A Multipurpose Sustainable Farming System for Tobacco Crops in the Mediterranean Area
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Materials, Experimental Treatments, Crop Management and Samplings
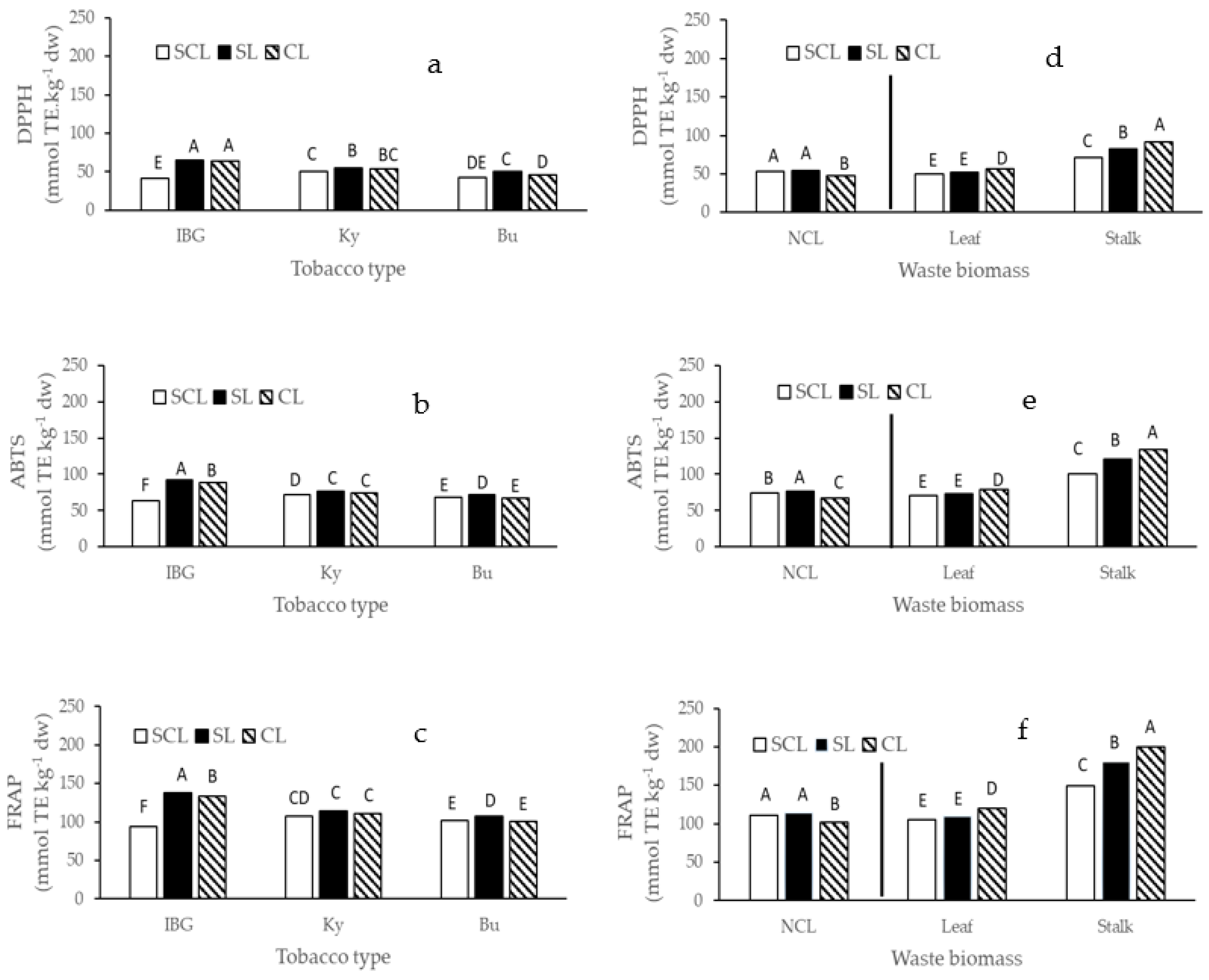
2.2. Polyphenols Determination, Orbitrap High-Resolution Mass Spectrometry Analysis and Antioxidant Activity (ABTS, DPPH and FRAP Assays)
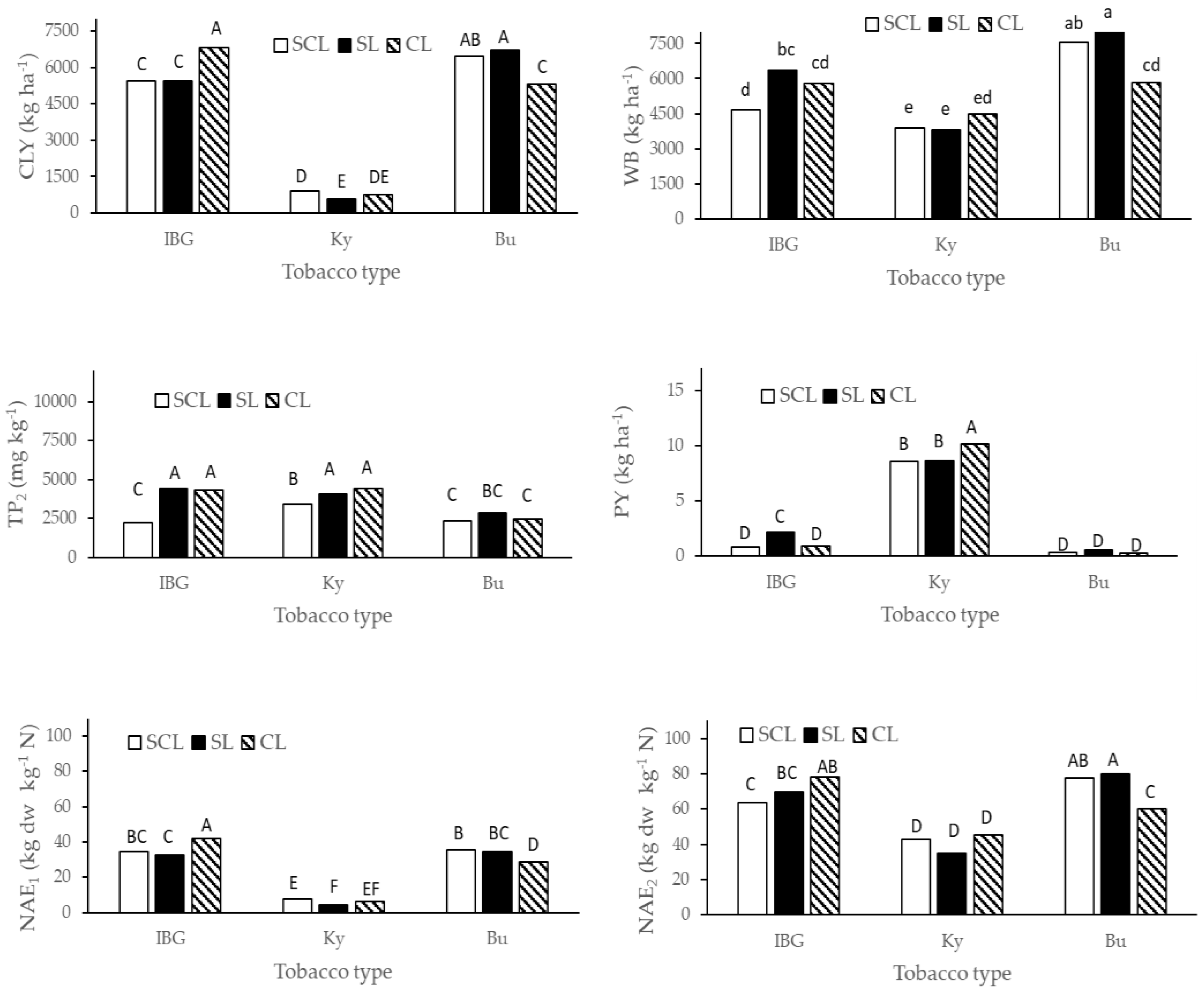
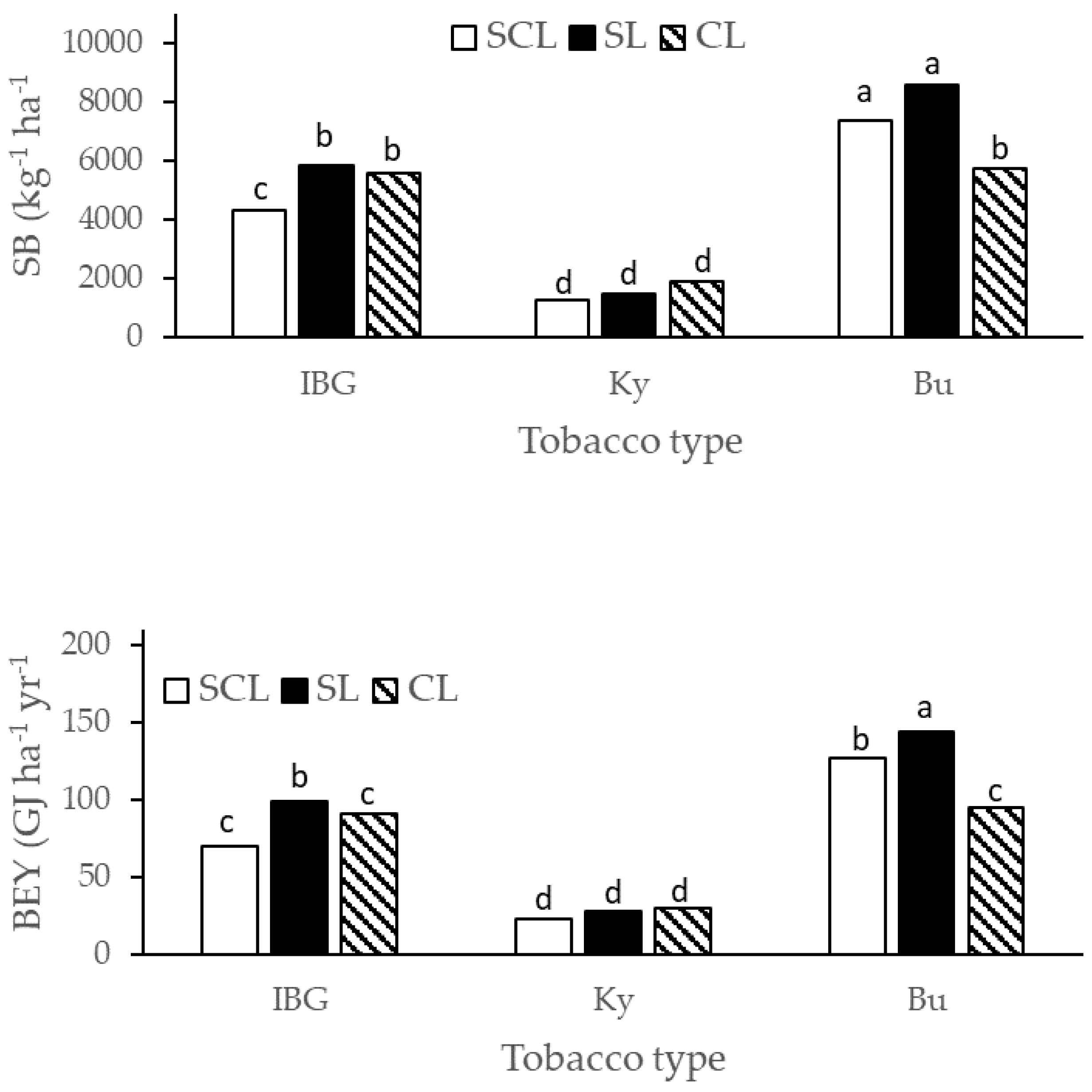
2.3. N Agronomic Efficiency Calculation and Calorific Measurements
NAE2 = (CLY + total WB)/N applied by fertilizer
2.4. Experimental Design and Statistical Analyses
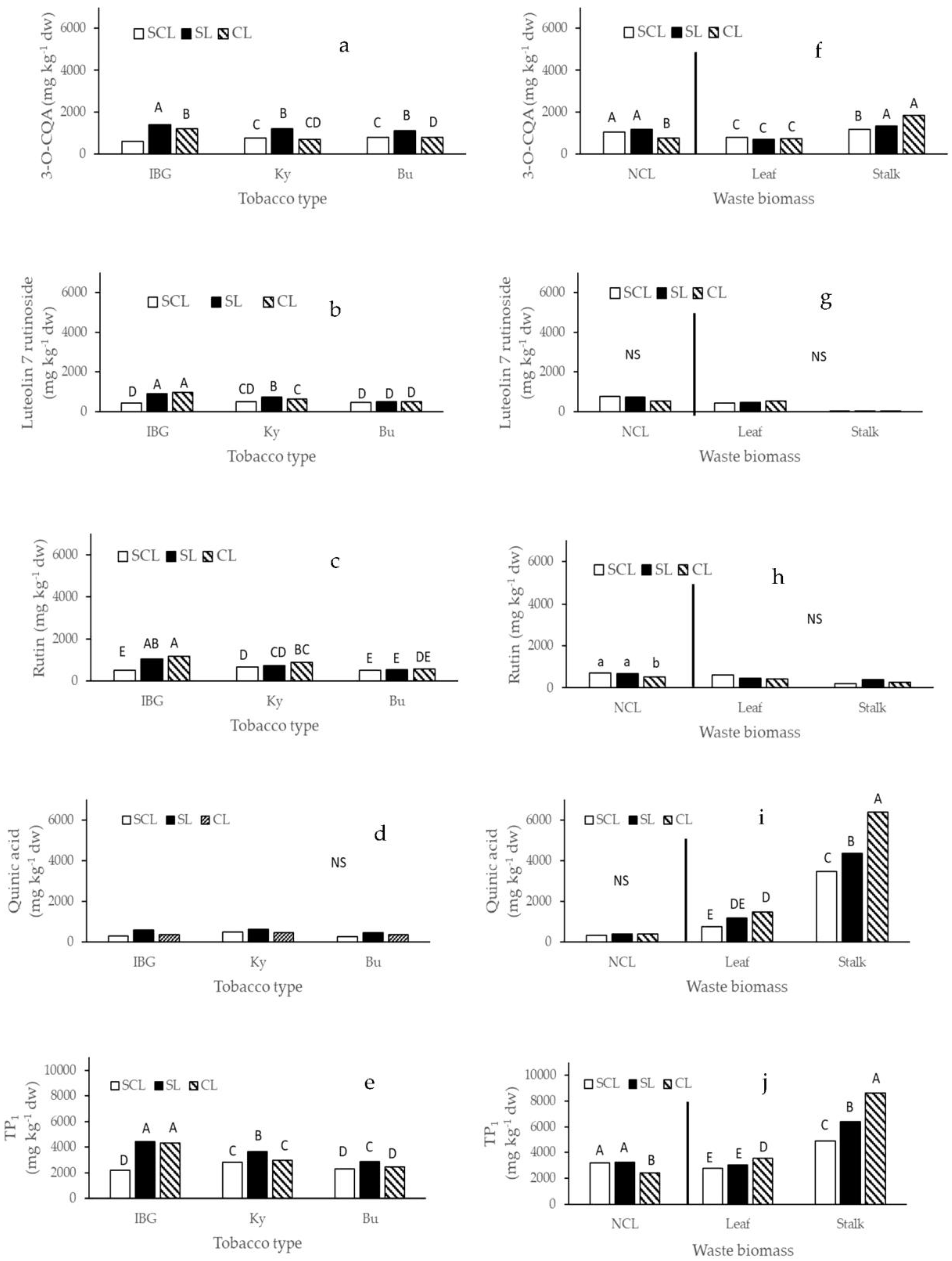
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| Abbreviation | Definition |
| IBG | Ibrid Badischer Geuderhetimer |
| Bu | Burley tobacco |
| Ky | Kentucky tobacco |
| SCL | Sandy Clay Loam soil |
| SL | Sandy Loam soil |
| CL | Clay Loam soil |
| WB | Waste Biomass |
| CLY | Commercial Leaves Yield |
| NAE | Nitrogen Agronomic Efficiency |
| TP | Total Polyphenols |
| PY | Polyphenols Yield |
| CV | Calorific Value |
| VM | Volatile Matter |
| BEY | Biomass Energy Yield |
References
- Renting, H.; Rossing, W.A.H.; Groot, J.C.J.; Van der Ploeg, J.D.; Laurent, C.; Perraud, D.; Stobbelaar, D.J.; Van Ittersum, M.K. Exploring multifunctional agriculture. A review of conceptual approaches and prospects for an integrative transitional framework. J. Environ. Manag. 2009, 90, S112–S123. [Google Scholar] [CrossRef]
