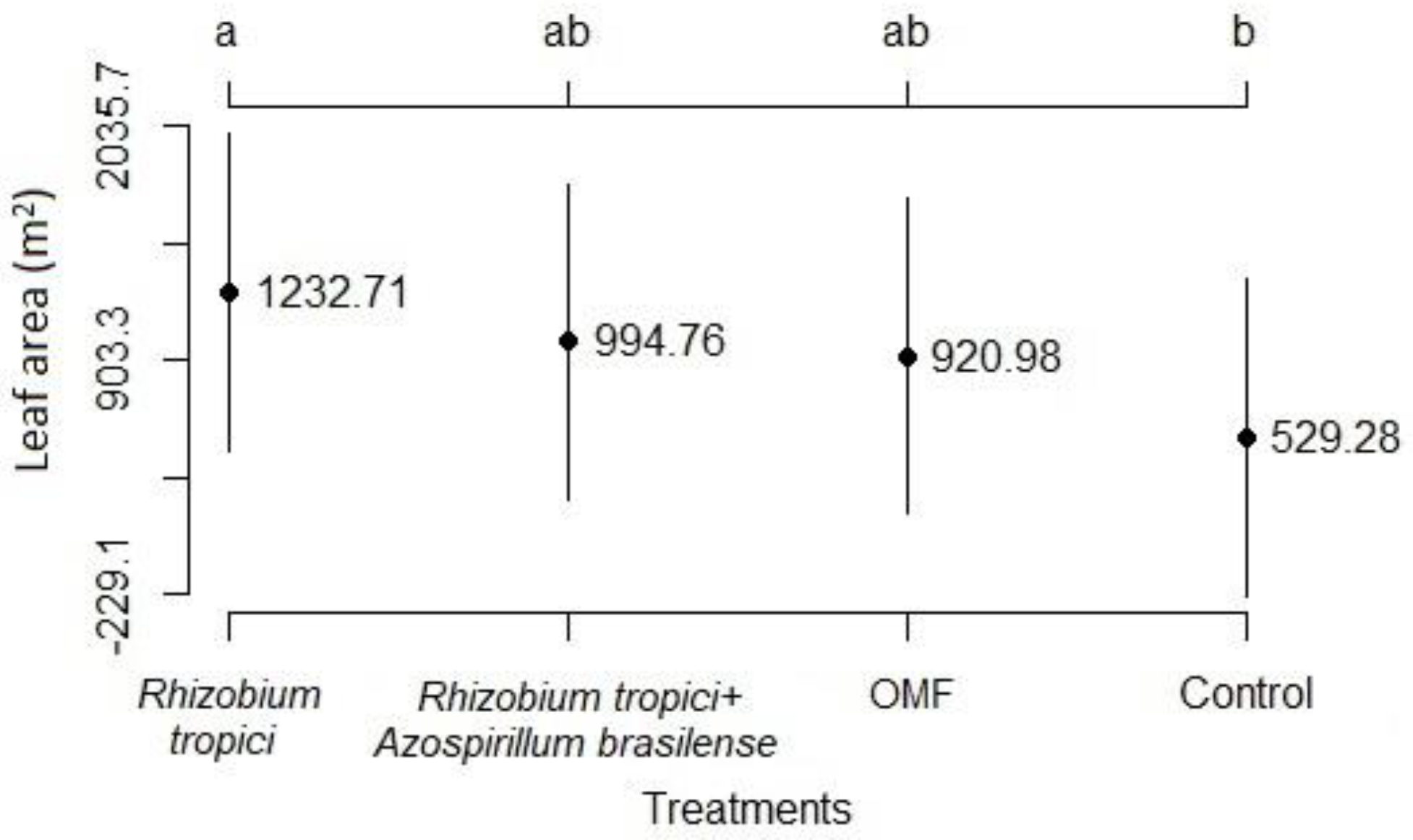
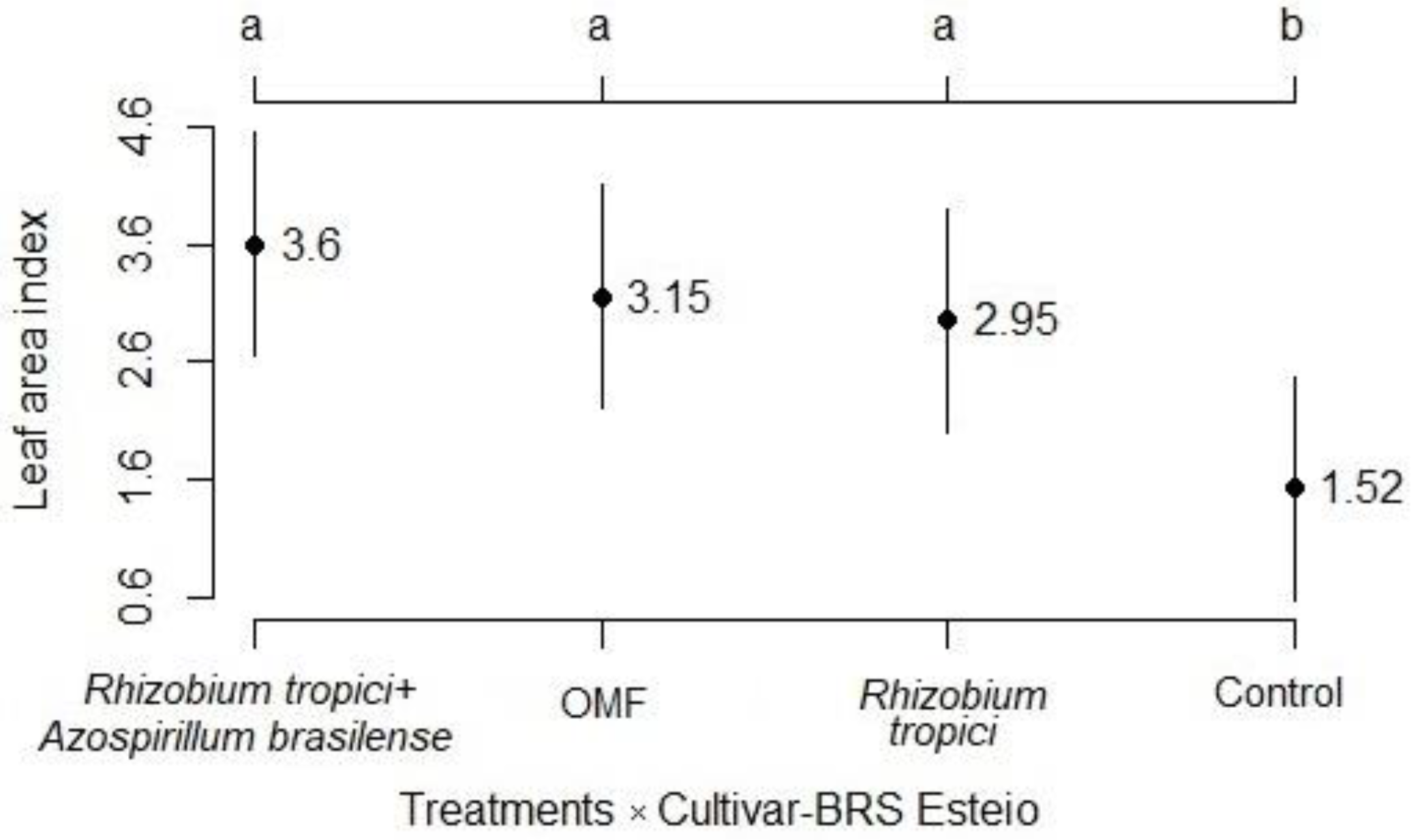
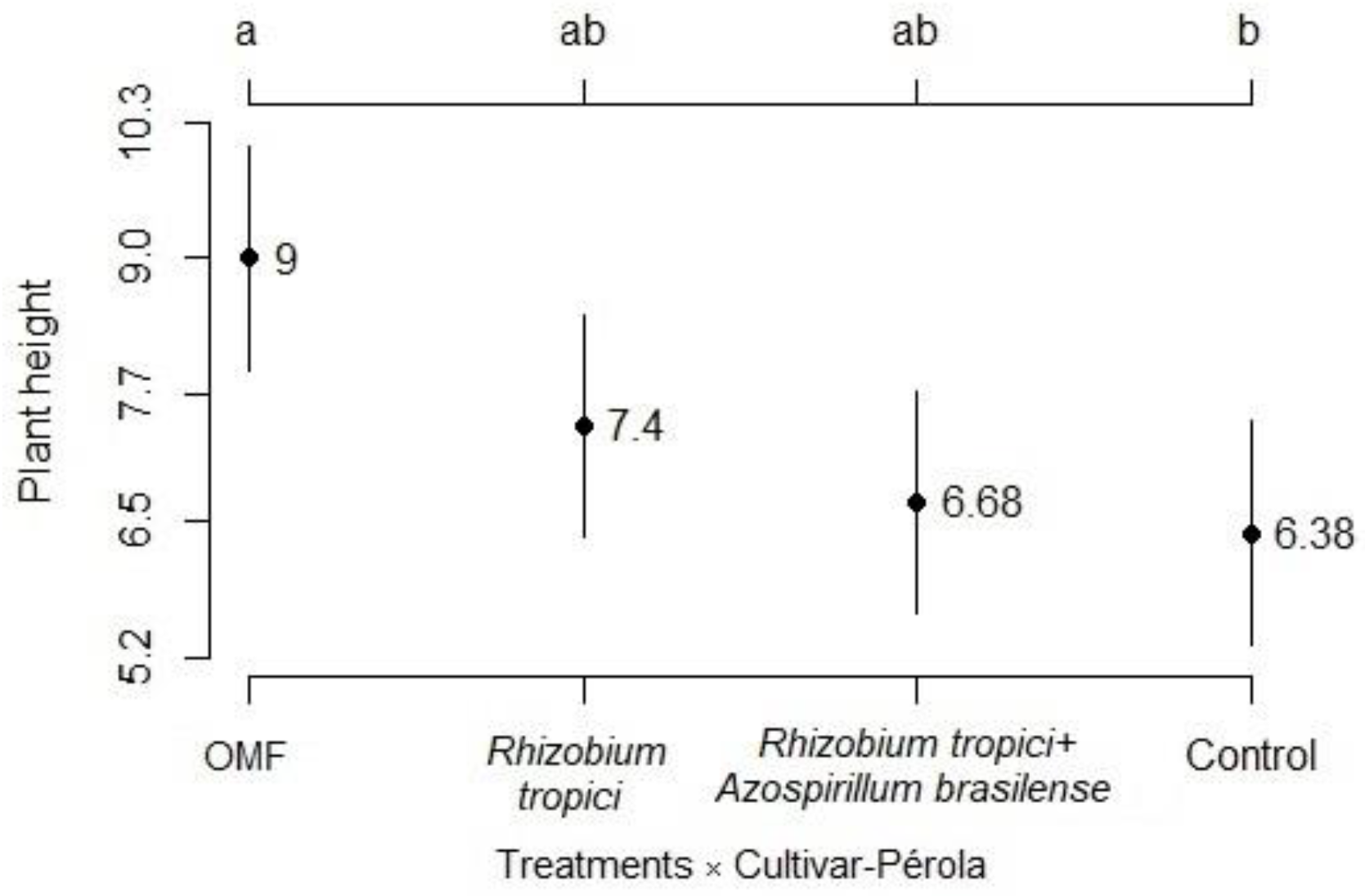
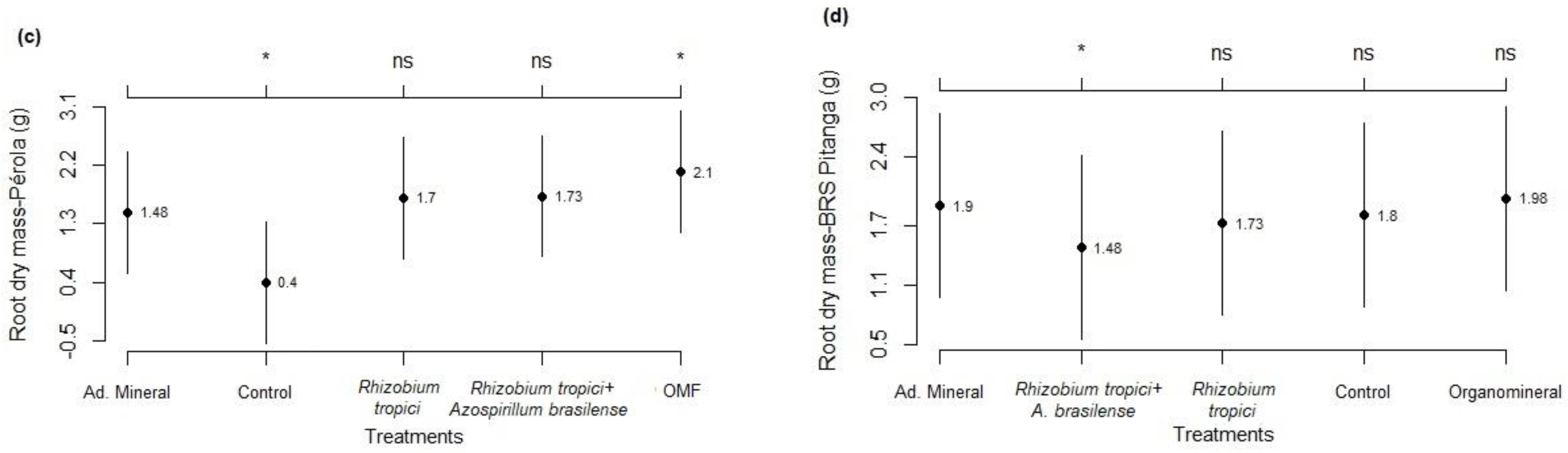
3.1. Development Analysis
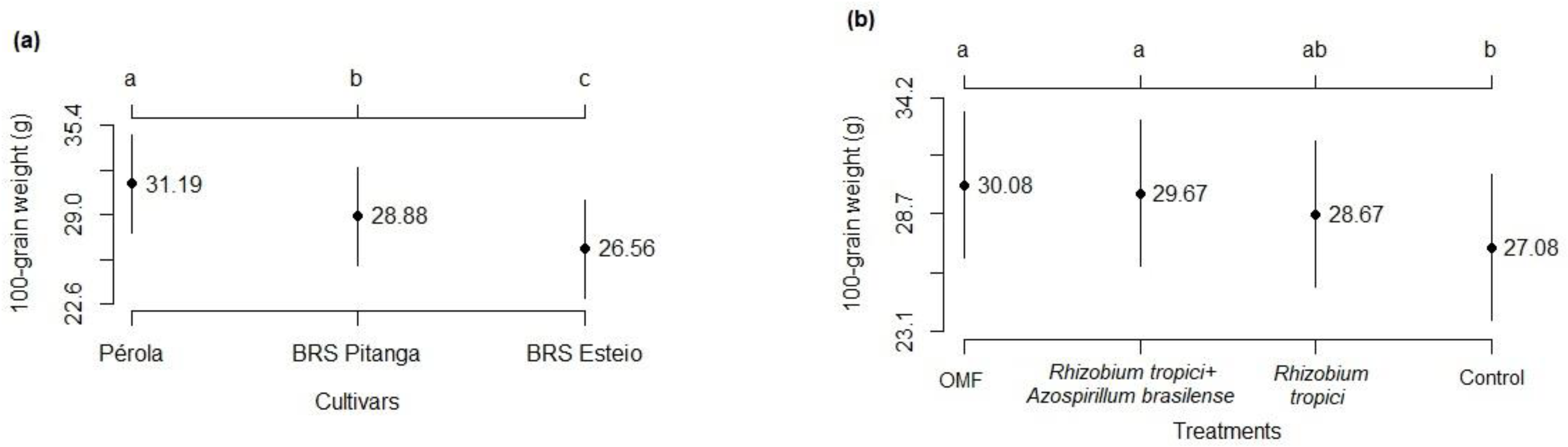
3.2. Components and Grain Yield
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Menegat, S.; Ledo, A.; Tirado, R. Greenhouse gas emissions from global production and use of nitrogen synthetic fertilizers in agriculture. Sci. Rep. 2022, 12, 14490. [Google Scholar] [CrossRef] [PubMed]