- Scaramuzzi, S.; Belletti, G.; Biagioni, P. Integrated Supply Chain Projects and multifunctional local development: The creation of a Perfume Valley in Tuscany. Agric. Food Econ. 2020, 8, 5. [Google Scholar] [CrossRef]
- Dodic, S.N.; Zekic, V.N.; Rodic, V.O.; Tica, N.L.; Dodic, J.M.; Popov, S.D. Situation and perspectives of waste biomass application as energy source in Serbia. Renew. Sustain. Energy Rev. 2010, 14, 3171–3177. [Google Scholar] [CrossRef]
- Motghare, K.A.; Rathod, A.P.; Wasewar, K.L.; Labhsetwar, N.K. Comparative study of different waste biomass for energy application. Waste Manag. 2015, 47, 40–45. [Google Scholar] [CrossRef] [PubMed]
- Espro, C.; Paone, E.; Mauriello, F.; Gotti, R.; Uliassi, E.; Bolognesi, M.L.; Rodrıguez-Padron, D.; Luque, R. Sustainable production of pharmaceutical, nutraceutical and bioactive compounds from biomass and waste. Chem. Soc. Rev. 2021, 50, 11191–11207. [Google Scholar] [CrossRef] [PubMed]
- Sifola, M.I.; Carrino, L.; Cozzolino, E.; del Piano, L.; Graziani, G.; Ritieni, A. Potential of Pre- harvest Wastes of Tobacco (Nicotiana tabacum L.) Crops, Grown for Smoke Products, as Source of Bioactive Compounds (Phenols and Flavonoids). Sustainability 2021, 13, 2087. [Google Scholar] [CrossRef]
- Amalina, F.; Razak, A.S.A.; Krishnan, S.; Sulaiman, H.; Zularisam, A.W.; Nasrullah, M. Biochar production techniques utilizing biomass waste-derived materials and environmental applications: A review. J. Hazard. Mater. 2022, 7, 100134. [Google Scholar] [CrossRef]
- Cárceles Rodríguez, B.; Durán-Zuazo, V.H.; Soriano Rodríguezv, M.; García-Tejero, I.F.; Gálvez Ruiz, B.; Cuadros Tavira, S. Conservation Agriculture as a Sustainable System for Soil Health: A Review. Soil Syst. 2022, 6, 87. [Google Scholar] [CrossRef]
- Spiertz, J.H.J. Nitrogen, sustainable agriculture and food security. A review. Agron. Sustain. Dev. 2010, 30, 43–55. [Google Scholar] [CrossRef]
- German, R.N.; Thompson, C.E.; Benton, T.G. Relationships among multiple aspects of agriculture’s environmental impact and productivity: A meta-analysis to guide sustainable agriculture. Biol. Rev. 2017, 92, 716–738. [Google Scholar] [CrossRef]
- Coppola, A.; Amato, M.; Vistocco, D.; Verneau, F. Measuring the economic sustainability of Italian farms using FADN data. Agric. Econ. 2021, 68, 327–337. [Google Scholar] [CrossRef]
- Tubiello, F.N.; Wanner, N.; Asprooth, L.; Mueller, M.; Ignaciuk, A.; Khan, A.A.; Rosero Moncayo, J. Measuring Progress towards Sustainable Agriculture; FAO Statistics Working Paper 21-24; FAO: Rome, Italy, 2021. [Google Scholar]
- Gomez, A.; Suete Kelly, D.E.; Syers, J.K.; Coughlan, K.J. Measuring Sustainability of Agricultural Systems at the Farm Level. Soil Science Society of America, 677. In Methods for Assessing Soil Quality; SSSA Special Publication 49; S. Segoe Rd.: Madison, WI, USA, 1996. [Google Scholar]
- Valizadeh, N.; Hayati, D. Development and validation of an index to measure agricultural sustainability. J. Clean. Prod. 2021, 280, 123797. [Google Scholar] [CrossRef]