- Kominko, H.; Gorazda, k.; Wzorek, Z.; Wojtas, K. Sustainable management of sewage sludge for the production of organo-mineral fertilizers. Waste Biomass Valor. 2018, 9, 1817–1826. [Google Scholar] [CrossRef]
- Soumare, A.; Diedhiou, A.G.; Thuita, M.; Hafidi, M.; Ouhdouch, Y.; Gopalakrishnan, S.; Kouisni, L. Exploiting biological nitrogen fixation: A route towards sustainable agriculture. Plants 2020, 9, 1011. [Google Scholar] [CrossRef] [PubMed]
- Tullio, L.D.; Nakatani, A.S.; Gomes, D.F.; Ollero, F.J.; Megias, M.; Hungria, M. Revealing the roles of y4wF and tid C genes in Rhizobium tropici CIAT 899: Biosynthesis of indole compounds and impacton symbiotic properties. Arch. Microbiol. 2019, 201, 171–183. [Google Scholar] [CrossRef] [PubMed]
- Hungria, M.; Vargas, M.A.T. Environmental factors affecting N2 fixation in grain legumes in the tropics, with an emphasis on Brazil. Field Crops Res. 2000, 65, 151–164. [Google Scholar] [CrossRef]
- Shamseldin, A.; Velázquez, E. The promiscuity of Phaseolus vulgaris L. (common bean) for nodulation with rhizobia: A review. World J. Microbiol. Biotechnol. 2020, 36, 63. [Google Scholar] [CrossRef] [PubMed]