- Young, M.D.; Ros, G.H.; De Vries, W. Impacts of agronomic measures on crop, soil, and environmental indicators: A review and synthesis of meta-analysis. Agric. Ecosyst. Environ. 2021, 319, 107551. [Google Scholar] [CrossRef]
- Egenolf, V.; and Bringezu, S. Conceptualization of an Indicator System for Assessing the Sustainability of the Bioeconomy. Sustainability 2019, 11, 443. [Google Scholar] [CrossRef]
- Yi, I.; Bruelisauer, S.; Utting, P.; McElroy, M.; Mendell, M.; Novkovic, S.; Lee, Z. Authentic Sustainability Assessment: A User Manual for the Sustainable Development Performance Indicators; UNRISD: Geneva, Switzerland, 2022. [Google Scholar]
- Bathaei, A.; Štreimikien, D. A Systematic Review of Agricultural Sustainability Indicators. Agriculture 2023, 13, 241. [Google Scholar] [CrossRef]
- Lebacq, T.; Baret, P.V.; Stilmant, D. Sustainability indicators for livestock farming: A review. Agron. Sustain. Dev. 2013, 33, 311–327. [Google Scholar] [CrossRef]
- Tuğrul, K.M. Soil Management in Sustainable Agriculture. In Sustainable Crop Production; Hasanuzzaman, M., Filho, M.C.M.T., Fujita, M., Nogueira, T.A.R., Eds.; IntechOpen: London, UK, 2019. [Google Scholar]
- Oenema, O.; Brentrup, F.; Lammel, J.; Bascou, P.; Billen, G.; Dobermann, A.; Erisman, J.W.; Garnett, T.; Hammel, M.; Haniotis, T.; et al. EU Nitrogen Expert Panel. Nitrogen Use Efficiency (NAE)—An Indicator for the Utilization of Nitrogen in Agriculture and Food Systems. Wageningen University, Alterra, PO Box 47, NL-6700 Wageningen, Netherlands. 2015. Available online: www.eunep.com (accessed on 1 August 2023).