- Sousa, W.S.; Teixeira, I.R.; Campos, T.S.; Silva, G.C.; Silva, M.B.; Moreira, S.G. Supplementary reinoculation in a top dressingof in common bean crop: Effects on nodulation, morphology, and grain yield. J. Plant Nutr. 2021, 45, 1–7. [Google Scholar]
- Steiner, F.; Ferreira, H.C.P.; Zuffo, A.M. Canco-inoculation of Rhizobium tropici and Azospirillum brasilensein crease common bean nodulation and grain yield? Semin. Ciênc. Agrár. 2019, 40, 81–98. [Google Scholar] [CrossRef]
- Souza, J.E.B.; Ferreira, E.P.B. Improving sustainability of common bean production systems by co-inoculating rhizobia and azospirilla. Agric. Ecosyst. Environ. 2017, 237, 250–257. [Google Scholar] [CrossRef]
- Peres, A.R.; Rodrigues, R.A.F.; Arf, O.; Portugal, J.R.; Corsini, D.C.D.C. Co-inoculation of Rhizobium tropici and Azospirillum brasilense in common beans grown under two irrigation depths. Rev. Ceres 2016, 63, 198–207. [Google Scholar] [CrossRef]
- Google Earth. Google Earth Pro. Available online: https://earth.google.com/web/ (accessed on 11 June 2021).