- Montemurro, F.; Diacono, M. Towards a Better Understanding of Agronomic Efficiency of Nitrogen: Assessment and Improvement Strategies. Agronomy 2016, 6, 31. [Google Scholar] [CrossRef]
- Tian, H.; Lu, C.; Pan, S.; Yang, J.; Miao, R.; Ren, W.; Yu, Q.; Fu, B.; Jin, F.; Lu, Y.; et al. Optimizing resource use efficiencies in the food–energy–water nexus for sustainable agriculture: From conceptual model to decision support system. Curr. Opin. Environ. Sustain. 2018, 33, 104–113. [Google Scholar] [CrossRef]
- Liang, G.; Sun, P.; Waring, B.G. Nitrogen agronomic efficiency under nitrogen fertilization does not change over time in the long term: Evidence from 477 global studies. Soil Tillage Res. 2022, 223, 105468. [Google Scholar] [CrossRef]
- Mohammed, Y.A.; Gesch, R.W.; Johnson, J.M.F.; Wagner, S.W. Agronomic and Economic Evaluations of N Fertilization in Maize under Recent Market Dynamics. Nitrogen 2022, 3, 514–527. [Google Scholar] [CrossRef]
- Sifola, M.I.; Mori, M.; Ceccon, P. Biomass and nitrogen partitioning in sorghum (Sorghum vulgare L.) as affected by nitrogen fertilization. Ital. J. Agron. 1997, 2, 103–109. [Google Scholar]
- Raun, W.R.; Johnson, G.V. Improving nitrogen use efficiency for cereal production. Agron. J. 1999, 91, 357–363. [Google Scholar] [CrossRef]
- Sifola, M.I.; Postiglione, L. The effect of nitrogen fertilization on nitrogen use efficiency of irrigated and non-irrigated tobacco (Nicotiana tabacum L.). Plant Soil 2003, 252, 313–323. [Google Scholar] [CrossRef]
- Yan, X.; Ti, C.; Vitousek, P.; Chen, D.; Leip, A.; Cai, Z.; Zhu, Z. Fertilizer nitrogen recovery efficiencies in crop production systems of China with and without consideration of the residual effect of nitrogen. Environ. Res. Lett. 2014, 9, 095002. [Google Scholar] [CrossRef]
- Zhang, X.; Davidson, E.A.; Mauzerall, D.L.; Searchinger, T.D.; Dumas, P.; Shen, Y. Managing nitrogen for sustainable development. Nature 2015, 528, 51–59. [Google Scholar] [CrossRef] [PubMed]
- Brentrup, F.; Hoxha, A.; Christensen, B. Carbon footprint analysis of mineral fertilizer production in Europe and other world regions. In Proceedings of the 10th International Conference on Life Cycle Assessment of Food (LCA Food 2016), Dublin, Ireland, 19–21 October 2016. [Google Scholar]
- Menegat, S.; Ledo, A.; Tirado, R. Greenhouse gas emissions from global production and use of nitrogen synthetic fertilisers in agriculture. Sci. Rep. 2022, 12, 14490. [Google Scholar] [CrossRef]
- Holka, M.; Kowalska, J.; Jakubowska, M. Reducing Carbon Footprint of Agriculture—Can Organic Farming Help to Mitigate Climate Change? Agriculture 2022, 12, 1383. [Google Scholar] [CrossRef]
- FAOSTAT. 2021. Available online: https://www.fao.org/faostat/en/#data/QCL (accessed on 20 October 2023).
- ISTAT. 2021. Available online: http://dati.istat.it (accessed on 20 October 2023).
- Mipaaf 2019 a. Disciplinare di Produzione del Tabacco Kentucky. Covarelli e Ventura. Available online: https://www.ontitalia.com/wp-content/uploads/2015/08/Disciplinare_di_Produzione_Tabacco_Kentucky.pdf (accessed on 1 August 2023).
- Mipaaf 2019 b. Disciplinare di Produzione del Tabacco Dark Air Cured (DAC). Covarelli e Ventura. Available online: https://www.ontitalia.com/wp-content/uploads/2015/08/Disciplinare_di_Produzione_Tabacco_DAC.pdf (accessed on 1 August 2023).
- Mipaaf 2019 c. Disciplinare di Produzione del Tabacco Burley (Tradizionale e Cimato). Covarelli e Ventura. Available online: https://www.ontitalia.com/wp-content/uploads/2015/08/Disciplinare_di_Produzione_Tabacco_Burley.pdf (accessed on 1 August 2023).