- Borges, B.M.M.N.; Lucas, F.T.; Modesto, V.C.; Prado, R.M.; Silva, E.S.; Braos, B.B. Métodos de determinação da matéria seca e dos teores de macronutrientes em folhas de alface. Rev. Tróp. 2011, 5, 12–16. [Google Scholar]
- Pimentel-Gomes, F. Curso de Estatística Experimental, 13th ed.; Nobel: Piracicaba, Brazil, 1990. [Google Scholar]
- Moreira, L.P.; Oliveira, A.P.S.; Brito Ferreira, E.P. Nodulation, contribution of biological N2 fixation, and productivity of the common bean (Phaseolus vulgaris L.) inoculated with rhizobia solates. Aust. J. Crop Sci. 2017, 11, 644–651. [Google Scholar] [CrossRef]
- Marschner, P. Mineral Nutrition o Fighterplants, 3rd ed.; Academic Press: London, UK, 2011. [Google Scholar]
- Barros, R.L.N.; Oliveira, L.B.; Magalhães, W.B.; Pimentel, C. Growth an yield of common bean as affected by seed inoculation with rhizobium and nitrogen fertilization. Exper. Agric. 2016, 54, 16–30. [Google Scholar] [CrossRef]
- Silva, H.C.; Lima, L.C.; Camargo, R.; Lana, R.M.Q.; Lemes, E.M.; Cardoso. A.F. Effects of organomineral fertilizers formulated with biosolids and filter cake on common bean yield crop (Phaseolus vulgaris L.). Aust. J. Crop Sci. 2019, 13, 1566–1571. [Google Scholar] [CrossRef]
- Taliman, N.A.; Dong, Q.; Echigo, K.; Raboy, V.; Saneoka, H. Effect of phosphorus fertilization on the growth, photosynthesis, nitrogen fixation, mineral accumulation, seed yield, and seed quality of a soybean low-phytate line. Plants 2019, 8, 119. [Google Scholar] [CrossRef]
- Nadeem, M.A.; Karaköy, T.; Yeken, M.Z.; Habyarimana, E.; Hatipoğlu, R.; Çiftçi, V.; Nawaz, M.A.; Sönmez, F.; Shahid, M.Q.; Yang, S.H.; et al. Phenotypic characterization of 183 Turkish common bean accessions for agronomic, trading, and consumer-preferred plant characteristics for breeding purposes. Agronomy 2020, 10, 272. [Google Scholar] [CrossRef]
- Favero, V.O.; Carvalho, R.H.; Leite, A.B.C.; Santos, D.M.T.; Freitas, K.M.; Boddey, R.M.; Xavier, G.R.; Rumjanek, N.G.; Urquiaga, S. Bradyrhizobium strains from brazilian tropical soils promote increases in nodulation, growth and nitrogen fixation in mung bean. App. Soil Ecol. 2022, 175, 104461. [Google Scholar] [CrossRef]
- Fiori, A.K.; Gutuzzo, G.O.; Sanzovo, A.W.S.; Andrade, D.S.; Oliveira, A.L.M.; Rodrigues, E.P. Effects of Rhizobium tropici azide-resistant mutants on growth, nitrogen nutrition and nodulation of common bean (Phaseolus vulgaris L.). Rhizosphere 2021, 18, 100355. [Google Scholar] [CrossRef]
- Oliveira, A.P.S.; Sousa, C.M.; Ferreira, E.P.D.B. Performance of inoculated common bean in response to different cover crops and desiccation times. Caatinga 2017, 30, 642–652. [Google Scholar] [CrossRef]
- Sá, G.C.R.; Carvalho, C.L.M.; Hungria, M.A.; Nogueira, M.A.; Heinrichs, R.; Soares Filho, C.V. Biomass yield, nitrogen accumulation and nutritive value of Mavuno grass inoculated with plant growth-promoting bacteria. Commun. Soil Sci. Plant Anal. 2019, 50, 1931–1942. [Google Scholar] [CrossRef]
- Filipini, L.D.; Pilatti, F.K.; Meyer, E.; Ventura, B.S.; Lourenzi, C.R.; Lovato, P.E. Application of Azospirillum on seeds and leaves, associated with Rhizobium inoculation, increases growth and yield of common bean. Arch. Microbiol. 2021, 203, 1033–1038. [Google Scholar] [CrossRef] [PubMed]
- Bai, Y.; Zhou, X.; Smith, D.L. Enhanced Soybean Plant Growth Resulting from Coinoculation of Bacillus Strains with Bradyrhizobium japonicum. Crop Sci. 2003, 43, 1774. [Google Scholar] [CrossRef]
- Mumbach, G.L.; Gatiboni, L.C.; Bona, F.D.; Smitt, D.E.; Corrêa, J.C.; Gabriel, C.A.; Daniel, J.; Dall’Orsoletta, D.J.; Lochims, D.A. Agronomic efficiency of organomineral fertilizer in sequential grain crops in southern Brazil. J. Agron. 2020, 112, 3037–3049. [Google Scholar] [CrossRef]
- Bettiol, J.V.T.; Filla, V.A.; Leal, F.T.; Coelho, A.P.; Meirelles, F.C.; Lemos, L.B. Sustainable production of common beans: Inoculation, co-inoculation and mineral fertilization in early-cycle cultivars. J. Plant Nut. 2020, 44, 16–28. [Google Scholar] [CrossRef]
- Yadegari, M. Inoculation of bean (Phaseolus vulgaris) seeds with Rizobium phaseoli and plant growth promoting rhizobacteria. Adv. Environ. Biol. 2014, 8, 419–424. [Google Scholar]
- Chekanai, V.; Chikowo, R.; Vanlauwe, B. Response of common bean (Phaseolus vulgaris L.) to nitrogen, phosphorus and rhizobia inoculation across variable soils in Zimbabwe. Agric. Ecosyst. Environ. 2018, 266, 167–173. [Google Scholar] [CrossRef] [PubMed]
- Mortinho, E.S.; Jalal, A.; Oliveira, C.E.S.; Fernandes, G.C.; Pereira, N.C.M.; Rosa, P.A.L.; Nascimento, V.; Sá, M.E.; Teixeira Filho, M.C.M. Co-inoculations with plant growth-promoting bacteria in the common bean to increase efficiency of NPK fertilization. Agronomy 2022, 12, 1325. [Google Scholar] [CrossRef]
- Shumet, S.T.; Ayalew, T.; Roro, A.G.; Beshir, H.M. Intercropping and Rhizobium inoculation affected microclimate and performance of common bean (Phaseolus vulgaris L.) varieties. Scientifica 2022, 2022, 3471912. [Google Scholar] [CrossRef]
- Conab. Safra Brasileira de Grãos. 2022. Available online: https://www.conab.gov.br/info-agro/safras/serie-historica-das-safras/itemlist/category/905-feijao (accessed on 8 June 2022).
- Smith, W.B.; Wilson, M.; Pagliari, P. Organomineral fertilizers and their application to field crops. In Animal Manure: Production, Characteristics, Environmentalconcerns, and Management; Waldrip, H.M., Pagliari, P.H., He, Z., Eds.; Wiley: Hoboken, NJ, USA, 2020; pp. 229–243. [Google Scholar]
- Almeida, V.; Carneiro, G.; Almeida, R.; Souza, B.; Teixeira, I.; Vieira, J.; Leandro, W. Development and yield of common bean in response to organomineral fertilization based on filter cake. J. Plant Nutr. 2023, 46, 3292–3311. [Google Scholar] [CrossRef]
- Yagi, R.; Andrade, D.S.; Waureck, A.; Gomes, J.C. Nodulations and grain yields of common beans in response to nitrogen fertilization or seed inoculation with Rhizobium freirei. Rev. Bras. Ciênc. Solo 2015, 39, 1661–1670. [Google Scholar] [CrossRef]