- Sifola, M.I.; Postiglione, L. The effect of increasing NaCl in irrigation water on growth, gas exchange and yield of tobacco Burley type. Field Crops Res. 2002, 74, 81–91. [Google Scholar] [CrossRef]
- Sifola, M.I.; Postiglione, L. The effect of nitrogen fertilization and irrigation on dry matter partitioning, yield and quality of tobacco (Nicotiana tabacum L.) Burley type. Agric. Mediterr. 2002, 132, 33–43. [Google Scholar]
- Sifola, M.I.; Ianuario, S.; Carrino, L.; Cozzolino, E.; Lucibelli, A.; Coppola, A. A survey of N responses of Kentucky tobacco (Nicotiana tabacum L.) yield and quality for cigars manufacture in the Benevento province (Southern Italy). Beitr. Tabakforsch. 2018, 28, 14–29. [Google Scholar] [CrossRef]
- Kumar, K.; Goh, K.M. Crop residues and management practices: Effects on soil quality, soil nitrogen dynamics, crop yield, and nitrogen recovery. Adv. Agron. 2000, 68, 197–319. [Google Scholar] [CrossRef]
- Hirel, B.; Tétu, T.; Lea, P.J.; Dubois, F. Improving Nitrogen Use Efficiency in Crops for Sustainable Agriculture. Sustainability 2011, 3, 1452–1485. [Google Scholar] [CrossRef]
- Kerdraon, L.; Laval, V.; Suffert, F. Microbiomes and Pathogen Survival in Crop Residues, an Ecotone between Plant and Soil. Phytobiomes J. 2019, 3, 246–255. [Google Scholar] [CrossRef]
- Yu, J.; Ahmedna, M.; Bansode, R.R. Agricultural by-products as important food sources of polyphenols. In Polyphenols; Cobb, D.T., Ed.; Nova Science Publishers, Inc.: Hauppauge, NY, USA, 2014; ISBN 978-1-63117-857-3. [Google Scholar]
- Heimler, D.; Romani, A.; Ieri, F. Plant polyphenol content, soil fertilization and agricultural management: A review. Eur. Food Res. Technol. 2017, 243, 1107–1115. [Google Scholar] [CrossRef]
- Fan, D.M.; Zhao, Z.M.; Wang, Y.; Ma, J.H.; Wang, X.C. Crop-type-driven changes in polyphenols regulate soil nutrient availability and soil microbiota. Front. Microbiol. 2022, 13, 964039. [Google Scholar] [CrossRef] [PubMed]
- Manach, C.; Scalbert, A.; Morand, C.; Rémésy, C.; Jiménez, L. Polyphenols: Food sources and bioavailability. Am. J. Clin. Nutr. 2004, 79, 727–747. [Google Scholar] [CrossRef] [PubMed]
- Berbec, A.K.; Matyka, M. Biomass Characteristics and Energy Yields of Tobacco (Nicotiana tabacum L.) Cultivated in Eastern Poland. Agriculture 2020, 10, 551. [Google Scholar] [CrossRef]
- Bareschino, P.; Marrasso, E.; Roselli, C. Tobacco stalks as a sustainable energy source in civil sector: Assessment of techno-economic and environmental potential. Renew. Energy 2021, 175, 373–390. [Google Scholar] [CrossRef]
- Grisan, S.; Polizzotto, R.; Raiola, P.; Cristiani, S.; Ventura, F.; di Lucia, F.; Zuin, M.; Tommasini, S.; Morbidelli, R.; Damiani, F.; et al. Alternative use of tobacco as a sustainable crop for seed oil, biofuel, and biomass. Agron. Sustain. Dev. 2016, 36, 55. [Google Scholar] [CrossRef]
- Samuel, O.D.; Okwu, M.O.; Tartibu, L.K.; Giwa, S.O.; Sharifpur, M.; Jagun, Z.O.O. Modelling of Nicotiana Tabacum L. Oil Biodiesel Production: Comparison of ANN and ANFIS. Front. Energy Res. 2021, 8, 612165. [Google Scholar] [CrossRef]
- Tremblay, R.; Wang, D.; Jevnikar, A.M.; Ma, S. Tobacco, a highly efficient green bioreactor for production of therapeutic proteins. Biotechnol. Adv. 2010, 28, 214–221. [Google Scholar] [CrossRef]
- Wang, H.; Zhao, M.; Yang, B.; Jiang, Y.; Rao, G. Identification of polyphenols in tobacco leaf and their antioxidant and antimicrobial activities. Food Chem. 2008, 107, 1399–1406. [Google Scholar] [CrossRef]
- Docheva, M.; Dagnon, S.; Statkova-Abeghe, S. Flavonoid content and radical scavenging potential of extracts prepared from tobacco cultivars and waste. Nat. Prod. Res. 2014, 28, 1328–1334. [Google Scholar] [CrossRef]
- Shifflett, J.R.; Watson, L.; McNally, D.J.; Bezabeh, D.Z. Analysis of the Polyphenols of Tobacco Using Pressurized Liquid Extraction (PLE) and Ultra Performance Liquid Chromatography With Electrospray Ionization—Tandem Mass Spectometric Detection (UPLC-ESI-MS/MS). Beitr. Tabakforsch. 2017, 27, 195–207. [Google Scholar] [CrossRef]
- Oney-Montalvo, J.; Uc-Varguez, A.; Ramírez-Rivera, E.; Ramírez-Sucre, M.; Rodríguez-Buenfil, I. Influence of Soil Composition on the Profile and Content of Polyphenols in Habanero Peppers (Capsicum chinense Jacq.). Agronomy 2020, 10, 1234. [Google Scholar] [CrossRef]