| Mean Squares | |||||
|---|---|---|---|---|---|
| SV | DF | LA | LAI | PH | RL |
| Blocks | 3 | 281,614.34 | 0.3455 | 4.2232 | 22.9099 |
| Cultivars (A) | 2 | 201,460.8 NS | 0.3008 NS | 3.6618 ** | 12.80083 * |
| Treatments (B) | 3 | 1024,133.1 ** | 2.8318 ** | 0.9285 NS | 5.6335 NS |
| A × B | 6 | 64,204.18 NS | 0.8425 * | 3.5602 ** | 3.4325 NS |
| Addi. Treatment | 2 | 14,252.64 NS | 0.3025 NS | 1.1633 NS | 1.5925 NS |
| Treatments × addi. Treatment | 1 | 1,149,486.1 NS | 0.2470 NS | 2.6250 NS | 9.8820 NS |
| Residue | 42 | 229,949.04 | 0.3547 | 0.6950 | 3.6582 |
| CV (%) | 56.4 | 21.8 | 11.9 | 13.1 | |
| SV | DF | SDM | RDM | ||
| Blocks | 3 | 0.1063 | 0.6461 | ||
| Cultivars (A) | 2 | 3.7731 ** | 0.9058 ** | ||
| Treatments(B) | 3 | 10.6535 ** | 0.9568 ** | ||
| A × B | 6 | 0.6097 ** | 1.2058 ** | ||
| Addi. Treatment | 2 | 0.97 ** | 0.2158 ** | ||
| Treatments × addi. Treatment | 1 | 6.8343 ** | 0.1760 * | ||
| Residue | 42 | 0.0774 | 0.3413 | ||
| CV (%) | 11.9 | 12.1 | |||
| Mean Squares | |||||
|---|---|---|---|---|---|
| SV | DF | NPP | NGP | W100 | YIELD |
| Blocks | 3 | 1.0388 | 2.5333 | 55.0666 | 62,777 |
| Cultivars (A) | 2 | 63.8125 ** | 0.8125 NS | 85.5625 ** | 2,374,793.58 ** |
| Treatments (B) | 3 | 87.5833 ** | 0.2222 NS | 21.3611 ** | 2,536,109.13 ** |
| A × B | 6 | 1.3958 NS | 0.0347 NS | 8.8402 NS | 182,579.44 * |
| Addi. Treatment | 2 | 12.2500 NS | 0.2500 NS | 36.0833 ** | 1,213,846.58 ** |
| Treatments × Addi. treatment | 1 | 54.1500 NS | 0 NS | 40.0166 NS | 434,435.51 ** |
| Residue | 42 | 8.2508 | 0.2714 | 4.79286 | 56,048.44 |
| CV (%) | 33.2 | 17.3 | 7.6 | 22.6 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Reis, D.R.; Teixeira, G.C.S.; Teixeira, I.R.; Silva, G.R.; Ribeiro, B.B.A. Organomineral Fertilization Associated with Inoculation of Rhizobium tropici and Co-Inoculation of Azospirillum brasilense in Common Bean. Sustainability 2023, 15, 16631. https://doi.org/10.3390/su152416631
Reis DR, Teixeira GCS, Teixeira IR, Silva GR, Ribeiro BBA. Organomineral Fertilization Associated with Inoculation of Rhizobium tropici and Co-Inoculation of Azospirillum brasilense in Common Bean. Sustainability. 2023; 15(24):16631. https://doi.org/10.3390/su152416631
Chicago/Turabian StyleReis, Diana Rosa, Gisele Carneiro Silva Teixeira, Itamar Rosa Teixeira, Guilherme Romão Silva, and Brenda Bárbara A. Ribeiro. 2023. "Organomineral Fertilization Associated with Inoculation of Rhizobium tropici and Co-Inoculation of Azospirillum brasilense in Common Bean" Sustainability 15, no. 24: 16631. https://doi.org/10.3390/su152416631
APA StyleReis, D. R., Teixeira, G. C. S., Teixeira, I. R., Silva, G. R., & Ribeiro, B. B. A. (2023). Organomineral Fertilization Associated with Inoculation of Rhizobium tropici and Co-Inoculation of Azospirillum brasilense in Common Bean. Sustainability, 15(24), 16631. https://doi.org/10.3390/su152416631