- Prinsi, B.; Negrini, N.; Morgutti, S.; Espen, L. Nitrogen Starvation and Nitrate or Ammonium Availability differently affect Phenolic Composition in Green and Purple Basil. Agronomy 2020, 10, 498. [Google Scholar] [CrossRef]
- Hawa, Z.E.J.; Mohd, H.I.; Nur, F.M.F. Impact of Soil Field Water Capacity on Secondary Metabolites, Phenylalanine Ammonia-lyase (PAL), Maliondialdehyde (MDA) and Photosynthetic Responses of Malaysian Kacip Fatimah (Labisia pumila Benth). Molecules 2012, 17, 7305–7322. [Google Scholar] [CrossRef]
- Yang, L.; Wen, K.; Ruan, X.; Zhao, Y.; Wei, F.; Wang, O. Response of Plant Secondary Metabolites to Environmental Factors. Molecules 2018, 23, 762. [Google Scholar] [CrossRef] [PubMed]
- Pant, P.; Pandey, S.; Dall’Acqua, S. The Influence of Environmental Conditions on Secondary Metabolites in Medicinal Plants: A Literature Review. Chem. Biodivers. 2021, 18, e21003. [Google Scholar] [CrossRef] [PubMed]
- Bockstaller, C.; Guichard, L.; Makowski, D.; Aveline, A.; Girardin, P.; Plantureux, S. Agri-environmental indicators to assess cropping and farming systems: A review. Agron. Sustain. Dev. 2008, 28, 139–149. [Google Scholar] [CrossRef]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- Brand-Williams, W.; Cuvelier, M.; Berset, C. Use of a free radical method to evaluate antioxidant activity. LWT 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Benzie, I.F.; Strain, J.J. The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: The FRAP assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef]
- Moll, R.H.; Kamprath, E.J.; Jackson, W.A. Analysis and interpretation of factors which contribute to efficiency of nitrogen utilization. Agron. J. 1982, 74, 562–564. [Google Scholar] [CrossRef]
- Sifola, M.I.; Di Mola, I.; Cozzolino, E.; Ottaiano, L.; Piccirillo, G.; del Piano, L.; Mori, M. Yield Response, Quality Traits, and Nitrogen-Use Efficiency of a Burley Tobacco Crop Grown in Mediterranean Areas (Southern Italy) as Affected by Intensive N Management. Agronomy 2021, 11, 1837. [Google Scholar] [CrossRef]
- EN ISO 18134-2; Solid Biofuels—Determination of Moisture Content (Simplified Method). ISO: Geneva, Switzerland, 2015.
- UNI EN ISO 1716; Determination of the Gross Heat of Combustion (Calorific Value). ISO: Geneva, Switzerland, 2010.
- Civitarese, V.; Faugno, S.; Picchio, R.; Assirelli, A.; Sperandio, G.; Saulino, L.; Crimaldi, M.; Sannino, M. Production of selected short-rotation wood crop species and quality of obtained biomass. Eur. J. For. Res. 2018, 137, 541–552. [Google Scholar] [CrossRef]
- EN ISO 18122; Solid Biofuels—Determination of Ash Content. ISO: Geneva, Switzerland, 2016.
- Faugno, S.; Civitarese, V.; Assirelli, A.; Sperandio, G.; Saulino, L.; Crimaldi, M.; Sannino, M. Chip Quality as a Function of Harvesting Methodology. Chem. Eng. Trans. 2017, 58, 271–276. [Google Scholar] [CrossRef]
- Mstat, C. A Microcomputer Program for Design Management and Analysis of Agronomic Research Experiments; Michigan State University: East Lansing, MI, USA, 1991. [Google Scholar]
- Feng, G.Z.; Wang, Y.; Yan, L.; Zhou, X.; Wang, S.J.; Gao, Q.; Mi, G.H.; Yu, H.; Cui, Z.L. Effects of nitrogen and three soil types on maize (Zea mays L.) grain yield in Northeast China. Appl. Ecol. Environ. Res. 2019, 17, 4229–4243. [Google Scholar] [CrossRef]
- Ouansafi, S.; Abdelilah, F.; Kabine, M.; Maaghloud, H.; Bellali, F.; El Bouqdaoui, K. The effects of soil proprieties on the yield and the growth of tomato plants and fruits irrigated by treated wastewater. AIMS Agric. Food 2019, 4, 921–938. [Google Scholar] [CrossRef]
- Yim, B.; Ibrahim, Z.; Rüger, L.; Ganther, M.; Maccario, L.; Sørensen, S.J.; Heintz-Buschart, A.; Tarkka, M.T.; Vetterlein, D.; Bonkowski, M.; et al. Soil texture is a stronger driver of the maize rhizosphere microbiome and extracellular enzyme activities than soil depth or the presence of root hairs. Plant Soil 2022, 478, 229–251. [Google Scholar] [CrossRef]
- Darch, T.; Blackwell, M.S.A.; Hood, J.; Lee, M.R.F.; Storkey, J.; Beaumont, D.A.; McGrath, S.P. The effect of soil type on yield and micronutrient content of pasture species. PLoS ONE 2022, 17, e0277091. [Google Scholar] [CrossRef] [PubMed]
- Gessesew, W.S.; Elias, E.; Gebresamuel, G.; Tefera, W. Soil type and fertilizer rate affect wheat (Triticum aestivum L.) yield, quality and nutrient use efficiency in Ayiba, northern Ethiopia. PeerJ 2022, 10, e13344. [Google Scholar] [CrossRef]
- Wang, L.; He, Z.; Zhao, W.; Wang, C.; Ma, D. Fine Soil Texture Is Conducive to Crop Productivity and Nitrogen Retention in Irrigated Cropland in a Desert-Oasis Ecotone, Northwest China. Agronomy 2022, 12, 1509. [Google Scholar] [CrossRef]
- Moldoveanu, S.C.; Davis, M.F. Analysis of Quinic Acid and of myo-Inositol in Tobacco. Beitr. Tabakforsch. 2012, 25, 499–506. [Google Scholar] [CrossRef]
- Hepperly, P.R.; Omondi, E.; Seidel, R. Soil regeneration increases crop nutrients, antioxidants and adaptive responses. MOJ Food Process. Technol. 2018, 6, 196–203. [Google Scholar] [CrossRef]
- Zargoosh, Z.; Ghavam, M.; Bacchetta, G.; Tavili, A. Effects of ecological factors on the antioxidant potential and total phenol content of Scrophularia striata Boiss. Sci. Rep. 2019, 9, 16021. [Google Scholar] [CrossRef]
- Benard, C.; Gautier, H.; Bourgaud, F.; Grasselly, D.; Navez, B.; Caris-Beyrat, C.; Weiss, M.; Genard, M. Effects of low nitrogen supply on tomato (Solanum lycopersicum) fruit yield and quality with special emphasis on sugars, acids, ascorbate, carotenoids, and phenolic compounds. J. Agric. Food Chem. 2009, 57, 4112–4123. [Google Scholar] [CrossRef]
- Sałata, A.; Lombardo, S.; Pandino, G.; Mauromicale, G.; Buczkowska, H.; Nurzy´nska-Wierdak, R. Biomass yield and polyphenol compounds profile in globe artichoke as affected by irrigation frequency and drying temperature. Ind. Crops Prod. 2022, 176, 114375. [Google Scholar] [CrossRef]
- McKendry, P. Energy production from biomass (part 1): Overview of biomass. Bioresour. Technol. 2002, 83, 37–46. [Google Scholar] [CrossRef]
| SCL | SL | CL | |
|---|---|---|---|
| Sand | 49.5 | 70.5 | 36.0 |
| Clay | 25.5 | 6.5 | 39.5 |
| Silt | 25.0 | 23.0 | 24.5 |
| N (Kjeldhal, %) | 0.106 | 0.074 | 0.080 |
| NO3-N (mg kg−1) | 14.81 | 62.37 | 17.42 |
| NH4-N (mg kg−1) | 11.84 | 13.37 | 8.17 |
| OM (%) | 1.71 | 1.61 | 0.95 |
| OC (%) | 0.99 | 0.94 | 0.55 |
| C/N | 9.4 | 12.7 | 6.9 |
| EC1:5 (dS m−1) | 0.153 | 0.106 | 0.148 |
| pH | 7.56 | 7.83 | 7.67 |
| FC (%) | 31 | 18 | 38 |
| WP (%) | 18 | 9 | 24 |
| SCL | SL | CL | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Variables | IBG | Ky | Bu | IBG | Ky | Bu | IBG | Ky | Bu |
| NAE1 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 |
| NAE2 | 1.89 | 5.46 | 2.17 | 2.16 | 8.30 | 2.33 | 1.85 | 7.06 | 2.11 |
| Source of Variation | SB | MC | CV | BEY | VM | Ash |
|---|---|---|---|---|---|---|
| Soil (S) | ||||||
| SCL | 4325.2 b | 84.2 | 17.1 | 74.2 | 94.6 b | 5.4 a |
| SL | 5315.1 a | 83.5 | 17.4 | 90.6 | 96.0 a | 4.0 b |
| CL | 4397.0 b | 82.3 | 16.2 | 72.0 | 96.3 a | 3.7 b |
| Tobacco type (T) | ||||||
| IBG | 5251.8 B | 84.7 A | 16.6 | 86.6 B | 94.9 b | 5.1 a |
| Ky | 1551.1 C | 81.6 B | 17.3 | 29.9 C | 96.6 a | 3.4 b |
| Bu | 7234.2 A | 83.6 A | 16.9 | 122.3 A | 95.3 b | 4.7 a |
| ANOVA | ||||||
| S | * | NS | NS | NS | * | * |
| T | ** | ** | NS | ** | * | * |
| S × T | * | NS | NS | * | NS | NS |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sifola, M.I.; del Piano, L.; Todisco, D.; Graziani, G.; Faugno, S.; Sannino, M.; Piscopo, R.; Salluzzo, A.; Cozzolino, E. A Multipurpose Sustainable Farming System for Tobacco Crops in the Mediterranean Area. Sustainability 2023, 15, 16636. https://doi.org/10.3390/su152416636
Sifola MI, del Piano L, Todisco D, Graziani G, Faugno S, Sannino M, Piscopo R, Salluzzo A, Cozzolino E. A Multipurpose Sustainable Farming System for Tobacco Crops in the Mediterranean Area. Sustainability. 2023; 15(24):16636. https://doi.org/10.3390/su152416636
Chicago/Turabian StyleSifola, Maria Isabella, Luisa del Piano, Daniele Todisco, Giulia Graziani, Salvatore Faugno, Maura Sannino, Rossella Piscopo, Antonio Salluzzo, and Eugenio Cozzolino. 2023. "A Multipurpose Sustainable Farming System for Tobacco Crops in the Mediterranean Area" Sustainability 15, no. 24: 16636. https://doi.org/10.3390/su152416636
APA StyleSifola, M. I., del Piano, L., Todisco, D., Graziani, G., Faugno, S., Sannino, M., Piscopo, R., Salluzzo, A., & Cozzolino, E. (2023). A Multipurpose Sustainable Farming System for Tobacco Crops in the Mediterranean Area. Sustainability, 15(24), 16636. https://doi.org/10.3390/su152416636








